Abstract
Mice deficient in the Syk tyrosine kinase showed severe petechiae in utero and died shortly after birth. The mechanism of this bleeding, however, remains unknown. Here it is shown that this bleeding is caused by morphologic defects of Syk-deficient endothelial cells during embryogenesis. Immunoblot and reverse transcriptase–polymerase chain reaction Northern blot analysis indicated that Syk is expressed in several endothelial cell lines. Immunocytochemical analysis also confirmed that Syk is expressed in the normal embryonic endothelial cells and is absent in Syk-deficient mice. Furthermore, electron microscopic analysis of Syk-deficient mice revealed an abnormal morphogenesis and a decreased number of endothelial cells. The results indicate a critical role for Syk in endothelial cell function and in maintaining vascular integrity in vivo.
Introduction
Syk protein-tyrosine kinase contains a C-terminal kinase domain and tandem N-terminal SH2 domains that bind phosphorylated immunoreceptor tyrosine-based activation motif and play critical roles in signaling through immune receptors.1,2Syk is expressed by all hematopoietic cells; it is essential for lymphocyte development and signal transduction via immune receptors in nonlymphoid cells.3,4 On the other hand, Syk-deficient mice show a lethal phenotype associated with severe petechiae, diffuse hemorrhage, and chylous ascites.5,6 This latter phenotype is suggestive of loss of vascular integrity although Syk expression has not been examined in vascular endothelial cells (ECs). Remarkably, the bleeding phenotype is transferable when Syk-deficient bone marrow is transplanted into wild-type mice.7,8 Classical tests of platelet function such as bleeding time are normal in radiation chimeras reconstituted with Syk-deficient platelets.7 9Thus, the mechanism of this bleeding is currently unclear. Here we report that Syk is expressed in ECs and the bleeding observed in Syk-deficient mice might be caused by EC dysfunction during embryogenesis.
Study design
Western blot and RT-PCR Northern blot analysis
Western blot and reverse transcriptase–polymerase chain reaction (RT-PCR) Northern blot analysis were performed as described previously.10
Immunohistology
Wild-type and Syk-deficient mice at embryonic day 18.5 were used for immunohistochemical analysis. Both embryos were fixed in 10% neutral buffered formalin followed by Bouins solution. Sections were immunostained using Serial sections 4 μm thick were cut and stained on glass slides. For double staining, the cells were incubated with the polyclonal anti-Syk antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and the monoclonal anti-CD31 antibody (Caltag, Burlingame, CA) for 2 hours at room temperature, washed 3 times with 0.2% Triton X-100 in phosphate-buffered saline, and then incubated with fluorescein isothiocyanate–labeled goat anti–rabbit immunoglobulin G (Sigma, St Louis, MO) and Alexa Fluor 594-labeled goat antirat immunoglobulin G (Molecular Probes, Eugene, OR) for 1 hour. The samples were washed as before, mounted using Slowfade Antifade kit (Molecular Probes), and analyzed using an Olympus (Tokyo, Japan) fluorescence microscope.
Electron microscopic analysis
Subcutaneous tissues from wild-type and Syk-deficient mice at embryonic day 18.5 were fixed for 3 days with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4). They were postfixed with 2% OsO4 in the same buffer. After dehydration with a graded series of ethanol, they were substituted by propylene oxide and embedded in epoxy resin. Silver to gold thin sections were doubly stained with uranyl acetate and lead citrate, examined with an H-7000 transmission electron microscope (Hitachi, Tokyo, Japan), and photographed at various magnifications as indicated.
Results and discussion
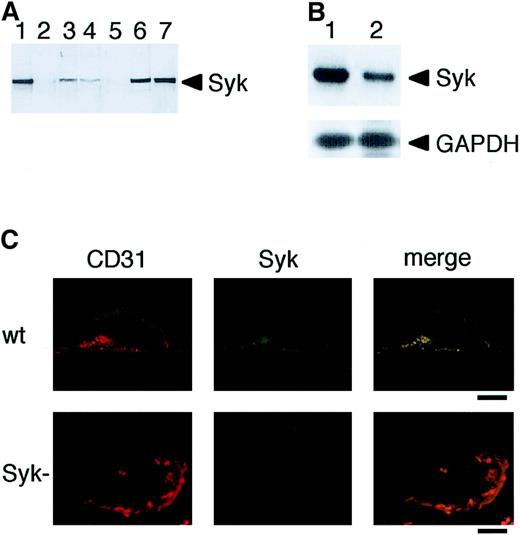
To examine whether Syk was expressed in vascular ECs, we performed immunoblot analyses using anti-Syk antibody. As shown in Figure1A, compared with the human Ramos B cells, a lower level of Syk expression was observed both in human umbilical vein endothelial cells (HUVECs) and bovine aortic endothelial cells (BAECs). The specificity of the antibody was evidenced by the abolition of a 72-kd band in lysates from Syk-deficient fetal liver and Syk-deficient DT40 avian B cells (Figure 1A, lanes 2 and 5). To confirm Syk expression in HUVECs, we performed RT-PCR analysis using messenger RNA (mRNA) prepared from these cells. This analysis demonstrated that Syk mRNA was expressed in HUVECs (Figure 1B). Furthermore, immunofluorescence microscopic analysis of microvasculature of wild-type and Syk-deficient mice showed that Syk is expressed in mouse embryonic ECs and is lacking from the endothelium of mutant mice (Figure 1C). These results provide the first evidence of Syk expression in ECs. It has recently been reported that Syk is also expressed, albeit at a low level, in various nonhematopoietic cells, including hepatocytes, neuronal cells, and fibroblasts.11-14 Thus, Syk may be a multifunctional protein tyrosine kinase involved in numerous signal transduction pathways.
Expression of Syk in vascular ECs.
(A) Immunoblot analysis of Syk in vascular ECs. Cell and tissue extracts (10 μg protein) were analyzed by immunoblotting with anti-Syk antibody: wild-type (lane 1) and Syk-deficient mice liver (lane 2), HUVECs (lane 3), BAECs (lane 4), Syk-deficient DT40 chicken B cells (lane 5), wild-type DT40 cells (lane 6), and human Ramos B cells (lane 7). (B) RT-PCR Northern blot analysis of Syk mRNA in Ramos B cell line (lane 1) and HUVECs (lane 2). The positions of Syk and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as control are indicated. (C) Expression of Syk in mouse embryonic ECs. Immunofluorescence microscopy of microvasculature of wild-type and Syk-deficient mice at embryonic day 18.5. ECs were double labeled with anti-Syk antibody and with anti-CD31 antibody as a marker for ECs. Bars = 1.0 μm
Expression of Syk in vascular ECs.
(A) Immunoblot analysis of Syk in vascular ECs. Cell and tissue extracts (10 μg protein) were analyzed by immunoblotting with anti-Syk antibody: wild-type (lane 1) and Syk-deficient mice liver (lane 2), HUVECs (lane 3), BAECs (lane 4), Syk-deficient DT40 chicken B cells (lane 5), wild-type DT40 cells (lane 6), and human Ramos B cells (lane 7). (B) RT-PCR Northern blot analysis of Syk mRNA in Ramos B cell line (lane 1) and HUVECs (lane 2). The positions of Syk and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as control are indicated. (C) Expression of Syk in mouse embryonic ECs. Immunofluorescence microscopy of microvasculature of wild-type and Syk-deficient mice at embryonic day 18.5. ECs were double labeled with anti-Syk antibody and with anti-CD31 antibody as a marker for ECs. Bars = 1.0 μm
To investigate whether the hemorrhage in Syk-deficient mice was caused by functional defect in microvascular ECs, we performed morphologic analyses of the microvasculature by electron microscopy using the subcutaneous tissues from the wild-type and Syk-deficient mice. Most surprisingly, electron microscopic analysis revealed the remarkable morphologic abnormality in microvasculature structure of Syk-deficient mice (Figure 2). ECs in particular showed unusually scanty cytoplasm (Figure 2B). The cytoplasm of ECs from the Syk-deficient mice barely traversed the inside of the vessel wall. It was very thin and thus appeared to be very fragile. As a consequence of the fragility of ECs, bleeding seemed to be caused by the collapse of blood vessel architecture (Figure 2D,E).
Electron microscopic analysis of the microvasculature of Syk-deficient mice.
Compared with panel A (wild-type [wt]), panel B shows remarkable morphologic abnormality and decreased numbers of ECs in Syk-deficient mice (Syk−). Arrowheads highlight the scanty cytoplasm. Compared with panel C (wt), panel D shows EC shrinkage (arrowheads) and bleeding in Syk-deficient mice. The sections in panels A and C and in B and D are different sites. (F) A higher magnification image of the hemorrhagic area in panel E, which shows an erythrocyte leaking out from the vessel wall. (G,H) Tight junction structure between vascular ECs in wild-type and Syk-deficient mice, respectively (arrowheads). Bars = 2.5 μm (A-F) and 0.5 μm (G,H).
Electron microscopic analysis of the microvasculature of Syk-deficient mice.
Compared with panel A (wild-type [wt]), panel B shows remarkable morphologic abnormality and decreased numbers of ECs in Syk-deficient mice (Syk−). Arrowheads highlight the scanty cytoplasm. Compared with panel C (wt), panel D shows EC shrinkage (arrowheads) and bleeding in Syk-deficient mice. The sections in panels A and C and in B and D are different sites. (F) A higher magnification image of the hemorrhagic area in panel E, which shows an erythrocyte leaking out from the vessel wall. (G,H) Tight junction structure between vascular ECs in wild-type and Syk-deficient mice, respectively (arrowheads). Bars = 2.5 μm (A-F) and 0.5 μm (G,H).
Notably, in several thin sections from Syk-deficient mice there was a significant decrease in the number of ECs when compared with the wild-type controls (Figure 2B and data not shown). Indeed, Figure 2D shows EC shrinkage, a typical characteristic of apoptotic necrosis and bleeding in Syk-deficient mice. Thus, the abnormal morphogenesis of vascular ECs in the Syk-deficient mice may be the result of excessive stretching of a smaller number of ECs that could not retain the normal mechanical stability in the face of physical force such as blood flow. Alternatively, it is possible that the cytoplasmic content of ECs was decreased by the loss of Syk function. In this case, Syk may play an important regulatory role in the organization of the cytoskeletal system, which in turn affects the cytoplasmic structure of vascular ECs. Indeed, Syk has been reported to be translocated from cytosol to cytoskeleton and phosphorylate cortactin, an F-actin–binding protein that is involved in signaling events for the reorganization of the microfilaments.15
Similar hemorrhaging was also observed in mice deficient in platelet-derived growth factor-B, where the defect was postulated to be a loss of pericytes and formation of microaneurysm without a significant change in EC morphology and number.16Pericytes form a part of the capillary wall and contribute to the mechanical stability of the capillary wall. In Syk-deficient mice, pericytes appeared well developed, without a decrease in their number (not shown), contrasting with phenotypes observed in platelet-derived growth factor-B–deficient mice. More importantly, however, there was no significant defect in tight junctions between vascular ECs in Syk-deficient mice (Figure 2H). Supporting this observation is that the point where the erythrocyte had leaked out from the vessel wall was not between ECs but the weak point between cell nucleus and cytoplasm, which had become structurally thin and obviously weak (Figure 2F). Taken together, our results suggest that the abnormal microvascular structure seen in Syk-deficient mice was mainly caused by the dysfunction of ECs and that Syk plays a critical role in physiologic regulation of ECs and contributes to maintaining vascular integrity in vivo.
We thank Dr T. Terashima and Dr T. Hirase for discussion. We also thank Dr S. Jahangeer for critically reading the manuscript.
Supported in part by the Uehara Foundation of Japan, Fellowships of the Japan Society for the Promotion of Science for Young Scientists (R.I.), Grant-in-Aid for the Research for the Future Program from the Japan Society for the Promotion of Science, Grant-in-Aids for Scientific Research, and Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hirohei Yamamura, Dept of Biochemistry, Kobe University School of Medicine, Chuo-ku, Kobe 650-0017, Japan; e-mail:yamamura@kobe-u.ac.jp.

![Fig. 2. Electron microscopic analysis of the microvasculature of Syk-deficient mice. / Compared with panel A (wild-type [wt]), panel B shows remarkable morphologic abnormality and decreased numbers of ECs in Syk-deficient mice (Syk−). Arrowheads highlight the scanty cytoplasm. Compared with panel C (wt), panel D shows EC shrinkage (arrowheads) and bleeding in Syk-deficient mice. The sections in panels A and C and in B and D are different sites. (F) A higher magnification image of the hemorrhagic area in panel E, which shows an erythrocyte leaking out from the vessel wall. (G,H) Tight junction structure between vascular ECs in wild-type and Syk-deficient mice, respectively (arrowheads). Bars = 2.5 μm (A-F) and 0.5 μm (G,H).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/9/10.1182_blood.v98.9.2869/4/m_h82111722002.jpeg?Expires=1769117581&Signature=GvapmhiYYtGIKGKL8bi6YlyGFXbLrMRtmRYvJS4sBTUhu6MwpQ~bX13SYpToxbRnq25ZTvCHK3GLzW-FnGzrolD1mNUA4hKaiuXJQ-jJJ6oaDGV54LUGSYzpEdmMoxtXAGmncWgCzbg0KL9Zlup4UNl3HIJgHxtmcRTytxVsP3noKLAVST2FK3JUYoV-42ozp4OeIW38x4Zl7qa7sCTPpxVAxAQrdVIH6D-MnBm3PKA1yRtLc7fVkAUGRNns30UaZqCa5QIhTLvZ2gXgI0mwwzhmPvZeX0R1h1rdXnQJefzEqa8ARPjW3lYRmeXlVqylJ7iI5F8c2PgjcMNNM8oO2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal