Thalassemia is a congenital hemolytic disorder caused by a partial or complete deficiency of α- or β-globin chain synthesis. Homozygous carriers of β-globin gene defects suffer from severe anemia and other serious complications from early childhood. The disease is treated by chronic blood transfusion. However, this can cause severe iron overload resulting in progressive organ failure. Some forms of α thalassemia are also associated with a similar clinical picture. Despite the difficulties associated with treatment, standards of care for thalassemic patients have improved in recent years, resulting in almost doubling of the average life expectancy. As a consequence, additional previously undescribed, complications are now being recognized. In particular, profound hemostatic changes have been observed in patients with β-thalassemia major (β-TM) and β-thalassemia intermedia (β-TI) and also in patients with α thalassemia (hemoglobin H disease). The presence of a higher than normal incidence of thromboembolic events, mainly in β-TI, and the existence of prothrombotic hemostatic anomalies in the majority of the patients, even from a very young age, have led to the recognition of the existence of a chronic hypercoagulable state in thalassemic patients. Despite the appearance of numerous publications on the frequent occurrence of thromboembolic complications in thalassemia, this complication has not been emphasized or comprehensively reviewed. This review summarizes the current literature and discusses possible mechanisms of the lifelong hypercoagulable state that exists in thalassemia.
Thromboembolic manifestations in β thalassemia
Cerebral thrombosis
There have been numerous reports of thromboembolic complications associated with thalassemia, many describing cerebral thrombotic events. As early as 1972, Logothetis et al,6 reviewing 138 cases of β-thalassemia major (β-TM) in Greece, described a “stroke syndrome” in 2 patients and neurologic deficits compatible with transient ischemic attacks in about 20% of the cases. An Italian multicenter study of 735 patients with β-TM reported 16 individuals with cerebral thromboembolic events accompanied by a clinical picture of headache, seizures, and hemiparesis.7 Cerebral thrombosis was also found in patients with β thalassemia/hemoglobin E disease and in α thalassemia.8,9 All these reports were from patients who were not given regular transfusions and were not associated with an individual blood transfusion. Other reports have described cases of hypertension, convulsion, and cerebral hemorrhage in thalassemic patients following blood transfusion.10-13 Asymptomatic brain damage has also been reported; results from magnetic resonance imaging (MRI) on 41 patients with β-thalassemia intermedia (β-TI) revealed asymptomatic brain damage including ischemic lesions as a frequent occurrence affecting 37% of patients.14Damage was inversely correlated with hemoglobin levels in patients with β-TI and increased with age.
Deep venous thrombosis and pulmonary embolism
Deep venous thrombosis (DVT), pulmonary embolism, and recurrent arterial occlusion have been described in patients with β-TM and β-TI from many countries.9,15-19 In most cases, thrombosis was spontaneous and there were no known risk factors, although some patients with thrombocytosis after splenectomy developed venous thrombosis. Following these sporadic reports, several multicenter studies were carried out to determine the incidence of thromboembolism in patients with β-TM and β-TI. In one Italian multicenter study, 32 of 735 patients (4.35%) experienced thromboembolic events. The incidence was 3.95% among 685 β-TM patients and 9.61% among 52 patients with β-TI.7 The same group reported a lower incidence (1.1%) of thromboembolic complications among 1146 patients with β-TM who were followed for 37 years.7 Another study showed a 5.3% overall incidence of thrombotic complications among 495 patients with thalassemia whose median age was 28 years.1,2 In this study, the prevalence of thromboembolic events was 3.3% among 421 patients with β-TM and 16.2% among 74 patients with β-TI, although 15.3% of these patients had predisposing congenital or acquired factors contributing to the hypercoagulability.2 Recently, Cappellini et al19 observed a high incidence of venous thromboembolic events (VTEs) in a group of 83 patients with β-TI who were followed for 10 years. Twenty-four patients (29%) developed either pulmonary embolism, DVT, or portal vein thrombosis, and recurrent VTEs occurred in 9 of these cases. All patients except one had undergone splenectomy.
