Abstract
Depsipeptide, FR901228, has demonstrated potent in vitro and in vivo cytotoxic activity against murine and human tumor cell lines. In the laboratory, it has been shown to be a histone deacetylase (HDAC) inhibitor. In a phase I trial of depsipeptide conducted at the National Cancer Institute, 3 patients with cutaneous T-cell lymphoma had a partial response, and 1 patient with peripheral T-cell lymphoma, unspecified, had a complete response. Sézary cells isolated from patients after treatment had increased histone acetylation. These results suggest that inhibition of HDAC is a novel and potentially effective therapy for patients with T-cell lymphoma.
Introduction
Postthymic T-cell lymphomas represent about 10% of all non-Hodgkin lymphomas. Peripheral T-cell lymphomas, unspecified, compose one subset. Patients with peripheral T-cell lymphoma may have a durable remission after they achieve a complete response with standard chemotherapy, with an overall survival rate of 25% at 5 years.1 However, most patients experience relapse and have a poor response to further treatment.2 Cutaneous T-cell lymphoma (CTCL) is another subset of postthymic lymphomas and accounts for approximately half of all lymphomas that arise in the skin. Among various forms of CTCLs, mycosis fungoides and the Sézary syndrome are the most common.3,4 Although patients with advanced stage CTCL respond to various forms of cytotoxic or biological therapies, these responses are typically short lived.5 In this report, we describe several patients with postthymic T-cell lymphoma that were treated on a phase I trial of a histone deacetylase (HDAC) inhibitor, depsipeptide.
Depsipeptide, FR901228, has been isolated fromChromobacterium violaceum and has demonstrated potent antitumor activity in vitro and against both human tumor xenografts and murine tumors in vivo.6,7 These include the human A549 lung adenocarcinoma, MCF-7 and ZR-75-1 breast adenocarcinoma, and LOX IMVI melanoma cell lines.6,7 Depsipeptide shows a lack of cross-resistance with several commonly used cytotoxic agents; however, it has been identified as a P-glycoprotein (Pgp) substrate.8 Depsipeptide is a member of a novel class of antineoplastic agents, the HDAC inhibitors (HDIs).9 HDIs have been shown to induce differentiation, decrease cell proliferation, and induce cell death. HDIs increase acetylation of histones, as well as other nuclear factors, modulating the expression of genes that play a role in the control of cell growth and differentiation.10
Study design
A phase I study of depsipeptide was conducted in the Medicine Branch, National Cancer Institute (NCI); details of the trial are being reported separately (V.S. et al, manuscript submitted). Four patients with T-cell lymphoma were enrolled and treated at the 12.7 or 17.8 mg/m2 dose level. The drug was administered by a 4-hour infusion on days 1 and 5 of a 21-day cycle. Responses were scored according to the World Health Organization criteria11 with skin responses scored as described by Zinzani et al.12 Toxicities were graded according to the NCI Common Toxicity Criteria. The major observed toxicities were fatigue, nausea, vomiting, granulocytopenia, thrombocytopenia, and hypocalcemia (V.S. et al, manuscript submitted).
Histone acetylation assays were performed on cytospins from patients' peripheral mononuclear cells. Slides were fixed with 95% ethanol/5% acetic acid, washed twice with phosphate-buffered saline (PBS), blocked with 8% bovine serum albumin (BSA), and then washed with PBS. The slides were then incubated overnight at 4°C with anti–acetyl histone H3 antibody (Upstate Biotechnology, Lake Placid, NY) diluted 1:200 in 2% BSA. After 2 washes, the slides were stained with horse anti–rabbit fluorescein isothiocyanate–conjugated secondary antibody (Vector Laboratories, Burlingame, CA), for 1 hour. After 3 washes, the slides were counterstained with 4′ 6-diamidino-2-phenylindole-2HCl containing antifade compound (Vector Laboratories).
Fine-needle aspirates were performed by a cytopathologist using a 23- or 25-gauge needle, with on-site evaluation of Diff-Quick–stained smears (Dade Diagnostics, Aguada, PR). The remainder of the sample was rinsed with RPMI 1640 (Gibco BRL, Grand Island, NY) and processed as Diff-Quick–stained cytospins.
Results
Patients 1 and 2 are men 63 and 53 years old, respectively, both diagnosed with Sézary syndrome. Neither patient responded to previous therapies, which included either cyclophosphamide, doxorubicin, vincristine, and prednisone or clyclophosphamide, vincristine, and prednisone. They were symptomatic with itching, and their physical examinations were significant for erythema and scaling of their trunks and extremities. Both had a white blood count (WBC) exceeding 60 × 109/L (60 000/μL). Flow cytometry identified a clonal population of T-helper lymphocytes in the peripheral blood. Computed tomography (CT) scans demonstrated enlarged lymph nodes. Following treatment with depsipeptide, there was a rapid decrease of circulating Sézary cells and improvement of skin erythema and edema. In addition, there was an increase in histone acetylation in peripheral mononuclear cells, which were, in these patients, composed predominantly of Sézary cells (Figure 1). Patient 1 was removed from the study after 4 cycles after developing multiple subcutaneous abscesses of methicillin-resistantStaphylococcus aureus. Patient 2 was removed from study after 5 cycles for pruritis and erythema that persisted despite therapy.
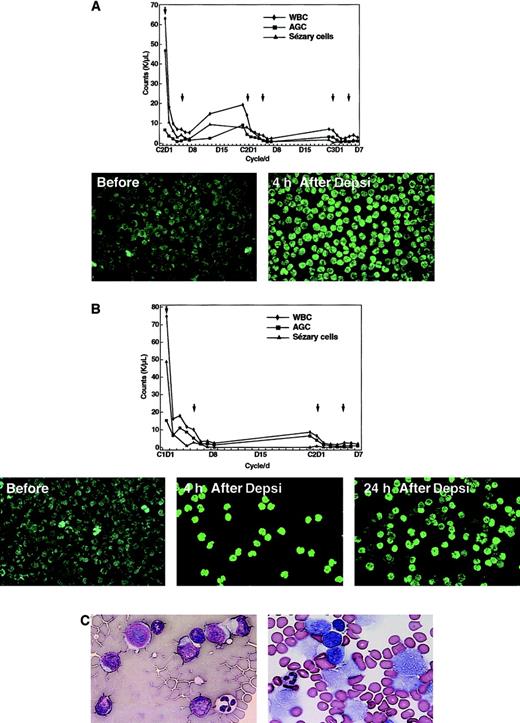
Effect of treatment on Sézary cell count, histone acetylation, and lymphoma cells.
The graphs represent the WBC, absolute granulocyte count, and Sézary cell counts of patient 1 (panel A) and 2 (panel B) in response to treatment with depsipeptide. The x-axis indicates the days of the cycle; the arrows in the chart indicate the days that depsipeptide was administered. Below are peripheral mononuclear cells following fluorescent labeling with anti–acetyl histone H3. The majority of the cells are Sézary cells taken from the patients prior to infusion of depsipeptide, at the completion of the 4-hour infusion and (in panel B) at 24 hours after initiation of the infusion. In panel C, the section on the left was from a fine-needle aspiration biopsy prior to the initiation of therapy and demonstrates atypical lymphocytes with enlarged hyperchromatic nuclei and scant deep basophilic cytoplasm (Diff-Quick × 400). The section on the right was obtained after administration of the second dose of the first cycle and has cells with vacuolization of cytoplasm and fragmentation of nuclei (Diff-Quick × 400).
Effect of treatment on Sézary cell count, histone acetylation, and lymphoma cells.
The graphs represent the WBC, absolute granulocyte count, and Sézary cell counts of patient 1 (panel A) and 2 (panel B) in response to treatment with depsipeptide. The x-axis indicates the days of the cycle; the arrows in the chart indicate the days that depsipeptide was administered. Below are peripheral mononuclear cells following fluorescent labeling with anti–acetyl histone H3. The majority of the cells are Sézary cells taken from the patients prior to infusion of depsipeptide, at the completion of the 4-hour infusion and (in panel B) at 24 hours after initiation of the infusion. In panel C, the section on the left was from a fine-needle aspiration biopsy prior to the initiation of therapy and demonstrates atypical lymphocytes with enlarged hyperchromatic nuclei and scant deep basophilic cytoplasm (Diff-Quick × 400). The section on the right was obtained after administration of the second dose of the first cycle and has cells with vacuolization of cytoplasm and fragmentation of nuclei (Diff-Quick × 400).
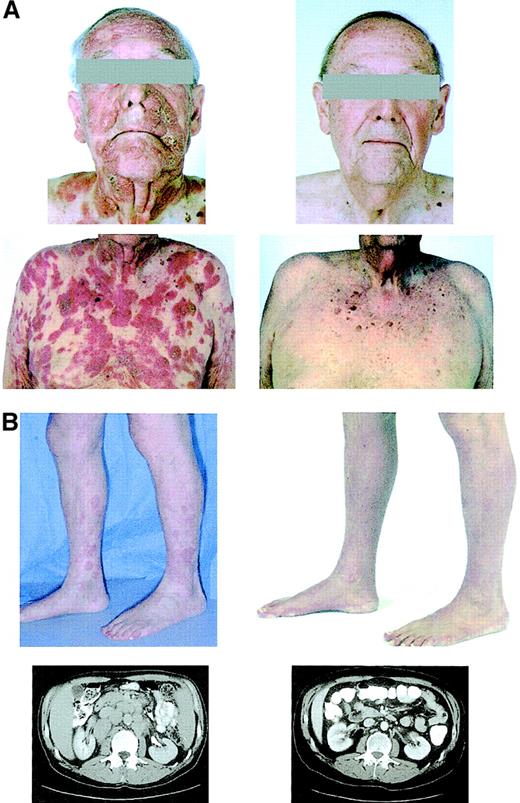
Patient 3 is a 78-year-old man who presented with tumor-stage CTCL. He was symptomatic with mild generalized pruritus, and his physical examination was notable for hundreds of tumors without the sparing of any skin areas. These lesions had developed within the previous 4 months and were not amenable to treatment with total-skin electron beam therapy or other topical therapies. After administration of the second dose of depsipeptide of the first cycle, fine-needle aspirations of 2 lesions were performed. A cytological examination showed many cells with cytoplasmic vacuolization and nuclear fragmentation, suggesting that a significant population of the tumor cells were affected by treatment (Figure 1C). There was clearing of almost all tumors after 6 cycles of treatment (Figure2A). Five lesions that had initially responded to treatment eventually progressed through therapy and were treated with radiation.
