Introduction
Interleukin-7 (IL-7) was initially isolated more than 10 years ago.1-4 Nevertheless, the complete set of physiologic roles for this cytokine, especially those involving lymphocyte homeostasis, have only recently been elucidated. After the initial descriptions of effects on B-cell precursors, recognition that IL-7 also has marked activity on immature5-7 and mature8 T cells soon followed. Information from gene-deleted mice showed IL-7 is a nonredundant cytokine for murine T and B lymphopoiesis.9,10 Mutations in the α chain of the IL-7 receptor in patients with severe combined immunodeficiency (SCID) confirmed that IL-7 is indispensable for T-cell development in humans. However, the presence of B cells in these individuals suggests important differences between the role of IL-7 in murine and human lymphocyte development.11 IL-7 also has potent effects on mature T cells. Recent work has shown that IL-7 is a critical modulator of low-affinity peptide-induced proliferation, which is a central feature of the homeostatic regulation of T-cell populations.12,13 Furthermore, circulating levels of IL-7 increase in response to T-cell depletion, suggesting a role in T-cell regeneration.14-16 Importantly, the primary sources of IL-7 are non–marrow-derived stromal and epithelial cells. Thus, IL-7 is a pleiotropic cytokine with central roles in modulating T- and B-cell development and T-cell homeostasis. The potency and breadth of effects suggest that IL-7 administration or neutralization of IL-7 may allow the modulation of immune function in patients with lymphocyte depletion, vaccine administration, or autoimmunity.
Genetics and structure
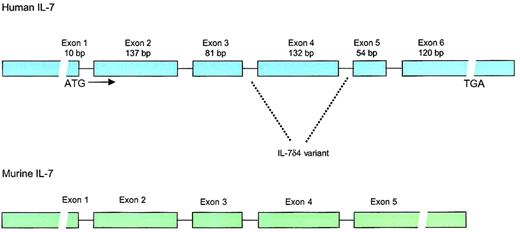
The gene for human IL-7 is located on chromosome 8q12-13,17 spans 6 exons, and has open-reading frame of 534 base pairs (177 amino acids), including a 25-amino acid signal peptide18 (Figure 1). Homology between the human and the murine IL-7 sequence is 81% in the coding regions and approximately 60% to 70% in the 5′ and 3′ noncoding regions. Although human IL-7 has activity in murine cells, murine IL-7 fails to stimulate human pre-B cells. The sequence of human IL-7 predicts a molecular weight of 17.4 kd, but glycosylation results in an active protein of 25 kd. IL-7 is classified as a type 1 short-chain cytokine of the hematopoietin family, a group that also includes IL-2, IL-3, IL-4, IL-5, granulocyte macrophage–colony-stimulating factor (GM-CSF), IL-9, IL-13, IL-15, M-CSF, and stem cell factor (SCF).
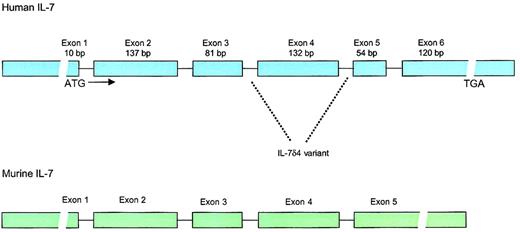
Structure of the human and murine IL-7 genes.
Human IL-7 locus consists of 6 exons and 9 introns with extensive 3′ and 5′ untranslated regions. The gene is located on chromosome 8q12-13. IL-7δ4 variant, which lacks exon 4, has been found in multiple tissues and lacks biologic activity. Whether this variant has any biologic significance is unknown. The murine IL-7 gene has approximately 80% homology to the human gene in the coding regions but lacks the 54–base pair (bp) exon 5.
Structure of the human and murine IL-7 genes.
Human IL-7 locus consists of 6 exons and 9 introns with extensive 3′ and 5′ untranslated regions. The gene is located on chromosome 8q12-13. IL-7δ4 variant, which lacks exon 4, has been found in multiple tissues and lacks biologic activity. Whether this variant has any biologic significance is unknown. The murine IL-7 gene has approximately 80% homology to the human gene in the coding regions but lacks the 54–base pair (bp) exon 5.
Sites and regulation of production
Production of IL-7 has been detected from multiple stromal tissues, including epithelial cells in thymus and bone marrow.19,20 Within the thymus, the predominant cell responsible for IL-7 production appears to be a major histocompatibility complex (MHC) class II+ epithelial cell that likely represents a cortical epithelial cell.21 Additional sites of IL-7 production include intestinal epithelium,22keratinocytes,23 fetal liver,24 adult liver,25 dendritic cells,26,27 and follicular dendritic cells.28 Importantly, IL-7 mRNA has not been detected in normal lymphocytes, though production by Epstein-Barr virus (EBV)–transformed lymphocytes has been reported.29 Thus, IL-7 is essentially a tissue-derived cytokine, with the primary sources stromal and epithelial cells in various locations, whereas bone marrow–derived dendritic cells appear to be relatively minor sources of IL-7. IL-7 has been shown to bind extensively to the extracellular matrix-associated glycosaminoglycan, heparan sulfate, and fibronectin—a feature that is likely to play an important role in the regulation of local tissue availability and IL-7–induced signaling within the microenvironment.30-32
Transforming growth factor-β (TGF-β) and IL-7 share a reciprocal relationship wherein each is capable of down-regulating the expression of the other. Indeed, the ability of TGF-β to inhibit IL-7–induced proliferation of pre-B cells was recognized soon after IL-7 was identified.33 In addition, TGF-β has been shown to down-regulate IL-7 mRNA and protein secretion from human bone marrow stromal cells.34 Interestingly, IL-7 has also been shown to down-regulate TGF-β production.35 36 Thus IL-7 shares an antagonistic relationship with TGF-β wherein TGF-β can down-regulate IL-7 production by stromal cells and IL-7 can down-regulate the production of TGF-β. Although the mechanisms and implications of this relationship are yet to be elucidated, the potency of both agents on a breadth of immune populations suggests that this represents an important level of immune regulation.
IL-7 receptor and signaling
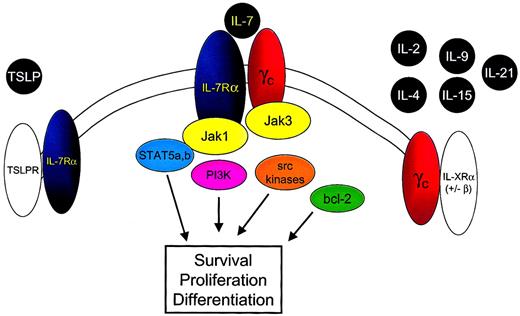
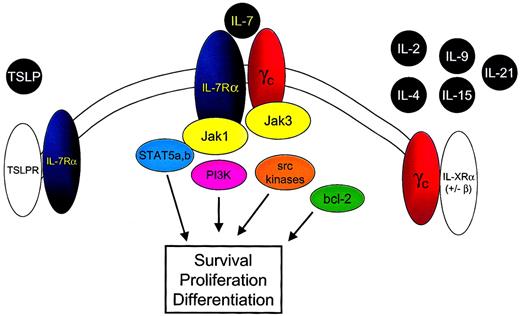
IL-7 is a member of the family of cytokines that signal through the common cytokine gamma chain (γc) (Figure2).37-39 A recent addition to this family is IL-21.40-42 IL-7 also uses a second component, the IL-7 receptor alpha chain (IL-7Rα) (CD127). Signaling through the IL-7R requires both IL-7Rα and the γc component. Because γc is expressed ubiquitously on lymphoid cells, the identification of IL-7Rα implies that IL-7 binding and subsequent signaling could occur. In general, IL-7Rα can be identified on immature B cells through the early pre-B stage, on thymocytes, and on most mature T cells with transient down-regulation upon activation.43 44
IL-7 shares the common cytokine γc with IL-2, IL-4, IL-9, IL-15, and IL-21 and the IL-7Rα chain with TSLP.
Binding of IL-7 to the IL-7Rα chain and γc leads to heterodimerization of these components and to juxtaposition of the intracellular signaling molecules Jak 3 and Jak 1. Phosphorylation of tyrosine residues within the cytoplasmic domain of the IL-7Rα chain and the Jak 1 molecule results in the activation of multiple downstream signaling pathways, including STAT5a and STAT5b, PI3-kinase (PI3K), and src kinases. In addition IL-7 signaling alters bcl-2 family member expression and localization, resulting in cell survival signals.
IL-7 shares the common cytokine γc with IL-2, IL-4, IL-9, IL-15, and IL-21 and the IL-7Rα chain with TSLP.
Binding of IL-7 to the IL-7Rα chain and γc leads to heterodimerization of these components and to juxtaposition of the intracellular signaling molecules Jak 3 and Jak 1. Phosphorylation of tyrosine residues within the cytoplasmic domain of the IL-7Rα chain and the Jak 1 molecule results in the activation of multiple downstream signaling pathways, including STAT5a and STAT5b, PI3-kinase (PI3K), and src kinases. In addition IL-7 signaling alters bcl-2 family member expression and localization, resulting in cell survival signals.
IL-7Rα is also used by thymic stromal-derived lymphopoietin (TSLP) as part of a complex that contains a second receptor chain that, thus far, appears to be used solely by TSLP.45-49 Indeed, the myriad subtle but significant differential effects observed in IL-7−/− versus IL-7Rα−/− mice may be attributed to the ablation of the effect of IL-7 alone in the IL-7−/− mice, whereas IL-7Rα−/− mice are deficient in IL-7 and in TSLP signals.
Like other members of the hematopoietin receptor family, IL-7Rα is a type 1 membrane glycoprotein folded to accommodate the binding of alpha helical cytokines. The 220–amino acid extracellular domain contains major regions of homology with other members of this family. In addition, there is a single 25–amino acid transmembrane region and a 195–amino acid cytoplasmic tail important in recruiting intracellular signaling molecules (reviewed in He and Malek50). Recruitment of kinases is required for signal transduction because the intracellular portion of IL-7Rα does not contain intrinsic tyrosine kinase activity.
IL-7 signaling involves a number of nonreceptor tyrosine kinase pathways that associate with the cytoplasmic tail of the receptor. These include the Janus kinase/signal transducer and activator of transcription (Jak/STAT) pathway, phosphatidylinositol 3-kinase (PI3-kinase), and Src family tyrosine kinases. Details of IL-7 signaling have been comprehensively reviewed elsewhere.51Of note, IL-7 shares intracellular signaling molecules with a number of other cytokines, and the exact mechanisms responsible for signaling specificity remain unclear.
In humans, mutations in γc52 and Jak353,54result in a SCID syndrome with defective T- and natural killer (NK)–cell generation similar to that observed in γc-deficient mice. Recently, patients with deficiencies in T cells, dysfunctional B cells, and normal to increased NK cells were identified as having mutations in IL-7Rα.11 Thus, whereas IL-7 is required for B-cell development in mice, it is not absolutely required in humans. It should be noted, however, that B-cell function remains severely impaired in these patients, and the mechanisms responsible for this immune dysfunction are not well understood. Table1 shows the phenotype of relevant knockout mice and corresponding human correlates from spontaneous mutations.
Based on the information available, the following model for IL-7–mediated signaling can be put forth (Figure 2). First, IL-7 binds to IL-7Rα, leading to dimerization with γc, which also has binding sites for IL-7.55 Jak3, associated with γc, phosphorylates tyrosine residues in the cytoplasmic portion of IL-7Rα, leading to recruitment of Jak1 and of STAT molecules. Although a number of cytokines use the components involved, specificity in signaling may be achieved by specific docking sites for particular STAT molecules or through the use of additional kinases as described above. It is important to point out that though the γc component is required for IL-7 signal transduction,39 this requirement appears to be based solely on the lack of intrinsic tyrosine kinase activity in IL-7Rα and the need for Jak3 to “trigger” phosphorylation of IL-7Rα–associated proteins.56Indeed, in a chimeric receptor system, an erythropoietin receptor containing Jak2 can substitute for γc-associated Jak3.57Thus, IL-7Rα appears to function as the driver of signaling once activated by dimerization with an appropriate receptor containing a trigger, which in the native setting involves γc.
B cells
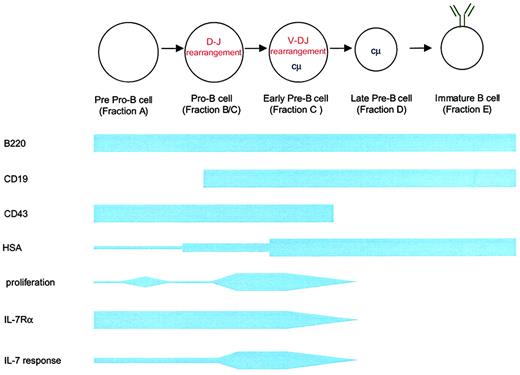
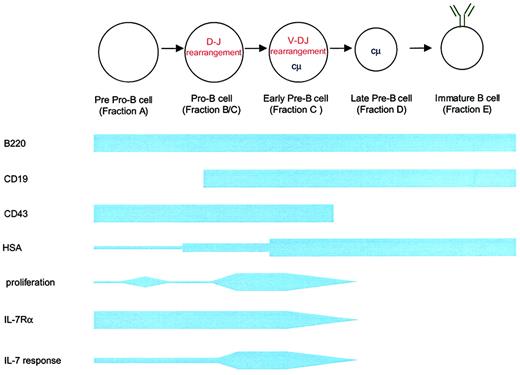
B-cell development can be divided into distinct phases in mice that can be characterized by surface phenotype (Figure3).58 IL-7 was first identified based on its capacity to induce the growth of immature B lymphocytes.1-3 The generation of IL-7–deficient10 and IL-7Rα–deficient mice9 and monoclonal antibody blocking experiments59 confirmed the requirement of IL-7 for B-cell development in mice. In IL-7−/− mice, a block in B-cell development occurs at the pro–B-cell to the pre–B-cell transition.10 Interestingly, in IL-7Rα−/−mice, the block in B-cell development occurs earlier, at the pre–pro–B-cell stage.9 This indicates the presence of a second molecule (such as TSLP) that uses IL-7Rα and regulates B-cell development at the pre–pro–B-cell stage.49 Transgenic IL-7 expression in lymphoid cells using an immunoglobulin promoter results in the dramatic expansion of immature and mature B cells.60 Mice that express IL-7 under an MHC class II promoter develop an expansion of immature B cells in the spleen, lymph nodes, and bone marrow with the eventual development of lymphoproliferative disorders bearing immature B-cell markers.61-63 Furthermore, the administration of exogenous IL-7 to normal mice leads to significant expansion of pre-B cells and mature B cells in normal and lymphocyte-depleted mice.64-66
Schematic of B-cell development in relation to IL-7Rα expression and IL-7 responsiveness.
B-cell development proceeds from a common lymphoid progenitor (not shown) that is characterized by the expression of IL-7Rα and c-kit but that lacks lineage-specific markers (eg, B220). The first identifiable progenitor committed to the B lineage is the pre–pro-B cell expressing B220 and low levels of heat stable antigen. Transition to the pro–B-cell stage involves a period of proliferation, probably in response to factors other than monomeric IL-7, and the beginning of immunoglobulin heavy chain rearrangement. Heavy chain rearrangement is completed at the early pre–B-cell stage. This stage also involves the expansion of successfully rearranged cells in response to IL-7 and other factors. By the late pre–B-cell stage, IL-7Rα expression ceases. Fractions listed correspond to those described by Hardy et al58 in the mouse.
Schematic of B-cell development in relation to IL-7Rα expression and IL-7 responsiveness.
B-cell development proceeds from a common lymphoid progenitor (not shown) that is characterized by the expression of IL-7Rα and c-kit but that lacks lineage-specific markers (eg, B220). The first identifiable progenitor committed to the B lineage is the pre–pro-B cell expressing B220 and low levels of heat stable antigen. Transition to the pro–B-cell stage involves a period of proliferation, probably in response to factors other than monomeric IL-7, and the beginning of immunoglobulin heavy chain rearrangement. Heavy chain rearrangement is completed at the early pre–B-cell stage. This stage also involves the expansion of successfully rearranged cells in response to IL-7 and other factors. By the late pre–B-cell stage, IL-7Rα expression ceases. Fractions listed correspond to those described by Hardy et al58 in the mouse.
Although there is no doubt that supraphysiologic levels of IL-7 potently expand B-cell progenitors in mice, leading to the expansion of the entire B-cell compartment, several questions remain regarding the exact physiologic role for IL-7 in regulating the proliferation, survival, and differentiation of developing B cells in normal mice. In particular, developing murine B cells show significant changes in the capacity and threshold for IL-7–induced proliferation, depending on the exact stage in B-cell development. Pre–pro-B cells display a high threshold for IL-7–induced proliferation, followed by a diminished threshold at the pro–B-cell stage with a return of a higher threshold at the pre–B-cell stage.67 Furthermore, IL-7–induced proliferation of pre-pro B cells (before immunoglobulin rearrangement) requires stromal contact,58,68 whereas IL-7 induces the proliferation of pro-B cells (D-J rearranged) in a contact-independent manner.33,69 One potential explanation for the stromal cell requirement in pre–pro-B cells was provided by the recent description of a heterodimeric “hybrid cytokine” formed by IL-7 and the β chain of hepatocyte growth factor termed pre–pro–B-cell growth-stimulating factor (PPBSF).70 PPBSF stimulates the proliferation and differentiation of pre-pro B cells in vitro, thus inducing receptivity to proliferation induced by monomeric IL-7 alone as the cells enter the pro–B-cell stage. Although a true low-affinity receptor on pre–pro-B cells has not yet been defined, it is postulated that PPBSF can signal through a low-affinity receptor on pre–pro-B cells, thus leading to the up-regulation of a high-affinity receptor and subsequent responsiveness to monomeric IL-7 on pro-B cells. In addition, TSLP may suffice for pre–pro-B cell proliferation because IL-7−/− mice generate pro-B cells whereas IL-7Rα−/− mice do not.
It was recently shown that assembly of the B-cell antigen receptor (BCR) complex regulates IL-7–induced proliferation because pro-B cells from RAG2−/− mice, which lack a pre–B-cell receptor, have an increased threshold for IL-7 responsiveness at the pro–B-cell stage and a failure to shut down IL-7 responsiveness at the pre-B cell stage.67 71 Thus, developing B cells appear to become transiently susceptible to IL-7–induced proliferative and trophic effects at the pre–B-cell stage associated with BCR rearrangement. However, subsequent to this point, tight control of IL-7–induced effects occurs by increasing the IL-7 signaling threshold in the presence of the BCR.
Whether IL-7 acts directly to induce BCR rearrangement or facilitates antigen receptor rearrangement indirectly by acting as a trophic factor that enhances the survival of cells undergoing BCR gene rearrangement has been a controversial area in B-cell development. Corcoran et al72 found impaired immunoglobulin gene rearrangements in IL-7Rα−/− mice, but D-J and V-D-J rearrangements of the heavy chain locus were detectable in IL-7−/−mice.73 However, the expression of cytoplasmic μ was reduced in IL-7−/−, γc−/−, and Jak3−/− mice, suggesting that IL-7 may be involved in cytoplasmic μ expression after rearrangement. A possible explanation for this discrepancy was that the lack of signaling of another putative factor that uses IL-7Rα was responsible for the impaired gene rearrangement in IL-7Rα−/− mice. Indeed, using an in vitro system, Corcoran et al74 demonstrated a direct role for IL-7Rα in promoting immunoglobulin gene rearrangement. By transferring mutated forms of the gene for IL-7Rα into IL-7Rα−/− mice, they identified a tyrosine residue on the IL-7Rα cytoplasmic domain that is required for PI3-kinase signaling. Mutations at this site abrogated proliferation but retained the ability to mediate immunoglobulin gene rearrangement as measured by μ protein expression. Therefore, it appears that signaling through IL-7Rα may play a mechanistic role in immunoglobulin rearrangement, and it remains possible that TSLP or another yet to be identified molecule can induce these effects in IL-7−/− mice. Furthermore, these studies demonstrated that at least 2 distinct IL-7Rα–mediated signaling pathways differentially regulate the proliferation of developing B cells and the mechanistic effects on BCR rearrangement.
In a number of cell types, including developing B cells, IL-7 can act as a trophic factor. Thus, in addition to the proliferative effect of IL-7 on developing B cells, IL-7 can maintain developing B cells by providing a survival signal. This effect appears to involve the modulation of bcl-2 family members, a group of intracellular, membrane-associated proteins that includes both proapoptotic and antiapoptotic members.75 Although this mechanism has been well established for T cells (as will be discussed later), the role of this pathway in developing B cells remains less clear. Transgenic expression of the antiapoptotic molecule bcl-2 was unable to restore B lymphopoiesis in γc−/− mice76 or IL-7Rα−/−.77 However, analysis of IL-7−/− mice indicated that the absence of IL-7 results in decreases in bcl-2, increases in bax, and increased apoptosis in developing B cells.78 Thus, the physiologic role of IL-7 as a trophic factor for developing B cells through the modulation of bcl-2 family members has not yet been determined definitively.
IL-7 synergizes with stromal-derived factor 1 (SDF-1)79and SCF80 in inducing the proliferation of developing B cells, and the combination of IL-7 and flt3 ligand induces dramatic expansions of B cells in vitro.81 Furthermore, IL-7 and flt3 ligand can support B-cell generation within the thymus.82 Thus, in the physiologic setting, it is likely that factors such as SDF-1, SCF, and flt3 ligand work in concert with IL-7 to regulate B-cell development. In summary, it appears as if the proliferative effect of IL-7 on pro- and pre-B cells is tightly regulated within the marrow environment by a complex interaction between antigen receptor assembly, responsiveness to IL-7, and action of other B-cell growth factors.
Mature B cells are generally incapable of responding to IL-7. However, recent work has demonstrated that a least a subset of peripheral B cells can become transiently IL-7 responsive. B-cell receptor antigen diversity is generated during development by immunoglobulin gene rearrangements mediated by recombinase-activating (RAG) genes. However, secondary rearrangements, termed receptor editing, can occur in IgM+IgD− immature B cells in vitro.83-85 Isotype switching, somatic hypermutation, and affinity maturation occur within germinal centers and result in modifications in antibody affinity, but because RAG expression was thought to cease in peripheral B cells, it was originally assumed that further immunoglobulin rearrangements did not occur. However, it is now known that the immunization of mice results in the re-expression ofRAG genes in B cells in lymphoid germinal centers and can result in functional V-D-J recombination in vitro and in vivo.86,87 Hikida et al88 showed that that IL-7Rα is also re-expressed in germinal center B cells and that IL-7 could induce RAG re-expression. Furthermore, the administration of an IL-7R α blocking antibody suppressed V-D-J recombination in the germinal centers of immunized mice. Therefore, IL-7 can act to restore the plasticity of B-cell antigen receptor specificity in mature B cells, implying that IL-7 may play a direct role in immunoglobulin rearrangement.
As discussed earlier, humans with SCID caused by IL-7Rα mutations show normal numbers of B cells.11 Furthermore, in an in vitro system, the generation of immature B cells from bone marrow–derived stem cells did not require the presence of IL-7.89 Nonetheless, there is abundant evidence that IL-7 can modulate B-cell development in humans. In the initial report on human IL-7, effects on human B cells derived from normal marrow were described.2 In one report, pro-B cells responded to IL-7 in the presence of stromal cells, whereas pre-B cells did not proliferate despite comparable IL-7Rα expression.90Thus, although human B-cell development does not appear to require IL-7, immature human B cells do proliferate in response to IL-7, and IL-7 treatment in mice leads to the expansion of immature B cells. Thus, it appears likely that pharmacologic doses or increased availability of endogenous IL-7 may affect B-cell generation in humans.
Developing T cells
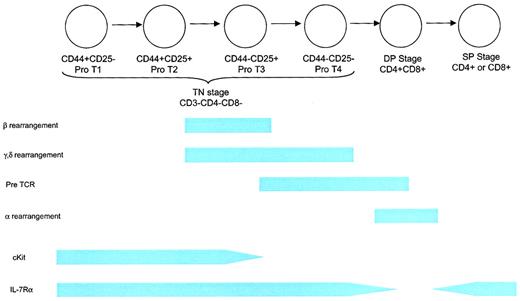
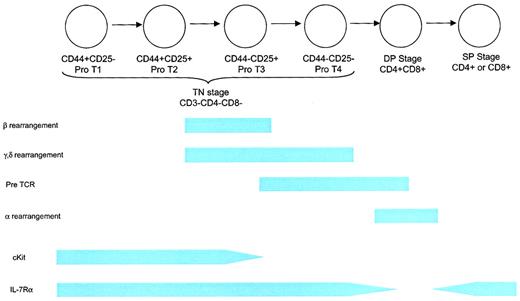
The development of T cells within the thymus proceeds through a complex series of stages (Figure 4). The first stage is represented by CD3−CD4−CD8− triple-negative (TN) immature thymocytes, followed by a CD4+CD8+ double-positive (DP) stage and, finally a CD4+ or CD8+ single-positive (SP) stage containing mature T cells. The TN, DP, and SP stages represent 5%, 80%, and 15% of the thymocyte pool, respectively. A complete description of thymic T-cell development can be found elsewhere.91 Because most (98%) T cells are lost to apoptosis during positive and negative selection, substantial numbers of T-cell progenitors are needed to generate a diverse repertoire of T-cell receptor (TCR) specificities in sufficient quantities.
Schematic of T-cell development.
The earliest T-lineage cell is the TN CD44+CD25− pro-T1 thymocyte. This cell can also give rise to B cells, NK cells, and dendritic cells. The next stage is a CD44+CD25+pro-T2 cell, which can give rise to T cells and probably dendritic cells. This stage involves proliferation in response to IL-7 and SCF. Rearrangement of the β, γ, and δ TCR chains begins at the end of this stage and is associated with diminished proliferation. The CD44−CD25+ and CD44−CD25− stages are characterized by the completion of rearrangement and the death of thymocytes that fail to undergo successful rearrangement, followed by a period of expansion. Thymus-derived γδ T cells arise from the CD44−CD25+ and possibly the CD44−CD25− stages. IL-7Rα chain is expressed throughout the TN stage, contributing to proliferation, survival, and rearrangement (at least for the δ locus) as described in more detail in “Developing T cells.” TN thymocytes comprise approximately 5% of the thymocyte fraction. Positive selection occurs during the DP stage, resulting in the death of most thymocytes and in self-MHC restriction. IL-7Rα expression is down-regulated during this stage. Rearrangement of the TCR-α component takes place during the DP stage. Clonal deletion of thymocytes expressing self-reactive TCRs begins toward the end of the DP stage and probably continues through the early SP stage. IL-7Rα chain is re-expressed at the SP stage and remains, at some level, throughout the life of a mature T cell. Eighty percent of thymocytes are DP, and 15% are SP.
Schematic of T-cell development.
The earliest T-lineage cell is the TN CD44+CD25− pro-T1 thymocyte. This cell can also give rise to B cells, NK cells, and dendritic cells. The next stage is a CD44+CD25+pro-T2 cell, which can give rise to T cells and probably dendritic cells. This stage involves proliferation in response to IL-7 and SCF. Rearrangement of the β, γ, and δ TCR chains begins at the end of this stage and is associated with diminished proliferation. The CD44−CD25+ and CD44−CD25− stages are characterized by the completion of rearrangement and the death of thymocytes that fail to undergo successful rearrangement, followed by a period of expansion. Thymus-derived γδ T cells arise from the CD44−CD25+ and possibly the CD44−CD25− stages. IL-7Rα chain is expressed throughout the TN stage, contributing to proliferation, survival, and rearrangement (at least for the δ locus) as described in more detail in “Developing T cells.” TN thymocytes comprise approximately 5% of the thymocyte fraction. Positive selection occurs during the DP stage, resulting in the death of most thymocytes and in self-MHC restriction. IL-7Rα expression is down-regulated during this stage. Rearrangement of the TCR-α component takes place during the DP stage. Clonal deletion of thymocytes expressing self-reactive TCRs begins toward the end of the DP stage and probably continues through the early SP stage. IL-7Rα chain is re-expressed at the SP stage and remains, at some level, throughout the life of a mature T cell. Eighty percent of thymocytes are DP, and 15% are SP.
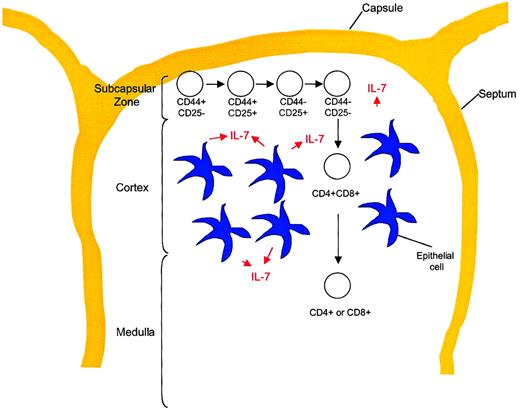
The process of T-cell development occurs within a relatively poorly understood microenvironment. Anatomically, TN precursors enter the thymus in or migrate to the subcapsular zone, then, as they mature, proceed centrally through the cortex to the medulla from which mature SP T cells emigrate to the peripheral circulation. Figure5 shows the anatomic locations within the thymus in the context of T-cell developmental stages. The supporting cells within the thymus include epithelial cells, dendritic cells, fibroblasts, and a variety of other cell types. These cells provide a network of growth factors and other molecules that are critical for T-cell development. IL-7 can be identified within the thymus of 13-day murine embryos coincident with the first wave of thymocyte expansion.19 The production of IL-7 has been identified in a subset of MHC class II+ epithelial cells21 that also express SCF, another important growth factor for early thymocytes.92 Interestingly, SCF synergizes with IL-7 in thymocyte proliferation, but it also acts at an earlier stage to up-regulate CD25 followed by IL-7– and SCF-mediated proliferation,93 suggestive of the massive proliferation of CD44+CD25+ thymocytes that is known to occur in vivo. Thus, the orderly development and selection of mature T cells occurs within the complex thymic microenvironment containing a variety of critical factors, including IL-7.
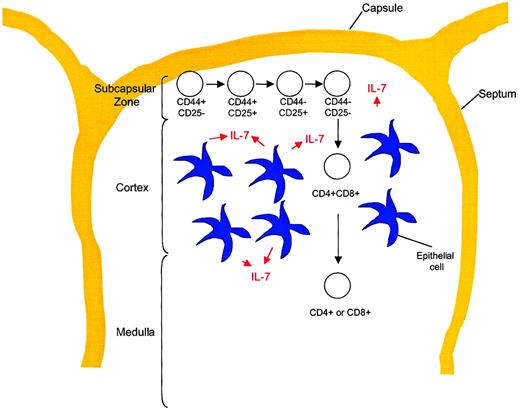
T-cell development stages in relation to thymic architecture.
Differentiation of TN thymocytes occurs within the subcapsular zone of the thymus. This region contains a network of epithelial reticular cells. At the DP stage, thymocytes migrate to the cortex, where they encounter cortical epithelial cells with long processes, fibroblasts, and macrophages. These cells are important for MHC class restriction and negative selection. Thymocytes then migrate to the medulla, where CD4 or CD8 lineage commitment occurs. This region contains medullary epithelial cells with shorter processes, dendritic cells, and macrophages. Mature T cells exit the thymus from the medullary region and enter the peripheral circulation.
T-cell development stages in relation to thymic architecture.
Differentiation of TN thymocytes occurs within the subcapsular zone of the thymus. This region contains a network of epithelial reticular cells. At the DP stage, thymocytes migrate to the cortex, where they encounter cortical epithelial cells with long processes, fibroblasts, and macrophages. These cells are important for MHC class restriction and negative selection. Thymocytes then migrate to the medulla, where CD4 or CD8 lineage commitment occurs. This region contains medullary epithelial cells with shorter processes, dendritic cells, and macrophages. Mature T cells exit the thymus from the medullary region and enter the peripheral circulation.
Soon after the identification of IL-7 as a growth factor for developing B cells, it was recognized that IL-7 also could induce the survival and proliferation of immature thymocytes in culture.94 As in B-cell development, differences in T-cell phenotype between IL-7−/− and IL-7Rα−/− mice has suggested that other molecules using the IL-7Rα chain are also important for early T-cell development. In IL-7−/− mice, thymic cellularity is decreased 20-fold. Analysis of thymocyte subsets showed a partial inhibition in TN differentiation with a relative accumulation of TN thymocytes.95 In IL-7Rα−/− mice, thymic cellularity is reduced to 0.01% to 10% of normal.9 Development of αβ T cells occurs in a subset of IL-7Rα−/− mice, but these cells do not function normally. Anti–IL-7 monoclonal antibody treatment for 12 weeks resulted in a greater than 99% decrease in thymic cellularity and an interruption before the CD44+CD25+ stage similar to that in IL-7−/− mice.96 Proportionally, there was an increase in the CD4−CD8− compartment because of an accumulation of CD3+CD4−CD8−αβ+cells.
Expression of IL-7 under an immunoglobulin κ light chain promoter resulted in increases in all mature T-cell subsets but no increase in pro-T cells.60 In a second transgenic line with an IL-7 transgene fused to an immunoglobulin heavy chain enhancer and promoter, there was a perturbation in thymic development with a profound decrease in DP thymocytes but a marked increase in mature T cells.97 Expression of the same IL-7 transgene in nude mice led to the restoration of mature T-cell numbers,98though it was not possible to distinguish between enhanced T-cell development and expansion of the small numbers of mature T cells in these mice. Finally, the expression of IL-7 under an MHC class II promoter resulted in a 30-fold increase in mature T cells, but thymic development was intact.99 Despite these conflicting reports, it appears that the overexpression of IL-7 results in increases in T-cell numbers attributed, at least in part, to increased thymic output.
The administration of IL-7 following T-cell depletion has been evaluated in murine models as a potential modulator of immune reconstitution. In a report by Abdul-Hai et al,100 IL-7 administered after syngeneic bone marrow transplantation (BMT) resulted in a 12-fold increase in thymic cellularity. In addition, RAG-1 expression and V-D-J recombination were increased in IL-7–treated animals. Bolotin et al101 showed that the administration of IL-7 after BMT resulted in a more rapid normalization in thymic cellularity and thymic subsets. Furthermore, increased numbers of thymus-derived mature T cells were seen following BMT with IL-7 treatment.102 Thus, exogenous IL-7 enhances thymopoiesis after radiation-induced lymphopenia.
The effects of IL-7 on developing thymocytes are multiple. Initial experiments using the fetal thymic organ culture system revealed that IL-7 could enhance the viability of thymocytes independent of a proliferative effect.103,104 von Freeden-Jeffry et al105 showed that bcl-2 protein is markedly decreased in CD44+CD25+ thymocytes from IL-7−/− mice, resulting in increased apoptosis. In addition, bcl-2 transgene expression in IL-7Rα−/− mice increased thymocyte numbers with substantial increases in peripheral T cells and restored mature T-cell function.77,106 The antiapoptotic effects of IL-7 also involve bax, a proapoptotic family member.107 This is further supported by the phenotype of bax-deficient mice that develop marked increases in thymocyte numbers.108 These results suggest that a substantial component of IL-7 action within the thymus involves a modulation of apoptosis through alterations in bcl-2 family members.
Thus, IL-7 maintains the survival of early thymocytes during the TN stage of development through the modulation of bcl-2 family members. In addition, in concert with other growth factors such as SCF, IL-7 contributes to the expansion of T-cell precursors. The end result is that sufficient numbers of T-cell precursors undergo TCR rearrangement before the massive cell loss that occurs during positive and negative selection. A lack of IL-7Rα signaling severely curtails this process, leading to a subsequent reduction in T-cell export.
IL-7 is absolutely critical for the development of γδ T cells. In 2 separate strains of IL-7Rα−/− mice, γδ T cells cannot be detected.109,110 Although the thymus is the predominant site of T-cell development, it has been suggested that T cells can also develop within extrathymic sites, most notably the intestine.111 Expression of an IL-7 transgene in the intestinal epithelium of IL-7−/− mice using a tissue-specific intestinal fatty acid-binding protein promoter rescued extrathymic T-cell development.112 Therefore, IL-7 plays an essential role in the generation and maintenance of thymus-derived γδ T cells and T cells derived from extrathymic pathways.
IL-7 also appears to be directly involved in the induction of TCR rearrangement. It has been difficult to definitively show whether IL-7 directly contributes to the process of gene rearrangement or simply maintains the survival of cells undergoing the rearrangement process because these effects are occur simultaneously (reviewed in113). Recently, it has been demonstrated that IL-7 regulates accessibility of the TCR γ locus by affecting histone acetylation through STAT5.114 115 Thus, IL-7Rα–mediated signals appear to be important for the rearrangement of the γ locus; however, for the other TCR loci, a mechanistic role for IL-7 in gene rearrangement is less clear.
Taken together, the information available suggests the following role for IL-7 during T-cell development in the thymus. After the migration of precursor cells to the thymic subcapsular zone, IL-7, in concert with other factors such as SCF, drives the proliferation TN precursors. The relative role of IL-7 in relation to other proliferative signals in vivo remains unclear. With the loss of CD44, these cells begin to undergo rearrangement of the TCR β, γ, and δ genes. During this phase, survival signals (bcl-2 family members) generated through IL-7Rα appear to be important. However, except for the γ chain, direct involvement of IL-7 in gene TCR rearrangement is less well established. Decreased expression of the IL-7Rα chain on DP thymocytes suggests that IL-7 may be less important at this stage. At the SP phase, IL-7Rα is re-expressed and is maintained (at least at some level) throughout the life of the T cell. The role of IL-7 in mature T cells is discussed in subsequent sections.
Two other reports are noteworthy regarding the role IL-7 plays in T-cell development. In terms of IL-7 signaling in the thymus, it was recently demonstrated that PIM1, a proto-oncogene that may be involved in pre–T-cell differentiation, partially restores thymic cellularity in IL-7−/− mice.116 Brugnera et al117 examined the role of IL-7 during the differentiation from DP to SP thymocytes, a step critical for lineage commitment because it involves the loss of expression of either the CD4 or the CD8 co-receptor. These authors suggest that IL-7 mediates the suppression of CD4 transcription in thymocytes destined to become CD8 SP cells. Thus, our understanding of the role of IL-7 in T-cell development, particularly as it relates to other developmental signals, continues to evolve and appears to extend beyond the TN stage.
IL-7 and thymic aging
Despite continued thymic T-cell development well into adulthood, there is a marked age-related decline in thymic function.118 The mechanisms underlying thymic atrophy remain unclear.119 IL-7 has been investigated in this regard because of its importance in T-cell development. Knowledge of the relative availability of IL-7 during the thymic aging process is critical to our general understanding of human T-cell development. In mice, the action of IL-7 in fetal and adult thymi may be distinct. Crompton et al120 demonstrated that the arrest in thymic maturation in IL-7Rα−/− mice was more marked in adult than in fetal thymi, suggesting different requirements for IL-7Rα signaling. Further analysis showed that IL-7Rα is required for proliferation, survival, and RAG expression of adult thymocytes. In contrast, in fetal thymocytes, IL-7Rα signals remain critical for the proliferation of thymocytes, but RAG expression and, perhaps, survival can occur in the absence of IL-7Rα signals. In a report by Aspinall,121 some strains of TCR transgenic mice did not develop age-associated thymic atrophy, suggesting that a diminished rate of TCR rearrangement plays a role in the thymic aging process. Based on the effects of IL-7 on TCR rearrangement, it was hypothesized that IL-7 deficiency may contribute to age-associated thymic involution. In recent studies, the treatment of aged mice with IL-7 led to significant increases in TN thymocytes with no appreciable change in the relative proportion of cells within in each subset.122Treatment with SCF did not result in the same effect. However, treatment of very aged mice with IL-7 does not lead to increases in thymic output (C.L.M., unpublished observations, June 1996). Furthermore, analysis of IL-7 mRNA expression in adult human thymi did not show an age-associated decline.123 Thus, though IL-7 may be able to enhance thymic function during aging, it appears unlikely that isolated IL-7 deficiency is the sole cause of thymic involution associated with aging.
IL-7 and mature T cells
Although IL-7 is best known for its effects on developing B-cell and T-cell populations, IL-7 also potently modulates mature T-cell function.124 First, IL-7 costimulates for T-cell activation by enhancing proliferation and cytokine production, especially in the setting of suboptimal TCR triggering. Although some of this effect is IL-2 dependent through the up-regulation of IL-2Rα by IL-7, murine and human studies have shown that at least some of the costimulatory effects of IL-7 are IL-2 independent.125,126Second, although IL-7 is not generally considered to play a central role in determining type 1 versus type 2 T-cell differentiation, IL-7 tends to induce type 1 immune responses because it potently up-regulates interferon-γ (IFN-γ) and IL-2 production, only weakly induces IL-4 production, and synergizes with IL-12 in inducing T-cell proliferation and IFN-γ production, in part by up-regulating the IL-12R on mature T cells.124,127,128 IL-7 also enhances expression of the chemokine receptor CXCR4, which is expressed on a subset of memory CD4+ T cells and may be important in T-cell homing to lymphoid tissues because of its binding to SDF-1.129
A third major effect of IL-7 on mature T cells is the inhibition of programmed cell death. Thus, IL-7 acts as a trophic factor for mature T cells, similar to the effects observed on developing B and T lymphocytes—partly through the up-regulation of bcl-2 family molecules130-132 and potentially through the up-regulation of the T-cell survival factor, lung Kruppel-like factor.133 Not surprisingly then, IL-7 enhances T-cell survival in long-term cell cultures, and, in some studies, IL-7 was shown to be superior to IL-2 in this regard.134 The combination of enhanced costimulation and programmed cell death inhibition by IL-7 is likely responsible for the role of IL-7 in facilitating memory T-cell differentiation in vivo. Unlike IL-15, which is absolutely required for the development of memory T-cell populations, the absence of physiologic levels of IL-7135leads to a significant reduction in the number of memory T cells generated following a primary antigenic stimulus in vivo.13 It is unknown whether supraphysiologic doses administered at the time of primary antigen exposure can actually increase the number of long-term memory cells generated in vivo, but such studies will be important in determining the potential role of IL-7 as a vaccine adjuvant.
The fourth major effect of IL-7 on mature T cells is the direct enhancement of lytic activity of classical CD8+CD3+ cytotoxic T lymphocytes (CTLs), NK lytic effectors, NKT cells,136,137 and CD4−CD8− γδ T cells.138 T cells maintained in the presence of IL-7 show enhanced antitumor and antiviral effects when adoptively transferred compared with similarly stimulated CTLs grown in the presence of IL-2 or IL-4.139,140 The mechanisms responsible for IL-7–induced increases in cytolytic activity of CD8+CD3+ CTLs are not entirely understood but likely involve the induction of pore-forming proteins and granules and the up-regulation of cytotoxic molecules such as IFN-γ.128,141 In addition, IL-7 also shows some capacity to induce lymphokine-activated killer (LAK) activity from resting NK cells, though IL-2 is more potent than IL-7 in this regard.141,142 Indeed, in vivo models suggest that immunomodulatory effects of IL-7 are largely independent of any action on NK cells.66,143 However, when IL-2 and IL-7 are used simultaneously, IL-7 potentiates the capacity of IL-2 for inducing NK/LAK cells, and cells harvested from patients treated with IL-2 show substantial increases in LAK activity following IL-7 treatment in vitro.144 Thus, although IL-7 as a single agent is a relatively weak inducer of LAK activity compared with IL-2, in the presence of IL-2, the effects of IL-7 on LAK cell induction and expansion are potentiated.
IL-7 and dendritic cells
IL-7 effects on the development and function of lymphoid populations are not limited to B cells and T cells. IL-7 also influences the development and function of dendritic cell populations and mobilizes myeloid populations.145 Indeed, IL-7 treatment of TN thymocytes induces the development of thymic dendritic cells (DCs)146 and thymic macrophages,147 and when IL-7 production is inhibited, the generation of thymic DCs is substantially reduced, suggesting that physiologic levels of IL-7 are important in the development of thymic DCs.148 Similarly, in mice, IL-7–containing cocktails are capable of generating thymic DCs from early thymic progenitors, and the use of IL-7 in such cultures precludes the requirement for GM-CSF.149 The combination of flt3-ligand and IL-7 are particularly potent at expanding early thymic progenitors and can dramatically enhance thymic B-cell numbers.82 Therefore, though it is well recognized that the potent effects of IL-7 on early thymocyte progenitors play a central role in primary T-cell development, emerging data also suggest that IL-7 may influence the development of thymic dendritic cells and potentially thymic B cells as well.
IL-7 treatment of mice leads to mobilization of hematopoietic progenitors from the marrow to the spleen, thus increasing splenic colony-forming units and diminishing megakaryocytic colony-forming units.65,150 Spleen and peripheral blood from such IL-7–mobilized mice can rescue lethally irradiated hosts, providing evidence that IL-7 is an effective hematopoietic mobilizing agent.151 It is also known that monocytes have receptors for IL-7 and, at high concentrations of IL-7 (10-100 μg/mL) monocytes, are induced to produce IL-6, tumor necrosis factor-α, and IL-1 and to become tumoricidal.152 Furthermore, if monocytes are incubated with GM-CSF and IL-7, they develop veiled processes and up-regulate co-stimulatory molecules in a manner similar to that induced by GM-CSF and IL-4,153 though GM-CSF/IL-7–generated myeloid DCs uniquely express CD21.153 Thus, IL-7 can be implicated as a co-factor for lymphoid dendritic cell development in the thymus and for monocyte dendritic cell development from myeloid precursors.
In addition to the capacity of IL-7 to induce dendritic cell development, some populations of normal dendritic cells also produce IL-7. This is particularly notable given that IL-7 is not produced by other normal cells of the hematolymphoid system but rather is produced primarily by stromal and epithelial cells. Langerhans cells do not produce IL-7,154 but both CD1a+ and CD14-derived dendritic cells, generated from cord blood, show mRNA for IL-7.26 In addition, analysis of low-density DCs isolated from human peripheral blood reveals that resting DCs do not produce IL-7 but that IL-7 is produced following overnight culture.27 Furthermore, when low DC numbers are used to stimulate antigen-specific responses in vitro, IL-7 neutralization inhibited the activation of responding T-cell populations, and IL-7 therapy has been noted to enhance dendritic cell function in vivo.132 Thus, IL-7 production by dendritic cells may contribute a co-stimulatory effect that may be of physiologic significance when other co-stimulatory molecules are limiting, such as might occur with low dendritic cell numbers. Despite evidence that marrow-derived DCs can produce IL-7, studies evaluating the role of IL-7 in the maintenance of T-cell homeostasis (detailed below) have shown that a marrow-derived source for IL-7 is not necessary for the induction of T-cell responses to low-affinity antigens following T-cell depletion, which is known to require IL-7.13 Thus, the predominant source for IL-7 appears to be non–marrow-derived populations, particularly stromal and epithelial cells. Importantly, follicular dendritic cells, which appear to be important sources for IL-7 within the lymphoid niche and which play a role in B-cell isotype switching, are not marrow derived but rather are of a distinct nonhematopoietic lineage.28 155
Modulation of immune responses in vivo with IL-7
Based on the myriad effects of IL-7 on mature T cells and antigen-presenting cell populations noted above, it is not surprising that IL-7 may serve to modulate immune responses in infectious disease or tumor models. Indeed, several investigators have shown that IL-7 is critical for in vitro expansion and maintenance of human and murine antigen-specific T-cell lines,134,139,140,156-160 and IL-7 was consistently as effective or more effective than IL-2 for maintaining these cells ex vivo. Similar effects were also observed on human cells in a unique model wherein human colon xenografts were implanted into immunodeficient mice and human allogeneic T cells were adoptively transferred with IL-7.161
In athymic T-cell–depleted hosts, the systemic administration of IL-7 dramatically enhances the number of T cells recovered following adoptive transfer.102,132 When IL-7 is administered in the context of male skin graft placement on athymic T-cell–depleted female mice, only 1% of the T-cell repertoire must be transferred to induce graft rejection, whereas 10% of the repertoire is required for graft rejection in the absence of IL-7.132 Thus, if the number of antigen-specific T cells is limiting in vivo, the addition of supraphysiologic levels of IL-7 can substantially reduce the required T-cell dose for a given effect. These effects are observed in athymic mice and require the transfer of mature T cells, thus illustrating the potent effects of IL-7 as an immune modulator independent of its effects on developing lymphocytes.
Systemic administration of IL-7 has also been used in the Renca renal cell carcinoma model in which a 75% reduction in pulmonary metastases was observed concomitantly with increases in the total body number of T cells, NK cells, B cells, and macrophages. Interestingly, this was not associated with an increase in LAK activity, suggesting that classical CTLs mediated these responses.66
The combination of IL-7–induced effects on mature T cells and the down-regulation of TGF-β production by IL-7,34-36detailed above, raises the prospect that local production of IL-7 within the tumor microenvironment might augment proliferative and cytolytic capacity of infiltrating lymphocytes, thus augmenting tumor immunity. Based on this, several investigators have used gene therapy techniques to increase local production of IL-7 within the tumor microenvironment. With this approach, Hock143,162 showed the induction of a CD4-mediated immune response that was capable of eradicating the J558L plasmacytoma, and CD8-mediated destruction of an IL-7–producing glioma163 and melanoma36 has been demonstrated. In these studies, IL-7–induced rejection led to antitumor immunity that was capable of resisting implantation of subsequent non–IL-7–transfected tumors.
To improve on these responses, costimulatory molecules have been co-transfected with IL-7 into tumor cells by Cayeaux et al.164,165 Here, the combination of B7/IL-7 transduction was shown to recruit activated CD8+CD28+CD25+ cells to the site of implanted tumors. In a murine lung cancer model, the co-administration of IL-7 gene-modified tumor and intratumoral dendritic cell injection induced superior tumor rejection compared with IL-7 gene modification alone.166 Thus, though IL-7 appears to enhance the reactivity of intratumoral lymphocytes when produced in the local tumor microenvironment, it cannot substitute for co-stimulatory molecule expression. In addition, some investigators have transduced IL-7 directly into dendritic cells with subsequent intra-tumor injection.167 Although reports discussed above have shown that some dendritic cell populations produce IL-7, this occurs generally at low levels, and antigen-presenting cell capacity can be enhanced by high-level production. Indeed, in a murine lung cancer model, the transduction of dendritic cells with B7- and IL-7–induced tumor regression, enhanced dendritic cell trafficking to the lymph nodes and spleen, and led to systemic protection to rechallenge.168
In summary, the myriad effects of IL-7 on mature T cells and the down-regulation of TGF-β induced by IL-7 make IL-7 an attractive molecule for inducing local tumor immunity. Figure6 illustrates the various mechanisms by which IL-7 may regulate antitumor immune responses. Gene therapy techniques that can induce IL-7 production in the tumor microenvironment in the presence of co-stimulatory molecules may be particularly potent at inducing antitumor immune responses. Thus far, all these studies have been performed in mouse models; future studies are indicated to determine whether these promising results can be translated for use in humans.
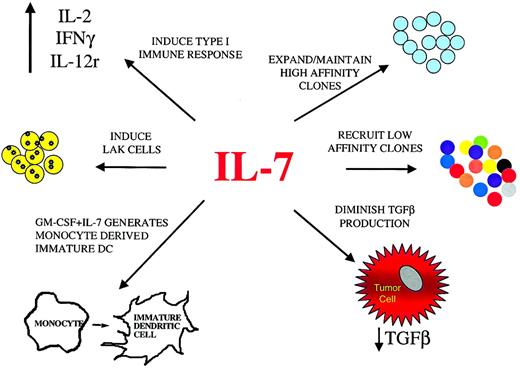
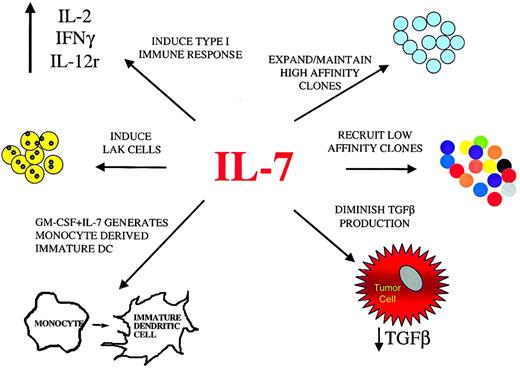
Potential effects of IL-7 on antitumor immune responses.
IL-7, administered systemically or as a local vaccine adjuvant, can potentially enhance immune responses against tumor through a variety of mechanisms. In addition to the expansion and maintenance of T cells expressing TCRs with high affinity for tumor antigens, IL-7 may also recruit low-affinity T cells clones, potentially broadening the immune response. This may have important implications for the control of tumor variants that lose expression of particular antigens. Enhanced generation of mature monocyte-derived dendritic cells by IL-7 combined with other factors, such as GM-CSF, is another mechanism through which IL-7 may enhance an antitumor immune response. Furthermore, IL-7, along with other cytokines, may contribute to the induction of a type 1 immune response and LAK cells. Finally, by diminishing TGF-β production, IL-7 can potentially down-regulate one mechanism through which tumors suppress local immune responses.
Potential effects of IL-7 on antitumor immune responses.
IL-7, administered systemically or as a local vaccine adjuvant, can potentially enhance immune responses against tumor through a variety of mechanisms. In addition to the expansion and maintenance of T cells expressing TCRs with high affinity for tumor antigens, IL-7 may also recruit low-affinity T cells clones, potentially broadening the immune response. This may have important implications for the control of tumor variants that lose expression of particular antigens. Enhanced generation of mature monocyte-derived dendritic cells by IL-7 combined with other factors, such as GM-CSF, is another mechanism through which IL-7 may enhance an antitumor immune response. Furthermore, IL-7, along with other cytokines, may contribute to the induction of a type 1 immune response and LAK cells. Finally, by diminishing TGF-β production, IL-7 can potentially down-regulate one mechanism through which tumors suppress local immune responses.
IL-7 as a regulator of T-cell homeostasis
Physiologic changes in T-cell–depleted hosts
Following T-cell depletion, physiologic changes in the immune milieu are invoked that exaggerate the peripheral mechanisms of T-cell homeostatic regulation as a means toward the restoration of T-cell numbers. Perhaps the most obvious evidence for altered immunobiology in T-cell depletion is the well-known observation that T-cell–depleted (TCD) hosts engraft and expand adoptively transferred T cells to a greater extent than do T-cell–replete hosts,169-171 a process that has been variably termed peripheral expansion and peripheral homeostatic expansion. This peripheral homeostatic expansion of mature T cells is primarily responsible for the restoration of T-cell homeostasis following T-cell depletion in athymic mice172 and in many patients with T-cell depletion.173-175
Recent work has shown that antigen drives peripheral homeostatic expansion, both in TCD and in T-cell–replete hosts.176,177 When high affinity or cognate antigen is supplied, responses are greatly exaggerated in TCD hosts compared to those observed in T-cell–replete hosts. However, TCD hosts also expand T cells in response to low-affinity antigens or self-antigens.12,178 Although the degree of expansion induced by low-affinity peptide is less than that induced by high-affinity peptide, it appears that proliferation to low-affinity antigens plays an important role in maintaining TCR repertoire diversity in T-cell depletion. When IL-7 is unavailable following T-cell depletion, proliferation and survival of T cells in response to low-affinity antigens is absent.12,13 Thus, IL-7 is required for the proliferation of T cells with TCRs specific for low-affinity antigens, which occurs in TCD hosts (reviewed in179).
IL-7 administration increases the rate of T-cell immune reconstitution after bone marrow transplantation or cytotoxic chemotherapy in mice.64,150,180 Part of this effect reflects the capacity of IL-7 for increasing thymopoiesis,101,102 but IL-7 also potently enhances the thymic-independent peripheral expansion of mature T cells following T-cell depletion.132 This is because of the combined effect of IL-7 in enhancing antigen-driven expansion of high-affinity clones and low-affinity clones in TCD mice treated with IL-7.102 Indeed IL-7 was the only cytokine tested capable of increasing homeostatic peripheral expansion in TCD hosts, whereas IL-2, IL-3, IL-6, and IL-12 were not active in this regard. IL-7 is also capable of inducing naive T-cell cycling, thus preserving T-cell repertoire diversity in the absence of cognate antigen.181,182 Furthermore, IL-7 therapy enhanced immune competence in TCD hosts, rendering them able to reject minor histocompatibility antigen-mismatched skin grafts despite profound T-cell depletion.132
In summary, IL-7 potently modulates T-cell immune reconstitution through the combined effects of increasing thymic output and enhancing homeostatic peripheral T cell expansion (Figure7). Furthermore, IL-7 is absolutely required for the recruitment of low-affinity ligands for peripheral homeostatic expansion, and supraphysiologic IL-7 levels enhance the magnitude of T-cell expansion to high-affinity, or cognate, antigens and to low-affinity, or self, peptides.
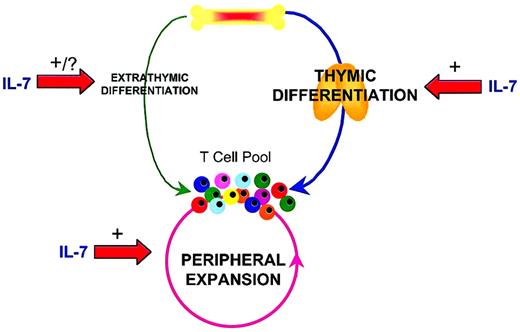
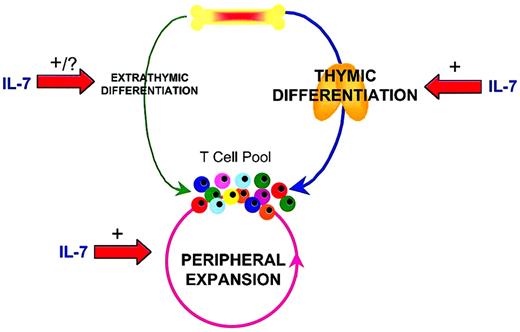
Modulation of T-cell regenerative pathways by IL-7.
Following T-cell depletion, regeneration of the peripheral T-cell pool can occur through multiple mechanisms. Thymic differentiation is the predominant pathway through which new T cells are generated if thymic capacity is sufficient. However, with diminished thymic function related to therapy-related toxicity, disease, or age related declines, the peripheral expansion of remaining mature T cells can substantially regenerate the T-cell pool. Extrathymic differentiation from bone marrow progenitors is a relatively minor pathway through which new T cells develop. IL-7 can profoundly increase thymic differentiation, peripheral expansion and, potentially, extrathymic differentiation pathways to T-cell regeneration.
Modulation of T-cell regenerative pathways by IL-7.
Following T-cell depletion, regeneration of the peripheral T-cell pool can occur through multiple mechanisms. Thymic differentiation is the predominant pathway through which new T cells are generated if thymic capacity is sufficient. However, with diminished thymic function related to therapy-related toxicity, disease, or age related declines, the peripheral expansion of remaining mature T cells can substantially regenerate the T-cell pool. Extrathymic differentiation from bone marrow progenitors is a relatively minor pathway through which new T cells develop. IL-7 can profoundly increase thymic differentiation, peripheral expansion and, potentially, extrathymic differentiation pathways to T-cell regeneration.
IL-7 elevations in patients with T-cell depletion
Studies of circulating IL-7 in TCD populations were initially performed by Bolotin et al,14 who found substantial elevations in circulating IL-7 in children after allogeneic BMT. Follow-up studies in multiple clinical cohorts with T-cell depletion have shown profound inverse relationships between circulating IL-7, as measured by a high-sensitivity enzyme-linked immunosorbent assay, and peripheral CD4 T-cell numbers in children and adults with T-cell depletion.15,16 In human immunodeficiency virus infection, elevated IL-7 levels decline as CD4 recovery occurs following effective antiviral therapy. In one cohort of patients with moderate total CD4 depletion but with more substantial depletion of the naive subset of CD4 cells, strong inverse correlations between circulating IL-7 and CD62L+CD45RA+CD4+ cells were observed that gradually disappeared with the recovery of the naive subset.15 Thus, isolated depletion of either total CD4+ T cells or the CD4+ naive subset appears capable of driving elevations in IL-7 levels. The strongest correlation exists between circulating IL-7 levels and CD4 counts, with weaker correlations between IL-7 and CD8+ T cells and B cells. It remains unknown whether isolated CD8 depletion might also be sufficient to raise circulating IL-7 levels. Similar relationships were not observed between circulating lymphocyte counts and IL-2, IL-4, IL-6, IL-12, or IL-15, suggesting that the relationship with IL-7 was unique.15
Inverse relationships were also observed in children and young adults treated with cytotoxic chemotherapy for cancer in whom circulating IL-7 increases following chemotherapy induced CD4 depletion and IL-7 levels returned to baseline following CD4+ recovery after the completion of therapy.15 In patients with idiopathic CD4 lymphopenia, which comprises a heterogeneous group of patients with CD4 depletion of uncertain etiology,183 less significant relationships between CD4 counts and circulating IL-7 levels were observed. Here it was observed that, compared with other patients studied, a subset of patients had inappropriately low levels of circulating IL-7 for the degree of CD4 depletion present, suggesting that low IL-7 levels contribute to the development of CD4 lymphopenia.15 Recently, in HIV infection, subsets of patients with unexpectedly low levels of circulating IL-7 for the degree of CD4 depletion have also been identified as having diminished capacity to restore peripheral CD4+ T-cell numbers following effective antiviral therapy.184 Further studies are necessary to confirm whether low IL-7 levels in the face of CD4 depletion correlate with diminished capacity for immune reconstitution and to identify the reason for low IL-7 levels in some patients with CD4 depletion.
Much work remains to be done to determine the mechanisms responsible for increasing circulating IL-7 levels in TCD hosts. It is not unknown whether IL-7 levels increase because of diminished adsorption by the reduced number of cells expressing IL-7R as a result of TCD, whether this reflects an increase in the production of IL-7, or both. Regardless of the mechanism, the physiologic effects of chronic IL-7 elevation in T-cell depletion are likely to be substantial.
In summary, recent studies have shown that IL-7, through its potent effects on mature T cells, plays a central role in modulating peripheral T-cell expansion in states of T-cell depletion. In the emerging model, T-cell depletion results in increased levels of stromally produced IL-7, which leads to increased T-cell proliferation in response high-affinity and low-affinity antigens. As a result, TCD hosts show increased peripheral homeostatic expansion that not only enhances immune competence to antigens encountered during this time period but potentially maintains a relatively diverse repertoire by limiting the contraction that would occur if responsiveness was limited to only high-affinity antigens. This potential capacity of IL-7 to break tolerance to low-affinity antigens might predispose to autoimmunity or lymphoproliferation in some patients, but it might also prove to be exploitable in the context of vaccine trials for cancer and other diseases.
Because of space limitations, we have tried to focus predominantly on more recent developments. Thus, we thank the many investigators who performed important work but who were not referenced. We thank Scott Durum for his careful review of the manuscript and his helpful suggestions. The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government.
References
Author notes
Terry J. Fry, Immunology Section, Pediatric Branch, National Cancer Institute, National Institutes of Health, Bldg 10, Rm 13N240, MSC 1928, 10 Center Dr, Bethesda, MD, 20892-1928; e-mail: tf60y@nih.gov.The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.