Multiple lines of evidence indicate that thrombopoietin (TPO) substantially impacts the number of hematopoietic stem cells and progenitors of all myeloid lineages. Nevertheless, tpoknock-out mice (T−) display thrombocytopenia only; blood erythroid and neutrophil levels are normal despite 60% to 85% reductions in stem and progenitor cells. The compensatory mechanism(s) for these deficiencies remains uncertain; lineage-specific cytokines such as erythropoietin or granulocyte colony-stimulating factor (G-CSF) have been postulated but never proven to be responsible. To directly test whether G-CSF can compensate for the myeloid progenitor cell reduction in the T−model of hematopoietic deficiency, T−and G-CSF–receptor knock-out(GR−) mice were crossed, and F1 animals bred to obtain doubly nullizygous mice(T−GR−). This experiment also allowed us to test the hypothesis that G-CSF contributes to the residual platelet production in T−mice. We found that T−GR−F2 mice displayed similar blood platelet levels as that seen inT−mice, indicating that G-CSF does not account for the residual megakaryopoiesis in T−mice. However, we also noted excessive perinatal mortality ofT−GR−animals, caused by infection due to a profound and significant decrease in marrow and peripheral blood neutrophils, far greater than that seen in eitherT−or GR−mice. These data indicate that in the additional absence of GR, T−mice cannot compensate for their 62% reduction in myeloid progenitors and become profoundly neutropenic, supporting the hypothesis that G-CSF can compensate for the myeloid effects of TPO deficiency by expanding the pool of cells between the granulocyte-macrophage colony-forming unit and mature neutrophil stages of granulopoiesis.
Introduction
A major step in our understanding of the regulation of blood cell production came with the cloning and characterization of hematopoietic cytokines and their receptors. Based on their activities in various in vitro assays and administration in vivo, the lineage specificity of these glycoprotein hormones was quickly determined. At present, the hematopoietic cytokines can be broadly divided into those that primarily affect the early aspects of hematopoietic development, working on multiple cell types with multilineage potential, and a subfamily of lineage-dominant cytokines that work primarily on cells committed to a single hematopoietic lineage.1 It is also clear that the most profound hematopoietic effects in vivo are exerted by the lineage-dominant cytokines erythropoietin (EPO), granulocyte colony-stimulating factor (G-CSF), and thrombopoietin (TPO).
There is little doubt that G-CSF is the primary regulator of steady state and reactive neutrophil production.2 The administration of the cytokine to normal animals leads to profound increases in neutrophil levels, and mice in which the G-CSFor G-CSF–receptor (G-CSF–R) genes have been genetically eliminated are reported to have neutrophil counts about 25% that of littermate controls.3,4 Likewise, the use of TPO in serum-free cultures of purified marrow progenitor cells and its administration to mice, rats, dogs, monkeys, and humans has clearly established that the hormone is the primary regulator of megakaryocyte (MK) and platelet production.5,6 Subsequent genetic studies have confirmed these conclusions; elimination of either the Tpo or Tpo receptor (known as Mpl) genes results in platelet counts about 10% to 15% of normal in mice7-9 and in the case of human Mpl leads to congenital amegakaryocytic thrombocytopenia.10
Although initially felt to represent a lineage-specific cytokine for MK and platelet production, it is becoming quite clear that TPO plays a major role in hematopoietic stem cell (HSC) biology. Analysis ofTpo−/− and Mpl−/−mice reveals greatly reduced numbers of both HSCs11and progenitor cells of all myeloid lineages,8 with such mice displaying only 15% to 40% of control levels of these cell types. However, despite the profound reduction in the numbers of hematopoietic stem and progenitor cells present in such mice, their red cell, granulocyte, and monocyte counts are normal. Thus, one important unanswered question, which this study addressed, is how the organism compensates for deficiencies in primitive hematopoietic cells. An important clinical arena in which this issue arises is following stem cell transplantation in animals and humans where, despite normal levels of mature blood cells, only small numbers of HSCs are present, at least initially.