Autopsy findings in patients with thalassemia have clearly demonstrated hypercoagulability as a pathologic feature.20,21 Autopsies on 17 splenectomized and 2 nonsplenectomized patients of 43 with β thalassemia/hemoglobin E disease showed atherosclerotic changes and obstructive lesions consisting of organized, recanalized thrombi in the pulmonary arteries and microvasculature. No evidence of thromboembolism was found elsewhere, although routine dissection of the veins in the legs was not performed.20 Similar findings of multiple microthrombi in the pulmonary arterioles, composed mainly of platelets, were found in autopsies performed on 2 thalassemic patients.21 Asymptomatic pulmonary vascular disease that could result from silent, recurrent thromboembolic events has been found in many patients with β-TM and β-TI. This was suggested by echocardiographic studies in 35 β-TM patients who had no clinical signs or symptoms of thromboembolic disease. Many of the patients showed pulmonary hypertension and right heart failure, which were more prevalent than left heart failure.22 In addition, reduced lung volumes and flow rates, hypoxemia, reduced carbon monoxide diffusion in the lung (DLCO), and pulmonary hypertension were found in these patients.22 In another study of 15 thalassemic patients, the mean total lung capacity, mean residual volume, and mean forced vital capacity were significantly reduced.23 In addition, the DLCO was low, and hypoxemia was present in 6 of 13 patients tested.23 These findings suggest that the early right ventricular dysfunction, which precedes left heart failure in many patients with β-TM and β-TI, may be due to pulmonary hypertension and not cardiomyopathy resulting from excessive iron deposition.24,25 In support of this idea, a Doppler echocardiography study in which pulmonary artery pressure was measured in 33 patients with β-TM (aged 2-24 years) showed that 28 patients had evidence of pulmonary hypertension.26 Pulmonary artery hypertension was also detected by M-mode and Doppler echocardiography in 15 of 16 children, aged 5 to 14 years, with homozygous β thalassemia and β thalassemia/hemoglobin E disease.27Right ventricular dysfunction was detected earlier than left ventricular dysfunction in these children, suggesting that the right heart failure and pulmonary hypertension seen in thalassemia could result from microembolization in the lungs. Indeed, autopsy findings revealed a high frequency of thrombotic lesions in the pulmonary arteries and the development of cor pulmonale consistent with a long-standing pulmonary vascular embarrassment.20,21 The venous and arterial thrombotic events have not received much attention and were not mentioned in comprehensive reviews on thalassemia.5
Hemostatic changes in thalassemia
Platelet activation
In 1978, Eldor28 and a year later Houssain et al29 found defective platelet aggregation in response to adenosine diphosphate, epinephrine, or collagen in β-TM patients. Most of the patients had undergone splenectomy and had high platelet counts. At that time, these anomalies were interpreted as signs of a mild bleeding disorder because many thalassemic patients experience frequent epistaxis as well as easy bruising.28 However, in 1981 Winichagoon et al30 found increased circulating platelet aggregates in 71% of splenectomized and 35% of nonsplenectomized patients with β-TI/hemoglobin E disease, an observation compatible with in vivo platelet activation and the existence of a hypercoagulable state. Supporting evidence for this finding came from platelet kinetic studies in β-TM and β-TI patients using autologous platelets labeled with indium In 111 oxine.31 A significant shortening of platelet life span was observed in 13 of 14 patients examined. The mean platelet life span in 10 patients (8 β-TM and 2 β-TI) who underwent splenectomy was 107 ± 36 hours compared to 248 ± 51 hours in healthy individuals who underwent splenectomy because of trauma (P < .001). The mean platelet life span in 4 nonsplenectomized patients (2 β-TM and 2 β-TI) was 102 ± 64 hours compared to 224 ± 23 hours in healthy individuals (P < .01). Analysis of the data suggested that the shortened platelet life span was caused by enhanced platelet consumption,31 a feature usually associated with active thrombotic disease, severe atherosclerosis, diabetes mellitus, and other chronic hypercoagulable states.
Further evidence for the existence of chronic platelet activation in thalassemia was provided by the measurement of urinary metabolites of thromboxane A2 (TXA2) and prostacyclin (PGI2). A study of 9 splenectomized patients with β-TM who were regularly transfused, 5 nonsplenectomized patients with β-TI who received occasional blood transfusions, and 20 healthy individuals3,32 found a significant 4- to 10-fold increase in the urinary excretion of 2,3-dinor-TXB2, 11-dehydro-TXB2, and 2,3-dinor-6-keto-prostaglandin (PG) F1α in patients with β-TM and β-TI compared to healthy controls. The concentration of metabolites in patients with β-TM and β-TI was not significantly different, and 6 patients who received aspirin (20 mg/d) for 7 days showed a significant decrease in their urinary concentrations of 2,3-dinor-TXB2 and 11-dehydro-TXB2 derived from platelets. In contrast, levels of urinary 2,3-dinor-6-keto-PGF1α, reflecting vascular production, and TXB2 and 6-keto-PGF1αoriginating from the kidney were not significantly changed.32 The results of this study are consistent with enhanced production of TXA2 due to chronic endogenous platelet activation and reflect the increased concentrations of urinary thromboxane metabolites found in other diseases associated with in vivo platelet activation including unstable coronary disease, severe atherosclerosis, and type II diabetes mellitus.33-36 In another more recent study,4 urinary prostaglandin metabolites were determined in a group of 62 β-TM patients comprising 26 children (aged 2-18) and 36 adults. All the thalassemic children (including the youngest, aged 2-8 years) and the adults had highly elevated levels of the urinary prostaglandin metabolites, 11-dehydro-TXB2 and 2,3-dinor-6-keto-PGF1α. None of the thalassemic children had experienced clinical signs or symptoms suggestive of venous or arterial thrombosis, indicating that platelet activation in thalassemic patients persists from early in childhood when clinical thrombotic events are extremely rare.
The existence of chronic platelet activation in thalassemia was further confirmed by flow cytometric studies, which demonstrated the presence of an increased fraction of platelets carrying the activation markers CD62P (P selectin) and CD63.37,38 In addition, morphologic changes in thalassemic platelets, elevated plasma platelet factor 3 (PF3), and increased spontaneous whole blood platelet aggregation were reported.39-41
The results from these studies show that, in addition to their increased number in splenectomized patients, chronic platelet activation is present in β-TM and β-TI. This may explain the weak response of thalassemic platelets to aggregation agonists reported previously28 as the activated platelets become refractory to additional stimulation.32 The presence of morphologic platelet abnormalities in splenectomized patients with β thalassemia/hemoglobin E disease may also contribute to an enhanced risk of vascular complications.42
Endothelial, monocyte, and granulocyte activation
The detection of elevated levels of endothelial adhesion proteins (intercellular adhesion molecule-1 [ICAM-1], E-selectin [ELAM-1], vascular cell adhesion molecule-1 [VCAM-1], von Willebrand factor [VWF], and thrombomodulin) in serum and plasma of thalassemic patients suggested that endothelial activation or injury may be a feature of the disorder.43,44 The adherence of red blood cells (RBCs) to endothelial cells (ECs) correlates with microvascular occlusions in sickle cell disease (SCD) and malaria and is considered a major contributor to microcirculatory disorders.45 RBCs from patients with β-TM and β-TI showed enhanced adhesion to cultured ECs (10- to 25-fold increase compared to normal RBCs).46 Similar findings were described in SCD45,47 where the interaction of sickle RBCs with ECs induced a state of oxidative stress leading to enhanced transendothelial migration of blood monocytes.48
Monocyte activation may also play a significant role in heightening endothelial activation or injury in both thalassemia and SCD. High serum levels of monocyte colony-stimulating factor and increased monocyte phagocytic activities (antibody-dependent cell cytotoxicity [ADCC]) toward RBCs were found in patients with hemoglobin H disease and β-TM.49 Recently, it was shown that ECs incubated with sickle mononuclear leukocytes were activated to a greater extent than those incubated with normal mononuclear leukocytes, as judged by the increased endothelial expression of adhesion molecules and tissue factor and the adhesion of polymorphonuclear leukocytes.50 Sickle monocytes, which had 34% more interleukin 1β and 139% more tumor necrosis factor-α per cell than normal monocytes, caused the nuclear translocation of endothelial nuclear factor κ B, an event indicating EC activation.50It is possible that a similar mechanism may operate in thalassemia.
Activated granulocytes could also contribute to the endothelial damage and the hypercoagulable state in thalassemia. Elevated granulocyte phagocytic function, as manifested by enhanced chemiluminescence, was observed in patients with β-TM, with greater prominence of the abnormality in patients older than 5 years.51 Removal of leukocytes from transfused blood with a Leukostop filter resulted in improved pulmonary function tests (forced expiratory volume in 1 second/forced vital capacity ratio) in 4 patients with β-TM, 6 months after the procedure.52 This clinical observation illustrates the deleterious effect that activated granulocytes can induce in the lungs of patients with thalassemia.
Coagulation factors and inhibitors
Studies of the coagulation proteins provide strong evidence for the existence of a chronic hypercoagulable state in thalassemia. Several investigators have reported profound changes in the levels of coagulation factors, coagulation factor inhibitors, and components of the fibrinolytic system.
In a study by Eldor et al,4 plasma prothrombin levels were significantly lower in adult patients (aged 19-30 years) with β-TM (68% ± 11.5%) compared to age-matched healthy controls (86.1% ± 12.3%), whereas the levels of factors V, VII, X, and plasminogen were similar. Similarly reduced levels of prothrombin (61.1% ± 6.5%) were observed in a group of children, aged 2 to 18 years, with β-TM, suggesting that this anomaly is related to the thalassemia rather than to hepatic dysfunction due to hemosiderosis, which is a rare occurrence in children.4
Low levels of the coagulation inhibitors, protein C and protein S, have been observed in patients with β thalassemia from a variety of ethnic backgrounds.4,19,53,54 In Israeli patients mostly of Kurdish Jewish, Yemenite Jewish, or Arabic origin, protein C (antigen and activity) and free protein S were significantly decreased in both adults and children.4 Mean protein C antigen levels were 51.2% ± 11.2% in adult β-TM patients and 46% ± 9.1% in β-TM children (aged 2-13 years); they were 94.1% ± 21% in healthy individuals (P < .001). Similar values were obtained for protein C activity: 52.3% ± 12.1% in β-TM adults, 48.8% ± 14.7% in β-TM children, and 99.2% ± 16.1% in healthy controls (P < .001). Levels of free protein S were 49.3% ± 9.6% in β-TM adults, 43.4% ± 8.7% in β-TM children, and 85.1% ± 18.2% in the control group (P < .001). The decreased levels of free protein S were not due to low C4b-binding protein levels that were similar in β-TM patients and the controls.4 No correlation was found between the levels of protein C and protein S and the levels of prothrombin or any other coagulation factors. Furthermore, protein C and protein S levels in β-TM patients were not related to levels of serum transaminases, γ-glutamyl transferase, or albumin, eliminating hepatic dysfunction as a cause of these anomalies.4Similar results were obtained in studies of β-TI patients in Italy19 and patients with α- or β-TM in Thailand and Turkey.53,54 Some thalassemic patients from Italy and Turkey had low antithrombin III (ATIII) levels in addition to protein C and protein S deficiencies, whereas no ATIII deficiency was found in the Israeli patients.4,19 55
Low levels of heparin cofactor II (HCII), known to be associated with increased thrombotic risk, have been found in thalassemic patients.56 Frequent blood transfusions resulted in a slow normalization of HCII levels, suggesting that the low HCII levels could be related to increased RBC turnover that had been suppressed by hypertransfusion.56
The possibility of a genetic basis for the hypercoagulable state in thalassemic patients seems unlikely because a study of 25 Israeli β-TM patients (18 adults, 7 children) found no increased prevalence of congenital thrombophilic mutations, including the factor V Leiden, MTHFR C677T, and prothrombin G20210A mutations. In addition, none of the patients showed evidence of anticardiolipin antibody.4
In conclusion, it seems that the low levels of protein C and free protein S seen in thalassemic patients may be acquired at an early age. The extent to which the imbalance between coagulation inhibitors and clotting factors contributes to the hypercoagulable state in thalassemia remains to be determined.
Plasma markers of hypercoagulability
The existence of a chronic and lifelong hypercoagulable state in thalassemia was further supported by the elevated levels of thrombin-ATIII (TAT) complexes found in about 50% of adults and children with β-TM.4 Increased TAT levels were detected on repeated examinations in many of the patients, of whom none had any clinical signs of overt thrombosis. In this group of patients the levels of the prothrombin fragment, F1.2, were normal.4 However, significantly elevated levels of F1.2 and fibrinopeptide A (FPA) were found in splenectomized patients with β-TI, and these patients also had high plasma D-dimer levels, a manifestation of enhanced fibrinolysis.19 Elevated TAT levels were also observed in patients with α thalassemia (unpublished results, January 2001).
Contribution of abnormal thalassemic RBCs to the hypercoagulable state
The mechanism of the hypercoagulable state in thalassemia has not been fully elucidated. However, evidence from studies of other types of hemolytic anemia, such as SCD and paroxysmal nocturnal hemoglobinuria (PNH), in which thrombosis is also a major clinical entity, may be helpful in understanding the etiology of the latter phenomena.57-59
A comparison of normal RBCs with those isolated from patients with β-TM or β-TI by our group suggests that thalassemic RBCs may provide a source of negatively charged phospholipids, which can increase thrombin generation, as measured by prothrombinase assay.60,61 These results were confirmed in a similar assay using RBCs from splenectomized β-TI patients as a source of phospholipids.19 The procoagulant effect of thalassemic RBCs seems to be due to an increased surface expression of anionic phospholipids such as phosphatidylethanolamine (PE) and phosphatidylserine (PS). This was demonstrated by experiments that showed that annexin V, which binds anionic phospholipids, could block the procoagulant effect of isolated thalassemic RBCs.61These data suggest that the procoagulant effect of thalassemic RBCs may contribute to the hypercoagulable state in thalassemia by amplifying thrombin generation and initiating platelet activation.
To substantiate these findings, we measured the ability of RBCs from thalassemia patients to bind annexin V using dual-color flow cytometry.38 Significantly higher (P < .01) fractions of fluorescein isothiocyanate (FITC)–annexin V–labeled RBCs were found in 30 β-TM patients (2.9% ± 1.9%; range, 0.5%-7.5%) and 6 β-TI patients (2.5% ± 2.8%; range, 0.9%-6.9%) compared to 25 healthy individuals (0.5% ± 0.3%; range, 0.1%-1.2%). Results of tests on patients with myelodysplastic syndrome and severe anemia were similar to those observed for a healthy control group.38 Moreover, in the thalassemic patients, a highly significant correlation (P < .001) was found between the number of RBC-bound annexin V molecules and the fraction of CD62P (P selectin) or CD63+ platelets.38 This association between annexin V binding and the expression of platelet activation markers was also found in individual thalassemic patients over time and was not dependent on whether the patients had undergone splenectomy. These results support the idea that the procoagulant surface of thalassemic RBCs promotes thrombin generation in vivo leading to platelet activation.38
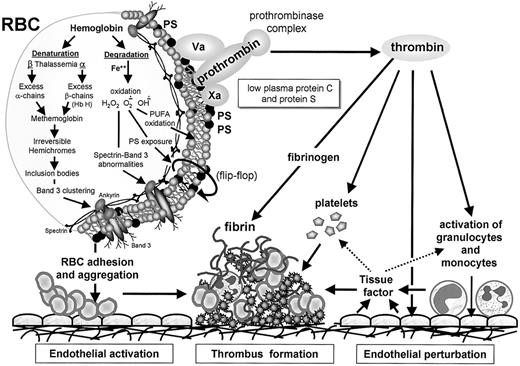
The asymmetrical distribution of membrane phospholipids seen in normal RBCs seems to be the result of a direct interaction of PS and PE with membrane skeletal proteins (mostly spectrin) and an adenosine triphosphate (ATP)–dependent unidirectional translocation of PS and PE from the outer toward the inner membrane leaflet (Figure1).62-65 This reaction is catalyzed by the aminophospholipid translocase enzyme that recognizes both PS and PE.63,64 Aged RBCs contain higher amounts of PS on the outer leaflet of their membranes compared to young cells and this may serve as a signal for their recognition and removal by the reticuloendothelial system.65 Deoxygenated (sickled) RBCs from patients with SCD show an abnormal distribution of membrane phospholipids that is caused by an accelerated trans-bilayer diffusion of phosphatidyl choline (PC) and a reduced rate of ATP-dependent transport of PS and PE.57,58 The existence of such membrane phospholipid asymmetry in the RBCs of patients with thalassemia was recently demonstrated.66 The membrane damage in thalassemic RBCs may be related to the primary abnormality in RBC lipids caused by lipid membrane peroxidation mediated by free iron.67 In fact, increased amounts of membrane-bound hemichromes and immunoglobulins were found in the RBCs of β-TM and β-TI patients and the membrane band 3 protein showed oxidative modifications such as aggregation and a decrease in sulfhydryl groups.68,69 The membrane phospholipid abnormalities in thalassemic and sickle RBCs may partly explain the increased adherence of PS-exposing RBCs to ECs (Figure 1). When human umbilical vein endothelial cell monolayers were incubated with PS-exposing RBCs, the ECs retracted and the RBCs adhered primarily in the gaps that had been opened between the ECs. Pretreatment of RBCs with annexin V significantly reduced adherence by shielding PS on the RBCs. This suggests an important contribution of the PS-exposing RBCs to the vascular damage observed in thalassemia and sickle cell anemia.45
The hypercoagulable state in thalassemia.
Thalassemia is associated with partial or complete deficiency of α- or β-globin chain synthesis, which leads to denaturation and degradation of the remaining globin chains. This process is associated with loss of the normal asymmetrical distribution of the RBC membrane phospholipids and translocation of PS to the external membrane leaflet (flip-flop). The membrane damage may be related to lipid peroxidation mediated by free iron and increased amounts of membrane-bound hemichromes and immunoglobulins and modifications in the membrane band 3 protein and spectrin. The membrane changes may partly explain the enhanced aggregation of PS-exposing RBCs, their increased adherence to ECs, and their capacity to enhance thrombin generation via the assembly of the prothrombinase complex. The enhanced thrombin generation leads to activation of platelets, monocytes, granulocytes, and ECs and expression of tissue factor, which further enhances the thrombotic process. The low levels of the coagulation inhibitors, protein C and protein S, further facilitate the resultant hypercoagulable state.
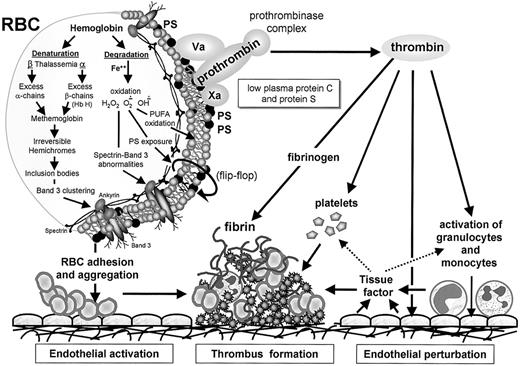
The hypercoagulable state in thalassemia.
Thalassemia is associated with partial or complete deficiency of α- or β-globin chain synthesis, which leads to denaturation and degradation of the remaining globin chains. This process is associated with loss of the normal asymmetrical distribution of the RBC membrane phospholipids and translocation of PS to the external membrane leaflet (flip-flop). The membrane damage may be related to lipid peroxidation mediated by free iron and increased amounts of membrane-bound hemichromes and immunoglobulins and modifications in the membrane band 3 protein and spectrin. The membrane changes may partly explain the enhanced aggregation of PS-exposing RBCs, their increased adherence to ECs, and their capacity to enhance thrombin generation via the assembly of the prothrombinase complex. The enhanced thrombin generation leads to activation of platelets, monocytes, granulocytes, and ECs and expression of tissue factor, which further enhances the thrombotic process. The low levels of the coagulation inhibitors, protein C and protein S, further facilitate the resultant hypercoagulable state.
Several studies have suggested that RBCs from thalassemic patients also demonstrate enhanced cohesiveness, which may contribute to the hypercoagulable state. Using a novel image analysis system to measure RBC aggregation in a flow chamber, an increased cohesion of TM RBCs was detected, demonstrated by the formation of large aggregates.70 Normal rouleaux formation was absent and higher shear stress was required to disperse the aggregates. It is noteworthy that RBC aggregate size was reduced to normal after patients received a blood transfusion and this observation was confirmed by in vitro experiments where the addition of normal RBCs to thalassemic RBCs resulted in reduced aggregation under flow.70 These in vitro findings could partly explain the recent clinical observation that patients with β-TI who do not receive transfusions regularly had a much higher incidence of thrombotic events compared to the incidence of such events in those receiving regular transfusions.19
The contribution of the abnormal RBCs to the thrombotic process has been also demonstrated in animal models of congenital hemolytic anemias.71-74 A lethal hypercoagulable state manifested by large thrombotic lesions in the heart and the liver and large venous thrombi was found in mice in which the expression of erythroid band 3 had been eliminated via targeted mutagenesis.71 The abnormal RBCs from these mice significantly shortened the Russell viper venom clotting time of normal plasma in a dose-dependent fashion, whereas RBCs from normal mice had no effect. These experiments suggested that the membrane of band 3 null RBCs provides a suitable surface for activation of the prothrombinase complex and, indeed, PS exposure on the outer membrane leaflet of the affected RBCs was demonstrated by increased FITC-annexin V binding.71 A high incidence of thrombosis in the heart and brain was also found in α-spectrin– and β-spectrin–deficient mice with hereditary spherocytosis.72 Thrombosis incidence in these animals was significantly reduced following the transfusion of normal RBCs or transplantation of normal bone marrow.73 The presence of normal RBCs in the peripheral circulation of these α-spectrin–deficient mice prolonged the survival of young animals and abrogated the development of thrombosis in adult animals.73
Similarity of thromboembolic manifestations and hemostatic changes in thalassemia and SCD
Similar thrombotic complications and hemostatic abnormalities, characteristic of a chronic hypercoagulable state, have been described in SCD (Table 1). Although much of the morbidity in SCD is caused by tissue ischemia and infarction (sickle cell crisis) resulting from microvascular occlusions caused by the abnormal sickling RBCs, these events are not associated with an activation of the hemostatic system.74-76 However, both adults and children with SCD are known to have an ill-defined but increased thrombotic risk associated with large blood vessels.76 Stroke occurs in 7% to 8% of children with SCD (hemoglobin SS) and is a major cause of morbidity.77-79 Silent brain lesions revealed by MRI are common and are associated with impairments of cognitive function.80,81 Sporadic cases of DVT, pulmonary embolism, portal vein thrombosis, aseptic necrosis of bone, leg ulcers, retinopathy, and miscarriage have also been reported.82-86Hemostatic abnormalities, including low protein C and protein S levels, and elevated plasma concentrations of TAT, F1,2, and D-dimer complexes have been found in sickle cell patients.61,75,87-89 In addition, chronic platelet activation was indicated by the elevated plasma levels of platelet factor 4 (PF4) and β-thromboglobulin (β-TG) and the expression of P selectin and enhanced binding of annexin V to the SCD platelets.89 These markers of platelet activation, thrombin generation, and enhanced fibrinolyisis were significantly elevated in asymptomatic subjects with SCD and further increased during episodes of pain.89
Evidence for endothelial activation in SCD was provided by the elevated urinary levels of 11-dehydro-TXB2 and the 2,3-dinor-6-PGF1α74 and increased numbers of circulating microvascular endothelial cells (CD36+) overexpressing ICAM-1, VCAM-1, E selectin, P selectin, and tissue factor found in these patients.89-91 Sickle RBCs expressing anionic phospholipid surfaces, manifested by enhanced annexin V binding, have been shown to have a procoagulant effect and are thought to be significant in the pathogenesis of the thrombotic manifestations.57,58,61,90 In support of this idea, significant reductions in the rates of stroke recurrence in sickle cell patients from 46% to 90% to less than 10% have been achieved by chronic blood transfusions and maintenance of hemoglobin S levels at less than 30%.92,93 Transfusion has also been shown to greatly reduce the risk of a first stroke in children with SCD who have abnormal results on transcranial Doppler ultrasonography.94
Thrombotic manifestations were also observed in 2 knockout-transgenic mouse models of SCD.95,96 These animals, which had exclusively human sickle hemoglobin, showed all the major features of human SCD, including thrombotic infarcts and vascular occlusions in the spleen, liver, and kidneys, and these thrombotic events were already observed at a relatively young age.95 96
Other congenital hemolytic anemias also carry an increased risk for thromboembolic events.59 PNH is reported to be associated with an increased tendency for DVT and portal vein thrombosis,97,98 and frequent thrombotic episodes were reported in 9 patients with hereditary stomatocytosis. Data on abnormal hemostatic parameters in these disorders are currently not available.99
Summary and conclusions
A range of laboratory tests has provided solid evidence for the existence of a chronic hypercoagulable state in thalassemia and, particularly, in splenectomized patients with β-TI who do receive regular transfusions. Thalassemic patients have low levels of protein C and protein S, show enhanced platelet consumption, and show ongoing platelet, monocyte, granulocyte, and endothelial activation. Increased plasma levels of activation peptides, TAT, F1,2, FPA, and D-dimer, are suggestive of continuous thrombin generation and enhanced fibrinolysis.
Thrombosis is typically an episodic complication associated with a temporary activation of hemostasis. In contrast, markers of platelet and coagulation activation are persistently and consistently elevated in most thalassemic patients (adults and children alike), even in the absence of overt thromboembolic events.4 The presence of a persistent hypercoagulable state combined with the infrequent occurrence of significant thrombotic events suggests that thrombosis is largely a subclinical process in thalassemia and has been associated with autopsy findings of platelet and fibrin thrombi in the microvasculature in the lungs and the brain.20,21 These thrombi could contribute to the pulmonary hypertension, low lung capacity, hypoxemia, and diffusion defects associated with right heart failure (cor pulmonale)22-27 and to the high frequency of ischemic brain lesions associated with asymptomatic brain damage as detected by MRI.14
Several etiologic factors may play a role in the pathogenesis of the hypercoagulable state in thalassemia. The specific changes in the lipid membrane composition of the abnormal RBCs and the hemosiderosis may contribute to the activation of the coagulation process and the activation of other blood cells, including the platelets, monocytes, and granulocytes, alone or together, and may induce activation of the vascular endothelium, which further contributes to the thrombotic process. The exact order of these events is still unclear, and the murine models of congenital hemolytic anemias may shed some light on the role of these anomalies in the induction of the hypercoagulable state.71-73
Venous thrombosis is more prevalent in β-TI patients who are not receiving regular transfusions and who have undergone splenectomy. These patients may be more susceptible to thromboembolism because they have more circulating damaged RBCs and increased platelet counts. The beneficial role of regular blood transfusions is illustrated by the observation that thromboembolic manifestations are more frequently recorded in less developed countries with limited transfusion resources and ex vivo and in vitro experiments that show that normal RBCs can eliminate the abnormal aggregation observed with thalassemic RBCs.70
The addition of prophylactic antithrombotic therapy has only recently been suggested for high-risk patients with β-TI who are exposed to transient thrombotic risk factors (eg, surgery, immobilization, pregnancy).19 Thalassemia major patients who had developed an acute thrombotic event should be considered for prolonged antithrombotic therapy, as for any patients with thrombophilia, in view of their profound hemostatic anomalies. What remains to be seen is whether lifelong treatment with antithrombotic agents is indicated in patients with thalassemia to prevent any subclinical thrombosis in the lungs and brain. It is noteworthy that some thalassemic patients responded to treatment with platelet inhibitor drugs (aspirin and dipyridamole) with a rise in their arterial oxygen content.21
Dr Amiram Eldor tragically passed away on Saturday, November 24, 2001, returning home from a scientific meeting in Germany; his many contributions to hematology will be remembered, and his presence in our community will be greatly missed.—Ed
Amiram Eldor died on November 24, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eliezer A. Rachmilewitz, Department of Hematology, Edith Wolfson Medical Center, PO Box 5, Holon, Israel; e-mail:rachmilewitz@wolfson.health.gov.il.