Abstract
A family with recessive X-linked thrombocytopenia affecting 4 males in 2 generations, characterized by macrothrombocytopenia, profound bleeding, and mild dyserythropoiesis, is described. Microsatellite linkage analysis identified a region of the X chromosome including theGATA-1 gene, which encodes a critical transcription factor involved in erythrocyte and megakaryocyte development. By sequencing the entire coding region of GATA-1, a 2-base mutation was detected that results in a single amino acid substitution (glycine 208 to serine) within a highly conserved portion of the N-terminal zinc finger domain. Restriction fragment length polymorphism confirmed that this novel mutation segregated with the affected males and female carrier. Although not required for DNA binding, Gly208 of GATA-1 is involved in direct interaction with Friend of GATA-1 (FOG), a cofactor required for normal megakaryocytic and erythroid development. These results demonstrate that the GATA-1–FOG interaction is partially disrupted by the mutation and that the greatest effect involves contact with the FOG zinc finger 9. These findings help describe a novel mutation of GATA-1 in humans as a cause of X-linked thrombocytopenia, and they confirm the vital role played by this transcription factor during in vivo megakaryocyte development.
Introduction
We evaluated a family with macrothrombocytopenia and severe bleeding disorder, affecting 4 males in 2 generations, to understand the genetic basis for the recessive X-linked thrombocytopenia. The presence of large platelets, the severity of the bleeding disorder, and the lack of associated immunodeficiency and eczema served to distinguish this rare condition from the sex-linked Wiskott-Aldrich syndrome, caused by mutation of the WASgene.1
Recent advances in understanding megakaryocytopoiesis at the molecular level have greatly enhanced our understanding of the transcription factors and signaling pathways used during normal lineage-specific differentiation.2 Because of its location on the X chromosome, GATA-1 was evaluated as a candidate gene for this disorder. Lineage-specific disruption of GATA-1expression has demonstrated its critical role in megakaryocyte and platelet development.3,4 These studies in genetically engineered mice have demonstrated that GATA-1 deficiency leads to hyperproliferation of megakaryocytes, incomplete nuclear and cytoplasmic maturation, and macrothrombocytopenia. Friend of GATA-1 (FOG) is a zinc finger protein that cooperates with GATA-1 to promote megakaryocyte and platelet differentiation and erythroid development.5 In the absence of FOG there is absolute thrombocytopenia, and megakaryocyte development is blocked at an earlier stage; hence, no recognizable megakaryocytes are produced.6
In the current study, we report a novel mutation of theGATA-1 gene that causes inherited thrombocytopenia by significantly impairing, but not altogether preventing, the interaction between GATA-1 and its essential cofactor, FOG. The mutation results in severe thrombocytopenia without measurable anemia, suggesting that terminal differentiation of megakaryocytes is more sensitive to partial disruption of the GATA-1–FOG interaction than is erythropoiesis.
Materials and methods
Recruitment and blood processing
Family members were enrolled in an approved human subject protocol at the University of Washington. After obtaining informed consent, venous blood was collected for automated complete blood cell count analysis (Serano Diagnostics, Allentown, PA). Blood was also used to make peripheral smears (modified Wright-Giemsa stain; Sigma, St Louis, MO) and for extraction of genomic DNA from buffy coat.
Thrombopoietin levels
Thrombopoietin levels were measured from platelet-poor plasma using a commercially available thrombopoietin enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Platelet-poor plasma was prepared by centrifugation of venous blood collected in anticoagulant citrate dextrose (Becton Dickinson, Franklin Lakes, NJ) buffer for 20 minutes at 300g to remove cellular layers. Plasma was further centrifuged for 10 minutes at 1550g to remove platelets and stored at −20°C. All samples were run in duplicate, and thrombopoietin concentrations were calculated using a log-log standard curve (absorbance vs thrombopoietin concentration).
Genomic linkage
X-chromosome markers from the ABI medium density marker set (panel 28; average distance, 10 cM) were used with each informative family member. Genotypes were determined using the ABI 310 genetic analyzer and Sequence Analysis software (PE Biosystems, Foster City, CA). Two-point lod scores were calculated using the MLINK program assuming X-linked recessive trait with 100% penetrance.
Amplification of human GATA-1 from genomic DNA
Total RNA and DNA were extracted from buffy coat cells using the SV Total RNA Isolation System (Promega, Madison, WI) and QIAamp DNA blood kit (Qiagen, Valencia, CA). RNA was converted to cDNA using Superscript IIRT (Gibco BRL, Gaithersburg, MD), and GATA-1was amplified by polymerase chain reaction (PCR) with 3 primer pairs: pair 1, 5′ctccgcaaccaccagcccagg3′ and 5′gggctcagccgctctgtcttc3′; pair 2, 5′aaaggcagcaccagcttcctgg3′ and 5′ggccgcaggcattgcacacggg3′; and pair 3, 5′gaccaccacgacactgtggcg3′ and 5′tcctctggaggccatgctctg3′. Amplified bands were excised from agarose gels, purified using glass beads (GeneCleanII; Bio101, Vista, CA), and sequenced using BigDye terminator system and an ABI 310 analyzer (PE Biosystems). Genomic DNA, amplified using primer pair 2, was subjected to digestion with Cel-II endonuclease (Boehringer Mannheim, Indianapolis, IN) for restriction fragment length polymorphism analysis.
Hematopoietic progenitor assays
Peripheral venous blood (50 mL) was collected from 2 affected family members with thrombocytopenia and 1 carrier. Light-density cells were isolated by centrifugation for 30 minutes at 400g on a cushion of LSM lymphocyte separation media, 1.077 g/mL (ICN Biomedicals, Aurora, OH). CD34+ cells were then isolated using immunomagnetic beads (CD34+ Isolation Kit; Miltenyi Biotec, Auburn, CA) and plated in methylcellulose culture (1 and 2 × 103/35-mm plate) with exogenous interleukin-3 (IL-3), IL-6, stem cell factor, erythropoietin, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor (Methocult H4435; Stem Cell Technologies, Vancouver, BC, Canada). After 12 days in culture, colonies from duplicate plates were scored as erythroid burst-forming units (BFU-Es), granulocyte-macrophage colony-forming units (CFU-GMs), and granulocyte-erythroid-macrophage-megakaryocyte colony-forming units (CFU-GEMMs). Megakaryocyte colonies (CFU-Mks) were assessed by plating 1 and 2 × 103 CD34+ cells/well in double-chamber slides using serum-free collagen matrix with supplemental TPO, IL-3, and IL-6 (Megacult; Stem Cell Technologies). After 14 days of incubation, the slides were dehydrated and immunostained to detect GPIIb/IIIa using a colorimetric system.
Expression vectors
The entire coding region of GATA-1 from one affected family member was amplified by reverse transcription–PCR and was cloned into the pCDNA3.1-Zeocin(+) vector (Invitrogen, Carlsbad, CA). After complete sequencing to verify PCR fidelity, the Gly208Ser mutation was reverted to wild-type sequence by site-directed mutagenesis (Quikchange; Stratagene, La Jolla, CA) and was verified by sequencing. Both the wild-type and the mutant forms ofGATA-1 were introduced into Ba/F3 cells by electroporation as previously described.7 Multiple cell lines were isolated by limiting dilution in the presence of mIL-3 and Zeocin selection (0.5 mg/mL; Invitrogen). Similar expression levels of GATA-1 were confirmed by Western blot analysis.
Electrophoretic mobility shift assay
Ba/F3 cells, engineered to express either the wild-type or the mutant form (Gly208Ser) of human GATA-1 were used to generate total cell lysates from cells in log-phase growth.8 Consensus DNA binding sites for both the single (5′-GACTCCGGCAACTGATAAGGATTCCCTG-3′) and the palindromic (5′-GCGCTATCAGATAAGGCCTTG-3′) GATA-1 recognition sequences were used to test for DNA-binding activity. Probes were labeled by the incorporation of α-32P-dCTP using Klenow reagent (Promega) and a short primer (5′-CAGGGAATC-3′) to fill in the 2nd strand. Centrifugation through a Sepharose column (Princeton Separations, Princeton, NJ) was used to remove unincorporated nucleotides. Cell lysate (40 μg/lane) was combined with radiolabeled probe (0.8 pmol) in a final volume of 15 μL, including 4 μg dIdC, and binding buffer: 15 mM HEPES, pH 7.9; 125 mM NaCl; 1 mM dithiothreitol; 0.15 mM EDTA; and 8% wt/vol glycerol. A 100-fold molar excess of unlabeled probe was used to demonstrate specificity of radioactive bands, and 2 μg GATA-1 antibody (N6; Santa Cruz Biotechnology, Santa Cruz, CA) or an unrelated antibody (α-Mpl antiserum; 2 μL; Zymogenetics, Seattle, WA) was added to some reactions to demonstrate supershift of the radiolabeled band and to identify GATA-1 as the transcription factor. The stability of the GATA-1–DNA interaction was further assessed by studying the dissociation rate of bound probe.9 After allowing binding of cell extract and labeled probe to achieve equilibrium at room temperature, the reaction was cooled to 4°C, and a 12.5-fold excess of unlabeled probe was added (both single and palindromic GATA-1 recognition sites were used). Aliquots were removed at various time-points and immediately subjected to electrophoresis under nondenaturing conditions in 8% polyacrylamide gels containing 5% wt/vol glycerol, 0.5× TBE for 3 hours at 100 V (running buffer, 0.5× TBE). Gels were dried and exposed to x-ray film to detect radiolabeled bands.
GATA-1 reporter assay
Cell culture manipulations were carried out using standard techniques. Briefly, NIH3T3 cells were cotransfected with 2 μg reporter construct, 250 ng wild-type or Gly208Ser mutantGATA-1 in pcDNA3, and 0, 50, 100, or 200 ng pMT2FOG5 using the calcium phosphate method as previously described.10 11 The reporter consisted of 3 AGATAA boxes upstream of the β-globin TATA box and a firefly luciferase reporter gene. Data shown are the results of duplicate experiments and have been normalized to Renilla luciferase levels derived from cotransfection with the control vector pRL-CMV (Promega).
Yeast 2-hybrid assay
Competent Saccharomyces cerevisiae HF7c yeast cells were transformed simultaneously with pGBKT7.GATA-1 N-finger and pGAD10.FOG-finger derivatives (2-Hybrid Matchmaker system; Clontech, Palo Alto, CA). PGBKT7.GATA-1 and pGBTK.GATA-1/Gly208Ser constructs encode residues 200 to 248 of GATA-1, or the same portion including the Gly208Ser mutation, fused to the Gal4 DNA-binding domain. The pGAD10.FOG finger constructs contained the following portions of FOG fused to the Gal4 activation domain: finger 1, 241 to 295; finger 6 residues, 677 to 760; finger 9 residues, 818 to 995.10Note the latter construct also contains fingers 7 and 8, but these domains have been shown to make no contribution to binding to GATA-1.10 Transformants were selected on -Leu,-Trp minimal media plates and were patched onto -Leu,-Trp,-His plates. After 48-hours incubation at 29°C, the plates were photographed.
