α- and β-tryptase genes encode serine proteases that are abundantly expressed by mast cells. Under physiologic conditions other myeloid cells are virtually tryptase negative. However, tryptases are also expressed in several myeloid leukemia cell lines. In this study, serum total tryptase levels were determined in 150 patients with acute leukemias (de novo acute myeloid leukemia [AML], n = 108; secondary AML, n = 25; acute lymphoid leukemia [ALL], n = 17) by fluoroenzyme immunoassay. In healthy subjects (n = 30), tryptase levels ranged between 2.0 and 12.6 ng/mL. Elevated tryptase levels (> 15) were detected in 42 (39%) of 108 patients with de novo AML and in 11 (44%) of 25 patients with secondary AML. No elevated tryptase levels were found in patients with ALL. In de novo AML, elevated tryptase levels were frequently detected in patients with French-American-British classification M0 (6 of 9), M2 (9 of 14), M3 (4 of 6), and M4eo (7 of 7), and less frequently in M1 (7 of 20), M4 (6 of 26), M5 (2 of 18), M6 (0 of 5), or M7 (1 of 3). The highest tryptase levels were found in M4eo. Immunohistochemical staining of bone marrow sections with anti-tryptase antibody as well as immunoelectron microscopy revealed tryptase expression in the cytoplasm of myeloblasts. As assessed by Northern blotting and reverse transcriptase–polymerase chain reaction, AML cells expressed α-tryptase messenger RNA (mRNA) but little or no β-tryptase mRNA. In AML patients with elevated serum tryptase before chemotherapy, who entered complete remission, tryptase levels returned to normal or near normal values. Blast cell persistence or regrowth was associated with a persistently elevated level or recurrent increase of tryptase. Together, tryptase is expressed in myeloblasts in a group of AML and may serve as a useful disease-related marker.
Introduction
Acute myeloid leukemia (AML) is characterized by clonal proliferation of immature myeloid (progenitor) cells without significant differentiation.1 The prognosis and clinical picture in AML varies, depending on the genes that underwent deregulation, cell type(s) involved, and the specific biological properties of the clone(s).1-6 In the past few decades, several disease-specific or lineage-associated markers indicating the presence and/or type of AML have been established. Some of these markers, like the breakpoint-specific gene-fusion products, represent useful tools for the diagnosis and monitoring of AML.7-10Other markers, like the CD molecules or distinct enzymes (myeloperoxidase, lysozyme), are useful for determining the subtype of AML.11-20
α- and β-tryptase genes encode serine proteases that are abundantly expressed by human mast cells and certain leukemic cell lines of myeloid origin.21-30 Peripheral blood basophils express only small amounts of tryptase (∼ 500-fold less than mast cells in tissue), and no mast cell tryptase is detectable in other blood leukocytes.22,26 Biochemical studies suggest that most of the α-tryptase product is not processed beyond the enzymatically inactive α-protryptase stage and is released spontaneously (constitutively) by mast cells.31,32 In contrast, most of theβ-tryptase product is processed to enzymatically active tetramers that are primarily stored in cytoplasmic granules and are only released during degranulation. Correspondingly, serum β-tryptase as well as total tryptase (α + β) concentrations are elevated in systemic anaphylactic (mast cell–associated) reactions, whereas only total tryptase levels (but not β-tryptase levels) are elevated in systemic mastocytosis (SM).32,33 This is most probably due to constant release of α-protryptase from (a high number of) mast cells in these patients. α- and β-tryptasegenes cluster on the short arm of human chromosome 16.27,28 30
So far, α- and β-tryptases were thought to be (almost) specific for mast cells. However, recent studies have shown that a number of myeloid leukemia cell lines also express substantial amounts of tryptases.23-25 Some of these cell lines are known to be related to the mast cell (HMC-1) or basophil (KU-812, LAMA-84) lineage. Other cell lines, like U-937 or MonoMac, show myeloid lineage characteristics without (other) apparent features of mast cells or basophils. The observation that AML-derived cell lines express substantial amounts of tryptases prompted us to investigate tryptase expression in acute (myeloid) leukemias. The results of our study show that mast cell tryptase is expressed in a subset of patients with AML and may represent a novel disease-related marker useful for the diagnosis and follow-up (monitoring) of these patients.
Patients and methods
Patients' characteristics
A total number of 150 consecutive patients with acute leukemias (median age, 60 years; range, 16-89 years; female-male ratio, 1:1) were analyzed. Of these patients, 108 had de novo AML (median age, 61 years; range, 16-89 years; female-male ratio, 1:1), 25 secondary AML (following myelodysplastic syndrome, previous chemotherapy, or radiation; median age, 68 years; range, 38-82 years), and 17 had ALL (median age, 36 years, range, 16-67 years). Leukemias were classified according to the proposal of the French-American-British (FAB) cooperative study group.34-37 The following AML subtypes (de novo AML) were diagnosed: M0, n = 9; M1, n = 20; M2, n = 14; M3, n = 6; M4, n = 26; M4eo, n = 7; M5, n = 18; M6, n = 5; and M7, n = 3. The patients' characteristics are summarized in Table1.
Measurements of tryptase and histamine
Serum total tryptase (α-protryptase + β-tryptase; referred to as tryptase in this article unless otherwise stated) concentrations were determined in 150 patients with acute leukemias and 30 healthy controls. In addition, 10 patients with SM were analyzed. In each case, informed consent was given. Tryptase levels were also measured in whole blood samples (leukemias, n = 128; SM, n = 10; healthy controls, n = 30) after lysis in distilled water (1:1) and freeze thawing. Moreover, tryptase levels were measured in supernatants and lysates of cultured bone marrow (bm) cells (AML). Total tryptase concentrations were determined by a commercial fluoroenzyme immunoassay (FIA; Pharmacia, Uppsala, Sweden).38 The detection limit of this assay was found to be 1 ng/mL. In selected cases with high levels of total tryptase, β-tryptase levels were measured by enzyme-linked immunosorbent assay (ELISA).39 Histamine was measured in whole blood lysates (generated by freeze thawing) by a commercial radioimmunoassay (Immunotech, Marseille, France).
Immunohistochemistry and immunocytochemistry
Immunohistochemistry was performed on bm biopsy specimens obtained from 23 patients (de novo AML, n = 20; secondary AML, n = 1; ALL, n = 2). Biopsies were taken from the iliac crest and fixed in ethanol (95%)/formaldehyde (37%) at 4:1, followed by fixation in 7.5% neutral-buffered formaldehyde and decalcification in EDTA. Paraffin-sections of 2 μm were cut, dewaxed, and treated with 0.3% methanol-H2O2 (vol/vol) (30 minutes) or 0.3% Tris-buffered saline (TBS)–H2O2 to block endogenous peroxidase. After each step, sections were rinsed twice in 0.05 M TBS at pH 7.5. Immunohistochemical staining was performed according to published techniques.21,40,41 In brief, sections were incubated with antibodies for 1 hour at room temperature (RT), washed in TBS, incubated with biotin-labeled horse antimouse antibody (30 minutes), washed, and then exposed to avidin-biotin-peroxidase complex (30 minutes at RT). The following first-step antibodies were applied: anti-tryptase monoclonal antibody (mAb) G3 (dilution, 1:5000; Chemicon, Temecula, CA), the basophil-specific mAb 2D742 (dilution, 1:1000), and anti-Kit mAb 1A2C543 (dilution, 1:300) kindly provided by Dr H.-J. Bühring (Tübingen, Germany). Antibody binding was made visible by 3-amino-9-ethylcarbazole. Sections were counterstained with Mayer Hämalaun and mounted in Aquatex (Merck, Darmstadt, Germany). Control slides were similarly treated with the primary antibody being omitted. For immunocytochemistry, peripheral blood or bm mononuclear cells (MNCs) from 6 patients with AML were spun on cytospin slides, fixed in acetone, washed in TBS, and incubated with antitryptase mAb G3 (1:5000) for 60 minutes. Then, slides were washed and incubated with biotinylated horse antimouse immunoglobulin G (IgG; 30 minutes). Slides were again washed and exposed to streptavidin-biotin-alkaline phosphatase complex (DAKO, Glostrup, Denmark) for 30 minutes at RT. Antibody reactivity was made visible with vector red alkaline phosphatase substrate. In control experiments, CD34+ cells purified from MNCs of normal bm (n = 3; one bm transplant donor and 2 patients with suspected hematologic neoplasm) were analyzed by immunocytochemistry by using biotinylated anti-tryptase mAb G3.
Immunoelectron microscopy
Immunoelectron microscopy was performed on primary leukemic bm cells (de novo AML, n = 9) according to established techniques.44,45 In brief, cells were fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, and 0.025% CaCl2in 0.1 M cacodylate buffer (pH 7.4) for 60 minutes. Then, cells were washed 3 times in 0.1 M cacodylate buffer, resuspended in 2% agar, and centrifuged. Agar pellets were postfixed in 1.3% OsO4buffered in 0.66 M collidine, and stained en bloc with 2% uranyl acetate in sodium maleate buffer (pH 4.4) for 2 hours at RT. Pellets were then rinsed, dehydrated in alcohol series, and embedded in Eponate 812 (Serva, Heidelberg, Germany). Thereafter, ultrathin sections were cut and placed on gold grids (Plano, Marburg, Germany). Grids were etched 3 times in 3% sodium metaperiodate and washed in phosphate-buffered saline (PBS) containing 50 mM glycine (pH 7.4). Sections were then preincubated in PBS containing 10% fetal calf serum (FCS) and 0.5 μg/mL Tween at RT (pH 7.4) for 30 minutes, washed, and then incubated with anti-tryptase mAb G3 (10 μg/mL) in 1% bovine serum albumin (BSA) for 4 hours. After incubation, samples were washed 4 times in PBS containing 0.5 μg/mL Tween (pH 7.4) and once in PBS containing 1% BSA and 0.5 μg/mL Tween (pH 8.0). After washing, grids were incubated with a goat anti-mouse antibody conjugated with gold (10 nM gold particles) for 3 hours at RT. Thereafter, grids were washed 3 times in PBS containing 0.5 μg/ml Tween (pH 7.4) and then contrasted in 1% uranyl acetate (5 minutes) and lead citrate (30 minutes). Sections were viewed in a JEOL 1200 AX transmission electron microscope (Tokyo, Japan). The staining reaction was controlled by using mouse IgG (10 μg/mL) instead of G3. In selected experiments, CD34+cord blood MNCs (blasts) were purified by magnetic cell sorting and flow cytometry46 47 and examined by immunoelectron microscopy.
Flow cytometric evaluation of tryptase expression
Cytoplasmic expression of tryptase was analyzed in AML blasts (tryptase+ AML, n = 3; tryptase− AML, n = 1; AML in complete remission [CR], n = 1), normal CD34+/CD45+ bm cells (n = 3 donors), HMC-1 cells (positive control), and the epithelial cell line A431 (negative control). In whole blood or bm samples (normal bm or AML), CD34+ cells were analyzed by multicolor flow cytometry using anti-tryptase mAb G3, a phycoerythrin (PE)-labeled CD34 mAb, and a peridinin chlorophyll protein (PerCP)–labeled CD45 mAb (Becton Dickinson, San Jose, CA). Prior to staining, erythrocytes were lysed by FACS Lysing Solution (Becton Dickinson). Cytoplasmic staining was performed according to published techniques.48 49 In a first step, cells were fixed in formaldehyde solution (15 minutes at RT). Cells were then washed in PBS and permeabilized with 0.1% saponin (Sigma, St Louis, MO) dissolved in HEPES buffer. Then, cells were incubated with anti-tryptase mAb G3 (diluted in saponin solution) for 30 minutes. After washing in saponin solution, a fluorescein isothiocyanate–conjugated second-step antibody was applied for 30 minutes. Cells were then saturated with mouse IgG (Becton Dickinson), washed once in saponin solution and once in PBS, and then were exposed to PE-CD34 plus PerCP-CD45. After washing, cells were examined by multicolor flow cytometry on a FACS Scan (Becton Dickinson). Isolated AML blasts and cell lines were examined by single-color flow cytometry. Staining reactions were controlled using isotype-matched antibodies.
Northern blot analysis
In 23 patients (M0, n = 3; M1, n = 4; M2, n = 3; M3, n = 4; M4, n = 1; M4eo, n = 4; M6, n = 1; secondary AML, n = 2; ALL, n = 1), bm MNCs were isolated using Ficoll and were prepared for Northern blotting. Northern blot analysis was carried out essentially as described.50 Total RNA was extracted from MNCs by the guanidinium isothiocyanate/cesium chloride method.51 Total RNA (10 μg) was size-fractionated on 1.2% agarose gels and blotted onto nylon membranes (Hybond N, Amersham, United Kingdom) using 20 × SSC (1 × SSC, 150 mM NaCl and 15 mM sodium citrate, pH 7.0) overnight. Then, RNA was cross-linked to membranes by UV irradiation (UV Strata-linker 1800; Stratagene, La Jolla, CA). Prehybridization was performed at 65°C for 4 hours in 5 × SSC, 7% sodium dodecyl sulfate (SDS), 10 × Denhardt solution (DhS; 1 × DhS consists of 0.02% wt/vol BSA, 0.02% wt/vol polyvinyl pyrolidone, 0.02% wt/vol Ficoll), 10% wt/vol dextran sulfate, 20 mM sodium phosphate (pH 7.0), sonicated salmon sperm DNA (100 μg/mL), and t-RNA. Hybridization was performed using32P-labeled synthetic oligonucleotide probes: α-tryptase (28-mer), 5′-CATGACCGTGTG GACGCGGCTGGAGATG-3′ (413-440); α-tryptase (32-mer), 5′-CAGTCTGGATGATGTAG AACTGTGGGTGCACC-3′ (341-372); β-tryptase (30-mer), 5′-GATCTGGGCGGTGTAGAA CTGTGGGTGCAC-3′ (342-371); β-tryptase (28-mer), 5′-GGTGACCGTGTGGACGTGGCTG GAGACC-3′ (413-440); β-actin (34-mer), 5′-GGCTGGGGTGTTGAAGGTCTCAAACATGA TCTGG-3′. Blots were washed in 5% SDS, 3 × SSC, 10 × DhS, and 20 mM sodium phosphate (pH 7.0) for 30 minutes (65°C), and once in 1 × SSC, 1% SDS (30 minutes at 65°C). Bound radioactivity was visualized by XAR-5 films at −70°C using intensifying screens (Eastman Kodak, Rochester, NY).