As already noted, G-CSF and G-CSF–R knock-out mice have reduced numbers of neutrophils, and Tpo andMpl nullizygous animals have reduced levels of platelets. However, these levels are approximately 15% to 25% of normal, but they are not zero. Thus, a second question to arise from analysis of hematopoietic cytokine– and cytokine receptor–deficient mice is what is responsible for the residual lineage-specific cell production. This question has already provoked much research. In an attempt to identify the cytokine(s) responsible for residual platelet production inMpl−/− mice, mice genetically deficient in other cytokine or cytokine receptors that have some effect on megakaryopoiesis have been crossed to Mpl−/−;however, the single combination of interleukin (IL)-3, IL-6–R, IL-11–R, or the combined signaling subunit of the IL-3–R, granulocyte-macrophage (GM)-CSF–R and IL-5–R (βC) andMpl failed to further reduce MK or platelet production below that seen with Mpl deficiency alone.12-15 Likewise, the genetic combination ofG-CSF with GM-CSF failed to further affect the neutropenia of G-CSF–R−/−mice.16 Although the combined elimination of theIL-6–R and G-CSF–R leads to a neutrophil count about half that seen in G-CSF–R−/− mice, it does not lead to absolute neutropenia.17 Thus, for both platelet and neutrophil production we do not have a complete understanding of all the essential components of their production. As such, the present study also sought to determine which cytokines might be responsible for the residual production of neutrophils and platelets in mice deficient in the G-CSF–R or in Tpo, respectively.
Materials and methods
Animal care
Mice made genetically deficient in Tpo were previously described18 and kindly provided by Fred de Sauvage at Genentech (South San Francisco, CA) and will be referred to as T−. Mice deficient in the G-CSF–R expression were previously described4 and generously provided by Daniel Link (Washington University, St Louis, MO) and will be referred to as GR−. Both strains had been bred onto the same genetic background (C57/Bl6) prior to their mating. Mice were housed in the specific pathogen-free (SPF) facility at the University of Washington and were maintained under normal animal husbandry conditions except that after some losses to infection the doubly nullizygous mice were administered antibiotics in the drinking water. Following mating of the 2 strains, obligate heterozygotes (F1) were bred to generate F2 mice that were assessed for wild-type and mutant alleles by polymerase chain reaction on preweaning tail DNA. The polymerase chain reaction primers utilized were GR sense 5′-GGTGAAGTAACTCAT CCAAG-3′, antisense 5′-GTAACTCCAGTCAGGGTG-3′;GRneoR sense 5′-GGTGAAGTAACTCATCCAAG-3′, antisense 5′-TTCCATTTGTCACGTCCTGC-3′; TPO sense 5′-GTCGACCCTTTGTCTATCCCT-3′, antisense 5′-GGTGAATGTAACCTGGGATAA-3′; andTPOneoR sense 5′-CTGCTGCGTGACTCCCAC-3′, antisense 5′-CTCGTGCTTTACGGTATCGC-3′. The University of Washington Animal Care Committee approved all protocols involving animals.
Hematologic analysis
Blood from the retro-orbital venous plexus was collected at weaning and in some animals at least 3 weeks prior to marrow analysis, in ethylenediaminetetraacetic acid–coated tubes and diluted 1:3 or 1:4 prior to Coulter analysis. Peripheral blood smears were prepared and stained with Wright-Giemsa and the leukocyte differential count performed by a single investigator (K.K.) on more than 100 cells. Bone marrow differentials were performed in a similar fashion except that more than 300 cell count differentials were performed. Only healthy-appearing animals were analyzed.
Hematopoietic assays
Single-cell suspensions of marrow cells from 6- to 10-week-old F2 mice of the genotypes T+GR+, T−GR+, T+GR−,and T−GR− were obtained by flushing femurs of mice following anesthesia and cervical dislocation. Assays for colony-forming unit (CFU)-GM, CFU-MK, and erythroid burst-forming unit (BFU-E) were performed in triplicate in semisolid medium as previously described using recombinant 50 ng/mL murine stem cell factor (mSCF) plus 1 ng/mL mIL-3, 50 ng/mL mSCF plus 10 ng/mL mTPO, and 100 ng/mL mSCF plus 1 U/mL human EPO, respectively, or with combinations of human G-CSF and mTPO in additional experiments.19
Neutrophil viability assay
Venous blood was collected from 5 healthy normal human volunteers (age range 25-43 years) using 0.2% K2ethylenediaminetetraacetic acid as anticoagulant. Neutrophils were isolated by sequential sedimentation in Dextran T-500 (Pharmacia LKB Biotechnology, Piscataway, NJ) in 0.9% NaCl, centrifugation in Histopaque-1077 (Sigma, St Louis, MO), and hypotonic lysis of erythrocytes, as previously described.20 The preparations contained more than 97% polymorphonuclear leukocytes, of which more than 95% were neutrophils. Cell viability immediately after isolation was more than 98% as determined by trypan blue exclusion. Neutrophil preparations were suspended in RPMI 1640 (BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal calf serum (BioWhittaker) or 0.1% bovine serum albumin, 10 mM HEPES, 0.2 mMl-glutamine, 25 U/mL penicillin, and 25 μg/mL streptomycin at a concentration of 1 × 106 cells per milliliter. Aliquots of 200 μL were incubated in the presence and absence of 10 ng/mL recombinant human TPO (a kind gift of Don Foster, ZymoGenetics, Seattle, WA) or recombinant human G-CSF (10 ng/mL; Amgen, Thousand Oaks, CA) in 96-well, flat-bottom cell culture plates (Corning Costar, Corning, NY) at 37°C in a humidified CO2incubator (5% CO2, 95% air). Cell viability was determined by an Alamar Blue–based metabolic assay according to the manufacturer's instructions (Trek Diagnostic Systems, Westlake, OH). At 0, 24, and 48 hours, 20 μL Alamar Blue reagent was added to each well and absorbance (ΔOD570 nm-600 nm) measured on an automated 96-well spectrophotometer after a defined time period for color development. All conditions were performed in duplicate. Data are expressed as the percentage of the control (time 0 hours) and reported as means ± SEMs (n = 5 independent normal donors). Statistical analysis was performed by analysis of variance (GraphPad InStat software, version 2.04a, San Diego, CA).
Statistical analysis of results
All comparisons were performed with a 2-sided Studentt test unless otherwise specified.
Results
Viability of T−GR−mice
Mice of the genotype T−GR−were generated by mating obligate heterozygousT+/−GR+/− F1 offspring ofT− and GR− mice. Of 504 pups born in 81 litters, only 13 (2.6%) were identified that were doubly homozygous at 4 weeks of age, compared with the 6.25% (1 of 16) expected according to mendelian ratios (P < .001; one sample binomial test). In addition, although initially appearing healthy, in comparison with mice of the single null phenotypes(T− and GR−), many doubly deficient mice were lost to infection soon after birth; complete blood counts revealed that compared with wild-type orGR− mice, the doubly null animals were severely neutropenic (Table 1). The use of antibiotics in the drinking water allowed us to generate sufficient mice for the experiments reported in this work. Nevertheless, the reduced viability of T−GR− mice was reflected in the mean litter size ofT−GR− × T−GR− matings: 7 such litters yielded a mean of 3.4 pups post-weaning, compared with 6.7 in doubly heterozygous F1 matings.
Hematologic analysis in T−GR−mice
From the surviving T−GR− mice and from another group of 22 doubly nullizygous mice we generated from matings of T−GR− mice, we found the neutrophil count to be 6.3% ofT+GR+, 7.7% ofT−, and 14% ofGR− mice (P < .001 for all values; Table 1). In contrast, the hematocrits were not significantly different than wild type, and the platelet counts were the same as that found in T− mice. Also of some interest, a small number of mice nullizygous for G-CSF–Rand heterozygous for TPO were analyzed; liketpo+/− mice that display platelet levels intermediate between that of wild-type and tpo-null animals,9 the neutrophil levels in miceT+/−GR−(142 × 109/L, n = 3) were intermediate between those of T+GR−(540 × 109/L) andT−GR−(75 × 109/L).
To further evaluate the pattern of hematopoiesis in the doubly nullizygous mice, in a second set of experiments we assessed peripheral blood hematocrits, neutrophil and platelet counts, marrow neutrophil frequency, and hematopoietic progenitor cell numbers in 8 matched mice of each of the 4 major genotypes in the study. As shown in Tables2 and 3, we found that although the hematocrits of all 4 groups of mice were virtually identical, the frequency of BFU-Es was significantly reduced in T−GR− mice (23%-38%;P < .001) compared with both control andGR− mice, values that were essentially the same as that found in T− mice (T−GR− BFU-Es were 69% ofT−; P = .21). This was also true if the total number of BFU-Es per femur were calculated from marrow cellularity. These data illustrate that despite substantially reduced numbers of progenitors committed to the erythroid lineage, bothT− and T−GR−mice, in which the EPO/EPO receptor system was intact, displayed normal erythrocyte levels.
Consistent with previous reports, the peripheral blood platelet count in T− mice was substantially reduced compared with that seen in wild-type mice (9% ofT+GR+; P < .001), as was the marrow frequency of CFU-MKs (26% ofT+GR+); the same was true of total femoral CFU-MKs. The additional elimination of the G-CSF–R in theT−GR− mice did not further affect these parameters; doubly nullizygous mice displayed 79% and 104% of the CFU-MK and platelet levels seen in T− mice (P = .44 and 0.71, respectively, for comparisons betweenT− and T−GR−;Tables 2 and 3).
The similarity in the developmental pattern of erythropoiesis and thrombopoiesis in T−GR+ andT−GR− mice was not true for granulopoiesis; the doubly nullizygous mice were severely neutropenic (T−GR− displayed 30%-39% pmn levels of T+GR+, T-GR+, and T+GR−mice; P < .05 for all comparisons). However, although (as previously reported) the numbers of CFU-GMs were reduced in T− mice to 49% of wild type in our analysis (P < .001), combining the T−and GR− genetic defects only slightly and nonsignificantly reduced marrow CFU-GMs;T−GR− mice displayed 38% of normal CFU-GMs (P = .16 for the comparisonT−GR+ vsT−GR−). To further explore the developmental level at which the defect inT−GR− mouse neutrophil production occurs, we sampled marrow from each of the 4 strains of mice and determined the level of myeloid maturation present; like for peripheral blood neutrophil levels, the frequency of marrow neutrophils was significantly reduced in doubly nullizygous mice compared with all 3 of the other genotypes studied (T−GR− mice displayed 32%-55% of the number of marrow neutrophils inT+GR+, T−GR+and T+GR− mice;P < .002 for all 3 comparisons). Thus, a maturation defect between the CFU-GM and marrow neutrophil stages of granulopoiesis was identified, a conclusion shared by previous studies of GR− mice.4
Marrow cell effects of TPO plus G-CSF in vitro
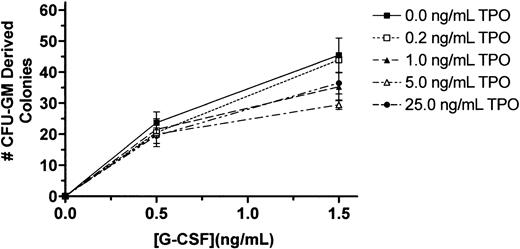
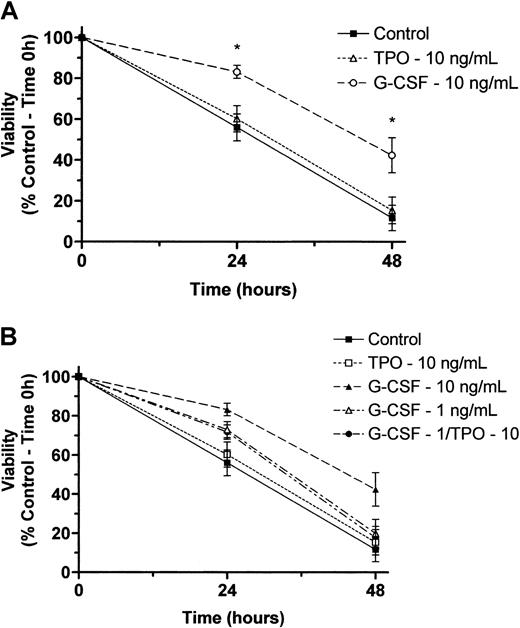
Our findings that T− mice have reduced granulocyte progenitors but normal marrow and blood neutrophil levels and that T−GR− mice have the same progenitor cell deficiency but additionally display reduced marrow and blood neutrophil levels could be explained by 1 of several possible effects of TPO on granulopoiesis: (1) The stem and progenitor cell deficiencies seen in the T−state are normally compensated for by G-CSF, resulting in preservation of normal neutrophil levels, so that in the doubly nullizygous state compensation fails and neutrophil production is reduced proportionately to the reduction in progenitor cells;(2) TPO has an effect on neutrophil maturation that acts in synergy with G-CSF; or (3) TPO acts in synergy with G-CSF to affect neutrophil survival. To distinguish among these possibilities, we tested for synergistic effects of TPO on granulocyte colony formation and survival in vitro. Despite varying cell numbers, concentrations of G-CSF, and concentrations of TPO in marrow cell colony-forming assays, we failed to detect any effect of TPO on G-CSF–induced proliferation of CFU-GMs (Figure1). A similar conclusion was reached using survival assays of peripheral blood neutrophils; we failed to see any effect of TPO on the viability of control or G-CSF–treated neutrophils regardless of the concentration of either of the 2 cytokines (Figure 2). Thus, it appears that G-CSF is required to expand the reduced numbers of CFU-GMs inT− mice to develop into a normal level of neutrophil production.
TPO fails to affect G-CSF–induced marrow cell colony formation.
Whole femoral marrow cells were obtained from BDF1 mice and plated in methylcellulose cultures containing the indicated concentrations of G-CSF and TPO; the number of CFU-GM–derived colonies on day 5 were enumerated by standard methods. The results reported are for a representative experiment of triplicate cultures; the experiment has been performed on 3 occasions.
TPO fails to affect G-CSF–induced marrow cell colony formation.
Whole femoral marrow cells were obtained from BDF1 mice and plated in methylcellulose cultures containing the indicated concentrations of G-CSF and TPO; the number of CFU-GM–derived colonies on day 5 were enumerated by standard methods. The results reported are for a representative experiment of triplicate cultures; the experiment has been performed on 3 occasions.
Effects of TPO and G-CSF on viability of normal human neutrophils maintained in culture.
(A) Neutrophils were maintained in culture at 37°C with and without recombinant human TPO (10 ng/mL) or recombinant human G-CSF (10 ng/mL) as designated above. At 0, 24, and 48 hours, cell viability was determined by an Alamar Blue–based assay as described in “Materials and methods.” Data are reported as the mean ± SEM of 5 separate experiments performed with neutrophils isolated from independent healthy donors. *A statistically significant difference in viability as compared with control cells maintained in culture for the time period indicated (P < .05). (B) Two additional experiments were performed using all combinations of TPO plus G-CSF. Attempts to demonstrate synergy between TPO and G-CSF were performed at a midoptimal dose of the latter (1 ng/mL).
Effects of TPO and G-CSF on viability of normal human neutrophils maintained in culture.
(A) Neutrophils were maintained in culture at 37°C with and without recombinant human TPO (10 ng/mL) or recombinant human G-CSF (10 ng/mL) as designated above. At 0, 24, and 48 hours, cell viability was determined by an Alamar Blue–based assay as described in “Materials and methods.” Data are reported as the mean ± SEM of 5 separate experiments performed with neutrophils isolated from independent healthy donors. *A statistically significant difference in viability as compared with control cells maintained in culture for the time period indicated (P < .05). (B) Two additional experiments were performed using all combinations of TPO plus G-CSF. Attempts to demonstrate synergy between TPO and G-CSF were performed at a midoptimal dose of the latter (1 ng/mL).
Discussion
The present study was designed to address 2 questions:(1) Are lineage-dominant cytokines responsible for homeostatic compensation of mature blood cell levels in states in which early hematopoiesis is deficient, and (2) are TPO or G-CSF responsible for the residual granulopoiesis and thrombopoiesis, respectively, in animals in which the lineage-dominant cytokine is absent. Our data indicate that G-CSF can compensate for a 62% reduction in myeloid progenitor cells and maintain neutrophil production at steady state levels but that neither G-CSF nor TPO are responsible for the residual thrombopoiesis or granulopoiesis inGR− and T− mice, respectively.
It has long been appreciated that a number of disorders of hematopoiesis are characterized, particularly in their early stages, by diminished numbers of primitive hematopoietic stem and progenitor cells but relatively preserved levels of mature blood cells. Because of our greater capacity to quantitate stem cell numbers in experimental animal models, our understanding of this discrepancy is best exemplified by several genetic defects of mice, including the W andSl mutants,21 mice given myelosuppressive or myeloablative radiation therapy,22 cats administered busulfan,23 and mice nullizygous for the Mplprotooncogene.11 In humans, children with congenital amegakaryocytic thrombocytopenia and patients soon after HSC transplantation also represent states in which stem and progenitor cell numbers are significantly reduced10 24 but all or most of the peripheral blood cell counts are normal. In such cases it has been postulated that hematopoietic cytokines compensate for the reduced number of progenitor cells by increasing the number of cell divisions each undergoes, allowing for a normal level of mature blood cell production. Several in vitro studies support this notion; for example, the number of cells that develop from an individual colony-forming cell is increased in the presence of high levels of hematopoietic cytokines. However, to our knowledge this form of physiologic compensation has never been proven to operate in animals.
The role of TPO in thrombopoiesis is well established; its administration to normal animals drives platelet production to massive levels, and its use in several settings of myelosuppression hastens platelet recovery. However, it has also been shown to play an important role in the survival and growth of primitive hematopoietic cells both in vitro and in vivo.8,11,25-27 Moreover, administration of the hormone to normal mice increased not only megakaryocytic progenitors, perhaps as expected, but also increased the number of erythroid and granulocyte-macrophage progenitors in the marrow and spleens of mice19 and humans.28 Despite this, the levels of erythrocytes and neutrophils in mice or humans administered TPO are normal; we hypothesized that the rise in progenitors is not translated into a rise in mature cells because the normal cytokine regulatory mechanisms for these lineages, EPO and G-CSF respectively, are intact in normal animals. Unfortunately, neither the knock-out nor administration studies could prove this hypothesis, because blood levels of TPO and G-CSF in mice are notoriously difficult to measure, especially in trying to determine if significant differences exist at the low levels circulating in animals with normal blood cell counts. Thus, we sought to genetically determine whether the normal neutrophil levels found in mice in which myeloid progenitors are reduced to about 40% of normal (ie, T− mice) are dependent on G-CSF. Crossing T− andGR− mice resulted in F2 animals that again exhibit about 40% of a normal level of myeloid progenitors but also display about 40% of the normal level of marrow and peripheral blood neutrophils. This result could also have been due the action of TPO on a late stage of myeloid cell development, acting much like G-CSF or IL-6, to enhance neutrophil maturation or survival. In support of this concept, Brizzi and colleagues showed that neutrophils are activated by TPO to phosphorylate signal transducers and activators of transcription-(STAT)1, release IL-8, and to prime an oxidative burst induced by the bacterial peptide f-Met-Leu-Phe.29 These data suggest that the TPO receptor may be present on mature neutrophils and thus likely also on maturing progenitor cells. Moreover, Sawai and coworkers showed that TPO augmented neutrophil production from CD34+ cells.30 However, numerous other investigators have failed to find any evidence that TPO affects granulopoiesis.31-33 Like those latter studies, TPO failed to affect G-CSF–induced neutrophil growth, maturation, or survival in vitro in our hands. Thus, because the additional genetic elimination of the G-CSF–R reduced neutrophil counts in T−mice to a level lower than that seen in eitherT− or GR− mice, a level that closely matches the reduced level of CFU-GMs inT−GR− mice, together these findings provide strong evidence that G-CSF compensates for the reduced numbers of myeloid progenitor cells in T− mice.
Although it is clear that TPO is the primary regulator of thrombopoiesis, the genetic elimination of TPO or its receptor in mice or man reduces platelet production to about 10% that in normal animals but does not eliminate it. Unfortunately, the cytokine(s) responsible for the remainder of thrombopoiesis remains uncertain despite a number of efforts to combine Mpl deficiency with that for IL-6, IL-11–R, and the cytokines that utilize the βcreceptor.12-15 A reasonable body of data exists suggesting that G-CSF may play a role in MK development, at least under certain conditions. MK precursor numbers are increased in G-CSF transgenic mice.34 The introduction of the signaling domain of the G-CSF–R into megakaryocytic progenitors by knock-in technology can lead to MK and platelet production,35 indicating that if the native receptor were expressed on MK progenitors then G-CSF could support thrombopoiesis in the absence of TPO. Of interest, G-CSF activates platelets,36 suggesting that its receptor may be expressed on MK progenitor cells. Finally, G-CSF is not strictly lineage-specific; numerous studies have shown that in combination with other early acting cytokines, G-CSF can affect other cell lineages.3 37 However, the combined elimination of theT− and GR− defects did not further reduce platelet levels below that seen inT− mice, indicating that G-CSF is not responsible for residual platelet production in animals lacking TPO or its receptor.
By combining genetic defects in the Tpo andG-CSF–R genes, we have shown that endogenous expression of a lineage-dominant cytokine, G-CSF, can compensate for the hematopoietic stem and progenitor cell deficiency characteristic ofTpo-deficient animals. However, our search failed to further account for the residual platelet production seen in Tpo-and Mpl-deficient mice and humans. These studies help to explain why human states of hematopoietic stem and progenitor cell deficiency seen after stem cell transplantation may be masked by cytokine-dependent compensatory mechanisms and perhaps provide a new opportunity to intervene in early states of marrow failure.
The authors thank Drs Thalia Papayannopoulou, Jan Abkowitz, and Grover Bagby for insightful discussions and Uyenvy Pham, Lori Cooper, and Alexis Kaushansky for excellent technical assistance.
Supported by National Institutes of Health grants R01 DK 49855, R01 CA 31615, and R01 HL 62995.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth Kaushansky, University of Washington School of Medicine, Divisions of Hematology and Infectious Diseases, Box 357710, 1959 NE Pacific St, Seattle, WA 98195; e-mail:kkaushan@u.washington.edu.