Glutathione-S-transferase fusion proteins and pull-down assays
The expression of glutathione-S-transferase (GST) fusion proteins was carried out in Escherichia coli BL21 as previously described.12 The GST fusion proteins contained fingers of FOG fused downstream of the GST protein contained in the vector, newpGEX2T.10 The portions of FOG contained were finger 1, 241 to 295; finger 6, 677 to 760; finger 7, 818 to 863, and finger 9, 945 to 995. 35S-labeled GATA-1 or GATA-1 containing the Gly208Ser mutation was prepared by in vitro translation from pcDNA3–GATA-1 or pcDNA3.1-Zeocin(+).GATA-1/Gly208Ser, respectively, using the TNT system (Promega). In vitro binding assays were performed in 0.3 mL buffer (150 mM NaCl, 20 mM Tris HCl, pH 7.5, 0.5% NP-40, 10 μM ZnSO4, 0.25% bovine serum albumin, 1 mM β-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride). One microgram fusion protein attached to glutathione agarose beads was incubated with 2 μL radiolabeled GATA-1 mix. After 1 hour of mixing at 4°C, the agarose beads were washed 4 times with 1 mL binding buffer. The beads were recovered, and bound material was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by imaging (Phosphorimager, Molecular Dynamics, Sunnyvale, CA).
Results
Clinical history
A summary of the complete pedigree is depicted in Figure1. Affected family members reported thrombocytopenia and severe bleeding since birth. The founder was a female carrier (I-2) without known bleeding tendency or abnormal platelets; she died of unrelated medical complications. All 3 male children (II-2, II-4, II-5) were severely affected. They were treated for presumptive immune thrombocytopenia with splenectomy, steroids, and dietary modifications without efficacy. Each of the 3 brothers had problems with persistent nosebleeds that necessitated packing and transfusions throughout their lives. They continue to have easy bruising, petechiae, and persistent bleeding after injury or minor surgery. Although the platelet counts have not improved, the frequency of serious bleeding has diminished in adulthood. II-2 died of gastrointestinal bleeding attributed to gastritis or peptic ulcer disease. None of the children of affected males have thrombocytopenia or persistent bleeding (III-1 through III-8). The only daughter of the founder (II-7) remains asymptomatic and has normal platelet levels, but her son (III-9) was born with severe thrombocytopenia and bleeding diathesis. Her daughter (III-10) is asymptomatic, and her carrier status is unknown. The pedigree is consistent with a recessive sex-linked trait with 100% penetrance.
Pedigree of X-linked thrombocytopenia.
The family under investigation is depicted with thrombocytopenic members represented by solid figures, asymptomatic carriers represented by cross-hatching, and healthy members represented by open figures. The numbers below each serve as unique identifiers throughout this manuscript (Roman numerals identify the generation, and Arabic numerals were arbitrarily assigned).
Pedigree of X-linked thrombocytopenia.
The family under investigation is depicted with thrombocytopenic members represented by solid figures, asymptomatic carriers represented by cross-hatching, and healthy members represented by open figures. The numbers below each serve as unique identifiers throughout this manuscript (Roman numerals identify the generation, and Arabic numerals were arbitrarily assigned).
Peripheral blood and thrombopoietin levels
Peripheral blood was analyzed from 3 affected family members, 3 known or predicted carriers, and 7 unaffected children or spouses. We found severe thrombocytopenia among affected persons, with normal platelet counts in unaffected persons and carriers (Table1). Although the difference did not meet statistical significance, there was a trend toward lower platelet numbers among carriers than among unaffected persons (range, 133 000-270 000 carriers; 206 000-317 000 unaffected). Direct examination of the peripheral blood smear revealed macrothrombocytes, some of which were too big for proper classification by the automated cell counter (Figure 2A-B). Smears from unaffected persons and carriers did not have giant platelets. By counting the average number of total platelets in 20 high-power fields (1000 ×) per patient, the true platelet count for affected persons was estimated at 27 000/μL. The calculated mean platelet volume (MPV) was higher in those with thrombocytopenia despite the fact that giant platelets were not included in this calculation. Although it failed to meet statistical significance, the MPV for carriers was intermediate between genotypically healthy and thrombocytopenic persons. Hematocrit, hemoglobin, mean corpuscular hemoglobin concentration (MCHC), and red cell distribution width levels were unchanged for affected family members when compared to carriers or unaffected family members (Table 1 and data not shown). The 2 thrombocytopenic adults (II-4, II-5) have nucleated red blood cells and macrocytosis (at least in part because of splenectomy), whereas the thrombocytopenic boy (III-9, age 8) has an intact spleen and a normal mean corpuscular volume (MCV). Neutrophils appeared normal in number and morphology. Thrombopoietin levels, assessed by ELISA from platelet-poor plasma, were elevated in affected family members (range, 91-555 pg/mL; n = 3) compared to unaffected noncarriers (range, 22-70 pg/mL; n = 6). This difference failed to achieve statistical significance (P = .27; 2 tailed t test assuming unequal variance), probably because of the small number of thrombocytopenic subjects.
Bone marrow evaluation
Marrow aspirate and biopsy specimen from one affected family member were available for review. Overall, the bone marrow was hypercellular and showed an increased frequency of large megakaryocytes (Figure 3A). Under high magnification, the megakaryocytes displayed subtle abnormalities, primarily reflected in peripheral location of the nucleus and lack of nuclear segmentation or nuclear fragmentation (Figure 3B-D). Erythroid precursors demonstrated mild megaloblastic changes with delayed nuclear maturation (Figure 3E).
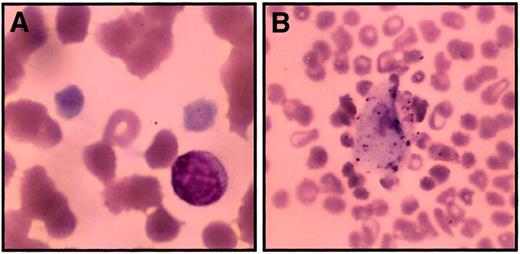
Peripheral smear from thrombocytopenic members.
Peripheral blood smears were stained with Wright-Giemsa stain and examined by light microscopy to determine platelet morphology. These photomicrographs demonstrate macrothrombocytes (A) (original magnification, 1000 ×) and a rare circulating megakaryocyte fragment (B) (original magnification, 200 ×). Red blood cell morphology in these images is distorted in part due to dyserythropoiesis and partially because of the age of the sample when smears were made (24 hours).
Peripheral smear from thrombocytopenic members.
Peripheral blood smears were stained with Wright-Giemsa stain and examined by light microscopy to determine platelet morphology. These photomicrographs demonstrate macrothrombocytes (A) (original magnification, 1000 ×) and a rare circulating megakaryocyte fragment (B) (original magnification, 200 ×). Red blood cell morphology in these images is distorted in part due to dyserythropoiesis and partially because of the age of the sample when smears were made (24 hours).
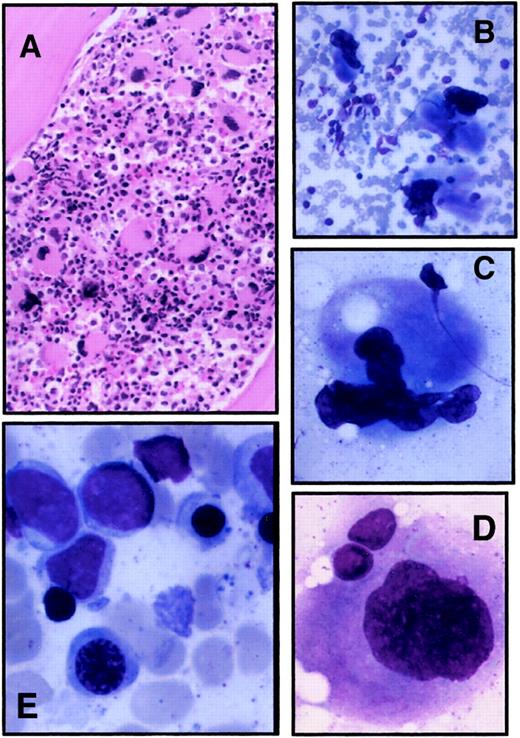
Bone marrow evaluation of a thrombocytopenic member.
Bone marrow aspirate and biopsy specimen were obtained from II-4 as part of his normal medical care. (A) Biopsy specimen; original magnification, 100 ×. (B-D) Typical megakaryocytes identified from bone marrow aspirate; original magnifications, 200 × (B), 500 × (C), 1000 × (D). (E) Erythroid precursors in bone marrow aspirate; original magnification, 1000 ×.
Bone marrow evaluation of a thrombocytopenic member.
Bone marrow aspirate and biopsy specimen were obtained from II-4 as part of his normal medical care. (A) Biopsy specimen; original magnification, 100 ×. (B-D) Typical megakaryocytes identified from bone marrow aspirate; original magnifications, 200 × (B), 500 × (C), 1000 × (D). (E) Erythroid precursors in bone marrow aspirate; original magnification, 1000 ×.
Linkage to chromosome X
The ABI medium density marker set on chromosome X was used to localize the abnormal gene. Based on recombination events in 4 informative meioses (children of carriers only), we were able to limit the area consistent with linkage to 85.5 cM (between markers D10 × 1214 and D10 × 1001). The maximum lod scores for markers D10 × 1106 and D10 × 990 were 0.868 and 0.723, respectively (theta = 0).
Mutation in GATA-1 gene Gly208Ser
Within this region of the human X-chromosome are 2 candidate genes that are known to play a role in megakaryocyte biologic function,WAS and GATA-1. Because the clinical phenotype of this family is distinct from Wiskott-Aldrich syndrome, we focused on the GATA-1 gene. Genomic DNA was amplified using primer pairs as described in “Materials and methods.” The entire coding region and intron–exon boundaries were sequenced and compared to the wild-type cDNA. We found a 2-base substitution at coding positions 622 and 623 (GG to TC), resulting in the change of glycine 208 to serine (Figure 4A). Cel-II digestion of genomic DNA amplified with primer pair 2 demonstrates that unaffected family members have only the wild-type restriction pattern; affected males have a new Cel-II restriction site (ie, 65 bp smaller than wild type), and a female carrier has both alleles (Figure 4B).
Two-base mutation in
GATA-1 segregates with thrombocytopenia. (A) cDNAs derived from an unaffected male (II-8, father), carrier female (II-7, mother), and affected male (III-9, son) were amplified withGATA-1 oligonucleotides (primer pair 2) and sequenced. The 2 bases mutated in this family (GG to TC) are highlighted in each sequence. (B) Genomic DNA samples from family members were amplified with GATA-1 primer pair 2 then digested with Cel-II endonuclease (recognition site, GCTNAGC). The larger band represents the wild-type allele (I-1, II-8), whereas mutant alleles contain a new Cel-II restriction site resulting in a 65-bp reduction of the largest DNA fragment (II-5, II-4, III-9). The presence of 2 bands (II-7) indicates carrier status with both alleles.
Two-base mutation in
GATA-1 segregates with thrombocytopenia. (A) cDNAs derived from an unaffected male (II-8, father), carrier female (II-7, mother), and affected male (III-9, son) were amplified withGATA-1 oligonucleotides (primer pair 2) and sequenced. The 2 bases mutated in this family (GG to TC) are highlighted in each sequence. (B) Genomic DNA samples from family members were amplified with GATA-1 primer pair 2 then digested with Cel-II endonuclease (recognition site, GCTNAGC). The larger band represents the wild-type allele (I-1, II-8), whereas mutant alleles contain a new Cel-II restriction site resulting in a 65-bp reduction of the largest DNA fragment (II-5, II-4, III-9). The presence of 2 bands (II-7) indicates carrier status with both alleles.
