The sequence of events and the mechanisms leading to the destruction of the thymus during human immunodeficiency virus (HIV) infection are still poorly characterized. Investigated here are the survival capacity on HIV-1 infection of the mature single-positive CD4+CD8−CD3+ (SP CD4+) and the intermediate CD4+ CD8−CD3− thymocytes previously shown to be able to replicate the virus in the thymic microenvironment. It is demonstrated that the mature SP CD4+ thymocytes exhibit a high survival capacity despite the production of a high yield of viruses. Interleukin-7, reported to be a crucial cofactor of tumor necrosis factor (TNF) to promote HIV replication, is shown here to counteract the apoptotic activity of TNF. Resistance to apoptosis of SP CD4+ cells is conferred by a high expression of the IL-7 receptor (IL-7R) associated with the capacity of IL-7 to permanently up-regulate Bcl-2. In addition, this high Bcl-2 level is further enhanced by infection itself. In contrast, intermediate thymocytes, which replicate the virus at a lower level, are more sensitive to apoptosis, and their differentiation into double-positive CD4+CD8+CD3− (DP CD3−) cells strongly increases their death rate on infection. This sensitivity is related to a lower expression of IL-7R and Bcl-2 in intermediate thymocytes, which further decreases at the DP CD3− stage. In addition, a decreased level of Bcl-2 is observed in this subset during infection. Altogether these data suggest that in vivo, HIV infection might create a persistent virus reservoir within the SP CD4+ thymocytes, whereas the later infection of intermediate cells might lead to thymopoiesis failure.
Introduction
Depletion of CD4 T lymphocytes is a characteristic feature of immune dysfunction during the progression of human immunodeficiency virus (HIV) infection. Multiple mechanisms, such as a permanent destruction1 and failure of reconstitution of this T-cell pool,1-3 likely contribute to this depletion. The contribution of the thymus to T-cell reconstitution during HIV infection has been suggested by the prevalence of abundant thymic tissue in 50% of HIV-1 infected adults4 and a larger increase in the number of naive T cells after HAART in these patients compared with those who have minimal thymic tissue.5,6 The determination by polymerase chain reaction of T-cell rearrangement excision circles (TRECs) has been proposed as a way to quantify recent thymic emigrants in peripheral blood.7 Using this approach, a low TRECs concentration was shown to be an important predictive factor for HIV-1 disease progression.8 The extent of thymic function is also dependent on disease stage, as shown in macaques infected by the simian immunodeficiency virus (SIV). For instance, a transient rebound in thymic activity, characterized by an increase in the influx of CD34+ precursors, was demonstrated during primary infection and was followed, at a later stage, by thymus damage.9Late thymic failure is reminiscent of the fact that, among patients with advanced disease administered antiretroviral therapies, CD4 cell counts often remain below normal despite long-term suppression of viral load.10 11
Furthermore, thymuses from pediatric patients who experienced accelerated disease processes12,13 or from adults with acquired immunodeficiency syndrome (AIDS)14,15 showed severe thymocyte depletion associated with a profound disorganization of the thymic epithelial network. Such alterations correlate with the presence of the virus in the thymuses of humans16-18 and animals, among them infected macaques19,20 and SCID-hu mice.21 22
As a possible explanation for thymocyte depletion, it has been suggested that CD34+ progenitors from HIV-infected patients have a reduced capacity to colonize the thymus.23 However, thymic infection also results in increased death rates of thymocytes in human and animal models. Nevertheless, there are some discrepancies in the literature regarding thymocyte subsets killed on infection of the thymus and on the mechanisms involved in their death. In SCID-hu mice, depletion of CD4+ thymocytes was attributed to the direct killing of infected cells by a mechanism distinct from apoptosis, whereas later stages of infection were characterized by increased apoptosis of the CD4− thymocytes.24 By contrast, in the same model, Su et al25 described an apoptotic mechanism in all thymocytes; however, only the CD4+CD8−CD3− cells are directly killed by infection. Conversely, in a human fetal thymic organ culture, HIV-1 infection induces a preferential loss of CD4+thymocytes by an apoptotic mechanism.26 In SIV-infected macaques, most of the infected thymic cells were located in the medulla, whereas apoptotic cells were in the cortex.20 In this model, double-positive CD4+CD8+ thymocytes were shown to be particularly sensitive to apoptosis.9
This paper aims at studying the mechanisms controlling the survival capacity of human thymocytes on HIV infection in the presence of factors of the thymic microenvironment. We previously demonstrated that the interaction of human thymocytes with thymic epithelial cells is crucial for HIV replication.27 This interaction leads to the cosecretion of 2 crucial cytokines for viral replication, tumor necrosis factor (TNF) and interleukin-7 (IL-7), that further synergize with IL-6, IL-1, and GM-CSF.27,28 However, this interaction is efficient only with mature single-positive CD4+CD8−CD3+ (SP CD4+) thymocytes.28 The restriction of viral replication in the other CD4+ cells was attributed to the lack of responsiveness to the required cytokines by double-positive CD4+CD8+CD3+/− and to the lack of TNF secretion during interaction of the intermediate CD4+CD8−CD3− thymocytes with epithelial cells.28 However, in the latter, the lack of TNF might be compensated for by the TNF secreted by activated macrophages that infiltrate the thymus during infection.16 18
We also showed that the deleterious effect of the virus on monocytic or lymphocytic cells was mediated by oxidative stress, leading to a decrease of Bcl-2 and, consequently, to apoptosis.29However, in cells in which persistent infection can be established, compensatory mechanisms take place to maintain or increase the Bcl-2 level.29 30
In this study, we focused on the survival capacity of 2 thymocyte subsets—mature SP CD4+ cells and intermediate cells able to replicate the virus in the presence of the required cytokines. We particularly studied the modulation of infection-induced cell death by TNF and IL-7, which are the crucial cytokines for HIV replication and which were shown to induce31,32 or to counteract33 34 apoptosis, respectively. We also investigated the Bcl-2 status of thymocytes resulting from the cytokine microenvironment and infection.
Materials and methods
Reagents
Antibodies used for the selection of the thymocyte subset.
The monoclonal antibodies (mAbs) used were CD3 (X35), CD3 (UCHT1), CD8 (B9.11), CD83 (HB15A), CD10 (ALB 1), CD34 (QBEND 10), all purchased from Beckman Coulter France S.A. Villepinte, and CD14 (MΦP9) from BD Biosciences (Erembodegem, Belgium).
Antibodies used for immunostaining.
To characterize thymic subpopulations, we used the following conjugated mAbs: Opticlone CD4-fluorescein isothiocyanate (FITC)/CD8-phycoerythrin (PE)/CD3-PE-CY5 (13B8.2/B9.11/UCHT-1), CD10-FITC (ALB 2), CD34-PE (QBEND-10) (Beckman Coulter France S.A), CD83-PE (HB15a), and CD14-FITC (MΦP9) (BD Biosciences). As negative control, we used Opticlone IgG1-FITC/IgG1-PE/IgG1-PE-CY5 (679.1Mc7) (Beckman Coulter France S.A). Expression of the IL-7 receptor α-chain was determined by using CDw127-PE antibodies (R34.34) and IgG1-PE (679.1Mc7) as a negative control (Beckman Coulter France S.A). For the antibodies cited, unconjugated control IgG1 antibody (679.1Mc7; Beckman Coulter France S.A) was used for treatment before immunostaining to avoid nonspecific binding of fluorochrome-labeled antibodies. Bcl-2 production was analyzed by using Bcl-2–PE antibody (Bcl-2/100) with the control IgG1-PE (MOPC-21) from BD-PharMingen (San Diego, CA). Measurement of intracellular p24 expression was performed with p24-FITC antibody (KC57) with the control MsIgG1-FITC (679.1Mc7) (Beckman Coulter France S.A). For triple immunostaining for CD4, CD8, and IL-7R or Bcl-2, anti–CD4-PC5 (13B8.2) anti-CD8–FITC (B9.11) antibodies and their control IgG1-PC5 and IgG1-FITC (both 679.1Mc7) were used (Beckman Coulter France S.A). Immunostaining was analyzed using an XL-4C cytofluorometer (Beckman Coulter France S.A).
Antibodies used for Western blot analysis.
Monoclonal anti–Bcl-2 (Bcl-2/100) from BD-PharMingen and anti–β-actin (AC-74) from Sigma (St Louis, MO) were used for Western blot analysis. Anti–mouse IgG conjugated with horseradish peroxidase (Amersham Pharmacia Biotech, Uppsala, Sweden) was used as a secondary antibody.
Cytokines.
All the human recombinant cytokines (R&D Systems, Minneapolis, MN) were used at the following concentrations: IL-6 and IL-1β at 10 ng/mL, TNF-α at 0, 1, or 1 ng/mL, and IL-7 at 0, 5, or 5 ng/mL.
Preparation of thymocyte subpopulations and culture
Thymocytes were isolated from fresh thymus fragments obtained during elective cardiac surgery on HIV-seronegative children (age range, 6 days to 24 months), as previously described.28,35The single positive CD4+CD8−CD3+(SP CD4+), the immature CD4−/+CD8−CD3− (as a pool of triple-negative CD4−CD8−CD3−[TN] and intermediate CD4+CD8−CD3−), and the immature double-positive CD4+CD8+CD3−thymocytes were obtained by negative selection as previously described.28 Thymocytes were then cultured in Mc-Coy 5A, 10% fetal calf serum, 1 mM L-glutamine, 10 mM HEPES, and antibiotics. Cytokines were added at the start of the culture and then every 3 or 4 days.
Infection of thymocytes
All infections were performed with the primary isolate HIV-1B-LAIp (p represents primary isolate)36at a multiplicity of infection of approximately 0.05, as previously described,28 and were cultured in the presence of cytokines. HIV-1 p24gag antigen concentration was determined in culture supernatants using the Coulter kit HIV-1 p24 antigen assay (Beckman Coulter France S.A.).
Measurement of apoptosis
Apoptotic cells were labeled with a YO-PRO-1 iodide solution (1/200 in phosphate-buffered saline) (Interchim-Molecular Probes, Eugene, OR) or by terminal dUTP nick-end labeling (TUNEL) using a kit from Roche Molecular Biochemicals (Mannheim, Germany). The percentage of apoptotic cells was also determined on the basis of the forward scatter–side scatter profiles as previously described.37 For all techniques, analysis was carried out by flow cytometry using a XL-4C cytofluorometer (Beckman Coulter France S.A.).
Western blot analysis
Preparation of total extracts of thymocytes and Western blot analysis were performed as previously described.29Densitometric analysis of the autoradiograms was carried out with a Gene Genius GG system imager (Ozyme-Syngène, Saint-Quentin Yveline, France).
Statistical analysis
Because of the variability in the studied parameters displayed by the different thymuses from different donors, we performed statistical analysis using the nonparametric Mann-Whitney Utest. Significance level was set at P < .05.
Results
Mature SP CD4+ thymocytes exhibited a strong capacity to survive during HIV-1 infection, in contrast to immature thymocytes
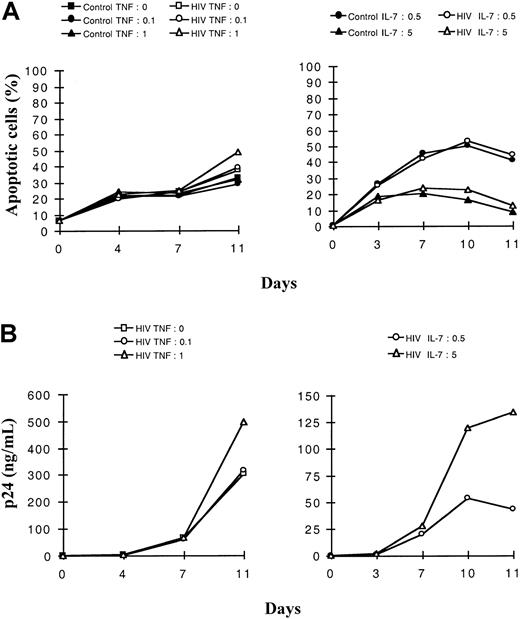
We aimed first at determining ex vivo the levels of constitutive and virus-induced apoptosis of the mature single positive CD4+ CD8−CD3+ (SP CD4+) and intermediate CD4+CD8−CD3− thymocytes, namely the 2 subsets able to produce the virus in the thymic microenvironment.28 Mature SP CD4+ and immature thymocytes, the latter isolated as a pool of intermediate and TN cells for technical convenience, were infected with a primary isolate, HIV-1B-LAIp. The cells were then maintained in culture in the presence of IL-1, IL-6, IL-7, and TNF. In this first experiment, the cytokines were used at the concentrations leading to the highest level of HIV replication in each subset. Apoptosis data presented here were obtained with the Yopro dye technique that was perfectly related to morphologic analysis of cells based on forward scatter–side scatter profiles. Similar results were obtained with TUNEL analysis (data not shown). As shown in Figure1A, the mature SP CD4+thymocytes displayed ex vivo a relatively low constitutive rate of apoptosis that was stable or slightly increased by infection. In contrast, the immature subset exhibited a higher constitutive rate of apoptosis, and these cells massively died within a few days of infection. On the basis of 5 independent experiments, we performed statistical analysis confirming that the difference of apoptotic levels between control and infected cells was significantly higher in immature than in SP CD4+ thymocytes (P < .01). Similar resistance of SP CD4+ cells to virus-induced apoptosis was observed using the HIV molecular clone NL4-3 (data not shown). Furthermore, the survival capacity of mature SP CD4+ was higher than that of immature cells in spite of higher virus production, as revealed by p24 analysis (Figure 1B, P < .01). The major determinant of this higher replication level in the SP CD4+ subset is the activation level induced by TNF and IL-7 in these cells.28 There is no implication of the expression level of the CXCR4 coreceptor used by HIV-1B-LAIp, which is even less expressed in SP CD4+ thymocytes than in immature thymocytes (38 39 and data not shown).
Kinetics of apoptosis and viral production in mature SP CD4+ and immature (intermediate and TN) thymocytes.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-7 (5 ng/mL), TNF-α (1 ng/mL), IL-1 (10 ng/mL), and IL-6 (10 ng/mL). Percentages of apoptotic cells were determined by the Yopro dye technique (A), and viral production was evaluated by p24 titration in the culture supernatants (B). Data are given as a mean of independent experiments carried out with thymuses from 5 different donors. ▪ indicates SP CD4+; ●, immatures ■, SP CD4+/HIV; ○, immatures/HIV.
Kinetics of apoptosis and viral production in mature SP CD4+ and immature (intermediate and TN) thymocytes.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-7 (5 ng/mL), TNF-α (1 ng/mL), IL-1 (10 ng/mL), and IL-6 (10 ng/mL). Percentages of apoptotic cells were determined by the Yopro dye technique (A), and viral production was evaluated by p24 titration in the culture supernatants (B). Data are given as a mean of independent experiments carried out with thymuses from 5 different donors. ▪ indicates SP CD4+; ●, immatures ■, SP CD4+/HIV; ○, immatures/HIV.
Immature thymocytes are permissive to HIV infection at the intermediate stage and die of apoptosis, particularly at the CD4+CD8+CD3−double-positive stage
Under our conditions of culture, we observed a differentiation process from the pool of TN and intermediate thymocytes. Therefore, we wondered whether the massive death observed in the immature subset on infection occurs at the intermediate stage or at a later stage of differentiation. We thus performed phenotypical analysis of the immature thymocytes at the time of their isolation and after 11 days of culture under control or infection conditions. The percentage of cells of each subset within the immature population was determined by flow cytometry analysis of the surface markers CD4, CD8, and CD3. We first confirmed that at the time of isolation (Figure2, day 0), the 2 main subsets are TN (CD4−CD8−CD3−) and intermediate (CD4+CD8−CD3−). However after 11 days in culture (Figure 2, day 11), under control conditions, we observed a decrease in the percentage of the TN and intermediate thymocytes and the appearance of a large subset of double positive CD4+CD8+CD3− (DP CD3−) and a few DP CD3low cells, demonstrating a differentiation process within the culture. However, this process was not induced to completion because the DP CD3high stage was not attained and the SP CD4 or CD8 stage was seen only for a low percentage of cells. Of particular interest, on HIV-1 infection, a strong decrease in the percentage of the DP CD3− subset occurred (P < .002), whereas an increase in the percentage of the TN subpopulation (P < .001) and no modification in the percentage of intermediate cells were observed (Figure 2, day 11). These data indicate that the proportion of TN cells among the pool of immature cells increased because the cells were not permissive to infection, whereas the percentage of intermediate cells was unchanged because of the equilibrium between a higher rate of differentiation from TN cells and a moderate rate of death from infection. A more thorough analysis of the intermediate thymocytes revealed a differential survival capacity inversely related to the level of expression of CD4 (data not shown). We observed that cells expressing the lowest levels of CD4 and closest to the TN stage survived infection best, in contrast to cells expressing the highest levels of CD4 and closest to the DP stage. However, we can likely rule out the possibility that the depletion of DP cells could have been caused by a blockade of differentiation by the virus at the intermediate stage because a high proportion of these cells was already present early in the course of infection—for instance, at 7 days (data not shown)—before the occurrence of virus-induced apoptosis (Figure1). In conclusion, the death rate of immature thymocytes strongly increased once differentiation into DP cells was achieved.
Phenotypic analysis of immature thymocytes, determined by analysis of CD3, CD4, and CD8 expression, in uninfected and HIV-infected cells.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-7 (5 ng/mL), TNF-α (1 ng/mL), IL-1 (10 ng/mL), and IL-6 (10 ng/mL). Immunostaining of surface antigens was analyzed by flow cytometry at the time of cell isolation (day 0) and after 11 days of culture with or without HIV-1 infection. Data are given as percentages of labeled cells as a mean of independent experiments carried out with thymuses from 7 different donors. ▪ indicates control; ■, HIV.
Phenotypic analysis of immature thymocytes, determined by analysis of CD3, CD4, and CD8 expression, in uninfected and HIV-infected cells.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-7 (5 ng/mL), TNF-α (1 ng/mL), IL-1 (10 ng/mL), and IL-6 (10 ng/mL). Immunostaining of surface antigens was analyzed by flow cytometry at the time of cell isolation (day 0) and after 11 days of culture with or without HIV-1 infection. Data are given as percentages of labeled cells as a mean of independent experiments carried out with thymuses from 7 different donors. ▪ indicates control; ■, HIV.
Tumor necrosis factor-α triggers apoptosis whereas interleukin-7 is protective in infected thymocytes
We then studied the roles of TNF and IL-7, known respectively for their cytotoxic or protective effect. We addressed the question of the interplay of these 2 cytokines on constitutive or HIV-induced apoptosis in the 2 studied thymocyte populations. For this purpose, the 2 subsets of HIV-1B-LAIp–infected thymocytes were exposed either to 2 different concentrations of TNF in the presence of a constant concentration of IL-7 or to 2 different concentrations of IL-7 in the presence of a constant concentration of TNF. As shown in Figure3A for the immature thymocytes, TNF and IL-7 exhibited antagonist effects on the levels of constitutive or virus-induced apoptosis. In a dose-dependent manner, TNF favored apoptosis (P < .05) whereas IL-7 counteracted this effect (P < .05). However, in SP CD4+ thymocytes, apoptosis slightly increased with TNF concentration exclusively on HIV infection (P < .05), whereas a high concentration of IL-7 protected efficiently both control and infected cells (P < .05), as shown in Figure4A. Increasing concentrations of IL-7, though they induced increased HIV replication (Figures 3, 4B,P < .05), conferred higher protection against apoptosis, especially in the mature thymocytes (Figure 4A). In contrast, TNF increased both HIV replication (Figures 3, 4B, P < .05) and apoptosis, especially in the immature thymocyte population (Figure 3A).
Dose-effect of TNF-α and IL-7 on the kinetics of apoptosis and viral production in immature (intermediate and TN) thymocytes.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-1 (10 ng/mL), IL-6 (10 ng/mL), IL-7, and TNF-α. In panels A and B, cells were exposed to 2 different concentrations of TNF-α (0.1 or 1 ng/mL) in the presence of a constant concentration of IL-7 (5 ng/mL) (left panels), or they were exposed to 2 different concentrations of IL-7 (0.5 and 5 ng/mL) in the presence of a constant concentration of TNF-α (1 ng/mL) (right panels). Percentage of apoptotic cells was determined by the Yopro dye technique (A), and viral production was evaluated by p24 titration (B). The experiment presented here is representative of independent experiments carried out with thymuses from 3 different donors.
Dose-effect of TNF-α and IL-7 on the kinetics of apoptosis and viral production in immature (intermediate and TN) thymocytes.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-1 (10 ng/mL), IL-6 (10 ng/mL), IL-7, and TNF-α. In panels A and B, cells were exposed to 2 different concentrations of TNF-α (0.1 or 1 ng/mL) in the presence of a constant concentration of IL-7 (5 ng/mL) (left panels), or they were exposed to 2 different concentrations of IL-7 (0.5 and 5 ng/mL) in the presence of a constant concentration of TNF-α (1 ng/mL) (right panels). Percentage of apoptotic cells was determined by the Yopro dye technique (A), and viral production was evaluated by p24 titration (B). The experiment presented here is representative of independent experiments carried out with thymuses from 3 different donors.
Dose-effect of TNF-α and IL-7 on the kinetics of apoptosis and viral production in SP CD4+ thymocytes.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-1 (10 ng/mL), IL-6 (10 ng/mL), IL-7, and TNF-α. In panels A and B, cells were exposed to 2 different concentrations of TNF-α (0.1 or 1 ng/mL) in the presence of a constant concentration of IL-7 (5 ng/mL) (left panels), or they were exposed to 2 different concentrations of IL-7 (0.5 and 5 ng/mL) in the presence of a constant concentration of TNF-α (1 ng/mL) (right panels). Percentage of apoptotic cells was determined by the Yopro dye technique (A), and viral production was evaluated by p24 titration (B). The experiment presented here is representative of independent experiments carried out with thymuses from 3 different donors.
Dose-effect of TNF-α and IL-7 on the kinetics of apoptosis and viral production in SP CD4+ thymocytes.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-1 (10 ng/mL), IL-6 (10 ng/mL), IL-7, and TNF-α. In panels A and B, cells were exposed to 2 different concentrations of TNF-α (0.1 or 1 ng/mL) in the presence of a constant concentration of IL-7 (5 ng/mL) (left panels), or they were exposed to 2 different concentrations of IL-7 (0.5 and 5 ng/mL) in the presence of a constant concentration of TNF-α (1 ng/mL) (right panels). Percentage of apoptotic cells was determined by the Yopro dye technique (A), and viral production was evaluated by p24 titration (B). The experiment presented here is representative of independent experiments carried out with thymuses from 3 different donors.