Detection of tryptase messenger RNA by reverse transcriptase–polymerase chain reaction
To confirm expression of tryptase messenger RNA (mRNA) in bm MNCs in AML (M1, n = 1; M2, n = 3; M3, n = 2; M4eo, n = 3) an established reverse transcriptase–polymerase chain reaction (RT-PCR) protocol47 was applied. The human mast cell line HMC-1 served as a positive control for tryptase, and cultured human fibroblasts (obtained from synovial tissue) served as a negative control. Total RNA was isolated by a modified guanidinium isothiocyanate–acid phenol extraction procedure using RNAzol B (Biotecx Laboratories, Houston, TX). Total RNA from 106cells was reverse transcribed into complementary DNA (cDNA) using a cDNA Synthesis kit (First-Strand c-DNA synthesis-kit; Pharmacia Biotechnology, Brussels, Belgium). Aliquots (6 μL) of the cDNA were used for PCR amplification in a final volume of 50 μL containing 1 × PCR buffer (PerkinElmer/Cetus ), 1.25 UTaq polymerase (PerkinElmer), 25 pM of each upstream and downstream primers specific for tryptase gene (5′ primer: 5′-GAGGCCCCCAGGAGCAAGTG-3′; 3′ primer: 5′-ACATCGCCCCAGCCAGTGAC-3′), and β-actin (5′ primer: 5′-AGGCCGGC- TTCGCGGGCGAC-3′; 3′ primer: 5′-CTCGGGAGCCACCAGCAGCTC-3′). Tryptase-specific primers were selected to amplify identical regions of α- and β-tryptase cDNA molecules that can be discriminated by restriction fragment polymorphism, ie, the β-tryptase–specific PCR product, but not α-tryptase–specific PCR product, contained a Dra III restriction site. Primers were purchased from MWG Biotech (Ebersberg, Germany). Samples were amplified by 32 PCR cycles (94°C for 1 minute, 63°C for 1 minute, and 72°C for 90 seconds; initial denaturation step at 95°C for 1 minute). To differentiate between α-tryptase and β-tryptase gene species, PCR products were digested with the restriction endonuclease Dra III (Boehringer Mannheim, Germany). For control purposes, α- and β-tryptase–specific PCR products were obtained from plasmid clones containing the α- and β-tryptase cDNA. After digestion, PCR products were subjected to gel electrophoresis and visualized by ethidium bromide staining.
Culture experiments using AML blasts
Isolated bm MNCs obtained from patients with de novo AML (n = 6) were cultured in RPMI 1640 medium supplemented with 10% FCS in 5% CO2 at 37°C for up to 30 days. Cultures were serially examined for the presence of total tryptase in cell lysates and cell-free supernatants. Lysates were prepared by centrifugation and freeze thawing. The amounts of tryptase generated/released by cultured AML cells were quantified by FIA.
Statistical analysis
To analyze the correlation between serum tryptase levels, whole blood tryptase levels, and other laboratory findings (white blood cell count [WBC], percentage of blasts, serum lactate dehydrogenase [LDH]), a linear correlation was applied.
Results
Detection of elevated serum tryptase levels in a group of patients with AML
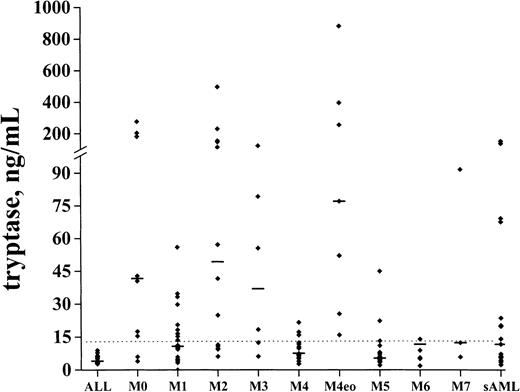
The levels of serum tryptase were determined in 108 patients with de novo AML, in 25 with secondary AML, and in 17 patients with ALL. In healthy controls (n = 30), the median serum tryptase level amounted to 5.1 ng/mL (mean ± SD, 5.3 ± 2.2; range, 2.0-12.6). Elevated serum tryptase levels (> 15 ng/mL) were detected in 42 (39%) of 108 patients with de novo AML and 11 (44%) of 25 patients with secondary AML. By contrast, in patients with ALL (n = 17), tryptase levels were consistently normal. Among de novo AML, elevated tryptase levels were particularly detectable in FAB groups M0 (6 of 9), M2 (9 of 14), M3 (4 of 6), and M4eo (7 of 7). The highest tryptase concentrations were found in AML-M4eo (up to 881 ng/mL) (Figure1). In M5 and M6, the majority of the patients had normal or near normal tryptase levels. In M1 (7 of 20), M4 (6 of 26), and M7 (1 of 3), a subgroup of patients had enhanced tryptase levels. No significant correlations between serum tryptase levels and other laboratory parameters (WBC, percentage of blasts, LDH) were found (r < 0.2). In 15 patients with AML in whom significantly elevated total tryptase levels (> 20 ng/mL) were detected, samples were also examined for the presence of β-tryptase by ELISA. In the majority of these patients (9 of 15), serum β-tryptase levels were < 1 ng/mL. In 6 of 15 patients, however, β-tryptase levels were also detectable (range, 1-14 ng/mL). Those β-tryptase+ AML cases (particularly those with AML-M4eo) were found to exhibit very high levels of total serum tryptase.
Serum tryptase levels in various FAB groups.
Serum tryptase levels were measured by FIA at the time of diagnosis. The median serum tryptase levels in each FAB category are indicated by horizontal bars.
Serum tryptase levels in various FAB groups.
Serum tryptase levels were measured by FIA at the time of diagnosis. The median serum tryptase levels in each FAB category are indicated by horizontal bars.
Detection of tryptase in leukemic blood cells
In healthy controls (n = 30) whole blood tryptase levels amounted to 4.6 ng/mL (range, 0.8-17.2) and thus showed a similar range compared with serum enzyme levels. In patients with AML, whole blood tryptase concentrations were elevated (> 20 ng/mL) in a group of patients (58 of 114 = 50.9%) and showed a significant correlation with serum enzyme levels (r = 0.86) (Figure2). Thus, in the majority of the (tryptase+) cases, whole blood enzyme levels were slightly higher or in the same range as compared with serum tryptase levels. By contrast, in patients with SM (n = 10) in whom the enzyme is derived from tissue mast cells (but not produced or stored in circulating blood cells) serum tryptase levels always exceeded whole blood tryptase levels (ratio whole blood tryptase/serum tryptase = 0.5-0.7). The presence of tryptase in circulating AML cells could be demonstrated by measuring the enzyme in isolated blood MNCs in patients with serum tryptase levels > 50 ng/mL. In those patients, circulating AML blasts were found to express significant amounts of tryptase (range, 0.03-7.7 ng/105 cells). No significant correlation between whole blood tryptase and whole blood histamine levels could be substantiated (r = 0.36). In fact, we were able to identify AML patients with high tryptase and high histamine levels, patients with high tryptase but low histamine levels, and also patients with high histamine but low tryptase levels (not shown).
Correlation between whole blood tryptase and serum tryptase.
Serum tryptase and whole blood tryptase levels were measured by FIA at the time of diagnosis. A significant correlation between serum tryptase and whole blood tryptase levels was found (r = 0.86).
Correlation between whole blood tryptase and serum tryptase.
Serum tryptase and whole blood tryptase levels were measured by FIA at the time of diagnosis. A significant correlation between serum tryptase and whole blood tryptase levels was found (r = 0.86).
Detection of the tryptase protein in AML blasts
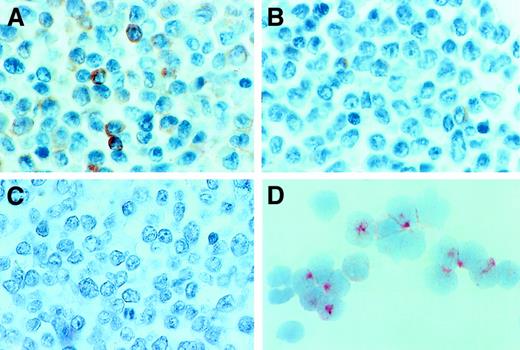
To further analyze tryptase expression in AML, immunohistochemistry, immunocytochemistry, and flow cytometry were performed by using the anti-tryptase mAb G3. Immunohistochemical staining experiments were conducted on bm sections (AML, n = 21; ALL, n = 2). As exemplified in Figure 3A,B, an increase in diffusely scattered tryptase+ cells was found in AML cases with significantly elevated serum tryptase levels (Table 2). Most of the tryptase-reactive material was localized in the cytoplasm of immature myeloid cells. The distribution of tryptase+ cells (blasts) in the bm always showed a diffuse pattern. In contrast, no focal (dense) accumulations of tryptase+ cells (like seen in mastocytosis) were found. In normal bm and cases with tryptase− AML (serum tryptase levels, < 15 ng/mL), only a few if any tryptase+ cells were detectable. Also, in cases with ALL, bm sections did not contain significant numbers of tryptase+ cells (Figure 3C). To evaluate lineage relationships, we also applied a basophil-specific antibody (2D7) and anti-Kit antibody (1A2C5). Interestingly, in cases with tryptase+ AML, a variable expression of 2D7 and/or Kit was demonstrable, whereas in most cases of tryptase− AML, leukemic blasts were also negative for 2D7 and Kit (Table 2). Immunocytochemical staining experiments (cytospin preparations) of bm MNCs confirmed the presence of tryptase-reactive material in myeloblasts in all patients with AML with clearly elevated serum tryptase levels (> 50 ng/mL) analyzed (n = 4) (Figure 3D). In contrast, no reactivity was found in AML blasts in patients with normal levels (≤ 15 ng/mL) of serum tryptase (Table 2). Also, normal CD34+ bm cells (normal bm; n = 3) were tryptase− (0 of 200 blast cells counted). Flow cytometric examination of tryptase expression disclosed corresponding results. In fact, in all cases with elevated enzyme levels (n = 3), blast cells were found to react with anti-tryptase mAb G3 whereas no reactivity was seen in a patient with tryptase− AML, a patient with tryptase+ AML in CR, and in CD34+/CD45+ progenitor cells obtained from normal bm (n = 3) (Figure 4).
Immunohistochemical and immunocytochemical detection of tryptase.
Expression of tryptase in AML blasts was demonstrated by immunostaining experiments using the anti-tryptase mAb G3 and bm sections or cytospin preparations (isolated MNCs). Immunostaining experiments were performed as described in the text. (A) A tryptase-stained bm section in a patient with AML M4eo (serum tryptase level, 881 ng/mL). As visible, many immature myeloid cells (blasts) in the bm reacted with anti-tryptase antibody. In other patients, only a few blasts were found to react with mAb G3. (B) The reactivity of blast cells with this mAb in a case of AML M2 (serum tryptase level, 41.6 ng/mL). In patients with AML without elevated tryptase and those with ALL, bm blasts appeared to be tryptase− by immunohistochemistry. (C) A bm section in a patient with ALL (serum tryptase level, 8.9 ng/mL) stained with mAb G3. Immunocytochemistry confirmed tryptase expression in AML blasts. (D) A cytospin preparation of bm MNCs prepared in a patient with AML M4eo (the same as in panel A). As visible, most of the AML blasts reacted with anti-tryptase mAb G3, confirming the immunohistochemical staining result (A). Note that most of the G3-reactive material is localized in the cytoplasm of AML blasts.
Immunohistochemical and immunocytochemical detection of tryptase.
Expression of tryptase in AML blasts was demonstrated by immunostaining experiments using the anti-tryptase mAb G3 and bm sections or cytospin preparations (isolated MNCs). Immunostaining experiments were performed as described in the text. (A) A tryptase-stained bm section in a patient with AML M4eo (serum tryptase level, 881 ng/mL). As visible, many immature myeloid cells (blasts) in the bm reacted with anti-tryptase antibody. In other patients, only a few blasts were found to react with mAb G3. (B) The reactivity of blast cells with this mAb in a case of AML M2 (serum tryptase level, 41.6 ng/mL). In patients with AML without elevated tryptase and those with ALL, bm blasts appeared to be tryptase− by immunohistochemistry. (C) A bm section in a patient with ALL (serum tryptase level, 8.9 ng/mL) stained with mAb G3. Immunocytochemistry confirmed tryptase expression in AML blasts. (D) A cytospin preparation of bm MNCs prepared in a patient with AML M4eo (the same as in panel A). As visible, most of the AML blasts reacted with anti-tryptase mAb G3, confirming the immunohistochemical staining result (A). Note that most of the G3-reactive material is localized in the cytoplasm of AML blasts.
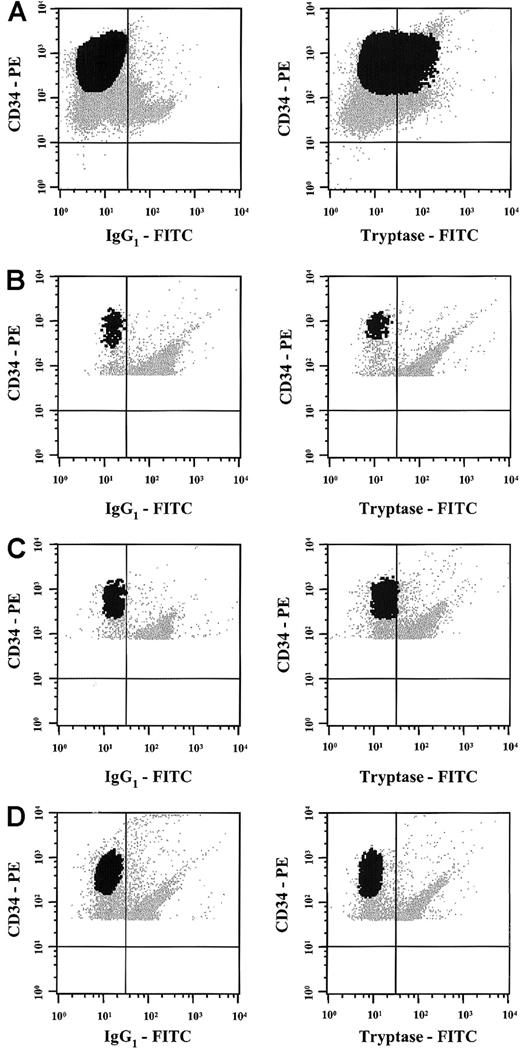
Flow cytometric detection of tryptase in AML cells.
Multicolor flow cytometry staining was performed in one patient with tryptase+ AML (A), 2 normal donors (B,C), and one patient with AML in complete remission (D) using antibodies against tryptase, a PE-labeled CD34 mAb, and a PerCP-labeled CD45 mAb. Multicolor staining for cytoplasmic tryptase (versus isotype-matched control) and surface antigens (CD34, CD45) was performed as described in the text. As visible, the CD34+ blast cells in the patient with AML showed clear expression of cytoplasmic tryptase (A). In contrast, tryptase was consistently negative in CD34+ bm cells in normal donors (B,C) or in the patient with tryptase+ AML in complete remission (D) without evidence of tryptase-positive (residual) subsets of cells.
Flow cytometric detection of tryptase in AML cells.
Multicolor flow cytometry staining was performed in one patient with tryptase+ AML (A), 2 normal donors (B,C), and one patient with AML in complete remission (D) using antibodies against tryptase, a PE-labeled CD34 mAb, and a PerCP-labeled CD45 mAb. Multicolor staining for cytoplasmic tryptase (versus isotype-matched control) and surface antigens (CD34, CD45) was performed as described in the text. As visible, the CD34+ blast cells in the patient with AML showed clear expression of cytoplasmic tryptase (A). In contrast, tryptase was consistently negative in CD34+ bm cells in normal donors (B,C) or in the patient with tryptase+ AML in complete remission (D) without evidence of tryptase-positive (residual) subsets of cells.
Detection of tryptase in leukemic blasts by immunoelectron microscopy
Immunoelectron microscopy was performed on bm MNCs obtained from 6 patients with tryptase+ AML (serum tryptase, > 15 ng/mL) and 3 with tryptase− AML (≤ 15 ng/mL). In cases with enhanced serum tryptase, a significant proportion of blasts were found to contain tryptase-immunoreactive material in their cytoplasmic compartment (Figure 5A). In most cells, the reactive material was located in granulelike structures (Figure5A). Sometimes, tryptase was also found loosely spread in the cytoplasm. The intensity of immunogold staining in blasts varied from donor to donor. In particular, in cases with high tryptase levels (> 100 ng/mL), AML blast cells were found to contain substantial amounts of tryptase (Figure 5A). In those with lower tryptase levels (< 100 ng/mL), we detected fewer tryptase+ particles in blast cells by immunogold labeling (Figure 5B). In the blast cells of patients with AML who exhibited normal serum tryptase levels, no tryptase-immunoreactive material could be detected in bm blasts. Also, no tryptase-reactive material was found in normal purified CD34+ (cord blood–derived) blasts.
Immunoelectron microscopy.
Immunoelectron microscopy was performed using isolated bm MNCs prepared in one patient with AML with a high tryptase level (A; AML M4eo; serum tryptase, 881 ng/mL) and in one patient with AML with a lower tryptase level (B; AML M4; serum tryptase, 224 ng/mL). Immunogold labeling was performed using anti-tryptase mAb G3 (for technical details see text). In the patient with elevated serum tryptase levels, G3-reactive material was detected in the (small) cytoplasmic compartment of AML blasts. Interestingly, tryptase was often found to localize to granulelike structures (A). Cases without elevated serum tryptase were consistently negative by immunoelectron microscopy (not shown).
Immunoelectron microscopy.
Immunoelectron microscopy was performed using isolated bm MNCs prepared in one patient with AML with a high tryptase level (A; AML M4eo; serum tryptase, 881 ng/mL) and in one patient with AML with a lower tryptase level (B; AML M4; serum tryptase, 224 ng/mL). Immunogold labeling was performed using anti-tryptase mAb G3 (for technical details see text). In the patient with elevated serum tryptase levels, G3-reactive material was detected in the (small) cytoplasmic compartment of AML blasts. Interestingly, tryptase was often found to localize to granulelike structures (A). Cases without elevated serum tryptase were consistently negative by immunoelectron microscopy (not shown).
Detection of tryptase mRNA in bm MNCs
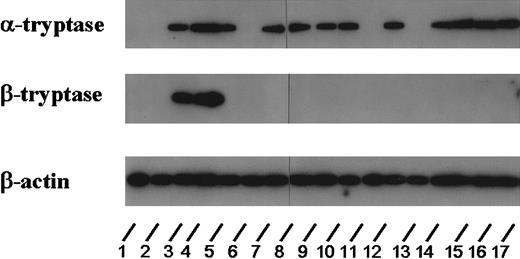
To analyze whether tryptase is expressed at the mRNA level in AML blasts, Northern blot experiments were performed using oligonucleotide probes specific for α- and β-tryptase. For this purpose, RNA of bm cells from 22 patients with AML and one patient with ALL was analyzed. Using oligonucleotide probes specific for α-tryptase, expression of mRNA was found in all AML cases in whom elevated serum tryptase could be detected (n = 16), whereas no transcripts for α-tryptase were detected in patients with AML with normal serum tryptase (n = 6) and in the patient with ALL. Figure 6 shows the results from 17 (of the 23) patients (ALL, n = 1; tryptase+ AML, n = 12; tryptase− AML, n = 4). With the use of probes specific for β-tryptase, only the 2 patients with AML-M4eo expressing very high serum tryptase concentrations (395 and 881 ng/mL, respectively) had detectable β-tryptase mRNA (Figure 6).
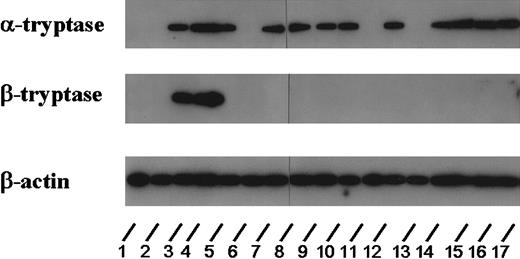
Expression of tryptase mRNA in AML cells.
Northern blot experiments were performed on leukemic blasts in 17 patients corresponding to lanes 1 (ALL, 2.7 ng/mL), 2 (M0, 3.9 ng/mL), 3 (M4eo, 395 ng/mL), 4 (M4eo, 881 ng/mL), 5 (M0, 120 ng/mL), 6 (M1, < 1 ng/mL), 7 (M3, 122 ng/mL), 8 (M4eo, 177 ng/mL), 9 (M1, 56 ng/mL), 10 (M2, 4.6 ng/mL), 11 (M4eo, 15.9 ng/mL), 12 (M2, 113 ng/mL), 13 (M6, 5.5 ng/mL), 14 (M4, 23.1 ng/mL), 15 (M3, 79.2 ng/mL), 16 (M4, 224 ng/mL), and 17 (M3 55.5 ng/mL). For detection of tryptase mRNA, an oligonucleotide probe specific for α-tryptase and a second probe specific for β-tryptase were applied. For control purposes, the membrane was hybridized with an oligonucleotide probe specific for β-actin. Using the α-tryptase probe, tryptase mRNA could be detected in all cases with elevated serum tryptase levels. By contrast, β-tryptase mRNA was detectable only in a small group of patients analyzed (ie, in AML M4eo). In the 2 AML cases with normal serum tryptase as well as in one case with ALL (normal serum tryptase level), no tryptase mRNA could be detected.
Expression of tryptase mRNA in AML cells.
Northern blot experiments were performed on leukemic blasts in 17 patients corresponding to lanes 1 (ALL, 2.7 ng/mL), 2 (M0, 3.9 ng/mL), 3 (M4eo, 395 ng/mL), 4 (M4eo, 881 ng/mL), 5 (M0, 120 ng/mL), 6 (M1, < 1 ng/mL), 7 (M3, 122 ng/mL), 8 (M4eo, 177 ng/mL), 9 (M1, 56 ng/mL), 10 (M2, 4.6 ng/mL), 11 (M4eo, 15.9 ng/mL), 12 (M2, 113 ng/mL), 13 (M6, 5.5 ng/mL), 14 (M4, 23.1 ng/mL), 15 (M3, 79.2 ng/mL), 16 (M4, 224 ng/mL), and 17 (M3 55.5 ng/mL). For detection of tryptase mRNA, an oligonucleotide probe specific for α-tryptase and a second probe specific for β-tryptase were applied. For control purposes, the membrane was hybridized with an oligonucleotide probe specific for β-actin. Using the α-tryptase probe, tryptase mRNA could be detected in all cases with elevated serum tryptase levels. By contrast, β-tryptase mRNA was detectable only in a small group of patients analyzed (ie, in AML M4eo). In the 2 AML cases with normal serum tryptase as well as in one case with ALL (normal serum tryptase level), no tryptase mRNA could be detected.
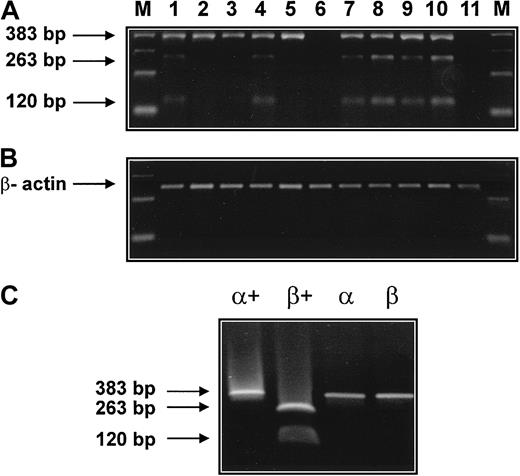
To further demonstrate the presence of mRNA specific for tryptase subtypes (α versus β) in AML cells, RT-PCR experiments with restriction fragment length polymorphism (RFLP) were performed (Figure7). RT-PCR was employed with oligonucleotides capable of amplifying α- and β-tryptase cDNAs of 383 base pair (bp) size. The presence of β-tryptase was confirmed by digestion of the cDNA with Dra III, yielding a 263-bp and a 120-bp fragment for β-tryptase. cDNA coding for α-tryptase 383 bp was amplified from 8 patients with AML (Figure 6A, lanes 1-5, 7-9) and from the human mast cell line HMC-1 (Figure 7A, lane 10) but not from human fibroblasts (Figure 7A, lane 11). The presence of cDNA coding for β-tryptase was demonstrable in 5 patients with AML (Figure 7A, lanes 1, 4, 7-9) and the HMC-1 cell line (Figure 7A, lane 10). Thus, in contrast to our (less sensitive) Northern blot experiments, the RT-PCR data suggest expression of both α- and β-tryptase mRNA in a significant group of patients with tryptase+ AML. This may be due to the higher sensitivity of the RT-PCR technique. In fact, in one patient with normal serum tryptase, RT-PCR showed expression of both α- and β-tryptase mRNA in leukemic blasts (Figure 7, lane 4). In another patient with AML with normal serum tryptase, however, blast cells were RT-PCR negative for tryptase (lane 6). In one patient with AML who had a slightly elevated serum tryptase (serum tryptase, 15.9 ng/mL), expression of tryptase mRNA was detectable by RT-PCR (lane 8) but not by Northern blotting. cDNA coding for β-actin could be amplified from all cell samples (Figure 7B).
Detection of α- and β-tryptase mRNA by RT-PCR.
(A) RT-PCR was performed with bm RNA from 9 patients with AML. The respective lanes are 1 (M1, 20.5 ng/mL), 2 (M2, 41.6 ng/mL), 3 (M2, 26.9 ng/mL), 4 (M1, 4.1 ng/mL), 5 (M3, 55.5 ng/mL), 6 (M3, 6.2 ng/mL), 7 (M4eo, 77 ng/mL), 8 (M4eo, 15.7 ng/mL), and 9 (M4eo, 881 ng/mL). In all cases with elevated serum tryptase (lanes 1,2,3,5,7,8,9) and in one patient with normal serum tryptase (lane 4), tryptase cDNA of 383 bp was obtained by RT-PCR. Restriction enzyme digestion (Dra III) revealed the presence of β-tryptase cDNA in 5 of 9 patients with AML (restriction fragments of 263 bp and 120 bp). The human mast cell line HMC-1 (lane 10) but not human fibroblasts (lane 11) was also found to express α- and β-tryptase mRNA. β-Actin cDNA was obtained from all samples (B). RT-PCR products for α- and β-tryptase obtained from plasmids containing α- and β-tryptase cDNA were subjected to electrophoresis before (α, β) and after (α+, β+) digestion with Dra III (C).
Detection of α- and β-tryptase mRNA by RT-PCR.
(A) RT-PCR was performed with bm RNA from 9 patients with AML. The respective lanes are 1 (M1, 20.5 ng/mL), 2 (M2, 41.6 ng/mL), 3 (M2, 26.9 ng/mL), 4 (M1, 4.1 ng/mL), 5 (M3, 55.5 ng/mL), 6 (M3, 6.2 ng/mL), 7 (M4eo, 77 ng/mL), 8 (M4eo, 15.7 ng/mL), and 9 (M4eo, 881 ng/mL). In all cases with elevated serum tryptase (lanes 1,2,3,5,7,8,9) and in one patient with normal serum tryptase (lane 4), tryptase cDNA of 383 bp was obtained by RT-PCR. Restriction enzyme digestion (Dra III) revealed the presence of β-tryptase cDNA in 5 of 9 patients with AML (restriction fragments of 263 bp and 120 bp). The human mast cell line HMC-1 (lane 10) but not human fibroblasts (lane 11) was also found to express α- and β-tryptase mRNA. β-Actin cDNA was obtained from all samples (B). RT-PCR products for α- and β-tryptase obtained from plasmids containing α- and β-tryptase cDNA were subjected to electrophoresis before (α, β) and after (α+, β+) digestion with Dra III (C).
The specificity of the RT-PCR/RFLP protocol was demonstrated using plasmids containing the cDNA coding for α- and β-tryptase (Figure7C). In particular, α- and β-tryptase cDNA were amplified from the corresponding plasmids (Figure 7C, lanes α, β). Restriction analysis with Dra III resulted in a single 383-bp band in case of α-tryptase, and in 2 bands of 263 bp and 120 bp in the case of β-tryptase (Figure 7C, lanes α+, β+).
Production of tryptase by isolated bm MNCs
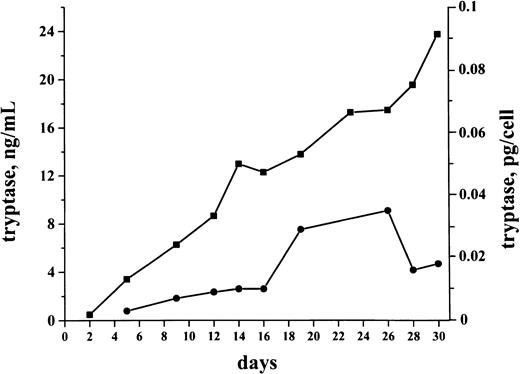
Cultures were generated by using bm MNCs obtained from 6 patients with tryptase+ de novo AML. Cells were cultured in RPMI 1640 plus 10% FCS for up to 30 days. Production and release of tryptase in cultured cells were analyzed by FIA. Baseline levels of tryptase were detected in both cell lysates and supernatants on days 2 and 5. During a first phase (days 2-18) in culture, the cellular levels of tryptase (pg/cell) increased in all cases (1.2- to 3.1-fold) with a maximum level of 1.2 pg/cell on day 23 measured in a patient with AML-M4eo. Accompanying this increase in cellular tryptase, there was a parallel increase in released tryptase detected in supernatants (1.2- to 5.1-fold increase comparing day 5 and day 23 levels). After day 25, cellular levels of tryptase decreased, whereas, in supernatants, tryptase remained at a high level (Figure8).
Spontaneous production and release of tryptase in cultured AML cells.
AML cultures were performed using isolated bm MNCs. The figure shows a representative culture experiment performed in a tryptase+AML patient (secondary AML, serum tryptase level, 30.4 ng/mL). Cells were cultured in RPMI 1640 medium supplemented with 10% FCS (37°C; 5% CO2) for 30 days. Tryptase levels (y axis) in cell-lysates (●) and supernatants (■) were measured serially by FIA. As visible, significant levels of tryptase were detected in these cultures. Cellular tryptase levels (pg/cell) as well as the tryptase levels in the supernatants increased during a first phase of culture. Thereafter, cellular levels of tryptase decreased, whereas, in the supernatants, tryptase remained at a high level.
Spontaneous production and release of tryptase in cultured AML cells.
AML cultures were performed using isolated bm MNCs. The figure shows a representative culture experiment performed in a tryptase+AML patient (secondary AML, serum tryptase level, 30.4 ng/mL). Cells were cultured in RPMI 1640 medium supplemented with 10% FCS (37°C; 5% CO2) for 30 days. Tryptase levels (y axis) in cell-lysates (●) and supernatants (■) were measured serially by FIA. As visible, significant levels of tryptase were detected in these cultures. Cellular tryptase levels (pg/cell) as well as the tryptase levels in the supernatants increased during a first phase of culture. Thereafter, cellular levels of tryptase decreased, whereas, in the supernatants, tryptase remained at a high level.