Hematopoietic colony assays
Although fresh bone marrow cells were unavailable, we studied hematopoietic progenitors isolated from peripheral blood to assess the number and quality of myeloid, erythroid, and megakaryocytic colonies. Colonies from one asymptomatic carrier and 2 thrombocytopenic family members were evaluated for number and character (Tables2, 3). In a methylcellulose culture system, those with thrombocytopenia had fewer BFU-Es, CFU-GMs, and CFU-GEMMs colonies than their asymptomatic sibling had (Table 2). Although colony appearance was unchanged, the relative decrease in BFU-Es from the thrombocytopenic brothers (5-fold) was more pronounced that those of CFU-GMs (1.5-fold) or CFU-GEMMs (2-fold). In contrast, the CFU-Mks grown from thrombocytopenic persons were distinctly abnormal. As shown in Table 3 and Figure5A, there was a marked increase in clusters of 3 to 8 small cells or cellular fragments that stained strongly positive for GPIIb/IIIa but were smaller than normal megakaryocytes. Typical medium and large megakaryocyte colonies were almost absent. In addition, there was a significant number of large, abnormal colonies that stained weakly positive for GPIIb/IIIa in both the thrombocytopenic and the carrier progenitors but not in a healthy control subject (Figure 5B). Whether these colonies represent abnormal megakaryocytopoiesis or another lineage with variable GPIIb/IIIa expression is unknown.
Megakaryocyte colonies from peripheral blood progenitors.
After 14 days of incubation, megakaryocyte colonies were visualized by immunostaining to detect GPIIb/IIIa-expressing cells (pink-red). (A) (right) Typical small (3-20 cells) CFU-Mks from an asymptomatic carrier (original magnification, 200 ×; II-7). (left) Cluster of small GPIIb/IIIa cells seen in cultures of thrombocytopenic control subjects (II-5). (B) Representative atypical colonies seen in thrombocytopenic members. Cells are large but weakly positive for GPIIb/IIIa (original magnification as indicated; II-4 and II-5).
Megakaryocyte colonies from peripheral blood progenitors.
After 14 days of incubation, megakaryocyte colonies were visualized by immunostaining to detect GPIIb/IIIa-expressing cells (pink-red). (A) (right) Typical small (3-20 cells) CFU-Mks from an asymptomatic carrier (original magnification, 200 ×; II-7). (left) Cluster of small GPIIb/IIIa cells seen in cultures of thrombocytopenic control subjects (II-5). (B) Representative atypical colonies seen in thrombocytopenic members. Cells are large but weakly positive for GPIIb/IIIa (original magnification as indicated; II-4 and II-5).
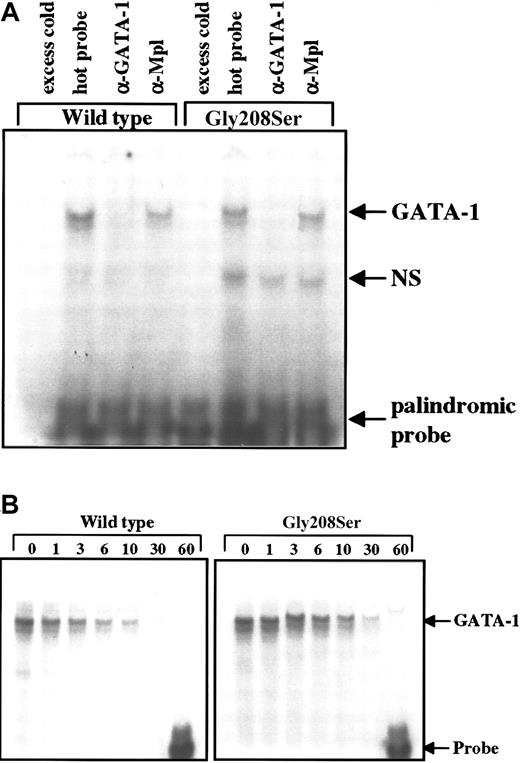
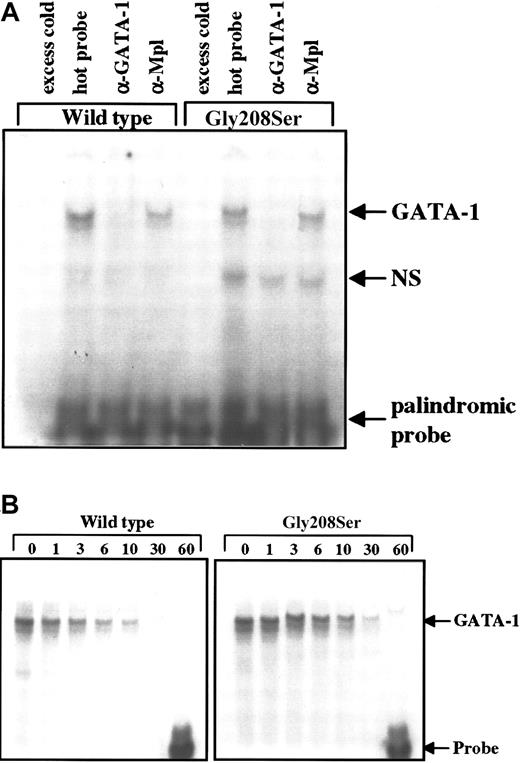
Gly208Ser does not affect GATA-1 DNA binding
Although G208 is not located on a protein face that would be expected to contact DNA,11 the N-zinc finger of GATA-1 plays a role in stabilizing DNA interaction, particularly with palindromic sites.9 To test for an effect on DNA binding, the wild-type and mutant forms of GATA-1 were expressed in Ba/F3 cells, and cell lysates were subjected to electrophoretic mobility shift assay. The amount of labeled probe that bound to GATA-1 was not altered by the single amino acid substitution in the N-terminal zinc finger when using either the single (data not shown) or the palindromic recognition sequence (Figure6A). The specificity of the interaction between GATA-1 and the labeled DNA probe was confirmed using excess unlabeled probe to block binding and using antibodies specific for GATA-1 or an unrelated protein (Mpl) in supershift assays (Figure 6A). Furthermore, the stability of the GATA-1–DNA interaction was assessed as the rate of dissociation in the presence of excess cold probe. We found that over 60 minutes, the rate of dissociation was identical for the wild-type and the Gly208Ser forms of GATA-1 using the palindromic probe, which should be most sensitive to mutations that disturb the N-zinc finger structure (Figure 6B).
Electrophoretic mobility shift assay.
(A) Total cell extracts were obtained from Ba/F3 cells engineered to express the wild-type or Gly208Ser mutant form of GATA-1. These lysates were then tested for the ability to bind a radiolabeled double-stranded DNA probe containing a palindromic GATA-1 binding site. The addition of 100-fold excess unlabeled probe 20 minutes before the radiolabeled probe was used to demonstrate specificity. GATA-1 specific antibody (N6) and nonspecific antibody (α-Mpl) were used to demonstrate supershift (in this case disruption) of the GATA-1 protein bound to labeled probe. These results are representative of 3 separate experiments. (B) Dissociation of radiolabeled probe from bound GATA-1 was studied by adding 12.5-fold excess unlabeled probe immediately after the initial sample (time = 0) was loaded onto the gel. The binding reaction was continued on ice (4°C) for 60 minutes, and equal amounts of the binding reaction were loaded onto the gel at the indicated times.
Electrophoretic mobility shift assay.
(A) Total cell extracts were obtained from Ba/F3 cells engineered to express the wild-type or Gly208Ser mutant form of GATA-1. These lysates were then tested for the ability to bind a radiolabeled double-stranded DNA probe containing a palindromic GATA-1 binding site. The addition of 100-fold excess unlabeled probe 20 minutes before the radiolabeled probe was used to demonstrate specificity. GATA-1 specific antibody (N6) and nonspecific antibody (α-Mpl) were used to demonstrate supershift (in this case disruption) of the GATA-1 protein bound to labeled probe. These results are representative of 3 separate experiments. (B) Dissociation of radiolabeled probe from bound GATA-1 was studied by adding 12.5-fold excess unlabeled probe immediately after the initial sample (time = 0) was loaded onto the gel. The binding reaction was continued on ice (4°C) for 60 minutes, and equal amounts of the binding reaction were loaded onto the gel at the indicated times.
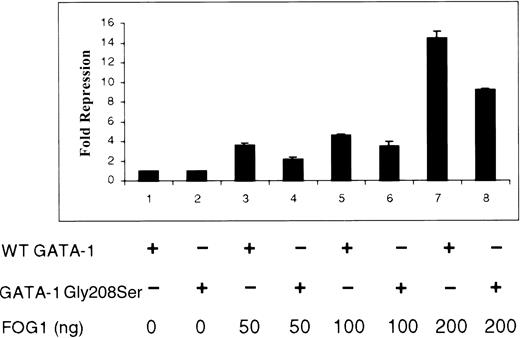
Gly208Ser diminishes the interaction between GATA-1 and FOG in vivo
The noncovalent interaction between zinc fingers of GATA-1 and FOG is critical for normal hematopoiesis.6 Depending on cellular promoter context, FOG has been shown to serve as either a coactivator or a repressor of GATA-1 activity.5,10,11,13,14 Using simple GATA-1–regulated promoters driving reporter genes, FOG most often functions to inhibit gene expression by recruiting the corepressor CtBP.10Therefore, we used a functional, in vivo measure of GATA-1–FOG interaction to test the effect of Gly208Ser substitution (Figure7). Baseline luciferase activities for wild-type and Gly208Ser forms of GATA-1 in the absence of FOG were equivalent and have been normalized to 1.0-fold repression. As previously reported, we found that FOG efficiently repressed the activity of a test promoter driven by wild-type GATA-1 (Figure 7; lanes 1, 3, 5, and 7). FOG was also able to repress a promoter driven by mutant Gly208Ser GATA-1 but less effectively (Figure 7; lanes 2, 4, 6, and 8). The previously described Val205Met mutation in GATA-1 was tested in this assay and diminished the FOG-induced repression more effectively than the Gly208Ser substitution (data not shown). These results demonstrate that both the Gly208Ser and the Val205Met mutations interfere with GATA-1–FOG interaction but that Gly208Ser does so to a lesser extent. This is consistent with the milder phenotype seen in this family.
Effect of Gly208Ser on the interaction of GATA-1 and FOG in vivo.
NIH3T3 cells were cotransfected with a luciferase reporter gene construct, the wild-type or Gly208Ser form of GATA-1, as indicated (250 ng in pcDNA3) and increasing amounts of a FOG expression vector (0, 50, 100, and 200 ng pMT2FOG, with a higher level for each pair of lanes from left to right). Results are expressed as fold repression of luciferase activity relative to that seen in the absence of FOG (baseline adjusted to 1.0).
Effect of Gly208Ser on the interaction of GATA-1 and FOG in vivo.
NIH3T3 cells were cotransfected with a luciferase reporter gene construct, the wild-type or Gly208Ser form of GATA-1, as indicated (250 ng in pcDNA3) and increasing amounts of a FOG expression vector (0, 50, 100, and 200 ng pMT2FOG, with a higher level for each pair of lanes from left to right). Results are expressed as fold repression of luciferase activity relative to that seen in the absence of FOG (baseline adjusted to 1.0).
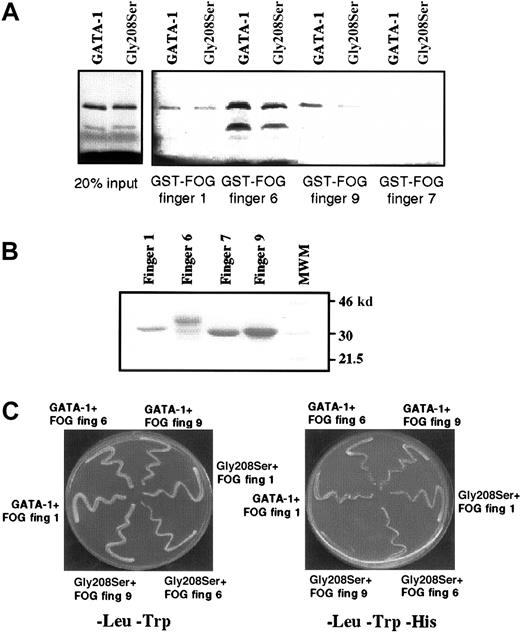
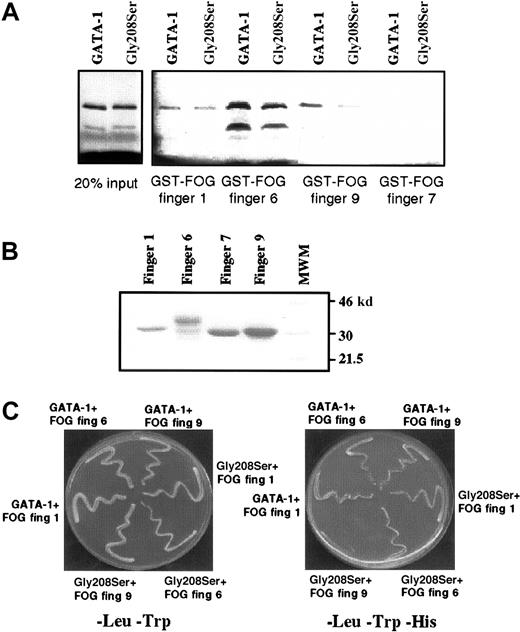
Gly208Ser substitution primarily affects binding to FOG zinc finger 9
It is known that the N-terminal zinc finger of GATA-1 can bind strongly to 3 separate zinc fingers within FOG, namely fingers 1, 6, and 9.10 The fact that the Gly208Ser substitution reduces but does not eliminate binding to FOG raises the possibility that the mutation might alter the ability of GATA-1 to bind some, but not all, FOG contact fingers. We therefore used an in vitro protein–protein interaction assay, the GST pull-down assay, to investigate how the Gly208Ser substitution affected binding to individual FOG fingers. As previously reported, we found that GST-FOG fingers 1, 6, and 9 all retain wild-type GATA-1, whereas a negative control GST-FOG finger 7 does not (Figure 8A). By comparing the amount of GATA-1 (wild type vs mutant) recovered in this pull-down assay, we found that FOG finger 1 retained an equal amount of wild-type and mutant GATA-1, finger 6 retained twice as much wild type as mutant, and finger 9 had a 4-fold difference (quantification by Phosphorimager). The 2 lanes on the left (Figure 8A) are controls showing that equal amounts of input wild-type and mutant GATA-1 were used, and Figure 8B is a control showing that comparable amounts of GST-FOG finger proteins were used. We also assessed the interactions between GATA-1 and individual FOG zinc fingers using the yeast 2-hybrid system. In this assay, yeast growth on minimal media lacking Leu, Trp, and His indicates a physical interaction between the 2 test proteins. As shown in Figure 8C (right panel), significantly reduced yeast growth is observed only when the Gly208Ser substitution is tested with a construct containing FOG finger 9. This result is consistent with the in vitro binding study. We have also tested the previously described Val205Met substitution15 in the yeast 2-hybrid assay and find that it dramatically affects the interaction with FOG-fingers 1, 6, and 9 (data not shown). This difference is interesting in light of the fact that the 2 mutations are associated with significantly different clinical conditions: the Val205Met mutation is associated with severe thrombocytopenia and anemia, whereas the Gly208Ser mutation is associated with thrombocytopenia without anemia.
Gly208Ser substitution affects GATA-1 interaction with finger 9 of FOG.
(A) GST pull-down assay showing retention of wild-type GATA-1 and the Gly208Ser mutant by different FOG fingers. On the left, 2 control lanes demonstrate that equal amounts of wild-type and Gly208Ser GATA-1 protein were used. Finger 7 has previously been shown not to bind GATA-1 and is included as a negative control. (B) Coomassie-stained gel indicating that approximately equal amounts of GST–FOG fusion proteins were used in the GST pull-down assay. (C) Yeast 2-hybrid experiment was used to study the interaction between wild-type or mutant GATA-1 and FOG fingers 1, 6, and 9. All yeast contain pGBKT7.GATA-1 bait vector and a pGAD10.FOG target vector as indicated. In the left panel, yeast are spread onto -Leu,-Trp media as a control for yeast growth. In the right panel, yeast are spread onto -Leu,-Trp,-His media. In this case growth depends on the physical interaction of the bait and target fusion proteins.
Gly208Ser substitution affects GATA-1 interaction with finger 9 of FOG.
(A) GST pull-down assay showing retention of wild-type GATA-1 and the Gly208Ser mutant by different FOG fingers. On the left, 2 control lanes demonstrate that equal amounts of wild-type and Gly208Ser GATA-1 protein were used. Finger 7 has previously been shown not to bind GATA-1 and is included as a negative control. (B) Coomassie-stained gel indicating that approximately equal amounts of GST–FOG fusion proteins were used in the GST pull-down assay. (C) Yeast 2-hybrid experiment was used to study the interaction between wild-type or mutant GATA-1 and FOG fingers 1, 6, and 9. All yeast contain pGBKT7.GATA-1 bait vector and a pGAD10.FOG target vector as indicated. In the left panel, yeast are spread onto -Leu,-Trp media as a control for yeast growth. In the right panel, yeast are spread onto -Leu,-Trp,-His media. In this case growth depends on the physical interaction of the bait and target fusion proteins.