Level of interleukin-7R expression on thymocytes correlates with their differential survival capacity on infection
The role of IL-7 as an antiapoptotic cytokine in infected thymocytes and the sensitivity to apoptosis of the distinct thymocyte subsets (Figure 2) suggested a difference in their responses to IL-7. We then investigated the expression of IL-7 receptor (IL-7R) in the DP CD3−, immature (TN and intermediate), and SP CD4+ thymocytes. To emphasize the detection of IL-7R, which is internalized or masked by IL-7 produced in the thymic microenvironment (data not shown), flow cytometry analysis was performed on cells cultured 24 hours after cell isolation in the absence of IL-7. As shown in Figure 5, the population of SP CD4+ thymocytes comprises 2 subsets, characterized as IL-7Rlow and IL-7Rhigh. The IL-7Rlow SP CD4+ are also characterized by a low expression of CD69 (data not shown), indicating that these cells have not attained complete maturation.40 41 These low-expressing cells were shown to die of apoptosis after 4 days in culture (data not shown). In contrast, the level of IL-7R, expressed as mean fluorescence intensity (MFI) in the IL-7Rhigh SP CD4+ cells, was particularly high compared to those in the DP CD3− or in the immature subpopulations (P < .05). Given that, in our conditions, intermediate cells were enriched with the TN cells as a pool of immature thymocytes, we performed double labeling for both CD4 and IL-7R to distinguish between both subsets. We observed a slightly but significantly higher level of IL-7R on TN than on intermediates (20% increase of MFI, mean of 4 assays; P < .03). In addition, intermediate thymocytes included both CD4low and CD4high cells, and the level of expression of CD4 was inversely correlated with the level of IL-7R (data not shown). Intermediate cells constituted the largest part of the pool of immature thymocytes (70% as median values for 4 assays). Thus, as shown in Figure 5, IL-7R expression was detected in a large proportion of the immature, mostly intermediate, cells at a higher level than in DP CD3− thymocytes (P < .05). Therefore, differences in the level of expression of IL-7R correlated with observed variations in the survival capacity of the studied subsets (Figures 1, 2 and text).
Expression of IL-7R on DP CD3−, immature (intermediate and TN), and SP CD4+ thymocytes.
Cells were isolated as described in “Materials and methods” and were maintained in culture in medium alone for 1 day. Immunostaining of IL-7R was analyzed by flow cytometry. Percentage of labeled cells and mean fluorescence intensity (MFI) are indicated in each quadrant. The experiment presented here is representative of independent experiments carried out with thymuses from 3 to 4 different donors. Dotted line indicates isotypic control; solid line, IL-7R.
Expression of IL-7R on DP CD3−, immature (intermediate and TN), and SP CD4+ thymocytes.
Cells were isolated as described in “Materials and methods” and were maintained in culture in medium alone for 1 day. Immunostaining of IL-7R was analyzed by flow cytometry. Percentage of labeled cells and mean fluorescence intensity (MFI) are indicated in each quadrant. The experiment presented here is representative of independent experiments carried out with thymuses from 3 to 4 different donors. Dotted line indicates isotypic control; solid line, IL-7R.
Level of Bcl-2 expression correlated with the level of expression of IL-7R in the distinct thymocyte subsets
We determined the expression level of Bcl-2 by flow cytometry analysis in DP CD3−, immature (TN and intermediate), and SP CD4+ thymocytes at the time of cell isolation and after 4 days of culture in the presence or absence of IL-7. As shown in Figure 6, under the stimulation conditions of the microenvironment (at day 0) or in the presence of IL-7, a major part of the SP CD4+ cells are Bcl-2high, and a smaller fraction is Bcl-2low. As for IL-7Rlow (Figure 5), these Bcl-2lowthymocytes constitute a pool of apoptotic cells (forward scatter–side scatter profile; data not shown) regardless of whether IL-7 is present. It is likely that the low constitutive rate of apoptosis observed within the SP CD4+ pool (Figure 1) is related to this minor fraction of less mature (CD69low) cells characterized by an IL-7RlowBcl-2low phenotype. In the absence of IL-7, Bcl-2 level decreased even in the SP CD4+ cells previously identified as Bcl-2high (at day 0 or under IL-7 treatment), leading to 2 peaks of low Bcl-2–expressing cells. The rate of survival decreased to 36% (forward scatter–side scatter profile). At day 0, the SP CD4+ Bcl-2high thymocytes exhibited a higher Bcl-2 level than immature cells or DP CD3− thymocytes (P < .05). As previously shown for IL-7R analysis, double labeling of immature subset for both CD4 and Bcl-2 performed at day 0 showed that Bcl-2 was expressed at a significantly higher level in TN than in intermediate thymocytes (87.9% labeled TN cells vs 72.8% labeled intermediate cells, 30% MFI decrease in intermediate cells compared with TN; mean of 4 assays;P < .03). Furthermore, among intermediate thymocytes, CD4high cells expressed a lower level of Bcl-2 than the CD4low population (data not shown). As shown in Figure 6 at day 0, immature, mostly intermediate, cells expressed a higher level of Bcl-2 than DP CD3− cells (P < .05). We also showed in Figure 6 that in all thymocyte subsets, Bcl-2 expression was decreased after 4 days in culture, unless IL-7 was added to the culture medium. This induction by IL-7 was underlined by a significant shift of the peaks of the Bcl-2–positive cells on the x-axis in the presence of this cytokine compared to their positions at day 0 (when IL-7 was not in saturating conditions) and at day 4 in the absence of IL-7 (Figure6). However, even in the presence of IL-7, the rate of survival cells was 51% for the immature and 33% for the DP cells (forward scatter–side scatter profiles). These data demonstrate that IL-7 is required to induce or sustain Bcl-2 in all thymocyte subsets and that this level of induction is directly related to the level of IL-7R expression (Figures 5, 6).
Expression of Bcl-2 in DP CD3−, immature (intermediate and TN), and SP CD4+ thymocytes and role of IL-7.
Cells were isolated as described in “Materials and methods.” Immunostaining of Bcl-2 was analyzed by flow cytometry at the time of isolation and after 4 days of incubation in the presence or absence of IL-7 (5 ng/mL). Percentage of labeled cells and MFI are indicated in each quadrant. The experiment presented here is representative of independent experiments carried out with thymuses from 2 (day 0 and day 4) to 3 (day 0) different donors. Dotted line indicates isotypic control; solid line, Bcl-2.
Expression of Bcl-2 in DP CD3−, immature (intermediate and TN), and SP CD4+ thymocytes and role of IL-7.
Cells were isolated as described in “Materials and methods.” Immunostaining of Bcl-2 was analyzed by flow cytometry at the time of isolation and after 4 days of incubation in the presence or absence of IL-7 (5 ng/mL). Percentage of labeled cells and MFI are indicated in each quadrant. The experiment presented here is representative of independent experiments carried out with thymuses from 2 (day 0 and day 4) to 3 (day 0) different donors. Dotted line indicates isotypic control; solid line, Bcl-2.
High expression level of Bcl-2 in mature SP CD4+IL-7RhighBcl-2high is further enhanced by HIV infection, whereas the low level of Bcl-2 observed in immature thymocytes is further decreased by HIV infection
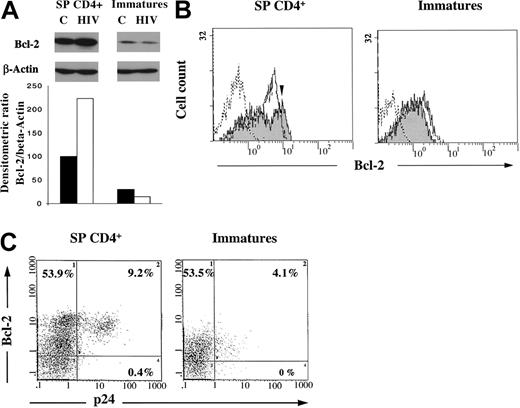
We demonstrated recently that infection itself was able to induce the up-regulation of Bcl-2 in a cellular model of HIV persistence.29 We now address whether such a mechanism occurs in mature SP CD4+IL-7RhighBcl-2high thymocytes (and not in immature cells), which might further strengthen their resistance to apoptosis on HIV infection. We then compared Bcl-2 status of the control or infected SP CD4+ population or immature thymocytes after 11 days in culture. At that time of culture, among the SP CD4+ thymocytes, only the IL-7RhighBcl-2high subset was selected, as pointed out earlier. As shown in Figure7A, Bcl-2 was analyzed by Western blot and was quantified as a ratio of Bcl-2/β-actin after densitometry analysis of the autoradiogram. First, as a confirmation of data shown in Figure 6, Bcl-2 is expressed at a much higher level in uninfected SP CD4+ than in the immature thymocytes (P < .01), especially after 11 days of culture, a time at which immature thymocytes consisted mainly of DP CD3−cells, as previously shown in Figure 2. However, the Bcl-2 concentration in SP CD4+ cells was further increased on HIV infection (P < .01). In contrast, in immature thymocytes, the low concentration of Bcl-2 was further decreased by HIV infection (P < .01). The increase in the Bcl-2 concentration observed in infected SP CD4+ cells suggested that the infection was able to induce an up-regulation of Bcl-2. To strengthen this hypothesis, we performed flow cytometry analysis of both intracellular Bcl-2 and p24 on HIV infection. This was carried out after 11 days of infection in both SP CD4+ and immature thymocytes as a control population dying from infection. As shown in Figure 7B, approximately 70% of control or infected SP CD4+ cells expressed Bcl-2. However, control cells displayed a homogeneous population for Bcl-2 expression (MFI, 4.8), whereas HIV-infected SP CD4+ thymocytes comprised a subpopulation (50% of Bcl-2–positive cells) in which Bcl-2 content was increased, as demonstrated by the shift (MFI, 8.8; indicated by the arrow) of the corresponding peak in Figure 7B. On the contrary, HIV-infected immature thymocytes exhibited a lower level of Bcl-2 than uninfected control cells. Of particular interest is the observation that p24-expression takes place in the cells overexpressing Bcl-2 under infection (Figure 7C), suggesting that infection itself might be responsible for a further increase in Bcl-2 level. This induction was not significantly observed in the immature subset. The higher level of virus production in the SP CD4+ subset was indicated by both a higher percentage of p24 positive cells and a higher level of p24 per cell (9.2% and MFI of 16.3 for SP CD4+ vs 4.1% and MFI of 3.5 for immature cells) (Figure 7C). This high level of viral replication likely accounts for the efficient induction of Bcl-2 in SP CD4+ thymocytes.
Expression of Bcl-2 and intracellular p24 in SP CD4+ and immature thymocytes.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-7 (5 ng/mL), TNF-α (1 ng/mL), IL-1 (10 ng/mL), and IL-6 (10 ng/mL) for 11 days. (A) Bcl-2 expression was analyzed by Western blot on cell extracts from both control (C) and infected cells (HIV) in comparison with β-actin as a protein of unchanged expression. Quantification was determined as a ratio of Bcl-2/β-actin after densitometry analysis of the autoradiogram. Data are presented as the mean of independent experiments carried out with thymuses from 5 different donors. (B) Bcl-2 expression was analyzed by immunostaining and flow cytometry in uninfected (white area) and infected thymocytes (gray area). Dotted line indicates isotypic control. The arrow indicates the shift of the peak of Bcl-2–positive cells on infection. The experiment presented here is representative of independent experiments carried out with thymuses from 2 different donors. (C) Bcl-2 and intracellular p24 expression was analyzed by double immunostaining and flow cytometry in HIV-infected thymocytes. Percentages of single- and double-labeled cells are indicated in each quadrant. The experiment presented here is representative of independent experiments carried out with thymuses from 2 different donors.
Expression of Bcl-2 and intracellular p24 in SP CD4+ and immature thymocytes.
Cells were isolated as described in “Materials and methods,” infected with a primary isolate HIV-1B-LAIp, and maintained in culture in the presence of IL-7 (5 ng/mL), TNF-α (1 ng/mL), IL-1 (10 ng/mL), and IL-6 (10 ng/mL) for 11 days. (A) Bcl-2 expression was analyzed by Western blot on cell extracts from both control (C) and infected cells (HIV) in comparison with β-actin as a protein of unchanged expression. Quantification was determined as a ratio of Bcl-2/β-actin after densitometry analysis of the autoradiogram. Data are presented as the mean of independent experiments carried out with thymuses from 5 different donors. (B) Bcl-2 expression was analyzed by immunostaining and flow cytometry in uninfected (white area) and infected thymocytes (gray area). Dotted line indicates isotypic control. The arrow indicates the shift of the peak of Bcl-2–positive cells on infection. The experiment presented here is representative of independent experiments carried out with thymuses from 2 different donors. (C) Bcl-2 and intracellular p24 expression was analyzed by double immunostaining and flow cytometry in HIV-infected thymocytes. Percentages of single- and double-labeled cells are indicated in each quadrant. The experiment presented here is representative of independent experiments carried out with thymuses from 2 different donors.
Discussion
In the current report we investigated the survival capacity on HIV infection of the SP CD4+ and intermediate CD4+CD8−CD3− thymocytes, which we have previously shown able to replicate the virus in the presence of cytokines of the thymic microenvironment.28 Our results clearly demonstrated that the mature SP CD4+ thymocytes display a high capacity to survive, whereas the pool of immature thymocytes (isolated as intermediate and TN thymocytes) massively die of apoptosis on infection. Because of differentiation in culture, the immature population evolves from TN CD4−CD8−CD3− and intermediate CD4+CD8−CD3− (CD4lowto CD4high) to double positive CD4+CD8+CD3− (DP CD3−) thymocytes. Considering the resistance to apoptosis of these subpopulations during the course of infection, we conclude that TN thymocytes (which are not permissive to HIV-1) are maintained at the highest proportion, whereas intermediate cells, especially CD4high cells, display lower resistance than mature thymocytes, and their differentiation into DP cells accelerates their death.
We then investigated the involvement of the factors of the thymic microenvironment on the modulation of apoptosis on infection. We focused on TNF and IL-7, which we showed to be crucial to sustain HIV replication.28 We observed that though these cytokines act synergistically to trigger virus replication, they exhibit antagonistic effects on apoptosis. TNF favors apoptosis whereas IL-7 counteracts its effect. Because the protection by IL-7 was high in the mature thymocytes and remained partial in the immature cells, we wondered whether this was due to a different level of expression of IL-7R. As expected, IL-7R is highly expressed in the major fraction of the SP CD4+ thymocytes at a level above that found in the immature subpopulations. Among immature thymocytes, the TN cells constitutively express a high level of IL-7R that is gradually down-regulated during differentiation into the intermediate and then the DP CD3−stage.
We then performed a detailed analysis of the coexpression of IL-7R and Bcl-2 in the human thymocyte subsets. Differential constitutive levels of Bcl-2 in the distinct subsets correlate with the levels of expression of IL-7R in these cells and with their survival capacity on infection. In the intermediate thymocytes, this relation is further strengthened by the fact that CD4low cells, which survive best on infection, express higher levels of IL-7R and Bcl-2 than CD4high cells. Furthermore, we strictly demonstrated that IL-7 is involved in the maintenance of a differential level of Bcl-2 in accordance with IL-7R expression in the different subsets. Therefore, Bcl-2 level appears to be of crucial importance as an underlying mechanism of IL-7–mediated protection against apoptosis in human thymocytes.
In addition, it is worthwhile noting that the high constitutive level of Bcl-2 in the mature SP CD4+ thymocytes is further increased on infection in a large fraction of thymocytes. It is interesting to point out that virus replication takes place in this particular fraction. Taken together, these data suggest that the virus itself acts to sustain the production of Bcl-2, conferring high resistance to apoptosis and then persistent infection in these cells. However, the number of cells detected for p24 expression is lower than the fraction of cells in which Bcl-2 is enhanced on infection. Two possible explanations are that p24 staining leads to an underestimation of the number of HIV-1–infected cells or that up-regulation of Bcl-2 also occurs in bystander cells. In contrast, in immature thymocytes, the lower level of Bcl-2 was further decreased on infection favoring apoptosis at various degrees according to stage of differentiation; the major effect occurred within DP cells.
In vivo studies are in agreement with the high resistance against apoptosis of TN during thymus infection.9,17,19,22 In contrast, the survival capacity of SP CD4+ thymocytes is controversial. In SCID-hu mice, the CD4+CD8−thymocyte subset was shown to be decreased,22unchanged,42 or occasionally increased43 on HIV infection. However, in these reports, analyses were performed with no consideration of the CD3 expression, thus preventing the distinction between the mature SP CD4+ and the intermediate thymocytes. In addition, the discrepancies observed in vivo might be dependent on the stage of infection. Indeed, the SP CD4+ population was shown to be decreased in the thymus of an HIV-1–seropositive infant already displaying a complete loss of corticomedullary demarcation.17 In macaques, this subset increases on SIV infection19 in the early course of infection. At a late stage, when morphologic evidence of severe thymic damage is present,19,20 it is likely that the insult to thymic stroma leads to an environment deprived of IL-7 and of a high level of TNF. Indeed, TNF might be brought by macrophages that infiltrate this compartment subsequent to infection, as shown in humans16-18 and in SIV-infected macaques.20This prolonged lack of balance toward TNF might lead to apoptosis of the mature thymocytes. Indeed, in prolonged culture with a high TNF concentration, we observed a significant percentage of apoptotic cells among HIV-infected SP CD4+ thymocytes (data not shown).
With regard to intermediate thymocytes in vivo, it was reported in the SCID-hu mouse model that apoptosis is induced by HIV.25 In the current paper, we provide new insights by demonstrating that intermediate thymocytes display partial resistance to infection with HIV-1, which is more pronounced in the CD4lowcells. Our results on the high sensitivity to apoptosis of DP cells observed in vitro are in agreement with a large number of in vivo studies demonstrating a massive reduction of this population on infection in patients17 or in animal models of AIDS.9,19,22,25,43,44 Furthermore, in SIV-infected macaques, a partial depletion of DP thymocytes correlates with a reduced expression of Bcl-2 and an increase of the apoptosis rate in these cells.9 It was unclear, however, whether this depletion was the consequence of cell death induced by a productive infection in these cells. We showed in a previous work that despite virus entry into the DP cells, HIV does not replicate because of a blockade of the activation in these cells.28 Virus entry in these cells does not modify their rate of apoptosis ex vivo (data not shown). We conclude here that infection occurs at the intermediate stage but that, after cell differentiation, cell death is particularly massive at the DP stage.
We also point out here that the mechanisms underlying the resistance to virus-induced apoptosis converge toward a maintenance of a high level of Bcl-2. First, a high level of Bcl-2, correlating with a high-level IL-7R, is required before infection to resist virus-induced cell death. This is reminiscent of our previous studies showing that survival of HIV-infected Jurkat T cells was directly dependent on the basic level of expression of Bcl-2 generated by transfection in these cells.29 This is perfectly compatible with the well-known role of Bcl-2, which is to prevent the disruption of the mitochondrial membrane induced by the oxidative stress45 occurring in HIV-infected cells.29,46 We actually demonstrated that this oxidative stress can be counteracted by a high level of Bcl-229, and this might occur in thymocytes, especially in the mature SP CD4+ subset.
The antagonist effect of IL-7 on TNF-induced apoptosis might also be attributed to the capacity of IL-7 to trigger the expression of the TNF receptor 2 (TNF-R2), as we previously reported for the mature SP CD4+ subset.28 TNF-R2 expression might be associated with a decreased sensitivity to TNF-induced apoptosis, as shown for peripheral T cells.47 Work is in progress in our laboratory to evaluate this possibility.
In addition to a high basic level of Bcl-2 in the mature SP CD4+ cells, HIV infection further increases this level in a substantial fraction of these cells. This is reminiscent of the up-regulation of Bcl-2 at the transcriptional level that we observed in the Jurkat T-cell clones on HIV infection.29 Studies are in progress to further elucidate whether the same mechanism is involved in the up-regulation of Bcl-2 on infection in mature thymocytes.
The current paper and our previous data27,28,35 lead us to propose a model of the sequential events leading to destruction of the thymus on HIV infection. At an early stage, mature SP CD4+thymocytes substantially produce HIV under the activation of cytokines cosecreted during their interaction with the thymic epithelial cells. Infected cells are protected from apoptosis because of their high capacity to respond to IL-7 and the subsequent high level of Bcl-2 further enhanced by infection itself. Thus, SP CD4+thymocytes constitute a persistent HIV reservoir and not a latent one, as reported by Brooks et al.48 Actually, there is no real discrepancy because the authors show nonnegligible HIV production in these thymocytes isolated from HIV-infected SCID-hu mice and left untreated for 3 days in culture.48 The infected SP CD4+ thymocytes may give rise to an increase in the peripheral viral load when these cells migrate as T lymphocytes into the blood. This is consistent with the strong correlation observed between the number of infected thymocytes in the thymic medulla and the level of peripheral viral load in SIV-infected monkeys.49At a later stage, because of an inflammatory response to infection, TNF produced by inflammatory cells, in combination with other cytokines, might induce HIV production in intermediate thymocytes. Subsequently, both CD4high intermediate and DP thymocytes will die of apoptosis because of reduced quantity of Bcl-2 that is further decreased by infection. Depletion of these subsets will lead to an interruption of thymopoiesis and finally to a failure of T-cell regeneration. This pattern is consistent with data recently reported in FIV-infected cats showing a high level of virus in mature thymocytes early after infection and the successive infection of immature thymocytes associated with inflammatory response and cortical atrophy.44 Such a sequence of events, which could participate in T-cell loss in the periphery, might be particularly favored in an active thymic microenvironment—for instance, in infants. In our experiments, the younger the donor, the more efficient are the HIV replication signals in thymocytes. This is consistent with the rapid progression to AIDS observed in some infected infants in the absence of antiretroviral therapy.13 On the other hand, under efficient therapy, the presence of active thymic tissue favors the recovery of the T-cell repertoire.6 Consistent with these observations, an increase of naive CD4 T cells in treated patients was shown to occur more precociously and at a higher rate in young children than in adults.50 51
Now that IL-7 is envisioned as immunotherapy for AIDS, it is important to emphasize the possible role of this cytokine in virus persistence in the thymus at the first stage of infection and later in the disruption of thymopoiesis by triggering HIV replication in apoptosis-sensitive immature thymocytes. In conclusion, even in association with antiretroviral therapy, the use of IL-7 as immunotherapy for AIDS warrants prudence and the need for testing first in a relevant animal model.
We thank Dr Sonia Berrih-Aknin (Hôpital Marie Lannelongue, Le Plessis-Robinson, France) and Prof Leca (Hôpital Necker, Paris, France) for providing us with thymuses. We particularly thank Dr Gianfranco Pancino (Unité de Biologie des Rétrovirus, Institut Pasteur) for helpful discussions and Dr David Ojcius (Laboratoire de Biologie des Interactions Cellulaires, Institut Pasteur) for careful reading of the manuscript.
Supported by the Agence Nationale pour la Recherche sur le SIDA (France). E.G. was the recipient of a fellowship from Ensemble contre le SIDA (Fondation pour la Recherche Médicale, Paris, France) and a fellowship from the Agence Nationale pour la Recherche sur le SIDA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicole Israël, Unité de Biologie des Rétrovirus, Institut Pasteur, 28 rue du Dr Roux, 75724 Paris Cedex 15, France; e-mail: nisrael@pasteur.fr.