Abstract
The potential role of unrelated donor cord blood transplantation (UD-CBT) in adults remains unclear. This study reports the results of UD-CBT in 22 adults with hematologic malignancies following conditioning with thiotepa, busulfan, cyclophosphamide, and antithymocyte globulin in 21, with thiotepa, fludarabine, and antithymocyte globulin in 1, and graft-versus-host disease (GVHD) prophylaxis with cyclosporine and prednisone. Median age was 29 years (range, 18-46 years), and median weight was 69.5 kg (range, 41-85 kg). HLA match was 6 of 6 in 1 case, 5 of 6 in 13 cases, and 4 of 6 in 8 cases. Median number of nucleated cells infused was 1.71 × 107/kg (range, 1.01 × 107/kg to 4.96 × 107/kg). All 20 patients surviving more than 30 days had myeloid engraftment, and only 1, who received the lowest cell dose, developed secondary graft failure. Median time to reach an absolute neutrophil count of at least 0.5 × 109/L was 22 days (range, 13-52 days). Median time to platelets numbered at least 20 × 109/L was 69 days (range, 49-153 days). Seven patients (32%) developed acute GVHD above grade II, and 9 of 10 patients at risk developed chronic GVHD, which became extensive in 4 patients. Twelve patients remained alive and disease-free 3 to 45 months after transplantation. Disease-free survival (DFS) at 1 year was 53%. Age strongly influenced DFS (P = .01). For patients aged 30 years or younger, the DFS at 1 year was 73%. These preliminary results suggest that UD-CBT should be considered a reasonable alternative in young adults with hematologic malignancy and no appropriate bone marrow donor.
Introduction
The potential role of unrelated donor cord blood transplantation (UD-CBT) in adults remains unclear. At present, information on the results of UD-CBT in adults comes only from 2 heterogeneous series of patients with different diseases from registries including mainly pediatric cases,1,2 from 2 heterogeneous series of adult patients recently reported by Eurocord3 and by 5 U.S. centers,4 from several small series of adults from a single institution,5-9 and from anecdotal case reports.10-16 This deficit is due mainly to grave concerns about the possibility of graft failure following UD-CBT in adults.17 None of the series of UD-CBT in adults have used a unique preparative and graft-versus-host disease (GVHD) regimen.
The major aim of this study was to evaluate the results of UD-CBT following a standardized preparative and GVHD regimen in a series of 22 consecutive adult patients with hematologic malignancies. All 20 evaluable patients engrafted. Thirteen patients remained alive and disease-free, with full donor chimerism, 3 to 45 months after transplantation.
Patients, materials, and methods
Patients and eligibility criteria
Between May 1997 and December 2000, 22 consecutive patients with hematologic malignancies underwent UD-CBT at our institution. Patients were eligible for enrollment if they met all of the 5 following criteria: (1) Allogeneic hematopoietic stem cell transplantation was expected to be the best therapy for their disorder. (2) There was no HLA-identical or one-antigen–mismatched related donor. (3) Using International Bone Marrow Donor registries, no potential HLA-identical matched unrelated donor (MUD) could be identified in time. (4) A UD-CB unit was available that fulfilled 2 criteria: more than 1 × 107 nucleated cells (NCs) per kilogram of the recipient's body weight in the cryopreserved CB unit and the sharing of at least 4 HLA antigens in common with the potential recipient (HLA class I antigens [A and B] determined by serologic or low-resolution DNA typing and class II antigens [DRB1] by high-resolution DNA typing). (5) There was signed informed consent of each patient. The local institutional review board approved the information given to patients and the provision of informed consent. In some cases, an autologous back-up of bone marrow or mobilized peripheral blood hematopoietic progenitors was available to deal with a potential graft failure.
Selection of a CB unit
All formal searches were conducted by the Spanish Registry of Bone Marrow Donors (Registro Español de Donantes de Médula Osea). After a preliminary search, the CB units that fulfilled the criteria specified above were prioritized on the basis of the degree of HLA match. When several units with the same degree of HLA match were available for a patient, we selected the one that contained the highest number of NCs. Whenever possible, mismatches within the same cross-reacting group of HLA antigens were preferred to major incompatibilities. In choosing between units with the same number of HLA disparities, no preference was given to mismatches for the class I or class II major histocompatibility complex antigens. For cases with more than one unit with the same degree of HLA mismatch and a similar number of NCs, high-resolution DNA typing of class I antigens (HLA-A, -B, and -C) and class II antigens (HLA-DRB1, -DQB1, and -DPB1) was performed to define the unit with the best match. All transplanted CB units tested negative for human immunodeficiency virus, hepatitis B and C viruses, and human T-cell lymphotropic virus type I. None of the units nor the mothers of the donors were positive for immunoglobulin M antibody to cytomegalovirus (CMV).
HLA typing of patients and donors
For confirmatory purposes, all patients and CB units were HLA typed in our laboratory (D.P.). HLA class I serologic typing was performed by a standard complement-dependent microlymphocytotoxicity technique.18 HLA-A, -B, -C, -DRB, -DQA1, and -DQB1 low- or high-resolution genotyping was performed by polymerase chain reaction with sequence-specific primers (SSP-PCR)19 and HLA-DPB1 high-resolution genotyping by PCR and reverse-hybridization with sequence-specific oligonucleotide probes (SSO-PCR).20
Transplantation procedure
Cryopreserved CB units were transported to the Centro de Trasfusión de la Comunidad Valenciana in a shipping container cooled by liquid nitrogen in vapor phase and stored in liquid nitrogen until use. The CB unit canister was left in the gas vapor phase for 5 to 10 minutes before proceeding. The canister was carefully opened, avoiding damage to the frozen blood bag, and the identifying information was verified. Thawing of CB units was performed by Rubinstein et al's method,21 with minor modifications as described elsewhere.9 Samples for cell counts, quantitation of CD34+ cells,22 cell viability, clonogenic assays,23 and microbiology were drawn directly from the bag before infusion.
Preparative regimen and GVHD prophylaxis
The conditioning regimen consisted of 21 cases on thiotepa (5 mg/kg per day on days −9 and −8), busulfan (1 mg/kg every 6 hours on days −7, −6, and −5), cyclophosphamide (60 mg/kg per day on days −4 and −3), and horse antithymocyte globulin (ATG; Lymphoglobuline, Merieux, Lyon, France) (15 mg/kg per day on days −5, −4, −3, and −2). One patient, who had failed to engraft after a MUD peripheral blood stem cell transplantation (PBSCT) performed 30 days before UD-CBT and conditioned with busulfan and cyclophosphamide, received fludarabine (30 mg/m2 per day on days −7, −6, −5, −4, and −3), thiotepa (5 mg/kg per day on days −7 and −6), and ATG (15 mg/kg per day on days −6, −5, −4, −3, and −2). To avoid ATG-associated infusion syndrome, patients concomitantly received paracetamol, chlorpheniramine, and methylprednisolone. Granulocyte colony-stimulating factor (Amgen, Thousand Oaks, CA) (5 μg/kg per day subcutaneously) was administered from day +7 until neutrophil engraftment. As GVHD prophylaxis, all patients received cyclosporine (3 mg/kg per day intravenously, followed by 3-5 mg/kg every 12 hours by mouth when oral intake was possible, reduced by 10% per week after day +180) and prednisone (0.5 mg/kg on days +7 to +14, 1 mg/kg on days +14 to +28, 0.8 mg/kg on days +29 to +56, 0.5 mg/kg on days +57 to +90, 0.2 mg/kg on days +91 to +120, and 0.1 mg/kg on days +121 to +180). Patients developing acute GVHD received high-dose methylprednisolone (20 mg/kg per day; halving the dose every 3 days until reaching 1 mg/kg per day, and then gradually tapered) as initial therapy, followed by ATG in refractory cases. Chronic GVHD was treated with prednisone 1 mg/kg per day for 6 to 9 months.
Supportive care
Patients were kept in reverse isolation under high-efficiency particulate air filtration. Ciprofloxacin, cotrimoxazole, and fluconazole were given as anti-infectious prophylaxis. The use of intravenous antibiotics and liposomal amphotericin B, and the transfusion policy adopted, were those usually employed when handling neutropenic patients. All transfused products were irradiated and depleted of leukocytes. As CMV prophylaxis, all patients received intravenous acyclovir (400 mg/m2 every 12 hours) from day −5 until engraftment, followed by intravenous ganciclovir (5 mg/kg per day) 3 to 5 days per week from engraftment until day +100. CMV infection surveillance and treatment has been described in detail elsewhere.9 Nonspecific intravenous immunoglobulin was administered to each patient, at a dose of 500 mg/kg weekly, through to day +100 and then monthly during the first year after transplantation.
Chimerism and minimal residual disease studies
The chimerism status after transplantation was studied by PCR-VNTR (variable number of tandem repeats),24-26typing of HLA antigen mismatches by SSP-PCR and SSO-PCR19,20 and, for transplants from the opposite sex, fluorescence in situ hybridization (FISH).27Detection of BCR/ABL transcripts in patients with Ph-positive leukemia was performed by reverse transcriptase (RT)–PCR following Cross et al's methods.28,29 The presence of BCR/ABL rearrangements was evaluated by FISH.30
Definitions and statistical analysis
Myeloid engraftment was defined as an absolute neutrophil count (ANC) of 0.5 × 109/L or greater, on 3 consecutive days, and platelet engraftment as a platelet count of 50 × 109/L or higher, without transfusion support, for 7 consecutive days. Time to myeloid or platelet engraftment was defined as the time required to reach the first day of engraftment of the relevant cell. Secondary graft failure was defined as the loss of an engrafted transplant. Acute and chronic GVHD were defined and graded according to standard criteria.31-33 A complete response to initial treatment of GVHD was defined as complete resolution of all signs and symptoms in all affected organs on day 28 after starting therapy without requiring secondary treatment. Patients who had a complete resolution of all signs and symptoms of acute GVHD but expired before day 28 were considered unevaluable for response. The remaining cases were considered as refractory to therapy. The same criteria were used for evaluating the response of acute GVHD to secondary treatment. Patients with chronic myelogenous leukemia (CML) in chronic phase and those with acute leukemia or myelodysplastic syndrome (MDS) in first/second complete remission (CR) at transplantation were considered to have early disease; patients with CML in accelerated phase or blast crisis and acute leukemia in greater than second CR were considered to have advanced disease. Apart from the patients with CML in first chronic phase, all the remaining patients were considered to be high-risk patients. Time to event curves were plotted by using the actuarial method of Kaplan-Meier,34 and differences between curves were analyzed by log-rank tests.35 In actuarial curves of time to engraftment, patients who died before achieving the end point (neutrophils > 0.5 × 109/L, platelets > 20 × 109/L) were considered as censored data at the time of death. All data were analyzed by February 28, 2001. All statistical analyses were carried out with software from the BMDP library.36
Results
Patient characteristics
The main characteristics of the patients are summarized in Table1. Sixteen patients had high-risk malignancies (6 CML not in first chronic phase, 9 high-risk acute leukemia, 1 high-risk MDS). The patient with de novo acute myeloblastic leukemia (AML) was considered to have high-risk AML due to hyperleukocytosis and achievement of CR only after salvage chemotherapy. The patient with MDS had an International Prognostic Scoring System score equal to 3.5,37 was in first CR after AML-type chemotherapy, and had engraftment failure after MUD-PBSCT. Eight patients with CML in the chronic phase had previously received AML-type chemotherapy for mobilization and harvest of hematopoietic progenitors from peripheral blood as part of an ongoing protocol for autologous PBSCT. Two patients with CML in blast crisis had received daunorubicin and cytarabine in an unsuccessful attempt to revert the disease to the chronic phase. Three patients with CML (2 who received transplants in chronic phase and 1 in blast crisis) had undergone previous autologous PBSCT 24 months, 33 months, and 34 months before UD-CBT, respectively. Five of the CML patients had previously received interferon without achieving a cytogenetic response. In the overall series, the median time from the start of the search for a CB unit to transplantation was 4 months (range, 0.2-12 months). In most instances, the reason for delaying transplantation after a CB unit had been selected was the need for achieving CR and/or the administration of consolidation chemotherapy prior to transplantation.
CB unit characteristics and degree of HLA mismatch
Eleven CB units were supplied by the Barcelona Cord Blood Bank, 3 by the Placental Blood Program at the New York Blood Center, 2 by the Milano Cord Blood Bank, 2 by the London Cord Blood Bank, and 1 each by the Düsseldorf Cord Blood Bank, the France Greffe de Moelle, the St Louis Cord Blood Bank, and the Colorado Cord Blood Bank. By means of serologic and/or low-resolution DNA typing for class I antigens and high-resolution DNA typing for class II HLA alleles, 8 CB units were matched for 4 of 6 HLA antigens, 13 for 5 of 6, and 1 showed a complete match. In 20 cases, HLA-A and -B antigens were typed by high-resolution DNA techniques. In 4 of them, 1 additional mismatch was present. The median number of NCs infused was 1.71 × 107/kg (range, 1.01 × 107/kg to 4.96 × 107/kg). Only 6 patients received more than 2 × 107 NCs per kilogram and in only 3 was the number of NCs more than 3.7 × 107/kg. The median number of cryopreserved NCs was 2.48 × 107/kg (range, 1.54 × 107/kg to 6.90 × 107/kg). Thus, the median proportion of NCs lost during thawing was 30% (range −5% to 61%). The median time that elapsed between storage of the CB unit and transplantation was 22 months (range, 7-57 months). There was a major ABO blood group mismatch in 6 cases and a minor mismatch in 3 cases (Table 2).
Engraftment
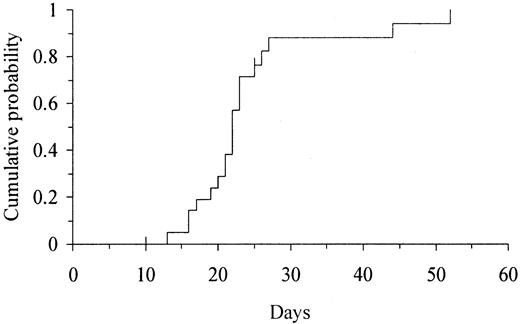
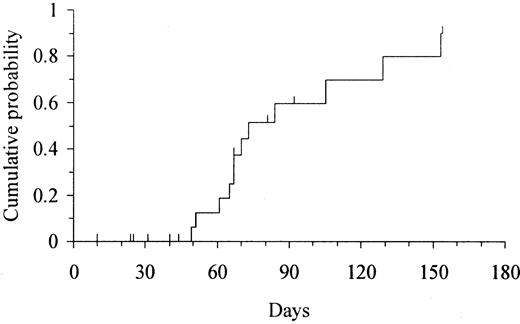
Two patients died on day +10 and +25 without evidence of myeloid engraftment. All 20 patients who survived for more than 30 days experienced myeloid engraftment. The median time to reach an ANC above 0.5 × 109/L and above 1 × 109/L was 22 days (range, 13-52 days) and 25.5 days (range, 14-64 days), respectively. The cumulative probability of ANC recovery above 0.5 × 109/L was 100% at 60 days (Figure1). The 2 patients with the slowest myeloid engraftment were (1) a patient who had undergone transplantation in a blast phase refractory to AML chemotherapy and who had a persistent massive splenomegaly and (2) a patient who received the lowest NC dose. In the former, the ANC returned to normal immediately after splenectomy. All 20 evaluable patients showed full donor chimerism at a median time of 14 days (range, 14-36 days) after transplantation. The patient who received the lowest NC dose experienced secondary graft failure shortly after engraftment. At that time, this patient had a mixed chimera (predominantly of the donor) based on PCR-SSP to HLA antigen mismatches but was evaluated as a full donor chimera by PCR-VNTR. Ten patients who died 10 to 154 days after transplantation had not experienced platelet recovery. The median time to achieve platelet counts above 20 × 109/L and above 50 × 109/L was 69 days (range, 49-153 days) and 105 days (range, 60-171 days), respectively. The cumulative probability of platelet recovery above 20 × 109/L and 50 × 109/L was 90% (95% confidence interval [CI], 72%-100%) at 150 days (Figure 2) and 100% at 180 days, respectively.
Kaplan-Meier estimate of the time to myeloid engraftment (ANC > 0.5 × 109/L) in 22 adults undergoing UD-CBT.
The cumulative probability of myeloid engraftment was 100% at 60 days.
Kaplan-Meier estimate of the time to myeloid engraftment (ANC > 0.5 × 109/L) in 22 adults undergoing UD-CBT.
The cumulative probability of myeloid engraftment was 100% at 60 days.
Kaplan-Meier estimate of the time to platelet engraftment (platelets > 20 × 109/L) in 22 adults undergoing UD-CBT.
The cumulative probability of platelet engraftment was 90% (95% CI, 72%-100%) at 150 days.
Kaplan-Meier estimate of the time to platelet engraftment (platelets > 20 × 109/L) in 22 adults undergoing UD-CBT.
The cumulative probability of platelet engraftment was 90% (95% CI, 72%-100%) at 150 days.
Acute and chronic GVHD
All but 1 patient developed acute GVHD. The clinical grading of acute GVHD was grade I in 5 cases, grade II in 9 cases, grade III in 3 cases, and grade IV in 4 cases. The median time to the development of acute GVHD was 9 days (range, 4-14 days). Skin involvement was observed in 21 cases (stage 1 in 2, stage 2 in 10, stage 3 in 7, stage 4 in 2), intestinal involvement in 6 cases (stage 1 in 2, stage 4 in 4), and liver involvement in 10 cases (stage 1 in 4, stage 3 in 4, stage 4 in 2). Seven patients had isolated skin involvement. Diagnosis of GVHD was histopathologically confirmed by skin biopsy in 16 cases, by liver biopsy in 2, and at autopsy in 3 cases. Twelve patients showed a complete response of acute GVHD after high-dose methylprednisolone, 6 were refractory (1 with grade II, 2 with grade III, 3 with grade IV acute GVHD), and 3 were considered unevaluable. Two of the 6 patients with steroid-refractory acute GVHD responded to salvage therapy for GVHD, 2 were refractory, and 2 were considered unevaluable. Nine of 10 patients at risk developed chronic GVHD. Chronic GVHD was limited in 5 cases and extensive in 4 cases. Chronic GVHD was responsive to prednisone and cyclosporine, except in 1 patient in whom chronic GVHD had progressed from refractory grade IV acute GVHD. The median time to the development of chronic GVHD was 121 days (range, 100-325 days). Notably, only 1 of the patients with chronic GVHD had hepatic involvement, whereas 3 had pulmonary involvement, which was suggestive of bronchiolitis obliterans of mild to moderate degree.
Outcome
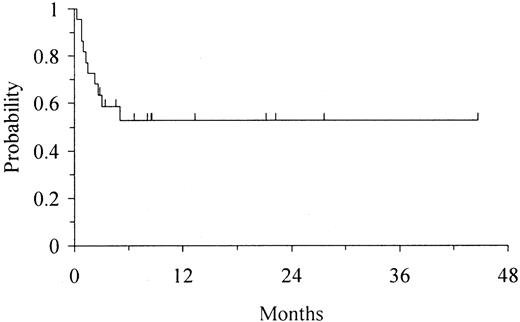
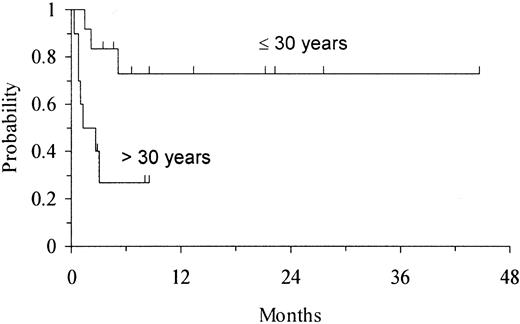
Twelve of 22 patients remain alive and disease-free, with full donor chimerism, 3 to 45 months after transplantation (median follow-up 8 months). Of these patients, 5 have CML in chronic phase (3 received transplants in first and 2 in second chronic phase), 3 ALL (2 received transplants in first remission [CR1] [1 with t(9;22) and 1 with t(4;11)], 1 in third remission [CR3]), 3 AML (2 with secondary AML, 1 with high-risk de novo AML), and 1 MDS. All 6 patients with Ph-positive disorders were BCR/ABL-negative by FISH and RT-PCR at last follow-up. The Karnofsky activity score in patients still alive ranges from 90% to 100%. The actuarial disease-free survival (DFS) in the overall group of patients was 53% (95% CI, 31%-75%) at 1 year (Figure 3). Age had a strong impact on outcome; 9 of 12 patients aged 30 years or younger remain alive and disease-free, with a projected actuarial DFS of 73% (95% CI, 45%-100%) at 1 year, whereas only 3 of 10 patients aged more than 30 years at transplantation remain alive and disease-free, with a projected DFS of 27% (95% CI, 0%-57%) at 3 months (P = .01) (Figure 4). Patients with early disease also showed some trend for a better DFS (P = .09). Cell dose, whether cryopreserved or infused, degree of HLA match, recipient CMV status, and risk category of the patient had no apparent effect on DFS.
Kaplan-Meier estimate of DFS in 22 adults undergoing UD-CBT.
The expected DFS at 1 year was 53% (95% CI, 31%-75%).
Kaplan-Meier estimate of DFS in 22 adults undergoing UD-CBT.
The expected DFS at 1 year was 53% (95% CI, 31%-75%).
Kaplan-Meier estimate of DFS according to age at transplantation in 22 adults undergoing UD-CBT.
The DFS for patients aged 30 years or less was 73% (95% CI, 45%-100%) at 1 year, whereas DFS for patients aged more than 30 years was 27% (95% CI, 0%-57%) at 3 months (P = .01).
Kaplan-Meier estimate of DFS according to age at transplantation in 22 adults undergoing UD-CBT.
The DFS for patients aged 30 years or less was 73% (95% CI, 45%-100%) at 1 year, whereas DFS for patients aged more than 30 years was 27% (95% CI, 0%-57%) at 3 months (P = .01).
Ten patients, including 7 with CML (3 who underwent transplantation in first chronic phase, 1 in accelerated phase, and 3 in blast crisis) and 3 with Ph-positive ALL (who underwent transplantation in CR1) died 10 to 150 days after transplantation. The cumulative probability of transplantation-related mortality (TRM) at 100 days was 43% (95% CI, 21%-65%). The main cause of death was infection in 4 cases, GVHD in 2, hemorrhage in 2, secondary graft failure in 1, and conditioning regimen toxicity in 1. When all contributing causes of death were accounted for, infection was present in 9 cases, GVHD in 4, hemorrhage in 3, conditioning regimen and/or cyclosporine toxicity in 2, and secondary graft failure in 1. Interestingly, all 3 patients with CML in blast crisis were BCR/ABL-negative by FISH, and 2 of them were negative by RT-PCR, at the time of death.
Discussion
This series of UD-CBT in adults with hematologic malignancies, the first to employ a standardized conditioning regimen, a unique GVHD prophylaxis, and a common supportive treatment, demonstrates short- and long-term hematopoietic engraftment and the prospect for cure in a substantial proportion of patients. This is particularly true for young adults who receive transplants in the earlier phases of their disease.
Despite its theoretical advantages over bone marrow transplantation (BMT) from unrelated donors,16 the lower number of hematopoietic stem cells in CB compared with bone marrow, together with preliminary data showing the importance of cell dose for the outcome of CBT,1,2 have been of major concern in adult patients. At present, it is almost impossible to evaluate the potential role of UD-CBT in adults. First, information about the results of UD-CBT in adults is only available through reports from registries1-3 (2 of them comprising mainly children1,2), a joint study from 5 U.S. centers reported in abstract form,4 small study series from single institutions5-9 (reported mostly only in abstract form5,6,8), or as anecdotal case reports.10-16 Secondly, in all but one instance9 the populations of patients and the conditioning regimens have been heterogeneous. The present series included only adult patients who received the same conditioning (except one case) and who were managed uniformly in terms of prophylaxis and treatment of GVHD as well as with respect to anti-infection and transfusion policy.
In the present series, the cell dose infused and the degree of HLA mismatch between recipients and CB units was very similar to that reported for adults by the New York Placental Blood Program,2 Eurocord,1,3 and single centers.6,8 As expected, the cell dose infused to the patients was clearly lower than that reported for children.1,2 In a recent report from Duke University,6 including 38 transplantations involving adults, only 2 patients experienced autologous recovery and 6 died from infection 18 to 89 days after transplantation with no evidence of engraftment. In the combined experience of UD-CBT in adults at 5 U.S. institutions, including 68 patients, the actuarial probability of neutrophil engraftment was 92%.4 The more extensive series of 108 adults undergoing UD-CBT reported by Eurocord had a myeloid engraftment rate of 81% at 60 days.3 In the present series, all 20 patients who survived more than 30 days had myeloid engraftment, and only 1 patient (interestingly, the one who received the lower cell dose) experienced loss of engraftment. Time to myeloid engraftment was shorter than expected for adults undergoing UD-CBT.3,4,6,8 In the 2 larger series studied by Eurocord3 and by the 5 U.S. centers,4 the median time to achieve an ANC above 0.5 × 109/L was 32 days and 27 days, respectively,3,4 whereas it was 22 days in our series. It is possible that the intensity of conditioning, as has been recently suggested to occur in children undergoing CBT from a related donor for hemoglobinopathy,38 and the use of ATG to further decrease the immune response in the recipient could have facilitated engraftment in our patients. The use of granulocyte colony-stimulating factor in all cases may have also played some role. In agreement with previous studies in both children and adults,1-3,6,8 platelet engraftment was delayed after UD-CBT. Nonetheless, it should be noted that although some patients died early without platelet engraftment, all patients with sufficient follow-up demonstrated a complete and sustained recovery of platelet counts to normal levels. Full donor chimerism, as in other series,4 was observed soon after transplantation, before day +21 in all but 2 cases. This chimerism status persisted in all but one case, where there was secondary graft failure.
Data from registries, including mainly children,1,2 and the results of a recent nonrandomized comparative study of UD-CBT and MUD-BMT in children with acute leukemia39 suggest that, despite a higher degree of HLA mismatch, the risk of acute and chronic GVHD after UD-CBT is lower than after unmanipulated MUD-BMT. In adults, although the reported incidence of acute and chronic GVHD after UD-CBT seems higher than in children, the preliminary data point in the same way.3,4,6 In the Duke University series, in which 35 of 38 patients were mismatched in 1 to 3 HLA antigens, only 13 patients (34%) developed grade II to IV acute GVHD.6 Among 108 adults with hematologic malignancies reported by Eurocord, of whom 102 had at least 1 HLA mismatch (1 in 38 cases, 2 in 51, 3 in 13 cases), the probability of developing grade II to IV acute GVHD was 41% and of developing grade III to IV acute GVHD was 26%; chronic GVHD occurred in 15 of 58 patients at risk.3 In the recently reported joint study by 5 U.S. institutions, with 71% of patients receiving CB units that were disparate for 2 or more HLA antigens, the probabilities of grade II to IV, grade III to IV acute GVHD, and chronic GVHD were 60%, 20%, and 38%, respectively.4 In the present series, most patients at risk experienced some degree of acute and chronic GVHD, and the probability of developing acute GVHD of grade II to IV and of grade III to IV was 63% and 32%, respectively. Apart from the small numbers, subjectivity in the application of the grading system for reporting acute GVHD among marrow transplant centers may account, at least in part, for these differences.40,41This variance could also be due to differences in the prophylaxis and treatment of acute GVHD and other factors. It also should be noted that 7 patients in the present series experienced acute GVHD of the skin only and that acute GVHD was responsive to therapy in most patients. However, although higher than in other reports of UD-CBT in adults, the incidence of acute GVHD in the present series was less than would be expected for adults receiving bone marrow from mismatched unrelated donors. As reported before by our group,9 the clinical pattern of chronic GVHD seems somewhat different than that usually seen after MUD-BMT. Only 1 patient had hepatic involvement, whereas 3 patients had pulmonary symptoms that were suggestive of bronchiolitis obliterans of moderate degree. Conceivably, unnoticed isolated lung involvement by GVHD may have contributed to the high death rate by pulmonary disease noted in a previous report on UD-CBT.2
Whether due to the slower speed of myeloid and platelet engraftment, GVHD, or defective immunologic reconstitution, TRM is the major drawback of UD-CBT. In our experience, TRM at day 100 was 43% and was mainly due to infection, bleeding, and GVHD. This figure is similar to that observed by others in both adults3,8 and children1,2 undergoing UD-CBT. As has occurred in the MUD-BMT setting,42,43 increasing experience with UD-CBT, including a better selection of CB units for transplantation, may reduce TRM. In fact, the preliminary data of UD-CBT in adults by Eurocord show a significant reduction in TRM at day 100 in transplantations performed after January 1998.3
Based on data from T-cell–depleted BMT44 and the strong inverse relationship between the development of GVHD and relapse risk in unmanipulated BMT,45 it has been speculated that the lower number of T cells in CB units coupled with the lower incidence of GVHD in UD-CBT could underlie a higher risk of relapse. Nonetheless, 3 recent reports comparing CBT and unmanipulated BMT from HLA-identical siblings46 and from unrelated donors39 47among children with leukemia have shown a similar risk of relapse. In our short experience, none of the patients have relapsed and all evaluable patients remain in molecular remission at the most recent follow-up. Furthermore, all 3 patients with CML in blast crisis at transplantation who died were in cytogenetic remission by conventional cytogenetics and FISH techniques, and 2 were in molecular remission by RT-PCR shortly before death.
At present, the long-term outcome of UD-CBT in adults cannot be properly evaluated, owing to small numbers, short follow-up, and heterogeneous patient populations. In the present series, using a standardized regimen, the estimated probability of DFS at 1 year was 53%. The cell dose infused per kilogram, measured as NCs per kilogram,1,2 colony-forming unit granulocyte macrophage (CFU-GM) infused per kilogram,48 or CD34+cells infused per kilogram,4 and the status of the disease at transplantation1,3,49 have emerged as strong indicators of survival after UD-CBT. The number of HLA mismatches have not demonstrated a significant relationship with survival in 2 large series,1,2 but a recent report has shown that it is a relevant factor for engraftment.50 We found that age at transplantation had a clear impact on DFS, with a poorer outcome for patients aged more than 30 years. Furthermore, there was some trend for a better DFS in patients with standard-risk disease status at transplantation. However, neither the cell dose cryopreserved or infused (measured as NCs, CFU-GM, and CD34+ cells per kilogram) nor the number of HLA disparities showed any apparent association with DFS. The more likely explanation for these somewhat surprising findings about the lack of impact of these 2 later variables may simply be that they are due to the small number of patients studied. Nevertheless, it could also be due to other reasons. For example, for adults, it is possible that the cell dose (assuming that a minimum threshold of NCs per kilogram is infused), although important, is not as critical for the outcome of UD-CBT as it is for children. It should be stressed that the cell dose infused per kilogram reflects not only the cell dose content of the CB units but, indirectly, other recognized prognostic factors for outcome after allogeneic stem cell transplantation, such as age,42,43 weight,43and disease status.9
Our data, in agreement with other reports, show that UD-CBT, despite the low cellular dose infused, is capable of promoting sustained hematopoietic engraftment in most adult recipients with hematologic malignancy. They also suggest that UD-CBT can result in long-term DFS in many of these patients, particularly in younger patients who receive transplants in earlier stages of their diseases. Because ongoing experience in the use of MUD-BMT has resulted in a significant increase in the success of this procedure, the same will probably hold true for UD-CBT. All of these preliminary results suggest that indications for UD-CBT in adults should be reviewed.
Supported in part by grant FIS-01/0066-01 from the Fondo de Investigaciones Sanitarias, Spanish Ministry of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Miguel A. Sanz, Servicio de Hematologı́a, Hospital Universitario La Fe, Av Campanar 21, 46009 Valencia, Spain; e-mail: msanz@uv.es.