Cellular trafficking of growth factor receptors, including cross-talk among receptors at the cell surface, may be important for signal transduction in normal hematopoietic cells. To test this idea, the signaling domain of Mpl (the thrombopoietin receptor) was targeted to the plasma membrane, or to the cytoplasm of murine marrow cells, and the ability of the cells to proliferate and differentiate in response to Mpl dimerized at the plasma membrane or free in the cytoplasm was assessed. Constructs encoding the signaling domain of Mpl linked to an FK506 binding protein domain (to permit dimerization by the membrane-permeable ligand AP20187) with or without a myristylation sequence (to target the receptor to the plasma membrane) and a hemagglutinin epitope tag were generated and introduced into murine marrow cells using a murine stem cell virus (MSCV)-based retroviral vector. Both populations of transduced marrow cells proliferated in Iscoves modified Dulbecco medium–10% FCS–100 nM AP20187 without exogenous growth factors for more than 100 days and achieved greater than a 107-fold expansion of cells by day 50 (n = 4 transductions). Growth was dimerizer dependent, and myeloid, erythroid, and megakaryocytic progenitors were generated. Activation of Mpl either at the plasma membrane or in the cytoplasm allowed for the terminal maturation of transduced progenitor cells. Introduction of membrane-targeted or cytoplasmic Mpl into fetal liver cells from homozygous JAK2 knock-out mice or wild-type littermates demonstrated that both forms of Mpl require JAK2 for signaling. These data show that the activation of Mpl independent of its normal plasma membrane location can support production of the full range of normal hematopoietic progenitor cells in vitro.
Introduction
Hematopoietic growth factor receptors are transmembrane glycoproteins located in the plasma membrane of the cell. Binding of growth factor causes receptor dimerization and recruitment and activation of signaling molecules, thus initiating signal transduction.1 After ligand binding, growth factor receptors cluster in clathrin-coated pits, become internalized, and are targeted for degradation or recycled to the cell surface. The cell surface location of hematopoietic growth factor receptors permits communication with the extracellular milieu. Other attributes of cell surface location of these receptors that may facilitate signal transduction include organization into specific microdomains on the cell surface,2-5 preassembled signaling complexes,6,7 cross-talk among heterologous cytokine receptors,8-10 and commencement of normal intracellular trafficking of the receptor-ligand complex.11-14
A stringent test of growth factor receptor function is the ability to support the proliferation and differentiation of normal hematopoietic cells. We hypothesized that the location of a hematopoietic growth factor receptor in the plasma membrane was important not only for communication with the extracellular milieu but also for expression of the full repertoire of receptor functions. The availability of membrane-permeable agents that can bind and dimerize FK506 binding protein 12 (FKBP12) domains provided a mechanism to test this hypothesis.15-17,49 50 We linked the 121-amino acid signaling domain of the murine thrombopoietin receptor Mpl to a modified FKBP12 and targeted the constructs to the plasma membrane or to the cytoplasm of normal murine marrow cells. We found that the activation of Mpl in the cell cytoplasm or at the plasma membrane supported long-term proliferation of the cells, generation of myeloid, erythroid, and megakaryocytic progenitor cells, and terminal maturation of the cells.
Interactions between growth factor receptors and signaling molecules are normally subject to precise spacial and temporal regulation within the cell,18 and there is considerable cross-talk among hematopoietic growth factor receptors. However, the current report documents that normal cellular localization and trafficking of Mpl are not required for proliferation or differentiation in primary hematopoietic cells.
Materials and methods
Plasmid construction
The construct encoding membrane-targeted Mpl, containing a myristylation sequence, a modified FKBP12 domain, the 121-amino acid signaling domain of murine Mpl, and a C-terminal hemagglutinin HA epitope tag, is shown in Figure1.16,17 The modified FKBP12 domain (FKBP12 with a phenylalanine-to-valine substitution at amino acid 36) allows for binding of the dimerizer AP20187.16,17,50 Membrane targeting is achieved by incorporating a 14-amino acid myristylation peptide from the amino terminus of Src.19 To generate the cytoplasmic version of Mpl, which lacks the myristylation peptide, polymerase chain reaction (PCR) was performed with the forward primer annealing downstream of the 5′-myristylation sequence, 5′-ACGCTCGACGCCACCATGCTCGAGGGCGTGCAGGTGGAGAC-3′), whereas the reverse primer flanked the HA epitope tag, 5′-GGGGATCCGAATTCTTAGTCGAGTGCGTAGTC-3′) (Operon Technologies, Alameda, CA). The resultant PCR product, incorporating a Kozak consensus sequence and an ATG, was digested with SalI andBamHI and was inserted into the XhoI andBglII sites of MSCVneo20 (a gift from Dr Robert Hawley, American Red Cross, Rockville, MD). To generate COS-1 expression vectors, the membrane-tethered and cytoplasmic Mpl cassettes were removed with EcoRI digests and inserted into theEcoRI site of pEGFP-N1 (Clontech, Palo Alto, CA).
Schematic diagram of constructs.
