Abstract
Acute promyelocytic leukemia (APL) blasts possess a unique sensitivity to the differentiating effects of all-transretinoic acid (ATRA). Multicenter trials confirm that the combination of differentiation and cytotoxic therapy prolongs survival in APL patients. However relapses still occur, and exquisite adaptation of therapy to prognostic factors is essential to aim at a possible cure of the disease. A heterogeneity was previously reported in the differentiation rate of patients' APL blasts, and it was postulated that this may reflect the in vivo heterogeneous outcome. In this study, it is demonstrated that patients of the APL93 trial whose leukemic cells achieved optimal differentiation with ATRA in vitro at diagnosis had a significantly improved event-free survival (P = .01) and lower relapse rate (P = .04). This analysis highlights the importance of the differentiation step in APL therapy and justifies ongoing studies aimed at identifying novel RA-differentiation enhancers.
Introduction
All-trans retinoic acid (ATRA), a naturally occurring compound derived from vitamin A, specifically induces acute promyelocytic leukemia (APL) blasts to differentiate in vitro and in vivo, and complete remission (CR) is obtained in more than 90% of APL patients treated with ATRA alone.1-3 All the multicenter trials confirmed the benefit of ATRA combined with chemotherapy, with only 10% to 20% of the patients relapsing at 2 years and 7% dying during CR.4 5 The achievement of longer survival and a possible cure of the disease urges for more adapted treatment designs.
Differentiation therapy, at least in APL, is a true example of targeted therapy, as effective differentiation by retinoids is observed only in leukemic cells that harbor the promyelocytic leukemia retinoic acid receptor α (PML-RARα) oncogene. We have previously shown that in vitro differentiation of APL blasts with ATRA correlated to in vivo achievement of complete remission6,7and have subsequently shown that the degree of differentiation induction obtained in vitro was closely related to specific features of the APL blast, such as differential sensitivity to retinoid,8 intracellular concentrations of ATRA,9 high levels of cellular retinoic acid binding protein II,10 and cytokine expression.11 In this study, we asked whether the in vitro sensitivity of APL blasts to ATRA could determine the long-term in vivo response of APL patients. We show for the first time that this parameter has a significant impact on patient outcome and should be used as a target for the validation of novel differentiation enhancers.
Study design
Description of the trial and patients
The multicenter clinical trial (APL93) has been detailed elsewhere5 and is only briefly reported here. Patients with de novo APL aged 65 years or younger and with initial white blood cell (WBC) count lower than 5 ×109/L were randomized between 2 induction regimens: Group A, ATRA followed by daunorubicin-AraC chemotherapy (CT), and Group B, CT plus ATRA (added from day 3). Patients with initial WBC count higher than 5 ×109/L (Group C) all received ATRA plus CT, while those aged 65 years or older (Group D) all received ATRA followed by CT. Once CR was achieved, all patients were randomly allocated to maintenance therapy for 2 years with continuous low-dose chemotherapy (6-mercaptopurine plus methotrexate), intermittent ATRA, or both. The 77 consecutive patients included in the APL93 trial were studied at diagnosis for blast cell differentiation with ATRA. The sample of patients with biological analysis had characteristics similar to those of the overall cohort of patients, with the exception of WBC count (Table 1).
In vitro differentiation of APL cells
Mononuclear cells from patients' bone marrow samples were prepared by Ficoll-Hypaque density gradient purification and cultured, as previously described,12 at 1 × 106/mL in the presence of ATRA (10−7 M). After 3 and 6 days, the percentage of differentiated cells was assessed by morphological criteria and the appearance of burst function (nitroblue tetrazolium test).13 Briefly, 5 × 105 cells were resuspended in 450 μL of 1 μg/mL nitroblue tetrazolium (Sigma, Saint Quentin Favier, France) in Hanks buffer plus 50 μL of 4 μg/mL Phorbo12-Myristate13-Acetate (Sigma). After 20 minutes of incubation at 37°C, cells were analyzed on cytospin slides. In our hands, the analysis has a 90% reproducibility, is correlated to CD11b positivity (unpublished results of our laboratory, 1990, and Charrad et al14), and testifies to the presence of a functional differentiated granulocyte.
Statistical analysis
The time-to-failure data analysis used Kaplan-Meier estimation and log-rank test with January, 1, 2000, as the reference date. A multivariable Cox model was used to jointly estimate the additive effects of each variable. Continuous variables were entered as binary covariates, with the median used as the cutoff point. Analysis used SAS 6-12 software (Cary, NC).
Results and discussion
The 77 confirmed APL samples from the APL93 cohort were analyzed in vitro for ATRA sensitivity at diagnosis. Differentiation of the leukemic population was assessed after 3 and 6 days of culture performed in the presence or absence of 0.1 μM ATRA. At day 6 of culture, as already reported, the majority of the APL samples (75%) are sensitive to ATRA, with 50% to 100% of the leukemic cells achieving differentiation (mean, 71%; SD, 30). The remaining samples (25%), however, show reduced sensitivity to ATRA (10% to 40% of cells differentiated) despite confirmed APL diagnosis and presence of t(15;17). At day 3 of culture, the percentage of differentiated cells is well correlated to day 6 (P = .0001). Nevertheless, a higher interpatient variability is observed, with values ranging from 10% to 100% (mean, 40%; SD, 30), testifying to differing levels of ATRA sensitivity.
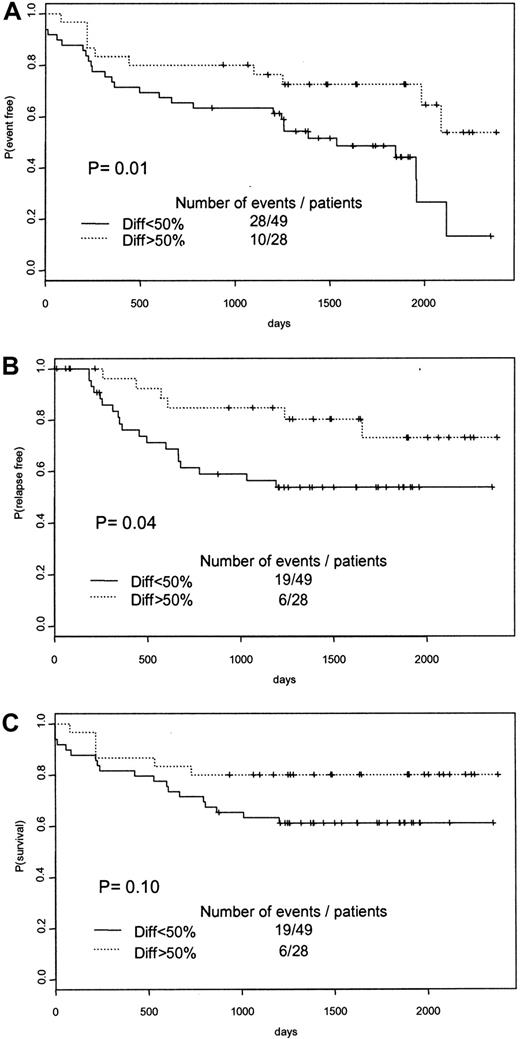
APL prognosis and therapy management take into account clinical (blood coagulation disorders, age) and biological features (high WBC count). The introduction of a novel therapy with ATRA needed to take into account the sensitivity of APL cells to ATRA, the role of ATRA syndrome in the CR rate, and ATRA-induced resistance in maintenance therapy. Besides the classical prognostic factors of APL (age, WBC count), other molecular parameters, such as CD215 and CD56,16,17 have been proposed. The PML-RARα isoforms are also linked to the patient's prognosis,18 but not independently of WBC count.19 The in vitro sensitivity of APL blasts to ATRA as an indicator of APL patient prognosis has never been studied. We therefore analyzed the APL93 cohort at 2 different end points (January 1, 1999, and January 1, 2000) and found evidence of an as yet unreported prognostic significance of in vitro ATRA sensitivity. At both end points (January 1, 1999, and January 1, 2000), APL patients whose leukemic clone achieved more than 50% differentiated cells with ATRA present with a better event-free survival (P = .01) (Figure 1A) and a shorter relapse rate (P = .04) (Figure 1B). A better overall survival was noted at the first analysis (January 1, 1999, end point;P = .04), though it was no longer statistically significant at the January 1, 2000, end point (P = .10) (Figure 1C). This novel prognostic parameter of APL, defined as the achievement of a rapid in vitro response to ATRA (more than 50% of cells differentiated at day 3 of culture), now allows the schematic separation of APL patients into 2 prognostic subgroups: good responders versus poor responders.
Survival curves of APL patients according to the level of in vitro differentiation of APL blasts at diagnosis.
Patients were separated into 2 groups according to the percentage of differentiated leukemic cells at day 3 of suspension culture in the presence of ATRA (continuous lines indicate a percentage lower than 50%; dotted lines, a percentage greater than 50). (A) Event-free survival. (B) Relapse-free survival. (C) Overall survival.
Survival curves of APL patients according to the level of in vitro differentiation of APL blasts at diagnosis.
Patients were separated into 2 groups according to the percentage of differentiated leukemic cells at day 3 of suspension culture in the presence of ATRA (continuous lines indicate a percentage lower than 50%; dotted lines, a percentage greater than 50). (A) Event-free survival. (B) Relapse-free survival. (C) Overall survival.
A multivariable analysis was performed to adjust for the effect of in vitro ATRA sensitivity on other known prognostic parameters of this leukemia subtype. Once adjusted for age and WBC count at diagnosis, the result of in vitro differentiation was still of prognostic significance for relapse rate (P = .05), event-free survival (P = .02), and overall survival (P = .10) although it was not statistically significant. When the adjustment was extended to include PML-RARα bcr subtype and randomization, in vitro differentiation remained predictive for overall survival (P = .08), relapse rate (P = .05), and event-free survival (P = .017). This corroborated the data we obtained in the univariate analyses showing that in vitro differentiation was not correlated to WBC count, age, CD2 or CD13 expression, or therapy arm (ATRA followed by CT, Groups A and D, versus ATRA with CT, Groups B and C) (data not shown). Indeed, when we analyzed the relevance of in vitro sensitivity in patients already considered at high risk of relapse (WBC count greater than 5 × 109/mL)5 20(n = 46), a higher rate of relapse (42% versus 27%; 12 of 28 versus 5 of 18 patients) was still observed in poor responders. In the univariate analysis, a correlation was noted between in vitro differentiation and the PML-RARα subtype (P = .03) and between in vitro differentiation and the AML3 French-American-British variant subtype (P = .02), which suggest that some inherent features of the APL cell predisposes to ATRA sensitivity.
In summary, this analysis provides evidence that the ATRA sensitivity of the leukemic clone at diagnosis is a determinant of patient outcome. When compared with other features of APL in a multivariate analysis, this in vitro differentiation sensitivity remained superior, showing it to be a novel independent factor in overall therapeutic efficacy. These findings reinforce the critical importance of differentiating therapy in the treatment of APL and underlines the importance of ongoing studies aimed at enhancing in vivo differentiation efficacy through optimization of retinoic acid activity on retinoic acid target genes or in combination with differentiation or chromatin modeling agents.21-23
The following participated in APL93 trial: S. Castaigne (Versailles), H. Dombret (Paris), R. Zittoun (Paris), E. Archimbaud (Lyons), P. Travade (Clermont Ferrand), C. Gardin (Clichy), A. Guerci (Nancy), A. M. Stoppa (Marseilles), F. Dreyfus (Paris), F. Stamatoulas (Rouen), F. Rigal-Huguet (Toulouse), H. Guy (Dijon), J. J. Sotto (Grenoble), F. Maloisel (Strasbourg), J. Reiffers (Bordeaux), J. M. Boiren (Pessac), A. Gardembas (Angers), D. Bordessoule (Limoges), N. Fegueux (Montpellier), F. Lefrere (Paris), T. Lamy (Rennes), M. Hayat (Villejuif), E. Deconinck (Bezançon), E. Guyotat (Saint Etienne), M. Martin (Annecy), E. Cony-Makhoul (Bordeaux), J. P. Abgrall (Brest), O. Reman (Caen), B. Desablens (Amiens), J. L. Harousseau (Nantes), Y. Bastion (Lyons), J. P. Pollet (Valenciennes), J. Pulik (Argenteuil), M. Lepeu (Avignon), M. Renoux (Bayonne), P. Morel (Lens), P. Henon (Mulhouse), N. Gratecos (Nice), P. Colombat (Tours), D. Machover (Villejuif), A. Dor (Antibes), P. Casassus (Bobigny), J. Donadiou (Castelnou), B. Salles (Chalon), B. Legros (Clermont Ferrand), P. Audhuy (Colmar), A. Dutel (Compiegne), N. Philippe (Lyons), B. Benothman (Meaux), C. Christian (Metz), C. Marguerite (Montpellier), F. Witz (Nancy), A. Pesce (Nice), A. Baruchel (Paris), L. Sutton (Paris), C. Quetin (Pointe à Pitre), B. Pignon (Reims), E. Vilmer (Paris), E. Bourquard (Saint Brieuc), J. P. Marolleau (Paris), P. Robert (Toulouse), B. Despax (Toulouse), G. Nedellec, P. Auzanneau (Paris), M. Janvier (Saint Cloud).
A complete list of participants appears in the .
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christine Chomienne, Laboratoire de Biologie Cellulaire Hématopoı̈étique, Institut d'Hématologie, Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, 75010 Paris, France; e-mail: lbch@chu-stlouis.fr.