After allogeneic hematopoietic stem cell transplantation (HSCT), mature transplanted T cells play a major role in restoration of the immune system. However, they can also induce a life-threatening complication: graft-versus-host disease (GVHD). Suicide gene therapy of GVHD aims to selectively eliminate alloreactive T cells mediating GVHD while sparing nonalloreactive T cells that should contribute to immune reconstitution. It was demonstrated previously that treatment with ganciclovir (GCV) can control GVHD in mice by killing donor T cells engineered to express the thymidine kinase (TK) suicide gene. TK allows phosphorylation of nontoxic GCV into triphosphate GCV, which is selectively toxic for dividing cells. Thus, in the TK-GCV system, the specificity of cell killing depends on the cycling status of TK T cells rather than allogeneic recognition. This is a potential drawback because in recipients of lymphopenic allogeneic HSCT, alloreactive and homeostatic signals drive the proliferation of donor T cells. It is shown here that the onset of alloreactive T-cell division occurs earlier than that of nonalloreactive T cells, thus establishing a time frame for GCV administration. A 7-day GCV treatment initiated at the time of HSCT allowed efficient prevention of GVHD, while sparing a pool of nondividing donor TK T cells. These cells later expanded and contributed to the replenishment of the recipient immune system with a diversified T-cell receptor repertoire. These results provide a rationale for designing the therapeutic scheme when using TK-GCV suicide gene therapy in allogeneic HSCT.
Introduction
The prognosis for a patient who undergoes allogeneic hematopoietic stem cell transplantation (HSCT) is influenced by the presence in the recipient of postthymic peripheral donor T cells that improve engraftment1 and T-cell reconstitution2 and that provide a graft-versus-leukemia (GVL) effect.3 In adult patients, such T cells mainly arise from the persistence and expansion of infused donor T cells.2,4 Indeed, the differentiation of donor HSCs in the adult recipient thymus is inefficient, as illustrated by the observation that adults grafted with T-cell–depleted (TCD) bone marrow (BM) often remain immunodeficient for several months after transplantation.4 In addition to the beneficial effects of infused donor T cells, activation of those specific for recipient alloantigens often results in a life-threatening complication: graft-versus-host disease (GVHD).5 Thus, a major challenge of allogeneic HSCT is to achieve good T-cell reconstitution without GVHD.
A strategy for genetic immunosuppression based on conditional elimination of dividing donor T cells expressing the herpes simplex type 1 thymidine kinase (TK) suicide gene was recently developed (for review, see Cohen et al).6TKallows phosphorylation of the nontoxic prodrug ganciclovir (GCV) into triphosphate GCV, which, by blocking DNA elongation, is toxic for dividing cells. Hence, on activation and division, TK T cells become sensitive to GCV.7,8 We previously validated this TK-GCV genetic immunosuppression approach in a murine model of GVHD. After allogeneic bone marrow transplantation (BMT) supplemented with TK T cells, animals survived without developing GVHD when GCV was administered from day 0 to day 6 after transplantation.9 10
The ultimate aim of this strategy is to selectively eliminate in vivo the T cells mediating GVHD while sparing T cells that contribute to immune reconstitution and GVL effect. Because in the TK-GCV system the specificity of cell killing depends on the cycling status of donorTK T cells but not directly on allogeneic recognition, this selective elimination should be achievable if only or mostly alloreactive T cells expand rapidly after HSCT in response to recipient alloantigens. However, when infused in a lymphopenic host, homeostatic regulation of T cell numbers drives donor T cells to undergo cell division on recognition of self-ligands.11 12Thus, after allogeneic HSCT in lymphopenic recipients, both alloantigen driven T-cell expansion of host-reactive donor T cells and homeostatic expansion of all T cells should occur.
Here we assessed the kinetics of T-cell expansion after semiallogeneic and syngeneic BMT and analyzed the origin of T-cell reconstitution after controlling GVHD with GCV. We show that, indeed, T cells of donor origin that have been spared by GCV contribute to the replenishment of the T-cell compartment and provide a diversified T-cell receptor (TCR) repertoire.
Materials and methods
Mice
FVB/N (FVB, H-2q), C57BL/6 (B6, H-2b) and [B6 × FVB]F1 (H-2qxb) mice were obtained from Iffa Credo (L'Arbresle, France). Double-transgenic [hCD4 × TK] mice were obtained by breeding the previously described line 40TK expressing the TK product in both mouse (m) CD4 and mCD8 T cells and line 38 human (h) CD4 transgenic mice expressing the hCD4 protein at the surfaces of mCD4 cells.8 13 Transgenic mice were bred in the animal facility of the Faculté de Médecine PitiéSalpêtrière (Paris, France). Mice were manipulated according to EEC guidelines.
Experimental GVHD
Experiments were performed as described14 unless otherwise stated. Briefly, hybrid F1[B6 × FVB] or FVB euthymic or thymectomized females (8-12 weeks of age) were lethally irradiated (11 Gy). TCD BM cells (1.0 × 107) from FVB mice plus 1.0 × 107 T cells from [hCD4 × TK] transgenic FVB mice were injected intravenously with 50 mg/kg per day GCV (Roche, Neuilly-sur-Seine, France) using 2001 Alzet mini-osmotic pumps (Alza, Palo Alto, CA). These pumps were implanted subcutaneously at the time of BMT under tribromoethanol (Avertin) anesthesia and delivered 1 ± 0.02 μL/h GCV. For shorter treatments, GCV was administered at the same dosage by intraperitoneal injections twice daily. This provides an experimental model of lethal GVHD that is efficiently controlled by early GCV administration.15
Immunocytofluorometry
Splenocytes from grafted animals were digested with collagenase and incubated with 2.4.G2 anti–Fc receptor monoclonal antibody. Cells were stained with combinations of the following monoclonal antibodies: phycoerythrin-labeled anti-CD4 (clone RM4-5; Pharmingen, San Diego, CA); quantum red-labeled anti-hCD4 (clone Q4120; Sigma, St Louis, MO); fluorescein isothiocyanate (FITC)-labeled anti-CD8 (clone CT-CD8a; Caltag Laboratories, San Francisco, CA); FITC-labeled anti-CD44 (clone IM7), anti-CD25 (clone 7D4), anti-CD62L (clone MEL-14), and anti-CD69 (clone H1.2 F3) (Pharmingen) or anti-CD45RB (clone 16A) (Cedarlane, Hornby, Ontario, Canada); and biotinylated anti-Vβ2 (clone B20.6), anti-Vβ3 (clone KJ25), anti-Vβ4 (clone CTVB4), anti-Vβ6 (clone RR4-7), anti-Vβ7 (clone TR310), anti-Vβ14 (clone 14.2) (Caltag), or anti-Vβ17a (clone KJ23) (Pharmingen). Biotinylated monoclonal antibodies were revealed with tricolor-labeled streptavidin (Caltag). Events were acquired on a FACScalibur (Becton Dickinson, San Jose, CA) and analyzed using CellQuest software (Becton Dickinson).
Assessment of donor T-cell proliferation
Splenocytes and lymph node cells from donors were stained with the 5- (and 6-) carboxyfluorescein diacetate succinimide ester (CFSE). Cells (1 × 107/mL) were incubated during 10 minutes at 37°C, 5% CO2, in a medium (RPMI 1640) containing CFSE at a concentration of 1.5 μM. Staining was stopped by the addition of fetal calf serum to reach a concentration of 20% of the total volume. Labeled cells were washed twice in phosphate-buffered saline, numbered, and injected intravenously in lethally irradiated mice. Splenocytes from grafted animals were collected at different times after BMT. Cell proliferation was studied as the sequential loss of CFSE fluorescence on cell division after FACS analysis of the hCD4 population.
T-cell receptor CDR3 length distribution
Experiments were performed as described.16,17Briefly, RNA was reverse transcribed, and a quantity of cDNA corresponding to 625 ng total RNA was amplified by polymerase chain reaction using a Vβ family-specific primer (same as in16 17 except for Vβ3-GCAAAGATGAGGTGTATCCCTG) and a Cβ-GCCCATGGAACTGCACTTGGC primer (Genset, Paris, France). Each Vβ-Cβ polymerase chain reaction product was then subjected to 10 run-off cycles primed with a ROX-labeled internal Cβ-GCCCATGGAACTGCACTTGGC primer (Genset). Each run-off product was denatured and loaded on a sequencing gel for fluorescence analysis on an ABI377 DNA sequencer (Perkin Elmer, Norwalk, CT). Data were analyzed with Immunoscope 3.01b software (Loginserm, Paris, France).
Statistical analyses
Statview software (Abacus Concepts, Berkeley, CA) was used for statistical analysis. Mann-Whitney U tests were used to evaluate T-cell reconstitution. P values are indicated only when differences between 2 groups were statistically significant.
Results
Experimental model
To identify the origin of T-cell reconstitution after BMT—ie, central thymic production versus peripheral expansion of mature donor T cells—we generated double-transgenic mice coexpressing the TK enzyme in CD4+ and CD8+ T cells18 and an hCD4 marker molecule at the surface of CD4+ T cells.13 T cells from [hCD4 × TK] double-transgenic mice (FVB, H-2q) were infused together with TCD BM cells from wild-type mice (FVB) in lethally irradiated syngeneic or allogeneic recipients. Tracking the hCD4 surface marker rather than the intracellular TK molecule, which is difficult to detect by available methods, readily allowed monitoring of infused mature donor CD4+ T cells independently of newly produced thymus-derived hCD4− T cells originating from nontransgenic HSCs. This system also permitted identification of infused donor T cells versus host T cells surviving after irradiation.
Early cell division of donor T cells after infusion in semiallogeneic lethally irradiated recipients
We first analyzed the kinetics of donor T-cell division after semiallogeneic BMT. For this, T cells from [hCD4 × TK] double-transgenic FVB mice were stained with CFSE and infused together with BM cells from wild-type FVB mice in lethally irradiated semiallogeneic recipients ([B6 × FVB], H-2bxq). Spleen cells from mice that received transplants were collected at different time points after BMT, and donor T-cell division was assessed by a CFSE fluorescence intensity decrease within the hCD4+population. In the absence of GCV, the first cell divisions for hCD4+ T cells occurred between hour 40 and hour 64 after grafting. At hour 88, almost all hCD4+ splenocytes had divided at least once (Figure 1A). When GCV was administered in this setting, most hCD4+ T cells were killed (see below), and the survivors were mostly cells that had not divided, indicating that dividing cells had been killed by GCV during this period (Figure 1A).
Early kinetics of division and donor T-cell expansion after
TK T-cell infusion in lethally irradiated hosts.[B6 × FVB]F1 (semiallogeneic host)-irradiated or FVB (syngeneic host)-irradiated euthymic mice received 1 × 107 FVB bone marrow cells supplemented with 1 × 107 CFSE-labeled mature T cells from [hCD4 × TK] double-transgenic FVB mice. In treated groups, GCV was administered from hour 0 to hour 88 by intraperitoneal injection twice daily. (A) CFSE intensity of hCD4+ splenocytes was analyzed at different time points after grafting. Each histogram is representative of 3 mice. The peak of highest intensity on the log scale identifies parent generation of infused donor T cells. Peaks with decreased CFSE intensity represent daughter generations that have undergone division. (B) The number of donor T cells in spleens of grafted animals (n = 3) was evaluated at different time points by their expression of the hCD4 marker. Coefficient of variation of triplicates was less than 5%. ●, GCV; ○, no GCV.
Early kinetics of division and donor T-cell expansion after
TK T-cell infusion in lethally irradiated hosts.[B6 × FVB]F1 (semiallogeneic host)-irradiated or FVB (syngeneic host)-irradiated euthymic mice received 1 × 107 FVB bone marrow cells supplemented with 1 × 107 CFSE-labeled mature T cells from [hCD4 × TK] double-transgenic FVB mice. In treated groups, GCV was administered from hour 0 to hour 88 by intraperitoneal injection twice daily. (A) CFSE intensity of hCD4+ splenocytes was analyzed at different time points after grafting. Each histogram is representative of 3 mice. The peak of highest intensity on the log scale identifies parent generation of infused donor T cells. Peaks with decreased CFSE intensity represent daughter generations that have undergone division. (B) The number of donor T cells in spleens of grafted animals (n = 3) was evaluated at different time points by their expression of the hCD4 marker. Coefficient of variation of triplicates was less than 5%. ●, GCV; ○, no GCV.
When similar experiments were performed in a lethally irradiated syngeneic host, the kinetics of CFSE-labeled T-cell division was delayed. When T-cell division began (hour 40) in a semiallogeneic host, no cell division could be detected (Figure 1A). At hour 88, when most T cells had already divided several times in semiallogeneic recipients, only a small fraction of hCD4+ cells had divided once in syngeneic hosts. As observed after semiallogeneic BMT, the small population of T cells that divided once could no longer be detected at hour 88 when GCV was administered in vivo (Figure 1A).
We next evaluated the expansion of donor T cells by counting absolute numbers of hCD4+ splenocytes in grafted mice. After semiallogeneic BMT, a strong T-cell expansion was observed between hour 40 and hour 88 (Figure 1B, P < .05) in accordance with CFSE experiments. When approximately 6 × 106hCD4+ cells were injected intravenously at the time of grafting, 12 × 106 hCD4+ cells were readily detected in the spleen at hour 88. Under GCV at hour 88, the number of hCD4+ cells was significantly decreased (1.3 × 106 cells) compared with untreated mice (P < .05), confirming an efficient elimination of proliferating T cells by the TK-GCV system. In contrast, when T cells were infused in syngeneic recipients, no differences between GCV-treated or untreated groups were observed (Figure 1B). This suggests that the modest rise in the absolute number of hCD4+ splenocytes observed at hour 88 was attributed to the homing of injected T cells to the spleen rather than to T-cell expansion. Together, these results demonstrate that, when injected in lethally irradiated mice, T cells proliferate more rapidly in semiallogeneic hosts than in syngeneic hosts.
Persistence and expansion of donor T cells after GCV-mediated control of GVHD
We next analyzed the persistence and expansion of donor T cells at later time points after GCV cessation. In the semiallogeneic BMT setting, mice that had not received GCV had dramatically reduced numbers of hCD4+ cells at day 7 (1.7 × 106cells, Figure 2) than at hour 88 (12 × 106 cells, Figure 1B, P < .02). At day 14, hCD4+ numbers were comparable to those observed at day 7, reflecting the absence of T-cell expansion that accompanies GVHD.19 When recipients were treated with GCV, an average of 2.4 × 106 hCD4+ splenocytes was found at day 7, representing 94% of the mCD4+ compartment (Figure2). This indicated that a pool of donor T cells had persisted in the recipient despite the 7-day GCV course. In the 7-day period after the cessation of GCV, hCD4+ splenic T cells had increased 5-fold (P < .002), revealing an important expansion of the remaining donor T cells. These cells still represented 81% of the mCD4+ compartment. Similar augmentations were observed in the mCD8+ compartment (Figure 2). These results demonstrate not only that a fraction of infused T cells persists despite GCV but also that these cells significantly expand after the cessation of GCV.
Persistence and expansion of donor T cells after GCV-mediated control of GVHD.
Lethally irradiated euthymic [B6 × FVB]F1 (semiallogeneic) or FVB (syngeneic) mice received 1 × 107 FVB BM cells supplemented with 1 × 107 mature T cells from [hCD4 × TK] double-transgenic mice. GCV was administered from day 0 to day 6 (●), whereas controls did not receive GCV (○). At day 7 and day 14 after grafting, splenocytes from grafted animals were collected. CD4 T cells originating from infused TK T cells are unequivocally identified as hCD4+ mCD4+cells (gated on lymphocytes by size criteria). The lymphoid compartment was analyzed in the spleen at day 7 (end of the GCV regimen) or at day 14. Results are given as mean ± SEM of total cell numbers after gating on the appropriate subsets (semiallogeneic/GCV, day 7, n = 9; semiallogeneic/GCV, day 14, n = 8; semiallogeneic/no GCV, day 7, n = 7; semiallogeneic/no GCV, day 14, n = 4; syngeneic/GCV, days 7-14, n = 4; syngeneic/no GCV, days 7-14, n = 5).
Persistence and expansion of donor T cells after GCV-mediated control of GVHD.
Lethally irradiated euthymic [B6 × FVB]F1 (semiallogeneic) or FVB (syngeneic) mice received 1 × 107 FVB BM cells supplemented with 1 × 107 mature T cells from [hCD4 × TK] double-transgenic mice. GCV was administered from day 0 to day 6 (●), whereas controls did not receive GCV (○). At day 7 and day 14 after grafting, splenocytes from grafted animals were collected. CD4 T cells originating from infused TK T cells are unequivocally identified as hCD4+ mCD4+cells (gated on lymphocytes by size criteria). The lymphoid compartment was analyzed in the spleen at day 7 (end of the GCV regimen) or at day 14. Results are given as mean ± SEM of total cell numbers after gating on the appropriate subsets (semiallogeneic/GCV, day 7, n = 9; semiallogeneic/GCV, day 14, n = 8; semiallogeneic/no GCV, day 7, n = 7; semiallogeneic/no GCV, day 14, n = 4; syngeneic/GCV, days 7-14, n = 4; syngeneic/no GCV, days 7-14, n = 5).
In the syngeneic BMT setting, the number of hCD4+ splenic T cells increased from 1.1 × 106 cells at day 7 to 3.6 × 106 cells at day 14 in mice that had not been given GCV (P < .01). At these time points, the hCD4+ compartment only represented 49% and 60%, respectively, of the mCD4+ subset. In GCV-treated mice, the number of hCD4+ cells also increased after GCV cessation, between day 7 and day 14 (P < .05). Nevertheless, compared with what was observed in semiallogeneic BMT, these numbers were significantly lower than those of nontreated mice (Figure 2,P < .02). Furthermore, 84% and 71% of mCD4+T cells were hCD4− at day 7 and day 14, respectively. The mCD8+ subset also decreased in GCV-treated mice compared with untreated mice (Figure 2, P < .02). These results, together with CFSE-staining data (Figure 1), indicate that a large fraction of infused donor T cells divides between day 3 and day 7 after syngeneic BMT. These cells are thus killed if GCV is administered, impairing further donor T-cell reconstitution (Figure 2).
Analysis of donor-type T-cell reconstitution after control of GVHD
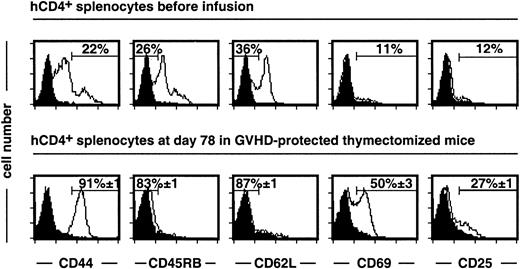
The above experiments were performed in euthymic mice and suggest rapid differentiation of HSCs in the recipient thymus. Indeed, at day 7 after a syngeneic graft and in the absence of GCV treatment, hCD4− cells represented half the recipient mCD4+ T cells. It is possible that rapid thymus-dependent T-cell differentiation, which is impaired in adult humans, could interfere with the expansion of infused mature T cells. We thus next analyzed the quality of long-term peripheral T-cell reconstitution in the absence of central production using thymectomized, irradiated recipients. At day 78 after semiallogeneic BMT, the hCD4+compartment of GCV-treated mice represented 84% of the mCD4+ subset, indicating donor-type T-cell reconstitution. Although most infused hCD4+ T cells had a naive CD4410/CD45RB+/CD62L+ phenotype, they acquired a CD44+/CD45RB−/CD62L− memory phenotype (Figure 3). In addition, 50% and 27% of these hCD4+ T cells still expressed the activation markers CD69 and CD25, respectively.
After semiallogeneic BMT, donor T cells display a memory-activated phenotype in GCV-treated thymectomized recipients.
Experiments were performed on splenocytes recovered at day 78 after BMT and GCV administration. 84% ± 1% of mCD4+ cells express the hCD4 marker. Memory-activation markers were analyzed on mCD4+ hCD4+-gated T cells from grafted mice (n = 9) and compared to a pool of donor T cells before infusion (n = 3). Isotype-matched negative control is represented by a black histogram. For the semiallogeneic BMT group, cell frequencies are given as mean ± SEM.
After semiallogeneic BMT, donor T cells display a memory-activated phenotype in GCV-treated thymectomized recipients.
Experiments were performed on splenocytes recovered at day 78 after BMT and GCV administration. 84% ± 1% of mCD4+ cells express the hCD4 marker. Memory-activation markers were analyzed on mCD4+ hCD4+-gated T cells from grafted mice (n = 9) and compared to a pool of donor T cells before infusion (n = 3). Isotype-matched negative control is represented by a black histogram. For the semiallogeneic BMT group, cell frequencies are given as mean ± SEM.
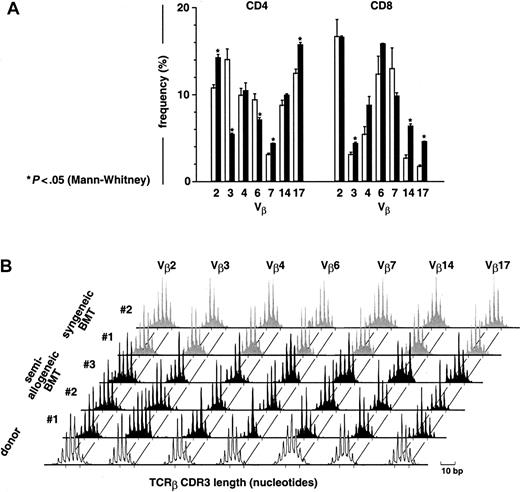
To evaluate T-cell diversity in the latter mice, TCR Vβ usage was assessed by flow cytometry. At day 78 after semiallogeneic BMT in GCV-treated mice, Vβ usage was significantly different in 5 of 7 Vβ families compared with donor T cells before infusion for the mCD4+ subset and in 3 of 7 Vβ families for the mCD8+ subset (Figure 4A). Nevertheless, all the Vβ families tested were represented in both mCD4+ and mCD8+ subsets (Figure 4A), and immunoscope analysis showed a broadly diversified distribution of TCRβ CDR3 transcript lengths (Figure 4B), confirming the polyclonality of the T-cell repertoire. However, individual peaks occasionally accumulated in some Vβ, suggesting preferential expansion of discrete T-cell specificities. After syngeneic BMT in thymectomized GCV-treated recipients, among the mCD4+subset 70% were hCD4+ and 50% were hCD4+CD44+ (not shown). Flow cytometric Vβ usage analysis proved polyclonality of the mCD4+ subset (not shown), whereas immunoscope analysis revealed mostly Gaussian-like TCRβ CDR3 length distribution (Figure 4B).
At day 78 after semiallogeneic BMT, donor T cells display a diversified repertoire in GCV-treated thymectomized recipients.
(A) At day 78, Vβ usage was determined by flow cytometry in mCD4+- and mCD8+-gated subsets using a series of 7 monoclonal antibodies covering approximately two thirds of the T-cell repertoire. Results are given as mean ± SEM for both the semiallogeneic BMT group (▪, n = 9) and a series of donor T cells before infusion (■, n = 5). (B) Diversity of the T-cell repertoire was determined in the spleen by analyzing the distribution of TCRβ CDR3 transcript lengths (immunoscope method16 17). Peaks were separated by a 3-nucleotide length, corresponding to in-frame transcripts. Representative data are shown for 3 of 7 thymectomized mice from the semiallogeneic BMT group treated by GCV (day 78, black), compared to the Gaussian-like distribution of donor T cells before infusion (white) and to a syngeneic BMT group of thymectomized mice treated by GCV (day 90, gray).
At day 78 after semiallogeneic BMT, donor T cells display a diversified repertoire in GCV-treated thymectomized recipients.
(A) At day 78, Vβ usage was determined by flow cytometry in mCD4+- and mCD8+-gated subsets using a series of 7 monoclonal antibodies covering approximately two thirds of the T-cell repertoire. Results are given as mean ± SEM for both the semiallogeneic BMT group (▪, n = 9) and a series of donor T cells before infusion (■, n = 5). (B) Diversity of the T-cell repertoire was determined in the spleen by analyzing the distribution of TCRβ CDR3 transcript lengths (immunoscope method16 17). Peaks were separated by a 3-nucleotide length, corresponding to in-frame transcripts. Representative data are shown for 3 of 7 thymectomized mice from the semiallogeneic BMT group treated by GCV (day 78, black), compared to the Gaussian-like distribution of donor T cells before infusion (white) and to a syngeneic BMT group of thymectomized mice treated by GCV (day 90, gray).
Discussion
Genetic immunosuppression for treating GVHD is aimed at selectively eliminating donor-dividing T cells responsible for GVHD while sparing nonalloreactive donor T cells. The latter pool of T cells would consequently persist at the end of the GCV treatment and should contribute to the reconstitution of the recipient's immune system. We and others previously demonstrated the efficiency of this approach to control GVHD in several genetic combinations of donor and recipient mice after BMT supplemented with TK T cells.8-10,20,21 In agreement, preliminary results of clinical trials using this strategy have demonstrated the possibility of controlling ongoing GVHD by GCV administration in recipients of donor TK T cells.22 23
It is also possible to efficiently prevent GVHD by depleting T cells from the HSC transplant. However, this strategy does not allow satisfactory T-cell reconstitution, yielding numerous infection complications.24 Thus, the TK-GCV system will offer a significant advantage over T-cell depletion only if it spares sufficient numbers of nonalloreactive T cells to allow T-cell reconstitution. Theoretically, an earlier onset of alloreactive T-cell division compared to that of nonalloreactive T cells should permit the scheduling of GCV administration so as to control GVHD but allow reconstitution of the recipient immune system. To investigate this possibility, we compared the kinetics of T-cell division after semiallogeneic BMT, in which both alloreactive and homeostatic T-cell expansion can occur, to the kinetics of T-cell division after syngeneic BMT, in which only homeostatic expansion takes place. For this, we analyzed splenocytes collected early after BMT. Indeed, after intravenous infusion, donor T cells rapidly home to the spleen, where they proliferate before their dissemination to target organs.25
When T cells were infused into syngeneic hosts, CFSE experiments indicated that only a small fraction of cells had already divided at hour 88. In contrast, in semiallogeneic hosts, most T cells had proliferated at least once at this time point. Concurrently, absolute hCD4+ splenocyte numbers increased moderately in syngeneic hosts versus approximately 20-fold in semiallogeneic hosts. The difference observed in the onset of division between host-reactive and non–host-reactive T cells validates the rationale of the TK-GCV approach. Indeed, it should be possible to induce preferential killing of host-reactive T cells using a short-term GCV treatment in the early graft period. The effect of GCV on donor T cells confirmed this hypothesis. In the syngeneic host, the small increase in hCD4+ splenocytes observed between hour 64 and hour 88 was not abrogated by GCV, likely reflecting homing into the spleen rather than proliferation of these cells. In contrast, GCV administration strongly lowered hCD4+ splenocyte numbers after semiallogeneic BMT, indicating proliferation (Figure 1B).
After semiallogeneic BMT, at the end of GCV treatment, donor hCD4+ T-cell counts were comparable between GCV-treated and untreated groups (day 7, Figure 2). This situation presumably arose from 2 completely different mechanisms. In the absence of GCV, alloreactive donor T cells that had rapidly proliferated likely underwent massive activation-induced cell death, in accordance with a previous report.26 Conversely, GVHD prevention by GCV spared a pool of nonalloreactive donor T cells that had not divided and therefore was readily detectable at the end of treatment.
After syngeneic BMT, at day 7, donor hCD4+ T-cell counts were reduced in GCV-treated mice as compared to untreated mice (Figure2). This presumably reflects that although significant homeostatic cell division of donor T cells was delayed compared to alloreactive cell division, it occurred between hour 88 and day 7 in the syngeneic recipients. One of the most intriguing findings of this study is the observation that in GCV-treated mice, the numbers of hCD4+cells are decreased 10-fold in syngeneic hosts relative to semiallogeneic hosts, on day 7 and on day 14. This result is counterintuitive because one could expect a similar time course for the homeostatic expansion in semiallogeneic and syngeneic hosts. The current data suggest that the homeostatic expansion began during the period of GCV administration (day 0 to day 7) in syngeneic hosts, whereas it was delayed in semiallogeneic hosts. It also suggests that, until they are killed by GCV, alloreactive T cells exert an inhibitory effect on the division of nonalloreactive T cells. This could be owing to a competition for T-cell niches19 or the secretion of inhibitory cytokines. This phenomenon deserves specific investigations that require an experimental model allowing distinction between alloreactive and nonalloreactive T cells. Whatever the mechanism involved, the follow-up of T-cell expansion from day 7 to day 14 (Figure 2), after GCV cessation, revealed that the best early T-cell reconstitution is achieved after semiallogeneic BMT provided that GVHD is efficiently controlled.
In adult humans, TCD efficiently prevents GVHD but is associated with poor immune reconstitution and infection because of poor thymic activity.24,27 Therefore, we analyzed the long-term T-cell reconstitution potential of infused mature donor T cells in thymectomized recipients. In mice in which GVHD was controlled, donor T cells were still present at day 78 after semiallogeneic BMT. They displayed a memory-activated phenotype, in agreement with previous observations.1 28 Furthermore, half of them expressed the CD69 activation marker, suggesting possible ongoing T-cell division in mice that remained lymphopenic. T-cell reconstitution of these mice, though numerically limited, provided a diversified T-cell repertoire in terms of both Vβ usage and TCRβ CDR3 transcript-length distribution. Some differences were found between this repertoire and that of donor T cells at the time of infusion (Figure 4), which could reflect the elimination of alloreactive T cells and discrete clonal T-cell expansions that were not seen after syngeneic BMT (Figure 4).
After allogeneic BMT, GCV-treated mice respond to a third-party alloantigen,9 to a foreign protein antigen,14and to primary infection with the lymphocytic choriomeningitis virus (LCMV).15 Of importance, using the same model of semiallogeneic BMT used in this report, we previously showed that donor antiviral memory can be conferred to BM-grafted GCV-treated hosts by the transfer of immunocompetent cells from LCMV-immune animals.15 Collectively, these data suggest an effective immune reconstitution from infused mature donor T cells. Thus, the TK-GCV strategy should permit introduction of a functional T-cell repertoire in the absence of GVHD, notably in the case of poor thymic function. Altogether, our results show that though the specificity of GCV-mediated cell killing does not directly depend on allogeneic recognition, the differences in the onset of cell division triggered by alloreactive or homeostatic signals allow preferential elimination of alloreactive T cells. The TK-GCV system not only efficiently controls GVHD after semiallogeneic BMT, it spares non–host-reactive donor T cells that contribute to T-cell reconstitution.
We thank L. Lejeune, S. Bruel, G. Gavory, M.-C. Burland, and V. Bon-Durand for their technical assistance, G. Boisserie and F. Baillet for the irradiation of mice, and B. Salomon and C. Frisén for helpful discussions during the revision of the manuscript.
Supported by Université Pierre et Marie Curie, Centre National de la Recherche Scientifique, Association Française contre la Myopathie, Association pour la Recherche contre les Déficits Immunitaires Viro-Induits, Génopoı̈étic S.A. J.L.C. is supported by the Fondation pour la Recherche Médicale.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David Klatzmann, CNRS/UPMC ESA 7087, Hôpital Pitié- Salpêtrière, 83 bd de l'Hôpital, F-75651 Paris Cedex 13, France; david.klatzmann@chups.jussieu.fr.

![Fig. 1. Early kinetics of division and donor T-cell expansion after. / TK T-cell infusion in lethally irradiated hosts.[B6 × FVB]F1 (semiallogeneic host)-irradiated or FVB (syngeneic host)-irradiated euthymic mice received 1 × 107 FVB bone marrow cells supplemented with 1 × 107 CFSE-labeled mature T cells from [hCD4 × TK] double-transgenic FVB mice. In treated groups, GCV was administered from hour 0 to hour 88 by intraperitoneal injection twice daily. (A) CFSE intensity of hCD4+ splenocytes was analyzed at different time points after grafting. Each histogram is representative of 3 mice. The peak of highest intensity on the log scale identifies parent generation of infused donor T cells. Peaks with decreased CFSE intensity represent daughter generations that have undergone division. (B) The number of donor T cells in spleens of grafted animals (n = 3) was evaluated at different time points by their expression of the hCD4 marker. Coefficient of variation of triplicates was less than 5%. ●, GCV; ○, no GCV.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2071/5/m_h81911581001.jpeg?Expires=1769532601&Signature=FaNhhBcUdxj~jKivAELDSiY9oYAVurlA4-SnOtWdn~R0FKJgMk3D08kplXoh-gnwAemRRurLOSs2suHIgtibvokcywkzEaz4yoTsXP4pZpO7m3v8f2zG2BC13ee1KoAq1RL00edW-q~hEQLGolsZyvtPcPx9TDLlNrLgYCS-CwgsR7hHl~DM2-9wXw4Pw9AnVw6DxS8UeM53tQkrB8f6nS1cAa57OGr-al8WDqUXF5MDdHhWsHtDKf19qoPD6zO5upKDlJpVlD-kDNYdxk4oSBLL39LovFZjelFDmlyUuj95Tx16XcPVczIMWnLFEEQU1sNCf1fXVeSIFcqcWizIEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Persistence and expansion of donor T cells after GCV-mediated control of GVHD. / Lethally irradiated euthymic [B6 × FVB]F1 (semiallogeneic) or FVB (syngeneic) mice received 1 × 107 FVB BM cells supplemented with 1 × 107 mature T cells from [hCD4 × TK] double-transgenic mice. GCV was administered from day 0 to day 6 (●), whereas controls did not receive GCV (○). At day 7 and day 14 after grafting, splenocytes from grafted animals were collected. CD4 T cells originating from infused TK T cells are unequivocally identified as hCD4+ mCD4+cells (gated on lymphocytes by size criteria). The lymphoid compartment was analyzed in the spleen at day 7 (end of the GCV regimen) or at day 14. Results are given as mean ± SEM of total cell numbers after gating on the appropriate subsets (semiallogeneic/GCV, day 7, n = 9; semiallogeneic/GCV, day 14, n = 8; semiallogeneic/no GCV, day 7, n = 7; semiallogeneic/no GCV, day 14, n = 4; syngeneic/GCV, days 7-14, n = 4; syngeneic/no GCV, days 7-14, n = 5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2071/5/m_h81911581002.jpeg?Expires=1769532601&Signature=VpZLru8RKBg8hcCjweA9PfM2V2QkuuCqVDRnbcyoZvub5DgCDOldEAttbtyMY2KDrDJFT6ujzzm8ir9VEd70H3nZP07vioXgW1ThIw61Dw4zPZ7sccRj2wz8E8JMKEe5kD0cBUiKVmrLPft7BTjfJZ9SE8rIleNfTlnqlOFy7Bftg75p-Hzxe6E01CyPONSeuMXzzqsNlewNgC~7UmPSsWuvy~7upqH3XwbxiRoxx4BtFrN2jcyidv5tLHAjVHVTQmHgQMHOOU4FD4VPXpWcEl38ocskb9kezhL5t7F~qHsBBnut3zDxyFIwlIG-Jo8DbCRrQysFH3G11Bne8mW7sQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 1. Early kinetics of division and donor T-cell expansion after. / TK T-cell infusion in lethally irradiated hosts.[B6 × FVB]F1 (semiallogeneic host)-irradiated or FVB (syngeneic host)-irradiated euthymic mice received 1 × 107 FVB bone marrow cells supplemented with 1 × 107 CFSE-labeled mature T cells from [hCD4 × TK] double-transgenic FVB mice. In treated groups, GCV was administered from hour 0 to hour 88 by intraperitoneal injection twice daily. (A) CFSE intensity of hCD4+ splenocytes was analyzed at different time points after grafting. Each histogram is representative of 3 mice. The peak of highest intensity on the log scale identifies parent generation of infused donor T cells. Peaks with decreased CFSE intensity represent daughter generations that have undergone division. (B) The number of donor T cells in spleens of grafted animals (n = 3) was evaluated at different time points by their expression of the hCD4 marker. Coefficient of variation of triplicates was less than 5%. ●, GCV; ○, no GCV.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2071/5/m_h81911581001.jpeg?Expires=1769532602&Signature=2F5-Jb7KzByEaW-a1DWS158fRBcSP4XMtpS1tUauq7C3xOFsM9iZz2a5PZE8AH-RyS5bM-SpKdBHlhtG0gqT3R0uFgOoSC3HkpDITVWQEOsj-1Rpv0UHiT-HzYGD56svvUzJMczPBOWMkelJZ1VwmksUYO~jHTs5SJlmUKPMPhhxDVauLnqk00pceIwBStsqznf5wJSS0vYUR4esqLEB1VbmQ9YH0qxFngsUWVOTqDkDN7z9viIwkGEYv7XeDlfuz878PMkGiFh6zWMxy5yo9Rj3yGXI3pMrOpwLizk-GUyrLHDIimGLIMMtndMdtLt6AbmcU610XJrTcZQqP4-0Pw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Persistence and expansion of donor T cells after GCV-mediated control of GVHD. / Lethally irradiated euthymic [B6 × FVB]F1 (semiallogeneic) or FVB (syngeneic) mice received 1 × 107 FVB BM cells supplemented with 1 × 107 mature T cells from [hCD4 × TK] double-transgenic mice. GCV was administered from day 0 to day 6 (●), whereas controls did not receive GCV (○). At day 7 and day 14 after grafting, splenocytes from grafted animals were collected. CD4 T cells originating from infused TK T cells are unequivocally identified as hCD4+ mCD4+cells (gated on lymphocytes by size criteria). The lymphoid compartment was analyzed in the spleen at day 7 (end of the GCV regimen) or at day 14. Results are given as mean ± SEM of total cell numbers after gating on the appropriate subsets (semiallogeneic/GCV, day 7, n = 9; semiallogeneic/GCV, day 14, n = 8; semiallogeneic/no GCV, day 7, n = 7; semiallogeneic/no GCV, day 14, n = 4; syngeneic/GCV, days 7-14, n = 4; syngeneic/no GCV, days 7-14, n = 5).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2071/5/m_h81911581002.jpeg?Expires=1769532602&Signature=kJd4z7BPXHQgdRI9VXZSjvTFAOE5IyzGu1eWtkM8txamWCh-OCf0d8YUtZBEsG9P-Vr0C~d0Mdyk1oArCdz9WFVzwqrDc~YpUAMJC-LWF9Oo6~xWXUuv9CJEOjdYCBFjNjsq~7hqV~xlsd~fQyFOnkz8~qts9bc-ov8sNFZkAHO4167Ks1hDjLHllPDYKJrAwHtha3mzZj81~B0k9ygWL5ILE42BZ4fr-fZ9-HycBg1KsC6xCC5yynwHfFgSJWtos9CnXHYq-R0dE6t-iLXPrGyuDoozNllTzR8h~oBg-qrDvdJE7fFQwR6MkjJCdjfd~i4uzX0oajZlcNyNNi0keQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)