Membrane-tethered Mpl construct encodes a fusion protein containing a myristylation motif derived from the first 14 amino acids of Src19 to target the fusion protein to the plasma membrane. The cytoplasmic mpl construct lacks a myristylation motif and is, therefore, predicted to be localized to the cytoplasm. Both constructs encode an FKBP12 domain harboring a phenylalanine-to-valine substitution at amino acid 36,15 allowing binding of the dimerizer AP20187 with a stoichiometry of 2:1. Red indicates myristylation peptide; hatched, FKBP12 domain; yellow, Mpl signaling domain; black, HA epitope tag.
Schematic diagram of constructs.
Membrane-tethered Mpl construct encodes a fusion protein containing a myristylation motif derived from the first 14 amino acids of Src19 to target the fusion protein to the plasma membrane. The cytoplasmic mpl construct lacks a myristylation motif and is, therefore, predicted to be localized to the cytoplasm. Both constructs encode an FKBP12 domain harboring a phenylalanine-to-valine substitution at amino acid 36,15 allowing binding of the dimerizer AP20187 with a stoichiometry of 2:1. Red indicates myristylation peptide; hatched, FKBP12 domain; yellow, Mpl signaling domain; black, HA epitope tag.
Retroviral producer lines
Retroviral producer lines were generated by transfecting the amphotropic packaging line PA317 with vector plasmid. Supernatant was collected 2 days later and was subsequently used for transduction of the ecotropic packaging line GpE+86. Producer clones were isolated in the presence of G418 and screened for virus titer based on end-point titration using NIH3T3 cells. Producer clones were isolated for each vector and yielded titers between 105 and 106colony-forming units (CFU) per milliliter. Genetic stability was confirmed by Southern blot analysis of clones and pools of producer cells using the restriction enzyme KpnI, which cuts once in each long terminal repeat, and a probe directed against theneo gene. Producer lines were documented to be free of helper-competent virus using a marker-rescue assay. Cell lines were cultured at 37°C in Dulbecco modified Eagle medium (DMEM; Gibco/BRL Life Technologies, Grand Island, NY) supplemented with 8% fetal calf serum (FCS), sodium pyruvate, nonessential amino acids, and 2 mM L-glutamine.
Retroviral transduction of Ba/F3 cells
Supernatant was collected from confluent retroviral producer cell clones packaging virus encoding either the membrane-targeted or the cytoplasmic form of Mpl. Retroviral supernatants were filtered through a 0.45 μm filter (Millipore), and 3 mL supernatant was added to a mixture of 2 × 106 Ba/F3 cells in 7 mL RPMI composed of 10% FCS and 5% conditioned media containing murine interleukin-3 (IL-3) (Stem Cell Technologies, Vancouver, BC, Canada). Polybrene was added to a final concentration of 8 μg/mL, and the cells were incubated for 48 hours at 37°C with 7% CO2. After the incubation period, cells were washed 4 times in 10 mL ice-cold phosphate-buffered saline and then plated by limiting dilution in RPMI composed of 10% FCS, 5% conditioned media containing murine IL-3, and 800 μg/mL G418.
Ba/F3 cell proliferation assays
Western blotting and signaling studies
Ba/F3 cells expressing wild-type Mpl or the membrane-tethered or cytoplasmic Mpl constructs were cultured in RPMI containing 10% FCS, penicillin, streptomycin, and murine IL-3 until a cell density of 5 to 7 × 105/mL was achieved with viability greater than 98% assessed by trypan blue exclusion. Cells were washed twice in RPMI and resuspended in RPMI with 0.5% bovine serum albumin (BSA) at a concentration of 2 × 106 cells/mL. After 6 hours of starvation, the cells containing wild-type Mpl were stimulated with thrombopoietin (20 ng/mL) and those containing dimerization motifs were exposed to AP20187 (100 nM) for 1 to 180 minutes. Cells were washed twice in ice-cold phosphate buffered saline, and total cell lysates were generated as previously described.23 Protein concentrations were measured using a modified Lowry assay (Bio-Rad, Hercules, CA). For JAK2 analysis, 1 mg total protein was immunoprecipitated with JAK2 antisera (2 μL; Upstate Biotechnology, Lake Placid, NY), and immune complexes were collected with Protein A–Sepharose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoprecipitated protein was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% acrylamide gels. After transfer to nitrocellulose membranes, the blots were probed with an antiphosphotyrosine antibody (4G10; Upstate Biotechnology) and visualized by chemiluminescent detection (New England Nuclear, Boston, MA) using a goat antimouse secondary antibody coupled to horseradish peroxidase (HRP; Bio-Rad). The blots were then stripped and reprobed with the same JAK2 antibody used for immunoprecipitation to confirm equal loading. To detect MAPK and STAT5 activation, 100 μg whole cell lysate was analyzed by Western blot using SDS-PAGE on 7.5% acrylamide gels. After transfer to nitrocellulose membranes, the blots were probed with antibodies specific for phospho-STAT5 (generous gift from David Frank, Boston, MA) or doubly-phosphorylated (active) MAPK (Promega, Madison, WI). Blots were visualized by chemiluminescence using goat anti–rabbit immunoglobulin G (IgG) conjugated to HRP (Bio-Rad). Membranes were stripped and reprobed with a STAT5B antibody (Santa Cruz) and Erk1/2 antibody (Upstate Biotechnology) to demonstrate equivalent loading of lysates in each lane.
Retroviral transduction of murine marrow cells
This study was approved by the Animal Care Committee of the University of Washington. 5-Fluorouracil (150 mg/kg) was injected intraperitoneally into female B6D2F1 mice (Jackson Laboratories, Bar Harbor, ME). Forty-eight hours later, marrow cells were harvested and cultured for 48 hours in DMEM containing 16% FCS (Intergen, Purchase, NY), 5% conditioned media containing murine IL-3, 100 ng/mL recombinant human IL-6, and 50 ng/mL recombinant murine stem cell factor at 37°C in a humidified incubator containing 5% CO2. After 48 hours of prestimulation with IL-3, IL-6, and stem cell factor, the cells were transferred onto irradiated (1500 cGy) retroviral producer cells and were cocultivated using identical growth conditions except for the addition of polybrene (8 μg/mL). Marrow cells were harvested after 48 hours of cocultivation and maintained in suspension culture. Transduction efficiency was assessed in colony assays (described below) using G418 (Gibco/BRL) at a concentration previously shown to prevent colony formation by nontransduced cells (800 μg/mL).
Suspension cultures
After retroviral transduction, marrow cells were cultured in Iscoves modified Dulbecco medium (IMDM) containing 10% FCS, 50 U/mL penicillin, and 50 μg/mL streptomycin either in the presence or absence of AP20187 (100 nM). AP20187 was a gift of ARIAD Pharmaceuticals (Cambridge, MA). Cell numbers were determined on the days indicated.
Hematopoietic colony assays
The numbers of erythroid (burst-forming units [BFU]-E), myeloid (CFU-granulocyte macrophage [GM]), and megakaryocytic (CFU-Meg) progenitor cells in the suspension cultures of the transduced marrow cells were quantitated in hematopoietic colony assays.24,25 Cells were washed free of AP20187 before plating. To quantitate BFU-E, the cells were plated in 1.4% methylcellulose (Dow Chemical, Midland, MI) in IMDM supplemented with 25% FCS, 1% BSA, 5 × 10−5 M β-mercaptoethanol (Sigma Chemical, St Louis, MO), 1% penicillin–streptomycin–fungizone (Sigma), rat stem cell factor (100 ng/mL; a gift from Amgen, Thousand Oaks, CA), and erythropoietin (2 U/mL).26 Plates were incubated at 37°C in a humidified incubator supplemented with 5% CO2, and BFU-E–derived colonies were counted on day 8. Myeloid colonies (CFU-GM) were quantitated by plating the cells in 1.4% methylcellulose in IMDM supplemented with 25% FCS, 1% BSA, and murine IL-3 (100 U/mL; a gift from Dr Ken Kaushansky, University of Washington, Seattle). Colonies were counted on day 5. Megakaryocytic colonies (CFU-Meg) were quantitated by plating the cells in 0.285% agar (Difco, Detroit, MI) in IMDM supplemented with 15% horse serum (Hyclone, Logan, UT), 5 × 10−5 M β-mercaptoethanol, murine thrombopoietin (10 ng/mL, a gift from Dr Ken Kaushansky), IL-3 (10 U/mL), and stem cell factor (10 ng/mL). Colonies were counted on day 5.
JAK2-deficient fetal liver cells
Fetal liver cells were obtained from homozygous JAK2-deficient or wild-type littermate day 12.5 embryos.27 Cells were transduced either with a retroviral vector encoding JAK227or with the MSCV vector encoding membrane-targeted Mpl, cytoplasmic Mpl, or a mock viral control, then placed in hematopoietic colony assays in the presence of IL-3 or AP20187. Similar studies were performed with fetal liver cells obtained from homozygous STAT5 A/B-deficient mice.28
Detection of Mpl fusions in murine marrow cells by confocal microscopy and by flow cytometry
Marrow cells transduced with either the cytoplasmic or the membrane-tethered Mpl construct were fixed in 0.5% paraformaldehyde and washed in saline. They were labeled with an anti-HA monoclonal antibody (10 μg/mL; BABCO, Richmond, CA) for 20 minutes in the presence of 0.05% Triton X-100, then washed and labeled with a biotin-conjugated goat anti–mouse IgG followed by streptavidin–fluorescein isothiocyanate (FITC) (both from Jackson Immunoresearch, West Grove, PA). Nuclei were stained with propidium iodide (5 μg/mL) in 0.1% citrate, 0.05% Triton X-100, and 10 μg/mL DNase-free RNase (Boehringer Mannheim, Indianapolis, IN).29 Fluorescence was analyzed using a confocal microscope (Radiance 2000;Bio-Rad).
Cellular fractionation and Mpl detection in COS-1 cells
Cellular fractionations were performed as reported by Koury et al.30 COS-1 cells were transfected with the membrane-targeted or the cytoplasmic Mpl constructs using the diethylaminoethyl dextran method,31 and 1 × 107 cells were harvested by trypsinization, washed in saline, and pelleted. Cell pellets were incubated on ice in swelling buffer (including protease inhibitors) and homogenized with 50 strokes in a prechilled dounce homogenizer. Lysed cells were adjusted to 0.25 M sucrose, and the nuclei were pelleted. The supernatant was ultracentrifuged, thereby pelleting membrane-associated proteins. After ultracentrifugation, the supernatant was used for immunoprecipitations of cytoplasmic proteins with the anti-HA monoclonal antibody, whereas the membrane pellet was floated on a sucrose step gradient encompassing 40%, 35%, 31%, 25%, and 8.5% sucrose concentrations. Plasma membrane-enriched fractions were harvested from the 35%–31% interface and the 31%–25% interface. These fractions were pooled, the sucrose was diluted, and the samples were ultracentrifuged. Pellets were solubilized in lysis buffer and analyzed on a 15% polyacrylamide gel. Proteins were electroblotted onto nitrocellulose (Bio-Rad) and detected with the primary HA antibody and secondary antibody goat anti–mouse HRP (Bio-Rad). Chemiluminescent detection (Amersham, Piscataway, NJ) was performed.
Results
Dimerization of Mpl at the plasma membrane or in the cytoplasm stimulates proliferation in Ba/F3 cells
G418-resistant Ba/F3 cell clones expressing either form of the Mpl fusion were isolated and tested for their ability to proliferate in response to the dimerizer AP20187. Proliferation assays were performed on individual clones. Mock-transduced Ba/F3 cells did not proliferate in response to AP20187 (data not shown). In contrast, the addition of AP20187 to cells expressing either form of Mpl stimulated a dose-dependent proliferative response (Figure2A). Ba/F3 cell clones expressing the cytoplasmic or the membrane-targeted form of Mpl proliferated over a range of concentrations of AP20187. Cells expressing either form of Mpl proliferated at an AP20187 concentration as low as 1 nM. Previous work by our laboratory has shown that high concentrations of a monomeric antagonist of the dimer can competitively inhibit the proliferative response by inhibiting dimerization.21 These results suggest that dimerization of Mpl, in the absence of membrane localization, is sufficient to activate proliferative signaling in Ba/F3 cells.
Proliferation assays and signaling studies comparing dose–response curves and signaling in Ba/F3 cell clones expressing the membrane-tethered or cytoplasmic Mpl fusion protein.
(A) Cell proliferation (MTT) assays of Ba/F3 cell clones expressing either the (i) cytoplasmic or the (ii) membrane-tethered form of Mpl. Proliferation data are represented as a fraction of the proliferative response of Ba/F3 cells to 5% conditioned media containing murine IL-3. (B) JAK2, MAPK, and STAT5 phosphorylation were assessed by Western blot analysis in Ba/F3 cell clones expressing either wild-type Mpl [MPL (WT)] or cytoplasmic [MPL (CYTO)] or membrane-targeted [MPL (MEM)] Mpl fusions. Wild-type Mpl was activated using thrombopoietin, whereas Mpl fusions were activated by adding AP20187 (100 nM) for the time indicated (in minutes). IP, immunoprecipitation; WCL, whole cell lysate.
Proliferation assays and signaling studies comparing dose–response curves and signaling in Ba/F3 cell clones expressing the membrane-tethered or cytoplasmic Mpl fusion protein.
(A) Cell proliferation (MTT) assays of Ba/F3 cell clones expressing either the (i) cytoplasmic or the (ii) membrane-tethered form of Mpl. Proliferation data are represented as a fraction of the proliferative response of Ba/F3 cells to 5% conditioned media containing murine IL-3. (B) JAK2, MAPK, and STAT5 phosphorylation were assessed by Western blot analysis in Ba/F3 cell clones expressing either wild-type Mpl [MPL (WT)] or cytoplasmic [MPL (CYTO)] or membrane-targeted [MPL (MEM)] Mpl fusions. Wild-type Mpl was activated using thrombopoietin, whereas Mpl fusions were activated by adding AP20187 (100 nM) for the time indicated (in minutes). IP, immunoprecipitation; WCL, whole cell lysate.
Signal transduction by membrane-tethered or cytoplasmic Mpl falls below the threshold of detection
Signal transduction experiments were performed with Ba/F3 cell clones expressing either version of Mpl to look for activation of known signaling intermediaries of the thrombopoietin–Mpl system. No detectable phosphorylation of JAK2, STAT5, or ERK1/2 in either of the modified forms of Mpl was observed; this is in sharp contrast to thrombopoietin-stimulated Ba/F3 cells expressing the wild-type receptor that activated these molecules efficiently (Figure 2B).
Ability of membrane-tethered or cytoplasmic Mpl to support proliferation of normal hematopoietic cells
Post-5-Fluorouracil murine marrow cells were cocultivated with retroviral producer cell lines for the membrane-tethered or the cytoplasmic versions of Mpl (Figure 1). Transduction efficiency rates (assessed by culturing the cells in the presence or absence of G418) were 52% for membrane-tethered Mpl and 57% for cytoplasmic Mpl. To determine whether the Mpl signaling domain expressed freely in the cytoplasm could support the proliferation of normal hematopoietic cells, these 2 populations of transduced cells were cultured in IMDM supplemented with 10% FCS and the dimerizer AP20187 (100 nM). Dimerization of cytoplasmic Mpl supported the proliferation of primary hematopoietic cells as effectively as dimerization of the membrane-tethered Mpl (Figure 3), resulting in more than a 107-fold increase in total cell numbers in a 50-day period in vitro.
Cytoplasmic Mpl supports proliferation of murine marrow cells indistinguishable from its membrane-tethered counterpart.
Murine bone marrow cells transduced with retroviral vectors encoding either membrane-targeted Mpl (closed circles) or cytoplasmic Mpl (open circles) were cultured in suspension in the presence or absence of AP20187 (100 nM). Cell growth failed to occur in the absence of AP20187 (data not shown). Results depict a single representative experiment from among 4 independent transductions.
Cytoplasmic Mpl supports proliferation of murine marrow cells indistinguishable from its membrane-tethered counterpart.
Murine bone marrow cells transduced with retroviral vectors encoding either membrane-targeted Mpl (closed circles) or cytoplasmic Mpl (open circles) were cultured in suspension in the presence or absence of AP20187 (100 nM). Cell growth failed to occur in the absence of AP20187 (data not shown). Results depict a single representative experiment from among 4 independent transductions.
To quantitate Mpl fusion protein expression, marrow cells expressing membrane-targeted Mpl, cytoplasmic Mpl, or a full-length form of Mpl with a C-terminal HA epitope tag were labeled with an anti-HA monoclonal antibody and a second antibody, as described above, and were analyzed by flow cytometry. Both the membrane-targeted Mpl fusion and the cytoplasmic Mpl fusion were expressed at approximately 50% of the level of the full-length Mpl. Murine marrow cells expressed approximately 1300 copies of full-length Mpl (assessed by Western blotting and compared with BaF3-Mpl cells, in which Mpl receptor display had been quantitated by Scatchard analysis of sodium iodide125I-thrombopoietin binding).32 These results indicate that the expression levels of the membrane-targeted Mpl fusion and cytoplasmic Mpl fusion—fewer than 1000 copies per cell—are within the range of Mpl expression in normal megakaryocytes (12 000 Mpl receptors per cell)33 and normal platelets (25-200 receptors per platelet).34 35
Growth rates of the cells expressing membrane-targeted or cytoplasmic forms of Mpl were identical. Sustained growth of both populations of cells was observed for more than 100 days (data not shown). Cell proliferation remained dimerizer dependent; when the cells were washed free of dimerizer and were resuspended in IMDM with 10% FCS, cell death ensued within 4 days. These results demonstrate that even with a low level of expression of the Mpl fusion proteins in normal hematopoietic cells (fewer than 1000 copies of the Mpl fusion protein per cell), dimerization of the Mpl signaling domain, engineered to be expressed free in the cytoplasm, can support long-term proliferation of normal hematopoietic cells.
Cellular localization of Mpl fusion proteins
Confocal microscopy was used to examine cellular localization of the 2 forms of Mpl in the transduced murine marrow cells (Figure4). Cells that had been in suspension culture for approximately 100 days in the presence of AP20187 were labeled with an anti-HA monoclonal antibody, then by a biotinylated goat antimouse antibody followed by streptavidin conjugated to FITC, and then viewed under a confocal microscope. Distinct patterns of fluorescence are evident. Cells transduced with the membrane-targeted Mpl construct show intense fluorescence at the plasma membrane (Figure4A), whereas cells transduced with the cytoplasmic Mpl construct show fluorescence in a punctate pattern throughout the cell cytoplasm, with fluorescence overlaying the nuclei and without fluorescence enhancement of the plasma membrane (Figure 4B).
Immunofluorescent localization of the Mpl fusion proteins in marrow cells.
Murine marrow cells transduced with either the membrane-targeted Mpl construct (left panel) or the cytoplasmic Mpl construct (right panel) were labeled with an anti-HA monoclonal antibody, biotinylated goat anti–mouse IgG, and streptavidin-FITC and then were analyzed by confocal microscopy. Nuclei were stained with propidium iodide.
Immunofluorescent localization of the Mpl fusion proteins in marrow cells.
Murine marrow cells transduced with either the membrane-targeted Mpl construct (left panel) or the cytoplasmic Mpl construct (right panel) were labeled with an anti-HA monoclonal antibody, biotinylated goat anti–mouse IgG, and streptavidin-FITC and then were analyzed by confocal microscopy. Nuclei were stained with propidium iodide.
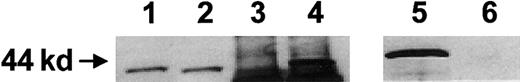
Low levels of expression of the Mpl constructs in normal hematopoietic cells impeded attempts to use standard cell fractionation techniques to further investigate the cellular distribution of the membrane-targeted and cytoplasmic Mpl proteins. To circumvent this problem, both 2 Mpl constructs were transiently expressed in COS-1 cells, and a high level of expression of the proteins was achieved (Figure5). Membrane-targeted and cytoplasmic versions of Mpl were expressed at similar levels in whole COS-1 lysates (Figure 5, lanes 1 and 2). The cytoplasmic version of Mpl was detected in the cytoplasmic fraction of the COS-1 cells (Figure 5, lane 4). However, though membrane-targeted Mpl was readily detected in the plasma membrane fraction of the COS-1 cells (Figure 5, lane 5), cytoplasmic Mpl was not detected (Figure 5, lane 6).