Discussion
GATA-1 and megakaryocyte development
GATA-1 expression is critical for normal erythroid and megakaryocytic development, and its forced expression in hematopoietic cell lines induces lineage-specific differentiation.16-18GATA-1 deficiency is lethal during embryogenesis because of arrested erythroid development at the proerythroblast stage and failure of megakaryocytes to undergo normal endomitosis and definitive differentiation despite increased proliferation.4,19,20Lineage-specific GATA-1 deficiency in megakaryocytes resulted in platelets that were abnormally large and round, displayed specific defects in aggregation, and disproportionately prolonged bleeding times,4 21 consistent with the degree of macrothrombocytopenia and severe bleeding tendency described in the current report.
GATA-1 and FOG interaction
Several years ago, an essential cofactor for GATA-1, FOG, was identified based on protein–protein interaction.5 FOG plays an essential role in both erythroid and megakaryocyte development, and its deletion in mice leads to embryonic death from anemia.6 Structural analysis through nuclear magnetic resonance spectroscopy and site-directed mutagenesis has revealed that GATA-1 and FOG interact through highly conserved zinc fingers.11,22,23 The N-terminal zinc finger of GATA-1 contains critical cysteine residues that coordinate zinc binding and several residues directly implicated in contacting FOG, including Glu203, Val205, Gly208, Ala209, His222, and Tyr223.11,22These amino acids, which are conserved in both human and murineGATA family members, are clustered on the molecular surface opposite the potential DNA-binding site.11,23 Substitution of one or more of these residues has been shown by yeast 2-hybrid assay and coimmunoprecipitation experiments to disrupt GATA-1–FOG binding11 and to block the maturation of erythroid cells.22 With respect to Gly208, published reports demonstrate that single amino acid substitutions (Gly208Val or Gly208Glu)22 and a double amino acid substitution (Gly208Gln, Ala209Thr)11 all cause loss of GATA-1–FOG interaction. These data suggest that any substitution for glycine at position 208 may disrupt FOG binding because of disruption of the contact surface on the zinc finger of GATA-1. Our functional studies of the ability of FOG to affect GATA-1–mediated gene activation (Figure7) and to bind GATA-1 in GST pull-down studies (Figure 8A) and yeast 2-hybrid experiments (Figure 8C) confirm that the current mutation, Gly208Ser, significantly but incompletely disrupts the interaction with FOG.
The effect of Gly208Ser substitution on erythropoiesis appears to be less severe than was observed in the recently described family withGATA-1 Val205Met mutation.15 Despite the fact that we found a 5-fold reduction in BFU-Es in peripheral blood from affected members, the degree of dyserythropoiesis was only mild to moderate in the bone marrow (Figure 3E), and those with thrombocytopenia were able to maintain normal hematocrit levels (Table1). Given the diminished erythroid progenitors in peripheral blood, it is possible that an affected person would display delayed recovery from physiologic stress (eg, blood loss, chemotherapy) despite the fact that the baseline hematocrit level is normal. Our data suggest that normal platelet production may be more sensitive than that of erythrocytes to incomplete disruption of GATA-1 and FOG interaction. At the molecular level, GATA Gly208Ser mutation causes disruption of binding primarily to FOG finger 9, whereas Val205Met substitution results in reduced binding to fingers 1, 6, and 9.
In each of the 5 informative meioses (found in children of heterozygous carriers), the mutant GATA-1 allele was inherited. This might have been the result of random chance. However, we cannot dismiss the possibility that the mutant form of GATA-1 may provide an embryonic survival advantage, similar to the proliferative advantage seen in megakaryocytes with diminished or absent GATA-1 activity.3,4 21
In addition to the recent report15 of a GATA-1mutation affecting Val205, 3 additional inherited GATA-1mutations (including the current family) were described in abstract form at the recent American Society of Hematology meeting (San Francisco, CA), all affecting different residues in the N-terminal zinc finger domain.24-26 One of these substitutions, GATA-1 Asp218Gly, results in macrothrombocytopenia without anemia and impaired expression of platelet surface glycoproteins GPIbβ and GPIX.24 A second GATA-1 mutation, Arg216Gly, causes milder thrombocytopenia and is associated with globin chain imbalance (thalassemia).27 Because Arg216 is located on the N-terminal zinc finger face opposite that of Gly208, the Arg216Gly amino acid substitution results in destabilization of binding to palindromic GATA recognition sites without affecting FOG interaction.25
Together with the current report, these data define a new type of X-linked thrombocytopenia caused by mutations of GATA-1. In the case of Gly208Ser substitution, the correlation of phenotype with molecular mechanism suggests that partial disruption of GATA-1–FOG interaction (especially involving the FOG zinc finger 9) may have a greater effect on megakaryocytopoiesis than erythrocyte development. It is likely that other families like these exist, but given the small numbers of affected persons the inherited basis of the disease may not be recognized. This could lead to unnecessary treatment for presumptive immune thrombocytopenia, such as splenectomy and immunosuppression. Because of the severe bleeding disorder, such families are good candidates for genetic therapy or stem cell transplantation to provide a normal GATA-1 allele.
We thank M. Sara Lowe for help with manuscript preparation and Cecelia Trainor for helpful discussions regarding the GATA-1–DNA dissociation experiment. We are indebted to the family members who participated in this study. Without their enthusiasm and help, our research would not have been possible.
Supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (J.G.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jonathan G. Drachman, Puget Sound Blood Center, 921 Terry Ave, Seattle, WA 98104; e-mail: jonathand@psbc.org.