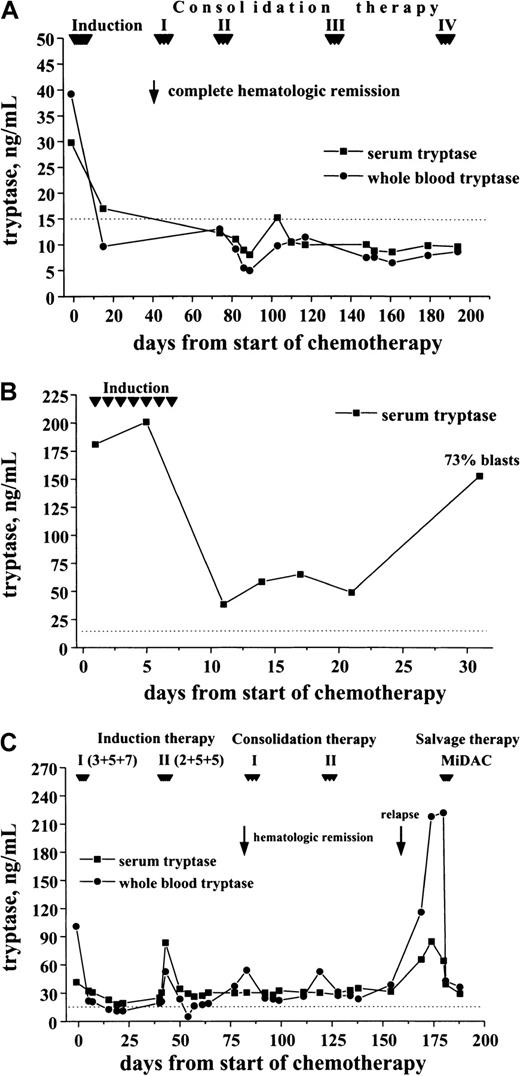
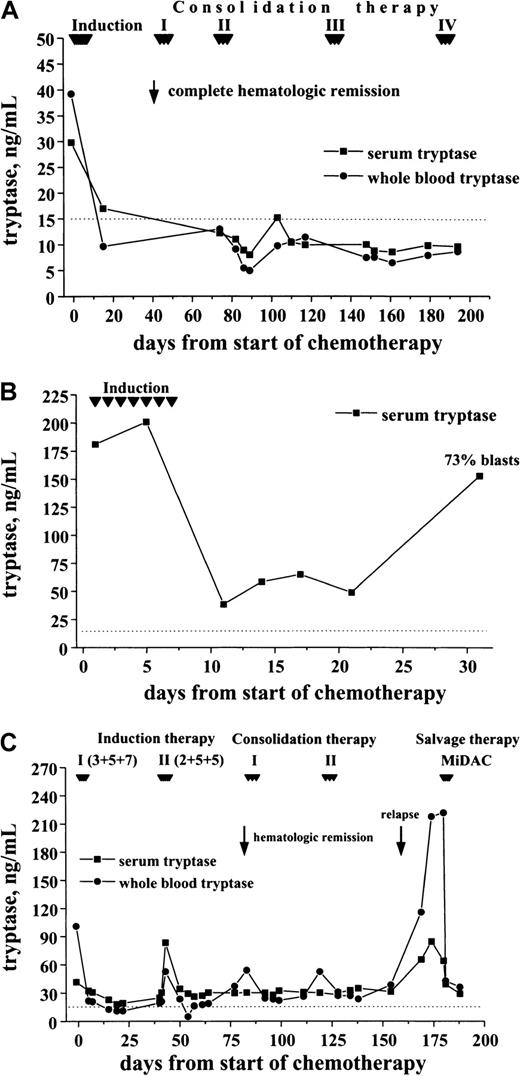
Correlation between clinical course and elevated tryptase levels
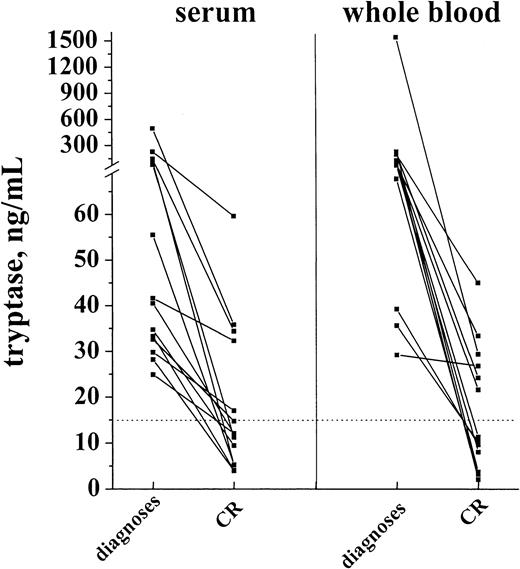
In a first step, tryptase levels measured at diagnosis and after achieving hematologic CR were compared. As visible in Figure9, induction treatment resulted in a decrease in serum tryptase levels in all cases. In the majority of patients with initially high levels, the serum tryptase concentrations were within the normal range at CR. Similarly, the whole blood tryptase levels decreased during therapy. In a smaller subset of patients, however, tryptase remained elevated despite hematologic CR. In most patients the time course of tryptase levels was analyzed. In patients who remained in CR during the observation period, tryptase levels remained ≤ 15 ng/mL (Figure 10A). By contrast, in cases with blast cell persistence or regrowth shortly after CR, tryptase levels remained elevated (Figure 10B). Figure 10C shows a patient with recurrence of disease during consolidation treatment. In this patient, tryptase levels decreased during induction treatment but remained above normal range at CR. The regrowth of blasts was associated with a marked increase in tryptase levels. The CR rate was similar in patients with normal serum tryptase compared with those with enhanced enzyme levels.
Serum tryptase and whole blood tryptase levels in patients with AML at diagnosis and at the time of complete hematologic remission (CR).
The levels of serum and whole blood tryptase were analyzed in patients with AML at diagnosis and after the patients had entered CR. As visible, tryptase levels decreased in response to chemotherapy, and, in the majority of the cases, the levels were within the normal range (< 15 ng/mL, dotted line) after patients had achieved a CR.
Serum tryptase and whole blood tryptase levels in patients with AML at diagnosis and at the time of complete hematologic remission (CR).
The levels of serum and whole blood tryptase were analyzed in patients with AML at diagnosis and after the patients had entered CR. As visible, tryptase levels decreased in response to chemotherapy, and, in the majority of the cases, the levels were within the normal range (< 15 ng/mL, dotted line) after patients had achieved a CR.
Follow-up of patients with AML with enhanced tryptase levels.
In patients with AML with elevated serum tryptase, enzyme levels were monitored during and after induction chemotherapy by serial measurements. In patients with continuous CR, serum tryptase levels remained in the normal range (A; M1, 29.8 ng/mL) By contrast, in patients with blast cell persistence or rapid regrowth of blast cells, tryptase levels often remained elevated or showed a recurrent increase. (B) This panel shows a patient (M0, 180 ng/mL) with blast cell persistence (refractory to induction chemotherapy) and persistently elevated serum tryptase. (C) This panel shows a patient with AML M2 (41.6 ng/mL) who entered CR after 2 induction cycles and relapsed during consolidation therapy.
Follow-up of patients with AML with enhanced tryptase levels.
In patients with AML with elevated serum tryptase, enzyme levels were monitored during and after induction chemotherapy by serial measurements. In patients with continuous CR, serum tryptase levels remained in the normal range (A; M1, 29.8 ng/mL) By contrast, in patients with blast cell persistence or rapid regrowth of blast cells, tryptase levels often remained elevated or showed a recurrent increase. (B) This panel shows a patient (M0, 180 ng/mL) with blast cell persistence (refractory to induction chemotherapy) and persistently elevated serum tryptase. (C) This panel shows a patient with AML M2 (41.6 ng/mL) who entered CR after 2 induction cycles and relapsed during consolidation therapy.
Discussion
Alpha- and β-tryptases have been used as specific markers for mast cells and mast cell–associated disorders (mastocytosis).21,26,32,33 However, a number of observations suggest that substantial amounts of tryptase are also expressed in neoplastic myeloid, non–mast cell lineage cells.23-25 In the present study, we have examined the expression of tryptase in patients with acute leukemias. The results of our study show that tryptase is aberrantly expressed in a group of patients with AML. Expression of tryptase in AML blasts could be demonstrated by Northern blotting and RT-PCR, immunohistochemistry, immunoelectron microscopy, FIA, and flow cytometry. Moreover, in patients with tryptase+ AML, elevated levels (> 15 ng/mL) of serum tryptase were measurable. By contrast, elevated levels of tryptase could not be detected in any of the patients with ALL, confirming specificity of mast cell tryptase for myeloid cells.
Two major mast cell tryptase genes have been identified and cloned, α- andβ-tryptase.27-30 In this study, we examined expression of mast cell tryptase species in AML. As assessed by Northern blotting, expression of α-tryptase mRNA was detectable in AML cells in all cases with elevated serum tryptase. In contrast, β-tryptase mRNA was detectable only in 2 patients (both AML-M4eo with very high serum tryptase levels). With the use of the more sensitive RT-PCR technique, however, β-tryptase mRNA expression could be demonstrated in a significant proportion of cases (5 of 9). All in all, these data suggest that, in most patients with tryptase+AML, blast cells express α-tryptase mRNA in excess over β-tryptase mRNA. Corresponding results were obtained from immunoassay experiments. In fact, measurable serum β-tryptase levels (> 1 ng/mL) could be detected only in 6 of 15 patients with AML having elevated total serum tryptase, and, in all cases analyzed, serum total tryptase levels exceeded β-tryptase levels by far. These observations suggest that the predominant form of tryptase expressed and released in AML cells is the α-protryptase type. Interestingly, in patients with SM, the predominant form of expressed tryptase also seems to be the α-protryptase type.32 Whether AML blast cells are also capable of expressing additional tryptases remains unknown. In this regard it is of interest to note that a third tryptase gene, called human mouse mast cell protease-7 like tryptase, has been described.30 However, little if any of the corresponding mRNA was detected in tryptase-producing mast cells or leukemic cell lines.52
To further examine tryptase production in AML, culture experiments were performed on isolated AML blasts. In these experiments, a spontaneous increase in extracellular tryptase levels was found, suggesting that AML blasts produce and release tryptase in vitro in a constitutive manner. Furthermore, we were able to detect the tryptase protein in isolated AML cells by immunocytochemistry and immunoelectron microscopy. As assessed by electron microscopy, tryptase could be localized to the (small) cytoplasmic compartment of AML blasts. An interesting aspect was that most of the immunoreactive material was detected in smaller or larger granulelike structures. These granules did not exhibit typical morphologic features of granules detected in mast cells or basophils. In fact, neither the scrolls and grating/lattice structures seen in mast cells53,54 nor the particulate-granula structures seen in basophils53 were detected in AML blasts. All in all, no definitive morphologic or ultrastructural signs of mast cell or basophil maturation could be detected in tryptase+ AML cells.
To analyze the distribution of tryptase+ cells in the bm of our AML patients, immunohistochemistry was performed. In these experiments tryptase was detectable in AML cells in bm sections of all cases with elevated serum tryptase, whereas no reactivity of leukemic cells was seen when serum levels of tryptase were normal. In all cases examined, the tryptase+ AML cells exhibited a diffuse infiltration pattern without focal dense infiltrates characteristic of mastocytosis.41 Thus, by histologic and immunohistochemical criteria, a primary mast cell disease was not present in our tryptase+ AML patients.
An interesting observation was that elevated serum tryptase levels cluster in distinct FAB groups of AML. In particular, high levels of the enzyme were detected in (most) patients with AML M0, M2, M3, and AML-M4eo, whereas most patients with M4, M5, and M6 had normal tryptase levels. The highest tryptase levels were detectable in AML-M4eo. In this regard it is noteworthy that the genes coding for human tryptases cluster on the short arm of chromosome 16.27,28,30 It is also of interest that a subgroup of patients with M4eo, M2, and M3 reportedly exhibit c-kit point mutations at position 816,55-57 a defect that is otherwise found specifically in SM. Finally, M4eo blasts express the CD2 antigen,58,59 a T-cell and natural kill cell marker that is also expressed specifically on neoplastic (but not normal) mast cells in patients with SM.60 Therefore, it may be tempting to speculate that tryptase expression in AML-M4eo is indicative of minimal differentiation along the mast cell pathway. The maturation arrest, however, would not allow for terminal differentiation and maturation.
To further examine lineage relationships, we also examined expression of histamine and other differentiation antigens (2D7, Kit) in our AML cases. In these experiments it was found that histamine is variably expressed in AML blasts without a significant correlation to tryptase expression. In particular, there were cases exhibiting high levels of histamine but normal tryptase levels and also cases of tryptase+ AML without significant expression of histamine. In a smaller group, however, both mediators were detectable. This finding is of particular interest because both mast cells and basophils express histamine, but only mast cells are capable of synthesizing larger amounts of tryptase.22,26 Thus, it is tempting to speculate that those AMLs with high amounts of both mediators involved a mast cell–committed progenitor and those expressing histamine in excess over tryptase involved a basophil-committed cell. It is also noteworthy that tryptase+ AMLs were sometimes labeled by 2D7 mAb that detects a basophil-related antigen.42 Another possibility would be aberrant expression of mediators and antigens. This may especially be true for cases expressing only one specific mediator (tryptase). Physiologic expression of tryptase in early development of (normal) myeloid progenitor cells seems unlikely, however. Notably, normal CD34+ bm progenitor cells were consistently tryptase negative by immunocytochemistry and flow cytometry without any evidence of subpopulations of tryptase-positive cells.
The functional or biologic significance of tryptase expression in AML remains unknown. Previous observations have shown that tryptases are potent mitogens for fibroblasts and endothelial cells.61-63 Because such cells are well known to produce hemopoietic growth factors (like granulocyte-macrophage colony-stimulating factor or stem cell factor) on activation, one may speculate on a paracrine function of tryptases produced by AML blasts. Another possibility could be an effect of AML-derived tryptases on bm angiogenesis known to be up-regulated in AML.64 65 A direct effect of tryptases on proliferation of AML blasts (autocrine loop) would be a third possibility. Studies are in progress to clarify the pathophysiologic role of tryptases expressed in AML blasts.
The most interesting aspect of our study was that whole blood and serum tryptase levels in AML showed a significant correlation with disease. In particular, a significant decrease in serum tryptase levels was seen during induction treatment, and at the time of CR the majority of the cases returned to normal values, whereas blast cell persistence was associated with a persistently elevated enzyme level. Moreover, in patients with hematologic CR and persistent elevation of serum tryptase, a hematologic relapse occurred in all cases. Therefore, tryptase may also be useful as a marker of disease in AML monitoring. The actual prognostic value of tryptase as an AML marker will be clarified in forthcoming studies.
In summary, we show that significant amounts of tryptase are expressed in blast cells in a group of patients with AML. Tryptase appears to be a myeloid-specific marker in acute leukemias and may be useful to detect and monitor minimal residual AML especially in patients who do not have another reliable (genetic) disease-related marker.
We thank Stefanie Wessel for skillful technical assistance.
Supported by Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich (FWF); grants P-14031 and F0506 by the ICP program of the Austrian Federal Ministry for Education, Science and Culture; and by grants AI20487 and AR45441 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wolfgang R. Sperr, Department of Internal Medicine I, Division of Hematology and Hemostaseology, University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria; e-mail:wolfgang.r.sperr@univie.ac.at.