Constitutively activating internal tandem duplication (ITD) and point mutations of the receptor tyrosine kinase FLT3 are present in up to 41% of patients with acute myeloid leukemia (AML). These FLT3/ITD mutations are likely to be important because their presence is associated with a poor prognosis. Both types of mutations appear to activate the tyrosine kinase activity of FLT3. We describe here the identification and characterization of the indolocarbazole derivative CEP-701 as a FLT3 inhibitor. This drug potently and selectively inhibits autophosphorylation of wild-type and constitutively activated mutant FLT3 in vitro in FLT3/ITD-transfected cells and in human FLT3-expressing myeloid leukemia–derived cell lines. We demonstrate that CEP-701 induces a cytotoxic effect on cells in a dose-responsive fashion that parallels the inhibition of FLT3. STAT5 and ERK1/2, downstream targets of FLT3 in the signaling pathway, are inhibited in response to FLT3 inhibition. In primary leukemia blasts from AML patients harboring FLT3/ITD mutations, FLT3 is also inhibited, with an associated cytotoxic response. Finally, using a mouse model of FLT3/ITD leukemia, we demonstrate that the drug inhibits FLT3 phosphorylation in vivo and prolongs survival. These findings form the basis for a planned clinical trial of CEP-701 in patients with AML harboring FLT3- activating mutations.
Introduction
The receptor tyrosine kinase FLT3 (FMS-like tyrosine kinase 3) appears to play an important role in the development of hematopoietic stem cells, dendritic cells, B progenitor cells, and natural killer (NK) cells, as demonstrated by targeted disruption studies.1,2 In hematopoietic cells, FLT3 expression is normally restricted to the CD34+ fraction of human bone marrow and a smaller fraction of CD34− cells destined to become dendritic cells.3,4 As a member of the platelet-derived growth factor receptor (PDGFR) tyrosine kinase subfamily, FLT3 shares the structural features of KIT, FMS, and PDGFR: 5 immunoglobulinlike domains in the extracellular region, a single transmembrane sequence, and, intracellularly, a short juxtamembrane portion followed by an interrupted kinase domain.5 Its activation signals are transduced through phosphorylation of itself and of cytoplasmic proteins in biochemical signaling pathways that promote cell growth and inhibit apoptosis.6 On binding FLT3 ligand (FL), the wild-type receptor dimerizes, activating its tyrosine kinase domain with resultant autophosphorylation.7 RAS-GAP, PLC-γ, PI3-kinase, STAT5, and ERK1/2 are important signaling proteins linked to activation of FLT3.8-11
FLT3 is aberrantly expressed on the malignant cells in the majority of patients with of acute myeloid leukemia (AML).12,13 Two distinct types of FLT3 mutations have now been identified in the subset of AML cases that expresses FLT3: internal tandem duplication (ITD) mutations within the juxtamembrane region and point mutations at aspartate 835 (Asp835) within the kinase domain.14,15 Both types of mutations constitutively activate the tyrosine kinase activity of FLT3 in experimental systems.15,16 The incidence of FLT3-activating mutations in AML appears to be up to 41% (17%-34% FLT3/ITD mutations, 7% Asp835 mutations).17 Current therapy using cytotoxic agents such as cytosine arabinoside (Ara C) and daunorubicin cures approximately 30% of patients with AML, but most published studies demonstrate that the FLT3-activating mutations are associated with a distinctly worse prognosis.15,16,18,19 In one study, the cure rate with a FLT3 mutation was reported to be as low as 7% compared to a cure rate of 44% without a mutation.20 New therapies are clearly needed for the estimated 200 000 cases of AML that occur each year worldwide.21 Recent clinical successes with the BCR-ABL inhibitor STI-571 (imatinib mesylate [Gleevec]; Novartis, Basel, Switzerland) in chronic myelogenous leukemia (CML) suggest that therapies targeted to specific activated kinases may be an effective approach in certain malignancies.22 FLT3 represents a rational potential target in AML.
Materials and methods
Reagents
CEP-701, stored as a 4-mM stock solution in dimethyl sulfoxide (DMSO) at −20°C, was diluted into medium just before use. For every experiment described in this report, each sample within the experiment contained identical amounts of DMSO (< 0.25%). For in vivo mouse experiments, CEP-701 was formulated in a vehicle of 40% polyethylene glycol 1000, 10% povidone C30, and 2% benzyl alcohol in sterile water. All antibodies used were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) except for antiphospho-STAT5 and antiphospho-STAT3 (Cell Signaling, Beverly, MA), and 4G10 (Upstate Biotechnology, Lake Placid, NY). Interleukin-3 (IL-3), FL, PDGF, stem cell factor (SCF), and KIT ligand (KL) were from Peprotech (Rocky Hill, NJ). All other reagents were from Sigma (St Louis, MO). Restriction enzymes were from Invitrogen (Rockville, MD).
Cell culture
Cell culture reagents were from Invitrogen except for fetal bovine serum (FBS), which was obtained from Gemini (Woodland, CA). All cells were cultured in a humidified incubator at 37°C with 5% CO2. MV4-11 cells were maintained in Iscoves modified Dulbecco medium (IMDM) with 20% FBS. All other cells (including patient samples) were maintained in RPMI with 10% FBS. Medium was supplemented with penicillin/streptomycin andl-glutamine.
Patient samples
Bone marrow samples were collected with informed consent from patients with de novo AML as part of a Johns Hopkins institutional review board–approved protocol. Mononuclear cells were separated and stored frozen as described previously.23
Mice
Ten-week-old male Balb/c mice (Harlan, Indianapolis, IN), were maintained 5 per cage under temperature- and humidity-controlled conditions with food and water ad libitum. Animals were quarantined for 1 week prior to experimental manipulation. For harvesting splenic leukocytes, mice were killed by cervical dislocation. Immediately after death, spleens were removed, transferred to ice-cold phosphate-buffered saline (PBS), and forced through a 40-μM nylon cell strainer (Becton Dickinson, Franklin Lakes, NJ). Erythrocytes were lysed in erythrocyte lysis buffer (0.155 M NH4Cl, 0.01 M KHCO3, 0.1 M EDTA), then the remaining leukocytes were washed in PBS and lysed in detergent buffer as previously described.23
Reverse transcription–polymerase chain reaction
Total RNA was isolated using RNeasy columns (Qiagen, Valencia, CA) and reverse transcribed. The resulting complimentary DNA (cDNA) (or control lacking reverse transcriptase) was used as a template for polymerase chain reaction (PCR) with primers for the FLT3/ITD assay or Asp835 assay.14,15 PCRs for the Asp835 mutation were digested with EcoRV. Products were resolved on 2.5% agarose gels. FLT3/ITD mutations were identified as bands migrating above the expected 365-bp size of the wild-type FLT3 fragment, whereas Asp835 mutations were identified as PCR products lacking digestion at the expected EcoRV site.15
BaF3 cell constructs
Total RNA was extracted from AML patient samples and reverse transcription–PCR (RT-PCR) was performed with the one-step RT-PCR kit (Invitrogen) using the forward primer 5′-CAATTG-(MunI)-TGTCGAGCAGTACTCTAAACA-3′ and the reverse primer 5′-GCATGC-(SphI)-ATCCTAGTACCTTCCCAAACTC-3′ at 94°C, 55°C, and 72°C (1 minute each) for 40 cycles with a final extension of 10 minutes at 72°C.3 14 The PCR products were resolved on 2.5% agarose gels with ethidium bromide staining and the FLT3/ITD DNA fragments were isolated and sequenced by the dideoxy method. FLT3/ITD fragments were digested with MunI andSphI and ligated to the MunI- andSphI-digested full-length FLT3 cDNA to replace the same region in the wild-type FLT3 cDNA. These cDNAs were subcloned into the pCIneo expression vector (Promega, Madison, WI) and used for transfection. BaF3 cells were transfected by electroporation at 300 mV/960 μF (Bio-Rad, Richmond, CA). Transfected cells were cultured in IL-3–containing medium for 48 hours and then selected in 1 mg/mL Gly418 (Invitrogen) for 2 weeks. Transfected cell lines were then subcloned by limiting dilution. CMV.fms, containing the cDNA for human FMS, was a generous gift from Linzhao Cheng (Johns Hopkins University, Baltimore, MD). The cDNA of human KIT, in the vector pcDNA3.1(+)c-kit, was a gift from Robert Arceci (Johns Hopkins University). The cDNA of human PDGFR-β, in the vector pcDNA3-PDGFR-β, was a gift from Frank Bohmer (Institute for Molecular Biotechnology, Jena, Germany).
Proliferation and apoptosis assays
Cell proliferation assays using 3-4,5-dimethylthiazol-2,5-diphenyltetrazolium (MTT) and apoptosis assays with annexin V were performed as described previously.23 Briefly, for the MTT assay, 50 μL of 2 × medium was aliquoted into triplicate wells of 96-well plates for each CEP-701 concentration point. All wells contained the same concentration of DMSO. Fifty μL cell suspension in medium was added to each well (50 000 cells/well for cell lines, 200 000 cells/well for AML blasts). Plates were incubated for 48 hours (cell lines) or 72 hours (AML blasts) at 37°C, 5% CO2, then the MTT assay was performed according to the manufacturer's instructions (Roche, Indianapolis, IN). For the annexin V apoptosis assay, cells were incubated for 48 hours in medium with increasing CEP-701 concentration, then annexin V binding was performed and assessed by flow cytometry according to the manufacturer's instructions (Clontech, Palo Alto, CA).
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously.11 Briefly, cells were incubated in culture medium with CEP-701 for 1 hour at 37°C, then washed in ice-cold PBS, and lysed in detergent buffer. Cytokines, if used, were added for the last 15 minutes of incubation. 500 μg clarified lysate per sample was incubated at 4°C overnight with anti-FLT3 antibody (S18; Santa Cruz Biotechnology), then protein A agarose (Upstate Biotechnology) was added for an additional 2 hours. After electrophoresis and transfer to Immobilon membranes (Millipore, Bedford, MA), immunoblotting was performed using antiphosphotyrosine antibody 4G10 to assess phosphorylated FLT3, or with anti-FLT3 antibody to measure total FLT3. Protein bands were visualized using chemiluminescence (ECL; Amersham, Piscataway, NJ) and scanned with an AGFA Arcus 1200 laser scanner. Densitometric analysis was performed on a Macintosh G4 computer using the public domain NIH Image program (developed at the US National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/).
Results
CEP-701 is a selective inhibitor of FLT3
To study the role of FLT3/ITD mutations in leukemia and to develop a screening assay for identifying FLT3 inhibitors, we generated FLT3/ITD model cell lines. Several FLT3/ITD mutations were cloned from RNA isolated from bone marrow samples from AML patients at our institution. These FLT3/ITD mutations were used to transfect BaF3 cells, a nonleukemic IL-3–dependent pro-B cell line derived from Balb/c mice, which lacks FLT3 expression.24 The resulting cloned cell lines (BaF3/ITD) proliferated in the absence of IL-3 and expressed constitutively phosphorylated FLT3. The BaF3/ITD cells were used to screen a library of small-molecule tyrosine kinase inhibitors to identify compounds with activity against FLT3. Cells were aliquoted into 96-well plates where each well contained a 100-nM concentration of different compounds. After 24 hours of incubation at 37°C, 5% CO2 in RPMI with 10% FBS, an MTT proliferation assay was performed to identify compounds that inhibited proliferation of the BaF3/ITD cells (data not shown).23 Selective inhibition of FLT3 in BaF3/ITD cells should revert the cells to IL-3–dependent growth. Therefore, to screen for compounds that were relatively selective for FLT3, the identical compounds were assayed on duplicate plates with the addition of 1 ng/mL IL-3. Rescue of growth by IL-3 demonstrates that normal proliferative pathways are still intact.
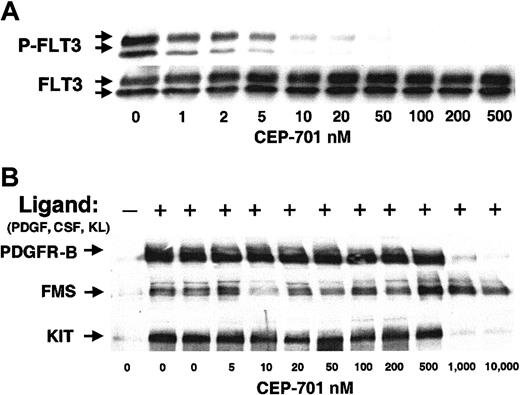
One compound that demonstrated selectivity in this assay was the indolocarbazole derivative, CEP-701. As an initial test of its potency as a FLT3 inhibitor in cell cultures, we carried out dose-response experiments in BaF3/ITD cells (Figure1A). Autophosphorylation of FLT3 is potently inhibited by CEP-701 with an inhibitory concentration of 50% (IC50) of approximately 2 nM, a result that correlates closely with in vitro kinase assays (3 nM; data not shown). In addition, we transfected BaF3 cells with FLT3 constructs containing Asp835Tyr and Asp835His point mutations. These constitutively activated FLT3 receptors were likewise inhibited by CEP-701 with a similar IC50 (data not shown). To confirm selectivity for FLT3 in the cell-based assay, we tested CEP-701 against the activity of the receptor tyrosine kinases most closely related to FLT3: PDGFR-β, FMS, and KIT (Figure 1B). In contrast to the potent inhibition of FLT3 tyrosine kinase activity, the inhibition of these receptors only occurred at CEP-701 concentrations of 500 to 1000 nM or greater. Thus, CEP-701 potently inhibits autophosphorylation of FLT3 and this inhibition is specific for FLT3 in comparison to these other receptors.
CEP-701 potently and selectively inhibits FLT3 autophosphorylation.
(A) BaF3/ITD cells were incubated with increasing concentrations of CEP-701 for 1 hour. Whole cell detergent extracts (500 μg/sample) were prepared and immunoprecipitated with antihuman FLT3 antibody, followed by separation in 8% polyacrylamide gel electrophoresis and immunoblotting with the antiphosphotyrosine antibody, 4G10 (upper row). The membrane was stripped and reprobed with anti-FLT3 antibody to demonstrate equal loading of FLT3 in each lane (lower row). (B) BaF3 cells transfected with cDNAs for human PDGFR-β, FMS, and KIT were activated with their cognate ligand (PDGF, CSF-1, or KL) and treated with increasing concentrations of CEP-701 for 1 hour. Cells were lysed and immunoprecipitated with the corresponding antibody followed by immunoblotting with antiphosphotyrosine antibody 4G10 as above.
CEP-701 potently and selectively inhibits FLT3 autophosphorylation.
(A) BaF3/ITD cells were incubated with increasing concentrations of CEP-701 for 1 hour. Whole cell detergent extracts (500 μg/sample) were prepared and immunoprecipitated with antihuman FLT3 antibody, followed by separation in 8% polyacrylamide gel electrophoresis and immunoblotting with the antiphosphotyrosine antibody, 4G10 (upper row). The membrane was stripped and reprobed with anti-FLT3 antibody to demonstrate equal loading of FLT3 in each lane (lower row). (B) BaF3 cells transfected with cDNAs for human PDGFR-β, FMS, and KIT were activated with their cognate ligand (PDGF, CSF-1, or KL) and treated with increasing concentrations of CEP-701 for 1 hour. Cells were lysed and immunoprecipitated with the corresponding antibody followed by immunoblotting with antiphosphotyrosine antibody 4G10 as above.
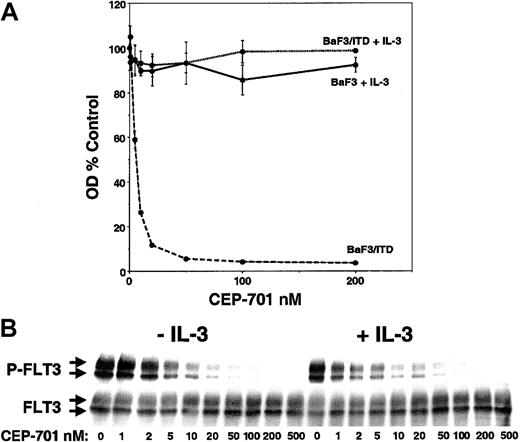
As another measure of selectivity for FLT3, the effect of CEP-701 on parental BaF3 cells and BaF3/ITD cells was examined (Figure2). CEP-701 inhibited the proliferation of BaF3/ITD cells in a dose-dependent fashion with an IC50of approximately 5 nM (Figure 2A). BaF3-derived cell lines expressing Asp835 or FLT3/ITD mutants were likewise inhibited (data not shown). In contrast to these results, parental BaF3 cells were not inhibited at concentrations as high as 200 nM. Furthermore, the addition of IL-3 rescued the growth of BaF3/ITD cells in the presence of CEP-701. In addition, the results of experiments measuring induction of apoptosis by CEP-701 showed dose-response curves similar to the MTT assay results in Figure 2A (data not shown). The immunoblot in Figure 2B shows that addition of IL-3 to the BaF3/ITD cells had no effect on FLT3 inhibition by CEP-701. Thus, IL-3 “rescue” of BaF3/ITD cells from CEP-701–induced cytotoxicity is not mediated through any effect on FLT3 phosphorylation. These results imply that CEP-701 does not interfere with IL-3–mediated signal transduction leading to proliferation and survival in BaF3 cells, but only affects FLT3-dependent signaling. Thus, CEP-701 is a relatively selective FLT3 inhibitor with cytotoxic effects on FLT3/ITD-transformed BaF3 cells.
Inhibition and IL-3 rescue of FLT3/ITD-transfected BaF3 cells treated with CEP-701.
(A) Triplicate samples of 50 000 cells were incubated in 96-well plates with increasing CEP-701 concentrations. BaF3 cells were cultured with IL-3 at 1 ng/mL (solid line), whereas BaF3/ITD cells were cultured with (dotted line) or without (dashed line) IL-3. After 48 hours of incubation, cell proliferation was assessed with the MTT assay, and the results, in the form of optical densities, were plotted as percent untreated (DMSO) control. (B) IL-3 has no effect on the inhibition of FLT3 by CEP-701. BaF3/ITD cells were incubated with CEP-701 with or without IL-3, then assayed for FLT3 phosphorylation as in Figure1A.
Inhibition and IL-3 rescue of FLT3/ITD-transfected BaF3 cells treated with CEP-701.
(A) Triplicate samples of 50 000 cells were incubated in 96-well plates with increasing CEP-701 concentrations. BaF3 cells were cultured with IL-3 at 1 ng/mL (solid line), whereas BaF3/ITD cells were cultured with (dotted line) or without (dashed line) IL-3. After 48 hours of incubation, cell proliferation was assessed with the MTT assay, and the results, in the form of optical densities, were plotted as percent untreated (DMSO) control. (B) IL-3 has no effect on the inhibition of FLT3 by CEP-701. BaF3/ITD cells were incubated with CEP-701 with or without IL-3, then assayed for FLT3 phosphorylation as in Figure1A.
CEP-701 is cytotoxic to FLT3-expressing leukemia cell lines
CEP-701 was tested on several human myeloid leukemia–derived cell lines that naturally express FLT3 (Figure3). RT-PCR was used as described to assay for the presence of FLT3 mutations in each cell line (data not shown).23 EOL-1 (eosinophilic leukemia)25 and BV173 (CML blast crisis)26 cells express wild-type FLT3 lacking any ITD or Asp835 mutation, and HEL cells (AML M6)27 completely lack FLT3 expression. MV4-11 cells (AML M5)28 express a typical FLT3/ITD mutation consisting of a 30-bp insertion at nucleotide 1857 (by RT-PCR and dideoxy sequencing; data not shown). Cell lines were assayed for the effect of CEP-701 on proliferation, apoptosis, and FLT3 phosphorylation. EOL-1 and MV4-11 cells displayed a cytotoxic dose-response to CEP-701 that closely paralleled the inhibition of FLT3 phosphorylation (Figure 3A,B). Cytotoxicity was observed once FLT3 phosphorylation was inhibited to a level less than roughly 30% of the untreated controls. BV173 cells demonstrated characteristic FLT3 inhibition in response to CEP-701, but showed no induction of apoptosis and only a modest inhibition of proliferation (Figure 3C). Thus, BV173 cells are not dependent on FLT3 signaling for survival. This may result from their expression of BCR/ABL,26 the phosphorylation of which is unaffected by 50 nM CEP-701 (data not shown). HEL cells, which lack FLT3 expression (Figure 3D), displayed no cytotoxicity to CEP-701 even at levels up to 500 nM (higher doses not shown). These data demonstrate that in some human leukemia cell lines that express FLT3, CEP-701 inhibits proliferation and induces apoptosis in a manner that parallels the inhibition of FLT3 phosphorylation.
CEP-701 inhibits FLT3 autophosphorylation, inhibits proliferation, and induces apoptosis in FLT3-expressing human myeloid leukemia–derived cell lines.
Cells were incubated with CEP-701 for 1 hour to assay for phosphorylation, or 48 hours to assay for proliferation and annexin V binding.23 Immunoprecipitation and immunoblotting were performed as in Figure 1. The results for all 3 assays for each cell line are plotted on the same graph. MTT proliferation results (solid lines) are expressed as percent untreated (DMSO) control. Annexin V assay results (dashed lines) are expressed as percent cells lacking annexin V binding (ie, as percent of nonapoptotic cells). The immunoblots are shown on the insets (upper row, phosphorylated FLT3; lower row, total FLT3), and the densitometric analysis results of the immunoblots of phosphorylated FLT3 are graphed [solid lines, labeled P-FLT3]) as percent untreated (DMSO) control. (A) EOL-1. (B) MV4-11. (C) BV173. (D) HEL (immunoblot not shown because no FLT3 protein was detected).
CEP-701 inhibits FLT3 autophosphorylation, inhibits proliferation, and induces apoptosis in FLT3-expressing human myeloid leukemia–derived cell lines.
Cells were incubated with CEP-701 for 1 hour to assay for phosphorylation, or 48 hours to assay for proliferation and annexin V binding.23 Immunoprecipitation and immunoblotting were performed as in Figure 1. The results for all 3 assays for each cell line are plotted on the same graph. MTT proliferation results (solid lines) are expressed as percent untreated (DMSO) control. Annexin V assay results (dashed lines) are expressed as percent cells lacking annexin V binding (ie, as percent of nonapoptotic cells). The immunoblots are shown on the insets (upper row, phosphorylated FLT3; lower row, total FLT3), and the densitometric analysis results of the immunoblots of phosphorylated FLT3 are graphed [solid lines, labeled P-FLT3]) as percent untreated (DMSO) control. (A) EOL-1. (B) MV4-11. (C) BV173. (D) HEL (immunoblot not shown because no FLT3 protein was detected).
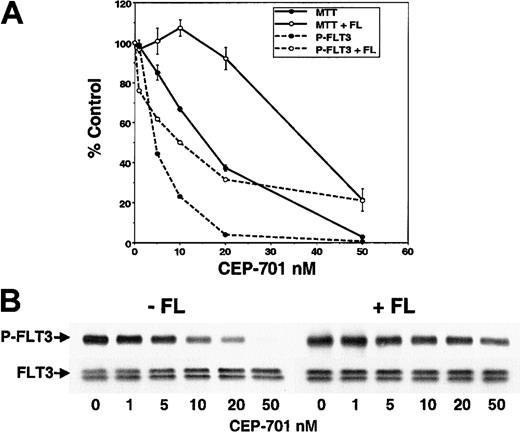
It is still possible that the cytotoxicity observed in EOL-1 and MV4-11 cells was mediated through inhibition of other unknown protein kinases. However, if these effects were due to FLT3 inhibition, then the addition of exogenous FL to the EOL-1 cells (which express wild-type FLT3 and are likely activated by endogenous expression of FL) should shift the CEP-701 dose-response curves toward the right. As predicted (Figure 4), both the cytotoxic effect and inhibition of FLT3 phosphorylation were shifted to a similar degree. This same shift was seen for apoptosis (data not shown). In the presence or absence of FL, cytotoxicity was observed in EOL-1 cells once CEP-701 inhibited FLT3 phosphorylation to below 30% of baseline (as measured by densitometry). Because FL will only stimulate FLT3, this provides further support that the cytotoxic effect of CEP-701 is mediated through direct inhibition of FLT3.
The addition of FL shifts the dose-response curve of EOL-1 cells treated with CEP-701.
(A) Graph displaying, as percent untreated control, proliferation (MTT; solid lines) and FLT3 phosphorylation (P-FLT3; dashed lines) in response to CEP-701 with (open circles) and without (solid circles) the presence of 100 ng/mL FL. Assays were performed as in Figure 3. (B) Immunoblot of phosphorylated FLT3 (P-FLT3; upper row) used for the graph in panel A. The same membrane stripped and reprobed with anti-FLT3 antibody is also shown (FLT3; lower row).
The addition of FL shifts the dose-response curve of EOL-1 cells treated with CEP-701.
(A) Graph displaying, as percent untreated control, proliferation (MTT; solid lines) and FLT3 phosphorylation (P-FLT3; dashed lines) in response to CEP-701 with (open circles) and without (solid circles) the presence of 100 ng/mL FL. Assays were performed as in Figure 3. (B) Immunoblot of phosphorylated FLT3 (P-FLT3; upper row) used for the graph in panel A. The same membrane stripped and reprobed with anti-FLT3 antibody is also shown (FLT3; lower row).
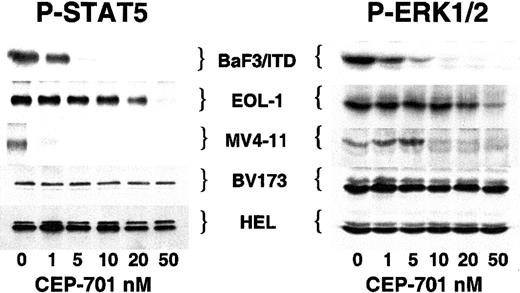
STAT5 and ERK1/2 are inhibited in response to FLT3 inhibition
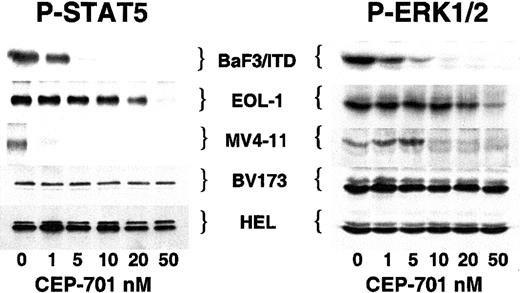
A number of proteins, including STAT5 and ERK1/2, have been identified as downstream components of the wild-type and mutationally activated FLT3 signaling pathway.9-11,29 These proteins are involved in the proliferative and antiapoptotic effects of FLT3 activation. To test whether or not activation of these effector proteins was affected by inhibiting FLT3 with CEP-701, we examined the phosphorylation status of STAT5 and ERK1/2 in each of the cell lines (Figure 5). The 3 CEP-701–responsive cell lines (BaF3/ITD, EOL-1, and MV4-11) all demonstrated a loss of ERK1/2 and STAT5 phosphorylation at increasing doses of CEP-701. In contrast, BV173 and HEL cells, which displayed minimal or no cytotoxic response to CEP-701, showed no change in STAT5 or ERK1/2 phosphorylation. This would be predicted for HEL cells, which lack FLT3 expression, and for BV173 cells, which express BCR/ABL (which results in STAT5 and ERK1/2 phosphorylation).30 31 These results further establish the relative selectivity of CEP-701 for FLT3 and illustrate a possible association between CEP-701–mediated cytotoxicity and inhibition of STAT5 and ERK1/2 activation.
CEP-701–mediated inhibition of FLT3 activity inhibits phosphorylation of STAT5 and ERK1/2.
Cells were incubated with increasing concentrations of CEP-701 prior to lysis as in Figure 1. For STAT5, 500 μg protein extract was immunoprecipitated with anti-STAT5 antibody followed by immunoblotting with antiphosphotyrosine antibody 4G10. For ERK1/2, 50 μg protein extract was loaded per lane and immunoblotting was performed with antiphospho-ERK antibody. Equal protein loading was confirmed (not shown) by stripping the blots and reprobing with anti-STAT5 and anti-ERK antibodies, respectively.
CEP-701–mediated inhibition of FLT3 activity inhibits phosphorylation of STAT5 and ERK1/2.
Cells were incubated with increasing concentrations of CEP-701 prior to lysis as in Figure 1. For STAT5, 500 μg protein extract was immunoprecipitated with anti-STAT5 antibody followed by immunoblotting with antiphosphotyrosine antibody 4G10. For ERK1/2, 50 μg protein extract was loaded per lane and immunoblotting was performed with antiphospho-ERK antibody. Equal protein loading was confirmed (not shown) by stripping the blots and reprobing with anti-STAT5 and anti-ERK antibodies, respectively.
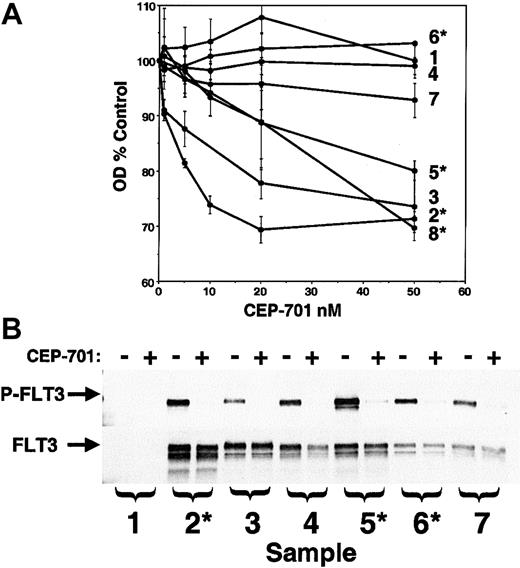
Effects of CEP-701 on primary AML samples
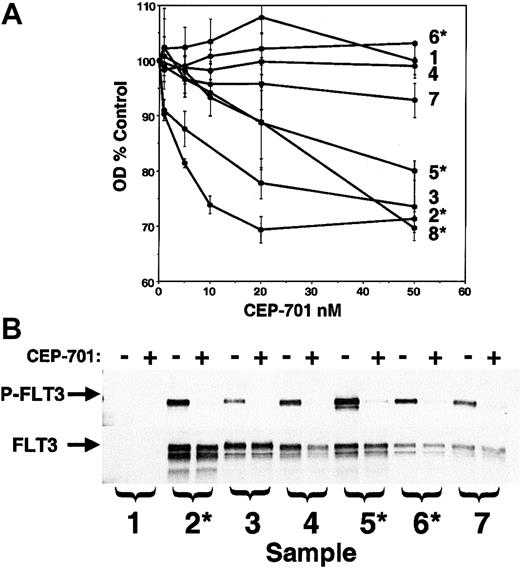
The ultimate goal of these studies is to assess the potential for use of CEP-701 in treating AML. Therefore, the next experiments were designed to study the effect of CEP-701 on primary human AML samples available from our institution's cell bank. RT-PCR analysis (data not shown; performed as described23) of the 8 samples chosen revealed that 4 of the samples expressed FLT3/ITD mutations and one contained a Asp835Tyr mutation. A CEP-701 dose-response MTT assay was performed in triplicate on all 8 samples (Figure6). Three of 4 FLT3/ITD samples displayed a cytotoxic response to CEP-701, as did a single non-ITD sample. One of the FLT3/ITD samples, along with the Asp835 mutant sample and the other 2 wild-type samples, did not respond to CEP-701. We also tested these samples for response to CEP-701 in the presence of 100 ng/mL FL and the responding samples were identical (not shown).
CEP-701 inhibits FLT3 phosphorylation and induces cytotoxicity in primary AML samples.
(A) MTT dose-response assays on patient samples performed in triplicate as described previously.23 Those samples found by RT-PCR to harbor a FLT3/ITD mutation are denoted with an asterisk. RT-PCR on sample 7 revealed the presence of an Asp835 point mutation. (B) Samples for which adequate cells were available were assayed for phosphorylated FLT3 (upper panel) by incubating them for 1 hour in the absence (−) or presence (+) of 50 nM CEP-701 prior to lysis and immunoprecipitation/immunoblotting. The blot was stripped and reprobed with anti-FLT3 antibody (lower panel) to verify equal protein loading.
CEP-701 inhibits FLT3 phosphorylation and induces cytotoxicity in primary AML samples.
(A) MTT dose-response assays on patient samples performed in triplicate as described previously.23 Those samples found by RT-PCR to harbor a FLT3/ITD mutation are denoted with an asterisk. RT-PCR on sample 7 revealed the presence of an Asp835 point mutation. (B) Samples for which adequate cells were available were assayed for phosphorylated FLT3 (upper panel) by incubating them for 1 hour in the absence (−) or presence (+) of 50 nM CEP-701 prior to lysis and immunoprecipitation/immunoblotting. The blot was stripped and reprobed with anti-FLT3 antibody (lower panel) to verify equal protein loading.
FLT3 protein was present and phosphorylated in 6 of the 7 samples analyzed (Figure 6B). CEP-701 at 50 nM completely inhibited FLT3 phosphorylation in all of these samples, including the one that harbored a Asp835 mutation. An interesting result of this experiment was the observed lack of difference in FLT3 phosphorylation levels between leukemic cells expressing wild-type and mutant FLT3. Although we have not attempted to address the mechanism of FLT3 stimulation in the nonmutant primary samples, it is possible that the activity seen here was influenced by coexpression of FL by the blasts themselves.32
From these experiments on primary AML samples, we can draw some tentative conclusions: (1) CEP-701 potently inhibits FLT3 phosphorylation in vitro in the leukemic blasts from AML patients. This includes wild-type FLT3, as well as both ITD and Asp835 mutant forms. (2) CEP-701 is selectively cytotoxic to most samples of AML blasts harboring FLT3/ITD mutations, suggesting that patients with these mutations are good candidates for this therapy. This finding is consistent with the findings of our previous study using a less selective, less potent FLT3 inhibitor.23
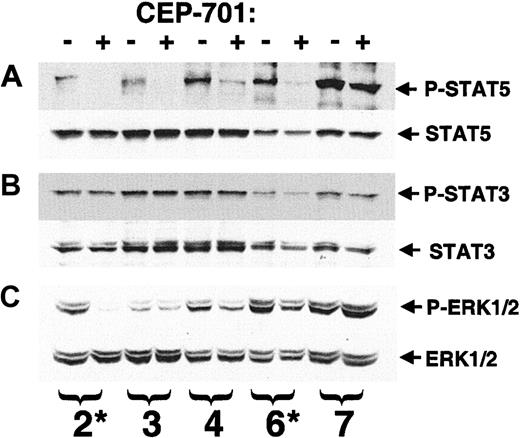
STAT5 and ERK1/2 are involved in FLT3 signal transduction and are constitutively activated in a significant fraction of AML samples, particularly in cases with FLT3/ITD mutations.29,33,34 In the leukemia cell lines we studied, a correlation was noted between CEP-701–mediated cytotoxicity and inhibition of STAT5 and ERK1/2. For 5 of the 8 primary AML samples shown in Figure 6, we had sufficient protein extract to analyze the phosphorylation status of STAT3, STAT5, and ERK1/2 by immunoblotting (Figure 7). STAT5 phosphorylation (Figure 7A) was present and inhibited by 50 nM CEP-701 in 4 of the 5 samples, including the responding samples. By comparison, STAT3 (Figure 7B), which mediates signaling from constitutively activated KIT in AML cells,35 was present in all 5 samples, but its phosphorylation was not affected. ERK1/2 phosphorylation (Figure 7C) was affected in some samples, but the degree of inhibition was less dramatic in all but a single sample. STAT5 may mediate survival signals in these blasts in a manner similar to that observed in BCR-ABL+ cells and erythroid precursors.36 37 Inhibition of STAT5 phosphorylation by CEP-701–mediated inhibition of FLT3 activation, therefore, may lead to a cytotoxic response in some blast samples.
Response of STAT5, STAT3, and ERK1/2 to CEP-701–mediated inhibition of FLT3 in primary AML blasts.
Whole cell extract (50 μg/sample) from the experiment shown in Figure6 was available for 5 of the 8 patient samples (numbering is the same as in Figure 6). Samples were electrophoresed and transferred to membrane, then the membrane was probed, stripped, and reprobed serially with the designated antibodies (A) upper, phosphorylated STAT5; lower, STAT5. (B) Upper, phosphorylated STAT3; lower, STAT3. (C) Upper, phosphorylated ERK1/2; lower- ERK1/2. Those samples found by RT-PCR to harbor a FLT3/ITD mutation are denoted with an asterisk.
Response of STAT5, STAT3, and ERK1/2 to CEP-701–mediated inhibition of FLT3 in primary AML blasts.
Whole cell extract (50 μg/sample) from the experiment shown in Figure6 was available for 5 of the 8 patient samples (numbering is the same as in Figure 6). Samples were electrophoresed and transferred to membrane, then the membrane was probed, stripped, and reprobed serially with the designated antibodies (A) upper, phosphorylated STAT5; lower, STAT5. (B) Upper, phosphorylated STAT3; lower, STAT3. (C) Upper, phosphorylated ERK1/2; lower- ERK1/2. Those samples found by RT-PCR to harbor a FLT3/ITD mutation are denoted with an asterisk.
CEP-701 inhibits FLT3 in vivo and prolongs survival in a mouse model of FLT3/ITD leukemia
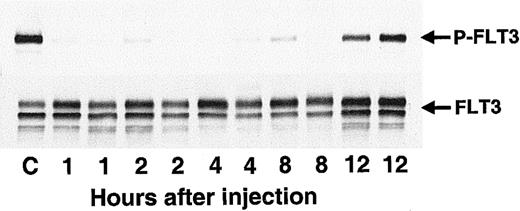
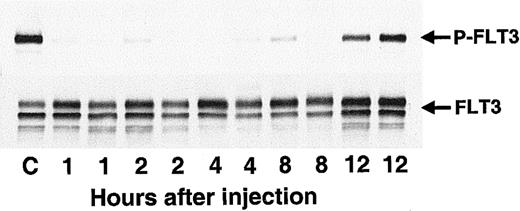
Having established CEP-701 as a potent and relatively selective FLT3 inhibitor with in vitro activity against leukemia cells, the next step was to test the compound for in vivo activity. Balb/c mice injected with cells expressing constitutively activated FLT3 provided a useful model of leukemia.11 These mice typically sicken and die, with large spleens full of leukemic cells, within 2 to 3 weeks following injection. Mice were injected with BaF3/ITD cells on day 1, and then were given a single subcutaneous dose of CEP-701 on day 12 (Figure 8). Two mice were killed at each time point following drug treatment. The spleens were harvested and the leukocytes within isolated for analysis of in vivo inhibition of FLT3 phosphorylation. In vivo inhibition of FLT3 occurred, with the effect lasting up to 8 hours after the single dose of CEP-701 (Figure 8).
CEP-701 inhibits FLT3 phosphorylation in vivo.
Ten-week-old Balb/c mice were injected with 2 × 106BaF3/ITD cells in PBS saline via tail vein on day 1. On day 12 at T = 0 hours the mice were given a subcutaneous dose of 20 mg/kg CEP-701. At 1, 2, 4, 8, and 12 hours after dosing, pairs of mice were killed, and the spleens (approximately 40-fold normal size by weight) were removed for assay. Splenic leukocytes were immediately harvested, lysed, and stored at −80°C until the next day, when immunoprecipitation with antihuman FLT3 and immunoblotting with antiphosphotyrosine antibody (500 μg/sample, upper panel) was performed. The blot was stripped and reprobed with the same anti-FLT3 antibody (lower panel). The antibody used for immunoprecipitation does not cross-react with mouse FLT3. For the control sample (“C”), the mouse was injected with vehicle only and killed 6 hours later.
CEP-701 inhibits FLT3 phosphorylation in vivo.
Ten-week-old Balb/c mice were injected with 2 × 106BaF3/ITD cells in PBS saline via tail vein on day 1. On day 12 at T = 0 hours the mice were given a subcutaneous dose of 20 mg/kg CEP-701. At 1, 2, 4, 8, and 12 hours after dosing, pairs of mice were killed, and the spleens (approximately 40-fold normal size by weight) were removed for assay. Splenic leukocytes were immediately harvested, lysed, and stored at −80°C until the next day, when immunoprecipitation with antihuman FLT3 and immunoblotting with antiphosphotyrosine antibody (500 μg/sample, upper panel) was performed. The blot was stripped and reprobed with the same anti-FLT3 antibody (lower panel). The antibody used for immunoprecipitation does not cross-react with mouse FLT3. For the control sample (“C”), the mouse was injected with vehicle only and killed 6 hours later.
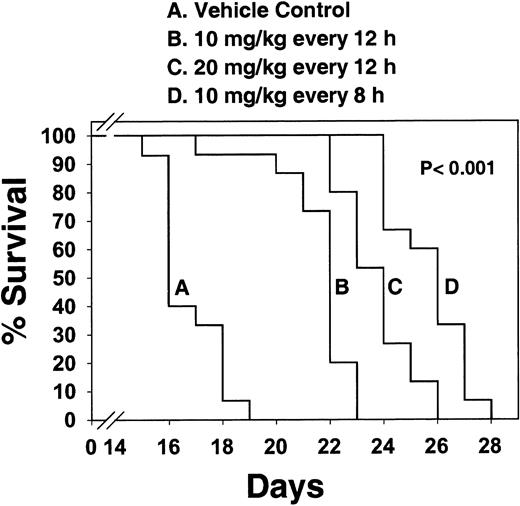
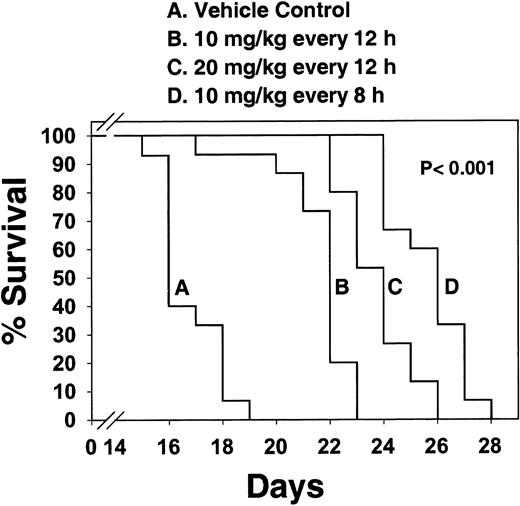
The next experiment was to see if CEP-701, administered on a daily basis, could prolong the survival of Balb/c mice injected with the BaF3/ITD leukemia cells. Based on the results from the single-dose studies shown in Figure 8, dosing intervals of 4 times per day would be optimal to maintain continuous FLT3 inhibition. However, practical considerations limited the dosing to a maximum of every 8 hours. Figure9 shows the survival curves of mice treated either with vehicle control or with increasingly intense dosing regimens. Survival was significantly prolonged (P < .001) in all 3 treated groups, with a clear dose-response effect. In a similar experiment, using EOL-1 cells injected into NOD-RAG mice, CEP-701 significantly (P < .001) prolonged the survival of treated mice (data not shown).
CEP-701 prolongs survival in Balb/c mice injected with BaF3/ITD cells.
Four groups of 10-week-old Balb/c mice (15 mice/group) were injected on day 1 with 2 × 106 BaF3/ITD cells. Subcutaneous injections of CEP-701 at 10 mg/kg every 12 hours, 20 mg/kg every 12 hours, 10 mg/kg every 8 hours, or vehicle control every 12 hours, were begun on day 1. Mice were monitored daily for survival. Moribund mice were euthanized. Percent survival is plotted on the y-axis, with days following BaF3/ITD cell injection indicated on the x-axis. Pwas calculated using a 2-tailed t test and is valid for each of the 3 treatment groups individually comparing median survival with that of the vehicle control group.
CEP-701 prolongs survival in Balb/c mice injected with BaF3/ITD cells.
Four groups of 10-week-old Balb/c mice (15 mice/group) were injected on day 1 with 2 × 106 BaF3/ITD cells. Subcutaneous injections of CEP-701 at 10 mg/kg every 12 hours, 20 mg/kg every 12 hours, 10 mg/kg every 8 hours, or vehicle control every 12 hours, were begun on day 1. Mice were monitored daily for survival. Moribund mice were euthanized. Percent survival is plotted on the y-axis, with days following BaF3/ITD cell injection indicated on the x-axis. Pwas calculated using a 2-tailed t test and is valid for each of the 3 treatment groups individually comparing median survival with that of the vehicle control group.
Discussion
In this report, we have described the characterization of CEP-701 as a FLT3 inhibitor. We have demonstrated its potency and relative selectivity for inhibiting wild-type and constitutively activated FLT3 in BaF3/ITD model cells and in myeloid leukemia–derived cell lines. CEP-701 is cytotoxic to some, but not all, FLT3-expressing cell lines. In BaF3/ITD, EOL-1, and MV4-11 cell lines, the drug was cytotoxic in a dose-responsive fashion that paralleled its inhibition of FLT3 phosphorylation. The cytotoxic effects began at approximately 5 nM and were maximal by 50 nM CEP-701. It is formally possible that the cytotoxicity observed could be mediated through inhibition of vascular endothelial growth factor receptor (VEGFR) 1 or 2, which are known to be up-regulated in AML and CML. However, the IC50 values of CEP-701 for these related receptor tyrosine kinases are 77 and 65 nM, respectively.38 Therefore, it is unlikely that the cytotoxic effects are mediated through inhibition of these VEGFRs. In BV173 cells, FLT3 phosphorylation was inhibited by CEP-701, but no cytotoxicity was observed, suggesting that these cells do not rely on FLT3 signaling for survival.
The variation in cytotoxic response to CEP-701 was also seen in the primary blasts from AML patient samples. CEP-701 inhibited the phosphorylation of wild-type, ITD mutant, and Asp835 mutant FLT3 in these primary AML blasts, and a cytotoxic response was seen in some samples with wild-type FLT3 and in most samples with FLT3/ITD mutations. We have now tested a total of 14 FLT3/ITD AML samples with FLT3 inhibitors (4 in this study, 8 in our previously published study using the tyrphostin AG1295,23 and 2 unpublished experiments, March 2001). Twelve of the 14 FLT3/ITD samples displayed a cytotoxic response, whereas a much smaller proportion of nonmutant samples responded.
The variable cytotoxic response brought about by CEP-701 raises several important issues regarding the use of FLT3 inhibitors in leukemia. It implies that FLT3 is often not the only signaling mechanism that must be inhibited to achieve a cytotoxic response. There are clearly other important signals in BV173 cells (presumably BCR/ABL) and in the nonresponsive blast samples that are unaffected by CEP-701. The inhibition of FLT3 phosphorylation probably results in the withdrawal of a strong antiapoptotic signal. However, it probably does not by itself introduce a proapoptotic stimulus. A combination of FLT3 inhibition and DNA damaging agents might well prove to be synergistic in killing leukemia cells.
The results from our animal experiments represent an important step in the preclinical development of CEP-701. In mice with FLT3/ITD leukemia, a single dose of the drug completely inhibited FLT3 phosphorylation in vivo for several hours, and dosing 2 to 3 times per day significantly prolonged survival. The lack of a complete cure of the mice by CEP-701 could be due to several factors. The in vivo phosphorylation study showed that FLT3 inhibition after a single dose only lasts up to 8 hours. Maintaining inhibition continuously (ie, 24 h/d) may be required to maximize efficacy. In addition, a number of mechanisms of resistance to tyrosine kinase inhibitors are known to exist (as has been seen with imatinib mesylate), any one of which may be operative in these experiments.39 40 Further studies will be necessary to determine which of these mechanisms might affect the efficacy of CEP-701.
CEP-701 is a derivative of the indolocarbazole K252, a microbial-derived compound.41 Other than FLT3, the only known tyrosine kinases inhibited by CEP-701 with similar potency are members of the TRK family (high-affinity nerve growth factor receptors).38 Preliminary dose finding (phase I) studies of orally administered CEP-701 have been completed (results in preparation). The drug was well tolerated by patients and there were no serious or irreversible toxicities. The pharmacokinetic data from these studies, coupled with the encouraging results demonstrating in vivo FLT3 inhibition in mice, suggest that a safe and tolerable oral dose of CEP-701 can achieve FLT3-inhibitory drug levels in the plasma of patients. The cytotoxicity displayed by leukemia cell lines and primary AML blasts in response to FLT3 inhibition lead to the prediction that CEP-701 will be an effective therapy in a significant number of patients with AML. The activity of imatinib mesylate in both the blast crisis phase of CML and Philadelphia chromosome–positive acute lymphocytic leukemia has demonstrated that a tyrosine kinase inhibitor can be an effective therapy in an acute leukemia.42 A clinical trial of CEP-701 in patients with AML is now underway.
Supported by grants from the National Cancer Institute (CA70970 to D.S., and K23 CA81262 to B.D.S.), Leukemia and Lymphoma Society (to D.S.), Children's Cancer Foundation (to D.S.), Cephalon, Inc. (to D.S.), and National Institutes of Health training grant 5T32CA09071 (to M.L.). Partial funding for the studies described in this report was provided by Cephalon, Inc.
D.S. is a paid consultant to Cephalon, Inc. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald Small, Johns Hopkins University School of Medicine, Bunting Blaustein Cancer Research Bldg, Rm 251, 1650 Orleans St, Baltimore, MD 21231-1000; e-mail: donsmall@jhmi.edu.



![Fig. 3. CEP-701 inhibits FLT3 autophosphorylation, inhibits proliferation, and induces apoptosis in FLT3-expressing human myeloid leukemia–derived cell lines. / Cells were incubated with CEP-701 for 1 hour to assay for phosphorylation, or 48 hours to assay for proliferation and annexin V binding.23 Immunoprecipitation and immunoblotting were performed as in Figure 1. The results for all 3 assays for each cell line are plotted on the same graph. MTT proliferation results (solid lines) are expressed as percent untreated (DMSO) control. Annexin V assay results (dashed lines) are expressed as percent cells lacking annexin V binding (ie, as percent of nonapoptotic cells). The immunoblots are shown on the insets (upper row, phosphorylated FLT3; lower row, total FLT3), and the densitometric analysis results of the immunoblots of phosphorylated FLT3 are graphed [solid lines, labeled P-FLT3]) as percent untreated (DMSO) control. (A) EOL-1. (B) MV4-11. (C) BV173. (D) HEL (immunoblot not shown because no FLT3 protein was detected).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/11/10.1182_blood.v99.11.3885/6/m_h81122634003.jpeg?Expires=1765896342&Signature=ncPjwYs5ZfebMdkHsSH0oHIc8bh8nhBZu~IWXdokUHdfrpDCEuQCXWhq4ZtElS3alvojhx-E24cbBEq1bC56j7M-pFiFMoUafXKdzWZJ8BdnqVkcGIWYpFg1NgYbdr3XRKhtXLdAOjaWYwnLunbLtQ~qdyjWuA7or76ADqunNtbQ6aMFDE88ibN4TNac2YPxMVDQB4ttr-JNBLEzMGhEvwTtp-6tiC0nVzqz0Fw7ns0Y93hmOkNOOHB0CCCQtd0KGJMplvKaJXw0xChGTdeL2Tf~UgRRdTFq4nMlDFDF4~FQjXZ0dvf~gd44ReFsjx45SvSwKVDcRTF5hO-0v~9aBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. CEP-701 inhibits FLT3 autophosphorylation, inhibits proliferation, and induces apoptosis in FLT3-expressing human myeloid leukemia–derived cell lines. / Cells were incubated with CEP-701 for 1 hour to assay for phosphorylation, or 48 hours to assay for proliferation and annexin V binding.23 Immunoprecipitation and immunoblotting were performed as in Figure 1. The results for all 3 assays for each cell line are plotted on the same graph. MTT proliferation results (solid lines) are expressed as percent untreated (DMSO) control. Annexin V assay results (dashed lines) are expressed as percent cells lacking annexin V binding (ie, as percent of nonapoptotic cells). The immunoblots are shown on the insets (upper row, phosphorylated FLT3; lower row, total FLT3), and the densitometric analysis results of the immunoblots of phosphorylated FLT3 are graphed [solid lines, labeled P-FLT3]) as percent untreated (DMSO) control. (A) EOL-1. (B) MV4-11. (C) BV173. (D) HEL (immunoblot not shown because no FLT3 protein was detected).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/11/10.1182_blood.v99.11.3885/6/m_h81122634003.jpeg?Expires=1766047892&Signature=Ykjjh43EyXtFaoQz7LDnZZ9rbA8VTlu1mfurEqW3IbBNBckvndZ5G6~C5Cr2JM3ORpGjjONvnd6Fb65umpmbakXsf0OyMgPlRJfDMuIKKdpmSLskND8t7Z-HYtAqagZLt-jm1rs6qc6bEq5VjegE0v0U6kRQSiRXXf4n2eGANPEpWYIGNHoIiC-D~NNfg8SDHduS51SvAlN5LwnMlCnhbXzh2mlvS1QRflShENo2cEvaSwvOV9QFlHvcVFAup9PCBxyz7kG~9yD-x11KlzyMSZg~DPXQ4ok0LXxku0IlZr35R0~jcbOA21wTnkwzidPcNGKg-kfy8gjIpFOGMsBQPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)