Clinical effects of depsipeptide.
Photographs of patient 3 (panel A) and photographs and abdominal CT sections of patient 4 (panel B) before (left) and after (right) treatment with depsipeptide. These demonstrate a response of the cutaneous tumors on the face and torso of patient 3 and of the erythematous plaques on the legs, and retroperitoneal lymphadenopathy of patient 4 after treatment with depsipeptide.
Clinical effects of depsipeptide.
Photographs of patient 3 (panel A) and photographs and abdominal CT sections of patient 4 (panel B) before (left) and after (right) treatment with depsipeptide. These demonstrate a response of the cutaneous tumors on the face and torso of patient 3 and of the erythematous plaques on the legs, and retroperitoneal lymphadenopathy of patient 4 after treatment with depsipeptide.
Patient 4 is a 53-year-old man with peripheral T-cell lymphoma, unspecified. Physical examination demonstrated erythemous plaques of the skin with nodules over the elbows, buttocks, and thighs and a large 3- to 4-cm mass over the plantar surface of the left foot. There was no bone marrow involvement. CT scan of the chest, abdomen, and pelvis showed mediastinal, retrocrural, axillary, retroperitoneal, pelvic, and inguinal adenopathy. After one cycle of etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin chemotherapy, the patient experienced progression of his subcutaneous nodules and erythema and was enrolled on the phase I depsipeptide study. After initiation of depsipeptide, there was clearing of his cutaneous lesions, skin nodules, and lymphadenopathy on CT scan (Figure2B). He was declared to be in complete remission (CR) after the eighth cycle.
Discussion
Depsipeptide is a member of the new class of antineoplastic agents, the HDIs. Depsipeptide, like other HDIs, has been shown to induce cell cycle arrest in both G1 and G2/M phases and to induce apoptosis in several cell lines.13Depsipeptide induces increased expression of p21, cyclin E, decreased expression of cyclin D1, and hypophosporylation of Rb.14Other molecular changes include a decreased expression of c-myc in fibroblasts13 and in T-cell hybridomas.15Other changes in the cellular milieu remain to be elucidated.
The case reports presented here suggest that depsipeptide, and potentially other HDIs, may be effective in T-cell lymphomas. Subsequent to these clinical observations, similar findings were also reported in laboratory models.16 Recent studies have shown that HDIs have activity against acute myeloid leukemia cell lines,17,18 an observation that may be related to chromosomal translocations that result in the synthesis of chimeric proteins with altered HDAC or histone acetyltransferase activity.18 Given the observed responses to depsipeptide, it seems plausible to hypothesize that alterations in HDACs or histone acetyltransferases may be involved in the pathogenesis of T-cell lymphomas.
On the basis of the observed responses, a phase II study testing depsipeptide in patients with cutaneous T-cell or peripheral T-cell lymphomas has been initiated. Since the patients with CTCL had some benefit from depsipeptide but did not achieve a CR, it will be important to identify agents that can be effectively combined with depsipeptide. Among the agents that it would be reasonable to consider are cytotoxic chemotherapeutic agents, Pgp inhibitors, retinoids, or interleukin-2 (IL-2) receptor–targeting agents. The tumors from patients 1, 3, and 4 were CD25−, a marker for the α-subunit of the IL-2 receptor. The circulating Sézary cells of patients 1 and 2 were CD25− at the start of therapy, but were CD25+ after several cycles of treatment and, in the case of patient 1, after the second dose of the first cycle (data not shown). It is noteworthy that a recent report demonstrated that Sézary cells treated with the HDI arginine butyrate had increased expression of the IL-2 receptor and increased sensitivity to IL-2 receptor–targeted therapy.19
In conclusion, this is the first report that demonstrates a clinical response to an HDI in a specific tumor type. In light of the poor therapy existing for patients with T-cell lymphoma, depsipeptide represents a promising new agent in the treatment of these patients.
Supported in part by research funding from the Fujisawa Pharmaceutical Company.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard L. Piekarz, 9000 Rockville Pike, Bldg 10, Rm 12n226, Bethesda, MD 20892; e-mail: rpiekarz@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal