The protective effect of von Willebrand factor (VWF) toward activated protein C (APC)–catalyzed inactivation of factor VIII (FVIII) has been attributed mainly to inhibition of FVIII binding to phospholipid. In the present study, we demonstrated that VWF-mediated FVIII protection from APC also results from direct inhibition of FVIII binding to APC. Inhibition of FVIII binding to anhydro-APC by VWF would be consistent with partial or complete overlap of the FVIII binding sites for APC and VWF. We examined, therefore, the inhibitory effects of 6 synthetic peptides spanning residues 1996 to 2028 around the previously localized APC binding region (FVIII residues 2009-2018). Peptide 2009 to 2018 inhibited FVIII binding to anhydro-APC by 83% (50% inhibition, 55 μM). Similarly, peptide 2013 to 2022 inhibited FVIII binding to VWF by 84% (50% inhibition, 25 μM). It was also found that peptides 2009 to 2018 and 2013 to 2022 optimally bound to anhydro-APC and VWF, respectively. A rabbit antipeptide IgG, raised against peptide 2009 to 2022, blocked the binding of both anhydro-APC and VWF to FVIII. This immunoglobulin G inhibited proteolytic cleavage of FVIII by APC. Our results indicate that the essential regions for the binding of APC and VWF to FVIII overlap and that the protective effect of VWF on APC-catalyzed FVIII inactivation includes competitive inhibition of APC binding to FVIII by VWF.
Introduction
Factor VIII (FVIII) is a glycoprotein cofactor that serves as a critical component in the intrinsic coagulation pathway.1 Insufficient expression of FVIII or expression of nonfunctional FVIII results in hemophilia A, one of the most common severe hereditary bleeding disorders. Mature FVIII is synthesized as a single-chain polypeptide consisting of 2332 amino acid residues.2,3 Based on internal sequence homologies, 3 types of structural domains have been identified, arranged in the order of A1-A2-B-A3-C1-C2.4 FVIII circulates in plasma as a heterodimer of a heavy chain, consisting of the A1 (1-372) and A2 (373-740) domains, together with heterogeneous fragments of partially proteolyzed B domains (741-1648), and a light chain (LCh) consisting of the A3 (1649-2019), C1 (2020-2172), and C2 (2173-2332) domains.3 4
Thrombin and factor Xa (FXa) are the major physiologic enzymes that activate FVIII. Both enzymes cleave FVIII at Arg372, Arg740, and Arg1689 and transform it to its active form, an A1/A2/A3-C1-C2 heterotrimer.5 Activated FVIII is incorporated into the FXase complex assembled on a phospholipid (PL) membrane surface and thus acts as an accelerator of FXa generation. Activated protein C (APC) inactivates activated FVIII by proteolytic cleavage at Arg336 in the presence of negatively charged PL.5 In this reaction, the 54-kd A1 fragment is cleaved into a 40-kd fragment with a loss of the carboxy-terminal acidic region. The APC binding site in the FVIII molecule has been located within the residues 2009 to 2018 in the A3 domain of the LCh.6 The site has not been confirmed in direct binding experiments, however.
FVIII circulates in plasma as a noncovalent complex with von Willebrand factor (VWF).7 VWF regulates the synthesis and cofactor activity of FVIII.8 Furthermore, VWF concentrates FVIII at the site of vascular injury.9 The absence of VWF or the presence of defective VWF results in a secondary decrease of FVIII activity and a bleeding tendency known as von Willebrand disease.7 Two major binding regions for VWF in the FVIII molecule have been reported, one within amino acid residues 1670 to 1689 in the amino-terminal region of LCh10,11 and the other within residues 2303 to 2332 in the carboxy-terminal region of the C2 domain.12,13 A3 fragments, composed of amino-terminal residues from 1672 to 1794, and LCh fragments lacking the amino-terminal acidic region of the C2 domain appear to have reduced affinities for VWF, indicating that both regions are essential for the high-affinity binding of VWF to FVIII.14 The heavy chain is not directly involved in VWF interaction with FVIII.15 Recently, Jacquemin et al reported that an anti-C1 monoclonal antibody (MoAb) inhibited FVIII binding to VWF, suggesting a possible role of C1 sequences in FVIII and VWF association.16
Activation and inactivation of FVIII is regulated by VWF, and this regulatory mechanism appears to be essential for the maintenance of normal circulating levels of FVIII activity. Upon activation by thrombin, FVIII dissociates from VWF and markedly enhances the activity of the FXase complex in the presence of PL in the coagulation cascade.15 VWF prevents premature interaction of FVIII with PL prior to FVIII activation.17 In addition, VWF protects FVIII from various PL-dependent proteases, such as factor IXa, FXa, and APC.18-20
Recently, we demonstrated that anti-FVIII autoantibodies, recognizing epitopes within the A3-C1 domains, inhibited APC-catalyzed FVIII proteolysis, resulting in normal levels of FVIII antigen in plasma.21 Although the autoantibodies did not react with the C2 domain, they inhibited FVIII binding to VWF. We hypothesized that inhibition of both VWF binding to FVIII and APC-catalyzed proteolysis of FVIII may result from an overlap of VWF and APC binding regions within the A3 domain of FVIII. To test this hypothesis, in the present study we used anhydro-APC, a catalytically inactive derivative of APC, to directly examine the mechanisms by which VWF inhibits binding of APC to FVIII.
Materials and methods
Proteins
FVIII was affinity-purified from human plasma using MoAb NMC-VIII/10 recognizing the A3 domain of FVIII. Elution from the MoAb affinity column was performed with 1 M potassium iodine (KI) and 40% ethylene glycol as previously reported.11 The specific activity of the purified FVIII was 2700 U/mg. Enzyme-linked immunosorbent assays (ELISAs) demonstrated that purified FVIII was free of VWF antigen.22 VWF was purified from a commercially available FVIII/VWF concentrate (Confact F, Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan) using gel filtration on a Sepharose CL-4B column (Amersham Pharmacia Biotech, Uppsala, Sweden). Residual FVIII was removed using immunobeads coated with immobilized anti-FVIII MoAb. The final preparation of VWF did not contain FVIII, as evidenced by previously described ELISA.22 Purified APC was kindly supplied by Chemo-Sero-Therapeutic Research Institute. Anhydro-APC, a catalytically inactive derivative of APC in which the active-site serine is replaced by dehydroalanine, was prepared as previously described.21 The purified anhydro-APC did not inactivate FVIII activity. PL vesicles were prepared by sonication of a phosphatidylserine/phosphatidylcholine mixture (20:80 molar ratio) as previously described.23
Synthetic peptides
Synthetic peptides were 10 to 12 residues long and represented overlapping sequences covering the amino acid residues 1996 to 2028 of FVIII. Peptides were prepared by the method of simultaneous multiple peptide synthesis as previously described.24 They were analyzed and purified by reversed-phase high-pressure liquid chromatography (purity > 95%). Peptides consisting of the same amino acid residues in random order were synthesized in a similar manner.
Antibodies
MoAb C5 recognizing residues 351 to 365 within the acidic region of the A1 domain was kindly provided by Dr C. A. Fulcher (Scripps Clinic Research Institute, La Jolla, CA).25 MoAb JR8 recognizing the FVIII A2 domain and MoAb APC against APC were obtained from JR8 Scientific (Woodland, CA) and Technoclone (Vienna, Austria), respectively. Antibodies were conjugated with peroxidase as previously reported.26 A polyclonal antibody was prepared in rabbits against the amino acid residues 2009 to 2022 of FVIII (HAGMSTLFLVYSNK) following coupling to keyhole limpet hemocyanin with glutaraldehyde using previously described methods.27 New Zealand White rabbits were immunized with 1 mg of the conjugated peptide in Freund adjuvant at weekly intervals. Complete Freund adjuvant was used for primary injections, and incomplete adjuvant was used for boosting injections. Immunoglobulin G (IgG) from rabbit antiserum was purified using protein G–Sepharose (Amersham Pharmacia Biotech) as previously described.28
ELISAs for measuring the inhibitory effect on FVIII binding
FVIII binding to anhydro-APC.
Binding of FVIII to anhydro-APC was measured as previously reported.21 Briefly, the wells of microtiter plates were coated with 30 μM anhydro-APC in 20 mM Tris, 150 mM NaCl, pH 7.4 (TBS), overnight at 4°C. After blocking with 4% human serum albumin (HSA), 100 nM FVIII diluted in TBS containing 2.5 mM CaCl2was added and incubated for 2 hours at 37°C. Bound FVIII was detected using peroxidase-conjugated MoAb JR8 at 492 nm (OD492). To determine the inhibitory effects of VWF, synthetic peptides, or rabbit antipeptide IgG on this binding, serial dilutions of each preparation were incubated with FVIII for 2 hours at 37°C prior to adding to the immobilized anhydro-APC. The OD492 in the absence of FVIII was regarded as nonspecific binding, and that in the absence of VWF, synthetic peptides, or antipeptide IgG was regarded as maximum binding. The percent of inhibition was calculated as follows: 100 − 100 × (bound − nonspecific OD492)/(maximum − nonspecific OD492).
FVIII binding to VWF.
Binding of FVIII to VWF was measured as previously reported.12 Briefly, microtiter wells were coated with 20 nM VWF in TBS and blocked with 4% HSA, followed by addition of 4 nM FVIII and incubation for 2 hours at 37°C. Bound FVIII was detected using peroxidase-conjugated MoAb JR8 as above. To determine the inhibitory effects of anhydro-APC, synthetic peptides, or antipeptide IgG, serial dilutions of each were incubated with FVIII for 2 hours at 37°C prior to adding to immobilized VWF.
ELISA for direct binding of anhydro-APC or VWF to FVIII synthetic peptides
Microtiter wells were coated with the FVIII synthetic peptides at 100 μM in 0.1 M sodium bicarbonate buffer, pH 9.6, overnight at 4°C. After blocking with 4% HSA, serial dilutions of anhydro-APC or VWF were added to each well and incubated for 2 hours at 37°C. Dilutions of anhydro-APC or VWF were prepared in TBS containing 4% HSA in the presence or absence of 2.5 mM CaCl2, respectively. The binding of APC or VWF to immobilized peptides was detected using peroxidase-conjugated MoAb APC or peroxidase-conjugated antihuman VWF (DAKO, Glostrup, Denmark), respectively. To confirm the binding specificity, each peptide at a concentration of 1 mM was mixed with anhydro-APC or VWF and incubated for 2 hours at 37°C prior to the addition to the corresponding immobilized peptide.
Effects of antipeptide IgG on APC-catalyzed FVIII inactivation
FVIII inactivation.
FVIII (10 nM) was incubated with serial dilutions of antipeptide IgG for 1 hour at 37°C in 50 mM sodium acetate, 7 mM sodium barbital, and 0.1 M NaCl containing HSA (dilution buffer). After the addition of 5 μM PL vesicles and 2.5 mM CaCl2 and incubation for 30 minutes, the reaction was initiated by the addition of 5 nM APC at 37°C. At selected time intervals, samples (10 μL) were taken from the mixture, and APC activity was immediately quenched by 500-fold dilution in 1 mM phenylmethylsulfonyl fluoride (PMSF) in dilution buffer at 4°C. Each sample was tested for FVIII coagulant activity using a one-stage clotting assay.29 Control experiments indicated that the presence of APC and PMSF in the diluted samples did not influence FVIII activity in the coagulation assay.
APC-catalyzed FVIII cleavage at Arg336.
Analysis of FVIII cleavage by APC was assessed as previously reported.21 Briefly, anti-FVIII MoAb JR8 (0.2 μM) was immobilized on microtiter wells. After blocking the wells with 4% HSA, 10 nM FVIII, incubated with serial dilutions of antipeptide IgG for 1 hour at 37°C, was added to each well, followed by addition of 5 nM APC, 10 μM PL vesicles, and 2.5 mM CaCl2. To quench the APC reaction at selected time intervals, the supernatant was removed from each well with the captured FVIII and 2 mM PMSF was added to each well. FVIII cleavage at Arg336 was detected by reduction in the binding of peroxidase-conjugated MoAb C5 (recognizing FVIII residues 351-365). The percent cleavage at Arg336 by APC was calculated as follows: 100 − 100 × (bound − nonspecific OD492)/(bound at time zero − nonspecific OD492). The OD492 in the absence of FVIII was considered as nonspecific binding of MoAb C5. Control experiments indicated that the MoAbs JR8 and C5 did not inhibit FVIII cleavage by APC.
Results
Effect of VWF on FVIII binding to anhydro-APC
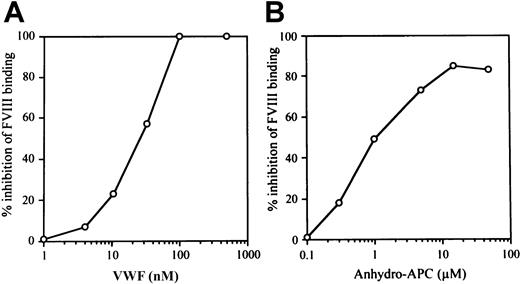
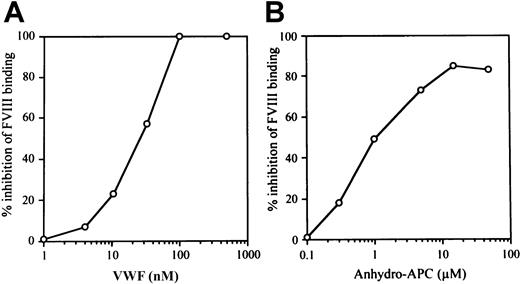
VWF inhibits APC-catalyzed FVIII inactivation.20Previously we have shown that anti-FVIII autoantibodies recognizing A3-C1 epitopes inhibit FVIII binding to anhydro-APC and to VWF,21 suggesting that binding sites for APC and VWF within the FVIII molecule overlap and that these ligands are likely to compete for binding to FVIII. In the current investigation, therefore, we focused on the effect of VWF on FVIII interaction with APC. We first investigated the effect of VWF on FVIII binding to anhydro-APC in an ELISA in the absence of PL. VWF inhibited FVIII binding to anhydro-APC in a dose-dependent manner. The concentrations of VWF required to produce 50% inhibition (IC50 value) and complete inhibition (> 95%) were 25 nM and 100 nM, respectively (Figure1A). We also tested the effect of anhydro-APC on FVIII binding to VWF. Anhydro-APC similarly inhibited FVIII binding to VWF in a dose-dependent manner. The IC50value was 1.0 μM, and maximum inhibition was achieved at 20 μM (Figure 1B).
Relationship between VWF and anhydro-APC in FVIII interaction.
(A) Effect of VWF on FVIII binding to anhydro-APC. Serial dilutions of VWF were incubated with FVIII (100 nM) for 2 hours at 37°C prior to the addition to immobilized anhydro-APC. Bound FVIII was detected using peroxidase-conjugated MoAb JR8 as described in “Materials and methods.” (B) Effect of anhydro-APC on FVIII binding to VWF. Serial dilutions of anhydro-APC were incubated with FVIII (4 nM) for 2 hours at 37°C prior to the addition to immobilized VWF. Bound FVIII was detected using peroxidase-conjugated MoAb JR8 as described in panel A.
Relationship between VWF and anhydro-APC in FVIII interaction.
(A) Effect of VWF on FVIII binding to anhydro-APC. Serial dilutions of VWF were incubated with FVIII (100 nM) for 2 hours at 37°C prior to the addition to immobilized anhydro-APC. Bound FVIII was detected using peroxidase-conjugated MoAb JR8 as described in “Materials and methods.” (B) Effect of anhydro-APC on FVIII binding to VWF. Serial dilutions of anhydro-APC were incubated with FVIII (4 nM) for 2 hours at 37°C prior to the addition to immobilized VWF. Bound FVIII was detected using peroxidase-conjugated MoAb JR8 as described in panel A.
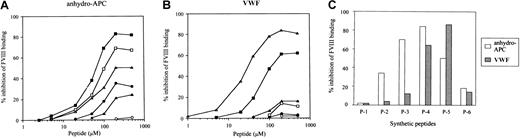
Effects of synthetic peptides on FVIII binding to anhydro-APC or VWF
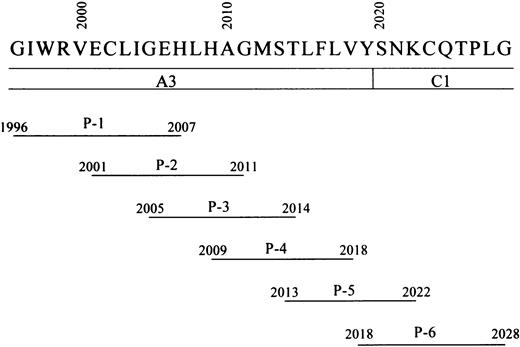
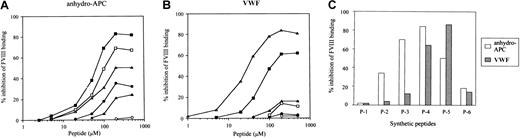
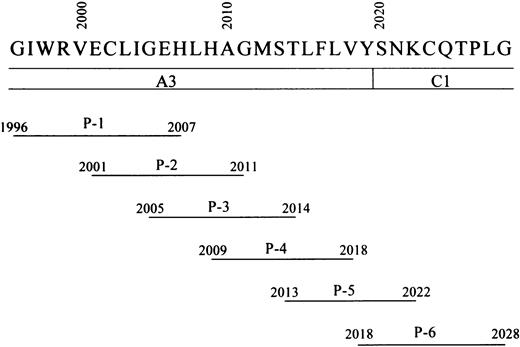
Residues 2009 to 2018 in the A3 domain of FVIII LCh have been demonstrated to contain the binding site for APC.6 To verify that this sequence optimally represents the APC binding site, we prepared 6 overlapping synthetic peptides spanning the A3 carboxy-terminus to the C1 amino-terminus of FVIII (residues 1996-2028) (Figure 2) and tested their effects on FVIII binding to anhydro-APC in an ELISA. One of the peptides, designated P-4 (residues 2009-2018), was the most effective in inhibiting FVIII binding to anhydro-APC by 83% at a concentration of 200 μM (Figure 3A). The inhibitory effect of peptide was dose dependent, with an IC50 value of 55 μM. The synthetic peptides P-3 (residues 2005-2014), P-5 (residues 2013-2022), and P-2 (residues 2001-2011) similarly inhibited binding of FVIII to anhydro-APC but to a lesser extent (69%, 51%, and 35%, respectively). P-6 (residues 2018-2028) and P-1 (residues 1996-2007) demonstrated weak or no inhibitory effect. These results indicated that the FVIII residues 2009-2018, contained in the P-4 peptide, were critical for FVIII binding to APC.
Schematic representation of synthetic FVIII peptides.
Amino acid residues from 1996 to 2028 of the FVIII A3-C1 domains are represented by their one-letter abbreviations. The lines below FVIII amino acid residues indicate the amino acid residues included in the synthetic peptides. The designation of each peptide (ie, P-1–P-6) is given below each sequence.
Schematic representation of synthetic FVIII peptides.
Amino acid residues from 1996 to 2028 of the FVIII A3-C1 domains are represented by their one-letter abbreviations. The lines below FVIII amino acid residues indicate the amino acid residues included in the synthetic peptides. The designation of each peptide (ie, P-1–P-6) is given below each sequence.
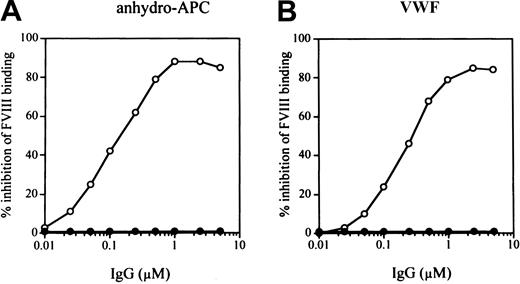
Inhibitory effects of synthetic peptides on FVIII bindings to anhydro-APC and VWF.
Serial dilutions of synthetic peptides were mixed with FVIII prior to adding to immobilized anhydro-APC (A) or VWF (B). Bound FVIII was detected using peroxidase-conjugated MoAb JR8. The symbols used are as follows: P-1, ○; P-2, ●; P-3, ■; P-4, ▪; P-5, ▵; P-6, ▴. (C) Percent inhibition of FVIII binding to anhydro-APC or VWF by synthetic peptide (P-1–P-6) at the concentration of 200 μM
Inhibitory effects of synthetic peptides on FVIII bindings to anhydro-APC and VWF.
Serial dilutions of synthetic peptides were mixed with FVIII prior to adding to immobilized anhydro-APC (A) or VWF (B). Bound FVIII was detected using peroxidase-conjugated MoAb JR8. The symbols used are as follows: P-1, ○; P-2, ●; P-3, ■; P-4, ▪; P-5, ▵; P-6, ▴. (C) Percent inhibition of FVIII binding to anhydro-APC or VWF by synthetic peptide (P-1–P-6) at the concentration of 200 μM
To determine whether the APC binding region of FVIII is involved in interaction with VWF, we tested the effects of the above peptides on FVIII binding to VWF. P-5 inhibited FVIII binding to VWF by 84% (Figure 3B). The inhibitory effect was dose dependent, and the IC50 value was 25 μM. The P-4 peptide, which was the most effective in blocking FVIII binding to anhydro-APC, also inhibited FVIII binding to VWF but to a lesser degree (63%) in comparison with P-5. Peptides P-1, P-2, P-3, and P-6 demonstrated weak or no inhibitory effects. These results indicated that the sequences encompassed by P-4 and P-5 peptides optimally represent the regions of FVIII involved in binding to APC and VWF, respectively. Figure 3C summarizes the inhibitory effects of all peptides on the binding of FVIII to anhydro-APC or VWF at peptide concentrations of 200 μM.
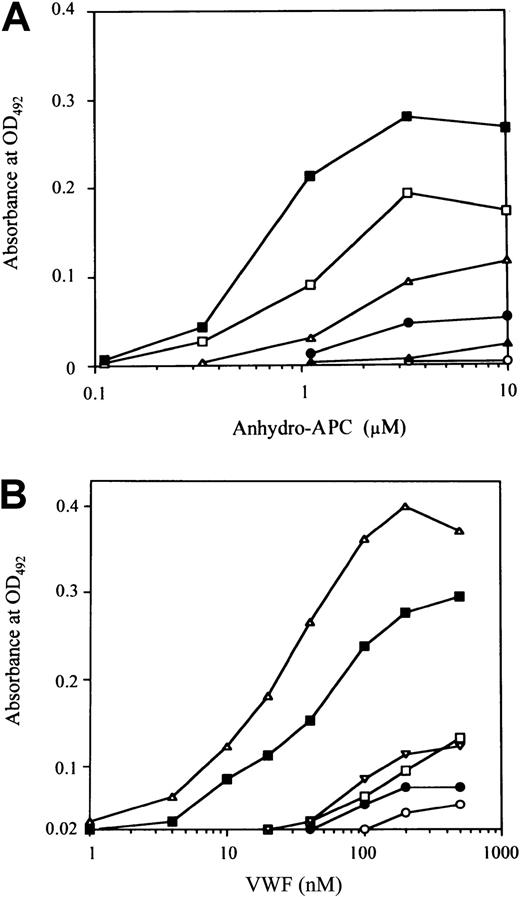
Direct binding of anhydro-APC or VWF to synthetic peptides
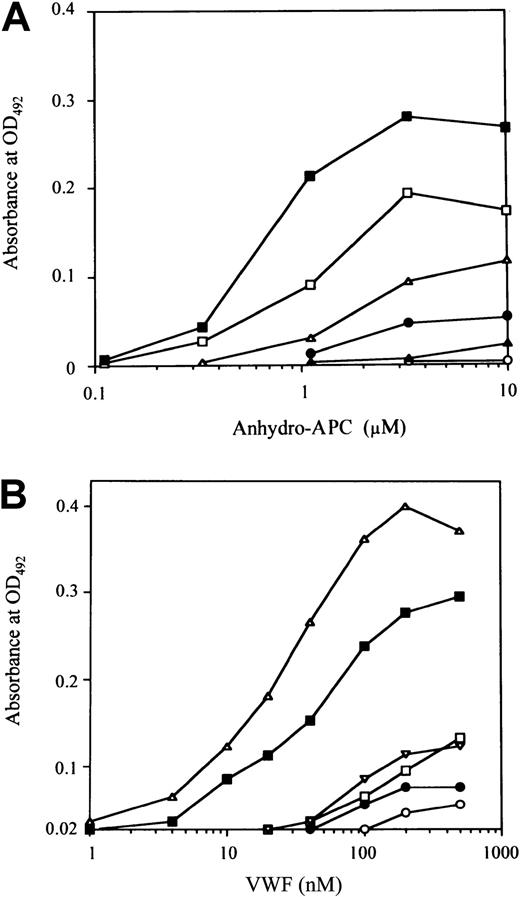
To confirm the role of residues 2009 to 2018 represented by the P-4 peptide in FVIII binding to APC, we examined the direct binding of anhydro-APC to the immobilized peptide. Anhydro-APC bound to immobilized P-4 in a dose-dependent manner (Figure4A). Dose-dependent binding patterns were also observed for P-3 and P-5 peptides, although in these instances the maximum binding levels were lower than that for P-4 (approximately 70% and 40% of P-4, respectively). Low or no binding of anhydro-APC was seen to P-2, P-6, and P-1. These results were consistent with the observed inhibitory effects of the peptides on FVIII binding to anhydro-APC. Preincubation of anhydro-APC with excess amounts of each soluble peptide (1 mM) completely abolished the subsequent binding of anhydro-APC to the respective immobilized peptide, confirming the specificity of the binding assays. Furthermore, anhydro-APC did not bind to immobilized peptides prepared from corresponding randomized sequences (data not shown).
Binding of anhydro-APC or VWF to immobilized synthetic peptides.
Serial dilutions of anhydro-APC (A) or VWF (B) were added to each immobilized synthetic peptide (100 μM). Anhydro-APC or VWF binding was detected with peroxidase-conjugated MoAb APC or peroxidase-conjugated rabbit antihuman VWF. The symbols used are as follows: P-1, ○; P-2, ●; P-3, ■; P-4, ▪; P-5, ▵; P-6, ▴.
Binding of anhydro-APC or VWF to immobilized synthetic peptides.
Serial dilutions of anhydro-APC (A) or VWF (B) were added to each immobilized synthetic peptide (100 μM). Anhydro-APC or VWF binding was detected with peroxidase-conjugated MoAb APC or peroxidase-conjugated rabbit antihuman VWF. The symbols used are as follows: P-1, ○; P-2, ●; P-3, ■; P-4, ▪; P-5, ▵; P-6, ▴.
Because P-5 and P-4 peptides inhibited FVIII binding to VWF, we examined whether VWF is able to directly bind to these peptides using ELISA. We found that VWF bound to immobilized P-4 and P-5 in a dose-dependent manner (Figure 4B), although the binding ability for P-4 was lower than that for P-5. The binding of P-4 was approximately 70% that of P-5. Low or no binding of VWF was detected for P-1, P-2, P-3, and P-6. These results were consistent with the observed inhibitory effects of the peptides on FVIII binding to VWF. As above, the specificity of the direct binding assays was confirmed by complete abrogation of VWF binding to immobilized peptides by preincubation of VWF with excess amounts of the respective soluble peptide (1 mM). In addition, VWF did not bind to immobilized peptides with corresponding randomized sequences (data not shown).
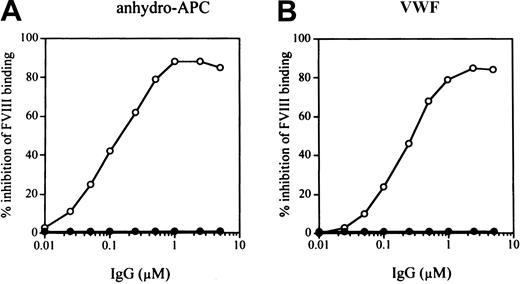
Inhibitory effect of rabbit antipeptide IgG on FVIII binding to anhydro-APC or VWF
To confirm that overlapping FVIII regions, encompassed by peptides P-4 (2009-2018) and P-5 (2013-2022), are indeed involved in FVIII binding to anhydro-APC and VWF, respectively, we synthesized a larger peptide (residues 2009-2022) containing both sequences and prepared a rabbit antipeptide antibody. The antipeptide IgG inhibited FVIII binding to both anhydro-APC (by 86%) and VWF (by 85%) at an IgG concentration of 2.5 μM. Inhibition was dose dependent, with IC50 values of 0.14 μM and 0.28 μM, respectively (Figure 5). In control experiments, normal rabbit IgG did not affect FVIII binding to anhydro-APC and VWF. These results confirmed that the sequences within the region 2009 to 2022 were essential for its binding to APC and VWF.
Inhibitory effect of rabbit antipeptide antibody IgG (residues 2009-2022) on FVIII bindings to anhydro-APC or VWF.
Serial dilutions of antipeptide IgG (○) or control rabbit IgG (●) were incubated with FVIII for 2 hours at 37°C prior to adding to immobilized anhydro-APC (A) or VWF (B). Bound FVIII was detected using peroxidase-conjugated MoAb JR8.
Inhibitory effect of rabbit antipeptide antibody IgG (residues 2009-2022) on FVIII bindings to anhydro-APC or VWF.
Serial dilutions of antipeptide IgG (○) or control rabbit IgG (●) were incubated with FVIII for 2 hours at 37°C prior to adding to immobilized anhydro-APC (A) or VWF (B). Bound FVIII was detected using peroxidase-conjugated MoAb JR8.
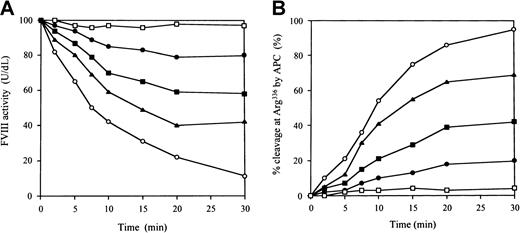
Inhibitory effects of antipeptide IgG on APC-catalyzed FVIII inactivation
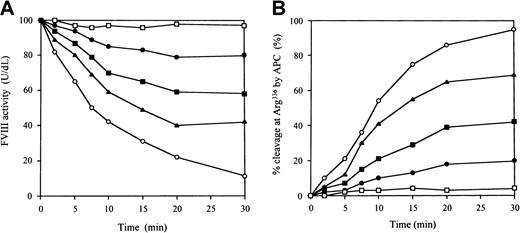
To investigate the inhibitory effects of the antipeptide IgG on APC-catalyzed FVIII inactivation, we examined FVIII inactivation by APC in a one-stage clotting assay. The IgG did not inhibit FVIII binding to immobilized PL (data not shown). Figure6A illustrates the time course of the inactivation of FVIII by APC. In control experiments in the presence of normal rabbit IgG, FVIII activity decreased to 15% after 30 minutes, with a half-life of 8 minutes. In contrast, FVIII activity remained at more than 80% after 30 minutes in the presence of antipeptide IgG at a concentration of 0.6 μM. This inhibition of FVIII inactivation was dose dependent. Because the antipeptide IgG did not inhibit FVIII and PL interaction, the effect of the antibody must have been derived from direct inhibition on APC binding, mimicking VWF. We further confirmed the inhibitory effects of the antipeptide IgG on APC catalysis by quantitative assay of FVIII proteolytic cleavage at Arg336 using ELISA as previously described.21 In the presence of the antipeptide IgG, the percent cleavage at Arg336 by APC was reduced to 21% in 30 minutes at an IgG concentration of 0.6 μM. The inhibitory effect was dose dependent. In contrast, the percent cleavage in 30 minutes remained more than 90% in the presence of control IgG (Figure6B). These results indicated that antipeptide IgG, which blocked FVIII binding to APC and VWF, inhibited APC-catalyzed FVIII inactivation independently of PL.
Inhibitory effects of antipeptide IgG on APC-catalyzed FVIII inactivation.
(A) FVIII inactivation. FVIII (10 nM) was preincubated with IgG for 1 hour at 37°C, followed by the addition of 5 nM APC, 5 μM PL vesicles, and 2.5 mM CaCl2. At timed intervals, inactivation was immediately stopped by 1 mM PMSF and FVIII activities were measured in a one-stage clotting assay as described in “Materials and methods.” The symbols used are as follows: normal rabbit IgG (0.6 μM), ○; antipeptide IgG (0.6 μM, ●; 0.2 μM, ▪; 0.06 μM, ▴); no APC added, ■. (B) FVIII proteolytic cleavage at Arg336 by APC. The IgG was added to 10 nM FVIII, which was bound to immobilized MoAb JR8 (0.2 μM). Then 5 nM APC, 10 μM PL vesicles, and 2.5 mM CaCl2 were added. At timed intervals, supernatants were removed and APC action was stopped by 2 mM PMSF. FVIII cleavage at Arg336 was detected with peroxidase-conjugated MoAb C5 as described in “Materials and methods.” The symbols used are as follows: normal rabbit IgG (0.6 μM), ○; antipeptide IgG (0.6 μM, ●; 0.2 μM, ▪; 0.06 μM, ▴); no APC added, ■.
Inhibitory effects of antipeptide IgG on APC-catalyzed FVIII inactivation.
(A) FVIII inactivation. FVIII (10 nM) was preincubated with IgG for 1 hour at 37°C, followed by the addition of 5 nM APC, 5 μM PL vesicles, and 2.5 mM CaCl2. At timed intervals, inactivation was immediately stopped by 1 mM PMSF and FVIII activities were measured in a one-stage clotting assay as described in “Materials and methods.” The symbols used are as follows: normal rabbit IgG (0.6 μM), ○; antipeptide IgG (0.6 μM, ●; 0.2 μM, ▪; 0.06 μM, ▴); no APC added, ■. (B) FVIII proteolytic cleavage at Arg336 by APC. The IgG was added to 10 nM FVIII, which was bound to immobilized MoAb JR8 (0.2 μM). Then 5 nM APC, 10 μM PL vesicles, and 2.5 mM CaCl2 were added. At timed intervals, supernatants were removed and APC action was stopped by 2 mM PMSF. FVIII cleavage at Arg336 was detected with peroxidase-conjugated MoAb C5 as described in “Materials and methods.” The symbols used are as follows: normal rabbit IgG (0.6 μM), ○; antipeptide IgG (0.6 μM, ●; 0.2 μM, ▪; 0.06 μM, ▴); no APC added, ■.
Discussion
In the present study we have demonstrated for the first time that VWF protects FVIII from APC catalysis by directly inhibiting of APC binding to FVIII. Because VWF inhibits PL binding to FVIII and APC-catalyzed FVIII inactivation is PL dependent,17,30 it has been previously assumed that the protective effect of VWF is entirely due to prevention of FVIII interaction with PL.20Fay et al compared the inhibitory effects of the homodimeric VWF fragment, SPIII (residues 1-1365), and monomeric fragment, SPIII-T4 (residues 1-272), on APC-catalyzed FVIII inactivation in fluid phase experiments.20 Although the protective effect of SPIII was retained, no protection was observed in a complex of the smaller SPIII-T4 with FVIII. They suggested, therefore, that the protective mechanism of VWF on APC-catalyzed FVIII inactivation was size dependent, whereas the possibility of direct VWF-mediated inhibition of APC interaction with FVIII was not considered. On the other hand, Koppelman et al used a panel of deletion mutants and VWF fragments in both solid phase and fluid phase experiments31 and demonstrated that the N-terminal 31-kd VWF fragment, T31, which is similar to SPIII-T4, was capable of protecting FVIII from inactivation by APC. Their results strongly suggested that protection of FVIII resulted from the binding to VWF. Therefore, the mechanism of inhibition of APC binding to FVIII by VWF has not been established. More recently, we demonstrated in direct binding experiments that FVIII inhibitor autoantibodies with only A3-C1 epitopes inhibited the binding of FVIII to both anhydro-APC and VWF.21 These antibodies neither reacted with the C2 domain nor inhibited FVIII binding to PL. Our results strongly indicated that FVIII regions responsible for the binding of APC and VWF are extremely close or even overlap within the A3-C1 domain of FVIII. Therefore, in the present study we investigated the possibility that VWF might directly compete with APC and thereby protect FVIII from APC-catalyzed degradation.
We have produced an anhydro-derivative of APC, which retains the ability to bind to FVIII but lacks proteolytic activity.21,32 It enabled us to perform direct competitive binding experiments for APC and FVIII, and we found that VWF dose-dependently inhibited FVIII binding to anhydro-APC in the absence of PL. Walker et al had previously identified an APC binding region within residues 2009 to 2018 using a panel of LCh mutants and synthetic peptides in coagulation-based assays.6 We confirmed the presence of APC binding sites within residues 2009 to 2018 by competitive direct binding assay using anhydro-APC. The concentrations of peptides in our binding assays were similar to those that were found to protect FVIII from APC-mediated inactivation in coagulation assays.6 Furthermore, specific FVIII peptides also bound to immobilized anhydro-APC, and an antipeptide IgG raised against residues 2009 to 2022 dose-dependently inhibited the binding of FVIII to anhydro-APC. The inhibitory effect of the IgG on binding of FVIII to anhydro-APC was comparable to that of anti-FVIII autoantibodies with A3-C1 epitopes.21 Notably, all of these autoantibodies were found to bind to the same peptides, supporting the view that the APC binding site is located within the defined peptide region 2009 to 2018.
The inhibitory effect of VWF on FVIII binding to anhydro-APC was confirmed in the absence of PL. The present findings indicated that VWF and APC binding sites are in close proximity to each other. As reported earlier, the major VWF binding sites in the FVIII molecule are located within residues 1670 to 1684 of the acidic region of A3 domain11 and the carboxy-terminus of the C2 domain, residues 2303 to 2332.14 Recent crystal analysis of a 3-dimensional model of the C2 domain revealed that the VWF binding site overlapped the possible PL binding site within the hydrophobic feet region comprising residues Met2199/Phe2200, Val2223, and Leu2251/Leu2252.33 In addition, an anti-C1 MoAb reactive against residue Arg2150 and autoantibodies with A3-C1 epitopes inhibited binding of FVIII to VWF,16,21 suggesting a role for the A3-C1 domain in this reaction. In the present investigation, we developed competitive and direct binding assays using overlapping synthetic peptides encompassing the APC binding site of FVIII, residues 1996 to 2028, and obtained additional evidence that a novel region involved in VWF binding is located within the A3-C1 domain. One of the peptides (P-5), corresponding to residues 2013 to 2022, inhibited VWF binding by 84%. The IC50 value was approximately 20-fold higher than that of the peptide 2303 to 2332, corresponding to the VWF binding site within the C2 domain of FVIII.14 Another peptide (P-4), corresponding to the FVIII residues 2009-2018, also inhibited binding (by 63%), whereas peptide P-6 (residues 2013-2028) had no inhibitory action. Furthermore, P-4 and P-5 bound directly to VWF, and the binding abilities correlated with their inhibitory effects on FVIII binding to VWF. It appears, therefore, that the residues 2013 to 2018 in the A3 carboxy-terminus constitute an important site responsible for VWF interaction. These data were further verified using a rabbit antibody raised against the peptide 2009 to 2022, which includes P-4 and P-5 sequences and inhibits binding of VWF to FVIII.
Although our present results strongly indicated that FVIII regions responsible for the binding of APC and VWF are extremely close within the A3-C1 domain of FVIII, different patterns of peptide inhibition were observed for VWF and APC binding to FVIII. This suggested that important sites for VWF and APC binding to FVIII are not identical and are encompassed by the residues 2013 to 2018 and 2009 to 2014, respectively.
In conclusion, we have obtained several lines of evidence to support the view that VWF protects FVIII from APC-catalyzed inactivation by inhibiting APC binding to FVIII. Furthermore, the results of the present study indicate that another region in the FVIII LCh is important for VWF interaction. This region has an overlap with the APC binding site within the carboxy-terminal portion of the A3 domain. Our data also imply that ligand interactions in the FVIII LCh are significantly more complex than previously thought. For instance, several regions of the LCh are involved in VWF binding. Moreover, binding of VWF to these regions regulates both FVIII activation and inactivation. This multifunctional role of the regions involved in VWF binding results in VWF-mediated inhibition of FVIII binding to diverse ligands, such as APC, PL, FXa, and low-density lipoprotein–related protein.17,19 34 Determination of the 3-dimensional structure of the FVIII LCh would help to clarify these VWF-dependent FVIII regulatory mechanisms.
We thank Dr John C. Giddings for helpful suggestions. We express our gratitude to Dr Natalya Ananyeva for linguistic assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Midori Shima, Dept of Pediatrics, Nara Medical University, 840 Shijo-cho, Kashihara City, Nara 634-8522, Japan; e-mail: mshima@naramed-u.ac.jp.