Subcellular localization of Mpl fusion proteins.
COS-1 cells were transiently transfected with constructs encoding either the membrane-targeted Mpl or the cytoplasmic Mpl fusion protein. Whole cell lysates, membrane fractions, and cytoplasmic fractions were obtained and analyzed by Western blotting with an antibody directed against the HA epitope tag. Lanes 1, 3, 5: membrane-targeted Mpl construct; lanes 2, 4, 6: cytoplasmic Mpl construct; lanes 1, 2: whole cell lysate; lanes 3, 4: cytoplasmic fraction; lanes 5, 6: membrane fraction.
Subcellular localization of Mpl fusion proteins.
COS-1 cells were transiently transfected with constructs encoding either the membrane-targeted Mpl or the cytoplasmic Mpl fusion protein. Whole cell lysates, membrane fractions, and cytoplasmic fractions were obtained and analyzed by Western blotting with an antibody directed against the HA epitope tag. Lanes 1, 3, 5: membrane-targeted Mpl construct; lanes 2, 4, 6: cytoplasmic Mpl construct; lanes 1, 2: whole cell lysate; lanes 3, 4: cytoplasmic fraction; lanes 5, 6: membrane fraction.
Generation of hematopoietic progenitor cells in the presence of membrane-targeted or cytoplasmic Mpl
To determine whether membrane-targeted and cytoplasmic Mpl constructs can support the generation of hematopoietic progenitor cells, aliquots of cells were removed from the liquid cultures at various time points, washed free of AP20187, and cultured in vitro in semisolid media in the presence of hematopoietic growth factors to detect erythroid (BFU-E), myeloid (CFU-GM), and megakaryocytic (CFU-Meg) progenitor cells. Both membrane-targeted and cytoplasmic Mpl supported the production of all 3 types of progenitor cells in a 1-month period in vitro (Table 1). There was no difference in the proportion of erythroid, myeloid, and megakaryocytic progenitor cells in the 2 suspension cultures. Dimerization of either the membrane-targeted Mpl or the cytoplasmic Mpl resulted in a 10 000-fold increase in the absolute number of hematopoietic progenitor cells in a 1-month period in vitro compared with the input number of progenitor cells.
Membrane-tethered or cytoplasmic Mpl can support the generation of progenitor cells
| Cellular compartment . | Colonies/105 cells plated . | ||
|---|---|---|---|
| CFU-GM . | BFU-E . | CFU-Meg . | |
| Mpl (membrane) | 2590 | 173 ± 17 | 10 ± 3 |
| Mpl (cytoplasmic) | 1867 | 105 ± 10 | 5 ± 1 |
| Cellular compartment . | Colonies/105 cells plated . | ||
|---|---|---|---|
| CFU-GM . | BFU-E . | CFU-Meg . | |
| Mpl (membrane) | 2590 | 173 ± 17 | 10 ± 3 |
| Mpl (cytoplasmic) | 1867 | 105 ± 10 | 5 ± 1 |
Marrow cells transduced with either membrane-targeted Mpl or cytoplasmic Mpl were cultured in suspension in the presence of AP20187 (100 nM), and on day 33 progenitor cell assays were performed. Data show the average of duplicate plates (CFU-GM) or the mean ± SEM of triplicate plates (BFU-E, CFU-Meg). Colony assays from a second independent transduction of marrow cells produced similar results.
Terminal maturation of hematopoietic progenitor cells
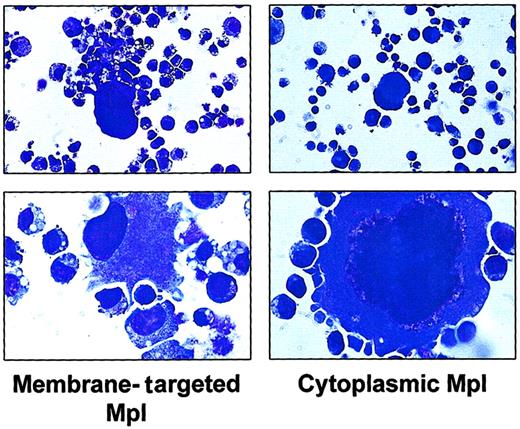
During the first 3 weeks of culture, neutrophils, macrophages, erythroid cells, and megakaryocytes were identified in the suspension cultures of cells transduced with either membrane-targeted Mpl or cytoplasmic Mpl (data not shown). At a later time point (102 days), mononuclear cells and megakaryocytes predominated (Figure6). These results are similar to those we have reported previously using the membrane targeted mpl49 50 and indicate that cytoplasmic Mpl can also support the terminal maturation of committed progenitor cells.
Photomicrographs of murine marrow cells expressing Mpl fusion proteins.
Cells transduced with either the membrane-tethered Mpl construct (left panel) or the cytoplasmic Mpl construct (right panel) were cultured in the presence of AP20187 (100 nM) for 102 days, then photographed. Magnification: top panels, × 40; bottom panels, × 100.
Photomicrographs of murine marrow cells expressing Mpl fusion proteins.
Cells transduced with either the membrane-tethered Mpl construct (left panel) or the cytoplasmic Mpl construct (right panel) were cultured in the presence of AP20187 (100 nM) for 102 days, then photographed. Magnification: top panels, × 40; bottom panels, × 100.
Requirement for JAK2 but not STAT5 signaling
Day 12.5 fetal liver cells obtained from homozygous JAK2-deficient mice27 were used to determine whether membrane-targeted Mpl or cytoplasmic Mpl requires JAK2 (Table2). JAK2 +/+ cells, but not JAK2−/− cells, formed CFU-Mix in response to IL-3, and the reintroduction of JAK2 enabled the JAK2−/− cells to produce CFU-Mix. Transduction of the JAK2 +/+ cells, but not the JAK2−/− cells, with either form of Mpl resulted in colony growth in the presence of AP20187 (Table 2). These results demonstrate that both membrane-targeted Mpl and cytoplasmic Mpl require JAK2 for signal transduction in primary hematopoietic cells. In contrast to the results with JAK2−/− fetal liver cells, both the membrane-targeted Mpl and cytoplasmic Mpl could support AP20187-dependent colony growth in STAT5 A/B-deficient fetal liver cells,28 hematopoietic colonies were found in the presence of AP20187 (data not shown). These results indicate that neither membrane-targeted nor cytoplasmic Mpl requires STAT5 A/B for signal transduction in normal hematopoietic cells.
Membrane-targeted Mpl and cytoplasmic Mpl require JAK2 for signaling in fetal liver cells
| Vector . | Colonies/105 cells . | ||
|---|---|---|---|
| Culture conditions . | JAK2 +/+ . | JAK2 −/− . | |
| None* | IL-3 | 198 ± 12 | 0 ± 0 |
| JAK2† | IL-3 plus EPO | 92, 105 | 20, 31 |
| Mpl (membrane)‡ | AP20187 | 9, 11 | 0, 0 |
| Mpl (cytoplasm)‡ | AP20187 | 8, 9 | 0, 0 |
| Vector . | Colonies/105 cells . | ||
|---|---|---|---|
| Culture conditions . | JAK2 +/+ . | JAK2 −/− . | |
| None* | IL-3 | 198 ± 12 | 0 ± 0 |
| JAK2† | IL-3 plus EPO | 92, 105 | 20, 31 |
| Mpl (membrane)‡ | AP20187 | 9, 11 | 0, 0 |
| Mpl (cytoplasm)‡ | AP20187 | 8, 9 | 0, 0 |
Results shown are the mean ± SD of the number of colonies per 105 fetal liver cells plated.
Fetal liver cells from either wild-type or JAK2-deficient day 12.5 embryos were cultured overnight with a JAK2 expressing retrovirus, then placed in colony assays. Results indicate the number of colonies per 105 cells plated from 2 animals.
Fetal liver cells from either wild-type or JAK2-deficient day 12.5 embryos were cultured overnight with a retrovirus encoding either membrane-targeted Mpl or cytoplasmic Mpl, then placed in colony assays. Results indicate the number of colonies per 105cells plated from 2 animals.
Discussion
The ability to dissociate Mpl function from its normal plasma membrane location demonstrates that membrane localization is not required for many aspects of Mpl function in normal hematopoietic cells. Remarkably, dimerization of the cytoplasmic version of Mpl in murine marrow cells resulted in the generation of myeloid, erythroid, and megakaryocytic progenitor cells in a 1-month period in vitro (Table1) and in the emergence of terminally differentiated mature blood cells (Figure 6). Cytoplasmic Mpl lacks one attribute of wild-type Mpl: the ability to modulate circulating levels of thrombopoietin.
Numerous reports indicate that cross-talk occurs among hematopoietic growth factor receptors embedded in the plasma membrane,8-10 including physical association and cross-phosphorylation of heterologous receptors. Generalization of this concept has recently been challenged by studies of mice engineered to lack both the common β chain shared by the IL-3, GM-CSF, and IL-5 receptors and the specific β chain of the IL-3 receptor.36 Marrow cells from these mice exhibit a normal proliferative response to G-CSF, erythropoietin (EPO) and stem cell factor, arguing that cross-talk involving the IL-3 receptor is not required by these cytokines. The current results suggest that interaction of Mpl with heterologous receptors within the plasma membrane also is not essential.
Cell surface proteins are not randomly distributed on the plasma membrane; rather, they are organized into microdomains that may be functionally important, including partitioning into or out of lipid-rich rafts,5 caveolae,4 and other cell surface zones.2 Ligand binding to transmembrane hematopoietic growth factor receptors alters the distribution of receptors on the cell surface,29 perhaps facilitating signal transduction. Moreover, certain hematopoietic growth factor receptors, including the EPO receptor, which is highly homologous to Mpl, are organized into preformed dimers or signaling complexes on the cell surface,6,7 which may serve to increase the effective concentration of receptors.37 EPO binding subtly alters the conformation of the preassociated receptors, triggering signal transduction. Thus, under normal circumstances, growth factor receptor display on the cell surface is subject to considerable organization. Despite these observations, the current report demonstrates that dimerization of the signaling domain of Mpl in the cell cytoplasm, independent of the normal topography of the plasma membrane, can support production of the full range of normal hematopoietic progenitor cells and their terminal maturation.
Like other cell surface hematopoietic growth factor receptors, Mpl is internalized after thrombopoietin binding.38,39 Normal cellular trafficking of receptors may be necessary for activation of the full complement of signaling pathways.11 For example, impairment of epidermal growth factor (EGF) receptor or insulin receptor internalization through overexpression of a dominant-negative dynamin suppresses activation of the mitogen-activated protein kinases (MAPKs) ERK1 and ERK2.12,13 Similarly, the intracellular routing of ErbB receptors affects signaling.40 The current report demonstrates that normal cellular trafficking of Mpl is not required for proliferative or differentiative signaling in primary hematopoietic cells.
These findings contrast with previous results using the T-cell receptor,41 fas,42 and the platelet-derived growth factor (PDGF) receptor.43 In the case of the PDGF receptor and other receptor tyrosine kinases, membrane recruitment of Src homology 2 (SH2) domain-containing cytoplasmic proteins such as the p85 subunit of phosphoinositide 3′ kinase, Shc, Grb2, and phospholipase Cγ is believed to be essential for signal transduction.44 Although a cytoplasmic PDGF receptor derivative became phosphorylated after dimerization, activation of p70 S6 kinase and MAPK failed to occur in the absence of membrane attachment. Similarly, the cellular compartment in which the epithelial growth factor (EGF) receptor is activated has important implications for signaling.45 Deletion of the membrane-anchoring region of EGF resulted in intracrine activation of the EGF receptor and altered the organotypic growth pattern of an epithelial cell line. Furthermore, in another study, the addition of a KDEL endoplasmic reticulum retention motif to IL-3 resulted in intracellular activation of IL-3 receptor and autocrine proliferation of a factor-dependent cell line.46 However, this study46 did not examine the ability of intracellularly activated IL-3 receptor to support proliferation and differentiation of normal hematopoietic cells.
JAK2 is essential for signal transduction by wild-type Mpl.27,47 The current report demonstrates that both membrane-targeted Mpl and cytoplasmic Mpl mimic wild-type Mpl in their JAK2 dependency in primary hematopoietic cells. Like their wild-type counterparts, neither membrane-targeted Mpl nor cytoplasmic Mpl requires STAT5 to induce cell growth. It is possible that cytoplasmic JAK2 activation alone is sufficient to induce self-renewal among primitive multipotent hematopoietic cells.48 Signaling studies in Ba/F3 cells expressing the membrane-tethered or the cytoplasmic Mpl fusion protein did not show activation of JAK2. We interpret these results to mean that the Mpl fusion proteins activate JAK2 below the limit of detection by Western blot analysis.
In summary, these results show that plasma membrane localization is not required for expression of the full range of Mpl functions in normal hematopoietic cells, and they suggest that the major purpose of receptor display on the plasma membrane is to enable communication with the extracellular milieu.
We thank T. Clackson (ARIAD Pharmaceuticals, www.ariad.com) for supplying AP20187, and James Yan and Hui Zeng for expert technical support.
Supported by National Institutes of Health grants R01DK52997, R01DK57525, P01HL53750, P01DK47754, and DK49855 and by an award from the Fanconi Anemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. Anthony Blau, Department of Medicine, University of Washington, Box 357710, 1959 NE Pacific St, Seattle, WA 98195; e-mail: tblau@u.washington.edu.


![Fig. 2. Proliferation assays and signaling studies comparing dose–response curves and signaling in Ba/F3 cell clones expressing the membrane-tethered or cytoplasmic Mpl fusion protein. / (A) Cell proliferation (MTT) assays of Ba/F3 cell clones expressing either the (i) cytoplasmic or the (ii) membrane-tethered form of Mpl. Proliferation data are represented as a fraction of the proliferative response of Ba/F3 cells to 5% conditioned media containing murine IL-3. (B) JAK2, MAPK, and STAT5 phosphorylation were assessed by Western blot analysis in Ba/F3 cell clones expressing either wild-type Mpl [MPL (WT)] or cytoplasmic [MPL (CYTO)] or membrane-targeted [MPL (MEM)] Mpl fusions. Wild-type Mpl was activated using thrombopoietin, whereas Mpl fusions were activated by adding AP20187 (100 nM) for the time indicated (in minutes). IP, immunoprecipitation; WCL, whole cell lysate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/7/10.1182_blood.v98.7.2077/5/m_h81911593002.jpeg?Expires=1765950236&Signature=ADB6L1sfMEH6qhVD0XvYq-Z7lX6JY3xv5X45kAz8BlOfJBgP7pch4soZIuHnRMZzwOg6aw6RkGIeZlvdJgj9co4e8PlZhOUO4X-2DXQj572~Xe5l2YupQtpA5vlm2I98x7TNuWt39cSKHFSzzZUvRfQ0IFG1Feem9qd-~wtULEgn4JHsr0I1LZKou0qo2-6hvTsrv8ntwYCVuSYk9SgAU2icMh8LwBHsZ9tFV64-EksLVY7-N8epIYPPfTyopbtPWmxENW6qGftTuj5t8LTD099HhZnIR~MPPJ0pLeS6pYHx9~HCaol62E82gmHEULadOilwH-kX9iCLsYQiztW6YA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal