Abstract
CD19 is a coreceptor that amplifies signaling initiated by antigen cross-linking of the B-cell antigen receptor (BCR). CD19 can also signal independently of BCR coligation. This study shows thatB-cell adaptor forphosphoinositide 3-kinase (BCAP), previously characterized as a substrate of the tyrosine kinases upon BCR engagement, is phosphorylated by cross-linking of CD19. Tyrosine phosphorylation of BCAP, mediated by Lyn, provides binding site(s) for phosphoinositide 3-kinase (PI3K), thereby participating in Akt activation. Thus, these results provide evidence that BCAP serves as an adaptor molecule for CD19 to activate the PI3K pathway in B cells.
Introduction
CD19 is a B-cell–specific transmembrane glycoprotein that is expressed from the pro–B cell until the plasma cell stage.1 On mature B cells, CD19 associates with 3 different molecules to form a tetrameric complex: CD21 (complement receptor type 2), CD81 (TAPA-1), and Leu13.2 3 These associations, together with the evidence that CD19, but not CD21, has a long cytoplasmic domain, have led to the concept that CD21 serves primarily as a ligand-binding subunit for complement components such as C3d, whereas CD19 functions as a signal-transducing subunit in mature B cells. On the other hand, in pro-B and pre-B cells, where CD19 is expressed in the absence of CD21, CD19 appears to function as a ligand-binding subunit and also as a signal transducer, although its ligand has not been identified.
Two different models for explaining the roles of the CD19/CD21 complex in B-cell function have been proposed. In one model, the CD19/CD21 complex serves as a costimulatory molecule for B-cell antigen receptor (BCR) signals when C3d fragments covalently bound to antigen coligate the CD19/CD21 complex with the BCR. Indeed, this coligation has been shown to lower the threshold for antigen stimulation of B-cell proliferation, thereby promoting more effective immune responses.4-6 A different model predicts that levels of endogenous ligands for the CD19/CD21 complex such as C3d could establish signaling thresholds for B cells in an antigen-independent manner through cross-linking the CD19/CD21 complex.7-13This model would explain why alterations in CD19 levels so profoundly affect all B cells, most of which should not be exposed to their appropriate antigens in vivo. Although direct data proving which model is more dominantly operating in vivo are not available, these stimulation modes could coordinately contribute to B-cell activation, depending upon differentiation stages of B cells and the natures of antigen and antibody.
The CD19/CD21 complex achieves these biologic responses by activating multiple intracellular signaling pathways through tyrosine phosphorylation of the cytoplasmic domain of CD19. Indeed, in a human Daudi B-cell line, ligating CD19 alone induced its tyrosine phosphorylation,14 presumably by Lyn, Fyn, or Lck associated with the unligated receptor.15 Among 9 potential phosphorylation sites, phosphorylated tyrosines on Tyr482xxM and Tyr513xxM motifs in CD19 were shown to bind to the tandem SH2 domains of the phosphoinositide 3-kinase (PI3K) p85 subunit.14,16,17 Although these biochemical data suggest that CD19 ligation alone induces PI3K activation, the direct in vivo evidence is still lacking. Moreover, even if this is so, given the existence of another YxxM motif–containing adaptor molecule,B-cell adaptor forPI3K (BCAP), in B cells,18 it remains elusive whether Tyr482xxM and Tyr513xxM motifs in CD19 are solely involved in the CD19-mediated PI3K activation. We now report that cross-linking of CD19 alone indeed induces PI3K activation, leading to up-regulation of Akt, and that this response requires YxxM motifs in BCAP, rather than those in CD19.
Materials and methods
Cells, constructs, and transfections
Wild-type and its derived mutant DT40 chicken B cells (BCR-, Lyn-, Syk-, Btk-, BCAP-deficient cells; Lyn-deficient cells expressing wild-type or kinase-negative Fyn; BCAP-deficient cells expressing wild-type or Y4F mutant BCAP) were cultured as described previously.18-22 Human Raji B cells were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, penicillin, and streptomycin. Primary splenic B cells from C57BL/6 mice were isolated as described previously.23
The mouse CD19 cDNA was isolated from murine splenic B cells by reverse transcriptase–polymerase chain reaction. In the Y2FCD19 mutant construct, mouse CD19 was mutated by oligonucleotide-directed mutagenesis to change Tyr (corresponding to 482 and 513 in the human CD19) to Phe. These cDNAs encoding wild-type and mutant CD19 were subcloned into the pAzeo expression vector.24 The resulting constructs were transfected into wild-type DT40 cells and its derived mutant cells by electroporation and selected in the presence of zeocin (1 mg/mL). Cell-surface levels of mouse CD19 were examined by flow cytometry analysis using biotin-conjugated antimouse CD19 monoclonal antibody (mAb) 1D3 and fluorescein isothiocyanate (FITC)–conjugated streptavidin.
Antibodies
Rabbit sera raised against chicken BCAP, mouse BCAP, the cytoplasmic domain of mouse CD19, chicken Syk, and chicken Lyn were described previously.18 19 The following antibodies (Abs) were purchased: biotin-conjugated antimouse CD19 mAb 1D3 (PharMingen, San Diego, CA), biotin-conjugated control rat IgG2a mAb (Immunotech, Marseille, France), antihuman CD19 mAb B4 (Immunotech), control mouse IgG1 mAb (PharMingen), rabbit anti–mouse IgG F(ab′)2fragment (Chemicon, Temecula, CA), antiphosphotyrosine mAb 4G10 (Upstate Biotechnology, Lake Placid, NY), anti-p85 Ab (Upstate Biotechnology), anti-Btk mAb (PharMingen), anti-Fyn Ab (Oncogene, Cambridge, MA), anti-Akt1 Ab (Santa Cruz Biotechnology, Santa Cruz, CA), and goat anti–mouse IgM F(ab′)2 fragment (Jackson ImmunoResearch Laboratories, West Grove, PA). Streptavidin was purchased from Chemicon.
Cell activation
Ligation of CD19 on purified splenic B cells and DT40 cells expressing mouse CD19 was achieved by incubation with 1 μg/mL biotin-conjugated antimouse CD19 mAb 1D3 or biotin-conjugated control IgG2a mAb at 37°C, followed by 20 μg/mL streptavidin at 37°C for varying times. Engagement of CD19 on human Raji B cells was conducted by incubation with 1 μg/mL antihuman CD19 mAb B4 or control IgG1 mAb at 37°C, followed by cross-linking with rabbit anti–mouse IgG F(ab′)2 fragment (20 μg/mL) at 37°C for the indicated times. For BCR stimulation, murine splenic B cells were incubated with 15 μg/mL anti–mouse IgM F(ab′)2 fragment at 37°C.
Immunoprecipitation and Western blot analysis
For immunoprecipitation, cells were solubilized in lysis buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Nonidet P-40) supplemented with protease and phosphatase inhibitors, as described previously.20 Lysates were precleared by incubation with protein A–agarose (except in the case of immunoprecipitation with anti-CD19 Ab) and subsequently incubated with proper Abs and protein A–agarose. Immunoprecipitates or whole-cell lysates were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA), and detected by the indicated Abs and enhanced chemiluminescence system (Amersham Pharmacia Biotech, Uppsala, Sweden).
In vitro kinase assay of Akt
In vitro kinase assay of Akt using histone H2B as an exogenous substrate was performed as described previously.18
Phosphoinositide analysis
Thin-layer chromatography (TLC) analyses of phosphoinositides were performed by 32P labeling of cells, extraction of lipids, and TLC analysis as described previously.18 The radioactivity of phosphatidylinositol-3,4,5-trisphosphate (PI[3,4,5]P3) spots was quantified by using a Fuji FLA2000 bioimaging analyzer (Fuji Photo Film, Tokyo, Japan) and was normalized to the total radioactivity in phospholipids of the samples. The resulting values were then normalized to the value at time zero of CD19 stimulation for each of 3 independent experiments and averaged.
Results
Cross-linking CD19 activates Akt in B cells
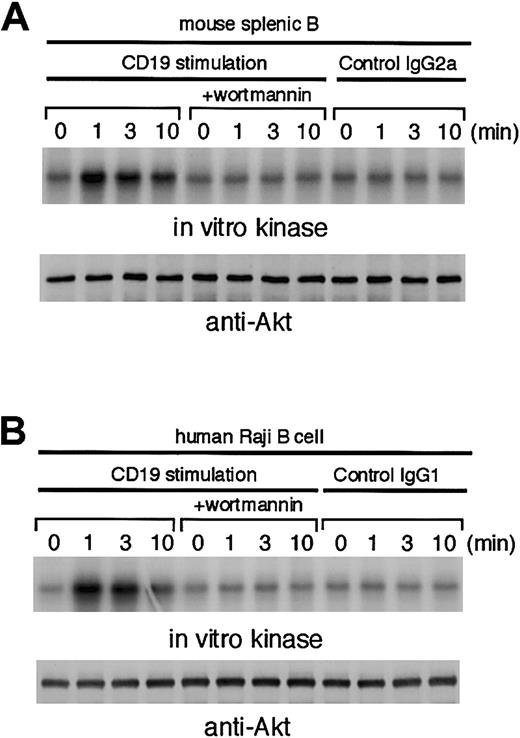
Although it is well documented that ligating CD19 induces the formation of a CD19/PI3K complex in human B-cell lines,14it is still unclear whether ligating CD19 alone can evoke PI3K activation or not. Thus, we first examined CD19-mediated activation of Akt, as a readout of PI3K activation, in murine splenic B cells. An in vitro kinase assay was performed on anti-Akt immune complexes isolated from unstimulated and stimulated B cells. As shown in Figure1A, CD19-mediated activation of Akt in primary B cells was maximal at 1 minute, after which activity declined. As a negative control, substitution of isotype-matched control mAb for anti-CD19 mAb did not elicit activation of Akt. This CD19-mediated activation of Akt was abolished by prior treatment with 50 nM wortmannin. The above observations are not specific for murine B cells because human Raji B cells also exhibited Akt activation upon CD19 cross-linking (Figure 1B). From these data, we conclude that engagement of CD19 indeed induces PI3K activation, which in turn up-regulates Akt.
Ligation of CD19 on mouse and human B cells results in Akt activation.
Treatment with 50 nM wortmannin was conducted 10 minutes before stimulation. Akt1 was immunoprecipitated, and the precipitates were divided. Half of them were used for Western blot analysis using anti-Akt1 Ab (lower panels). The remaining half were used for in vitro kinase assay using histone H2B as an exogenous substrate. The kinase reaction products were resolved on 15% SDS-PAGE, and their phosphorylation was quantified by autoradiography (upper panels). (A) Purified splenic B cells from C57BL/6 mice (1 × 107) were incubated with 1 μg/mL biotin-conjugated antimouse CD19 mAb 1D3 or biotin-conjugated control IgG2a for 3 minutes and subsequently cross-linked by using streptavidin (20 μg/mL) at 37°C for indicated times. (B) Human Raji B cells (5 × 106) were incubated with 1 μg/mL antihuman CD19 mAb B4 or control IgG1 for 3 minutes, followed by cross-linking with anti–mouse IgG F(ab′)2fragment (20 μg/mL) at 37°C.
Ligation of CD19 on mouse and human B cells results in Akt activation.
Treatment with 50 nM wortmannin was conducted 10 minutes before stimulation. Akt1 was immunoprecipitated, and the precipitates were divided. Half of them were used for Western blot analysis using anti-Akt1 Ab (lower panels). The remaining half were used for in vitro kinase assay using histone H2B as an exogenous substrate. The kinase reaction products were resolved on 15% SDS-PAGE, and their phosphorylation was quantified by autoradiography (upper panels). (A) Purified splenic B cells from C57BL/6 mice (1 × 107) were incubated with 1 μg/mL biotin-conjugated antimouse CD19 mAb 1D3 or biotin-conjugated control IgG2a for 3 minutes and subsequently cross-linked by using streptavidin (20 μg/mL) at 37°C for indicated times. (B) Human Raji B cells (5 × 106) were incubated with 1 μg/mL antihuman CD19 mAb B4 or control IgG1 for 3 minutes, followed by cross-linking with anti–mouse IgG F(ab′)2fragment (20 μg/mL) at 37°C.
BCAP is tyrosine phosphorylated upon CD19 engagement
Having demonstrated CD19-mediated activation of Akt in B cells, we reasoned that this activation could be accounted for by tyrosine phosphorylation of Tyr482xxM and Tyr513xxM motifs in CD19. Hence, we assessed the tyrosine phosphorylation status of CD19 after its ligation. Consistent with a previous report,16 CD19 by itself in murine splenic B cells was slightly tyrosine phosphorylated before stimulation, and this phosphorylation was marginally augmented by ligating CD19 (Figure2A). In addition, immunoprecipitates of CD19 were examined for its association with PI3K p85 from resting and CD19-activated B cells. As shown in Figure 2A, although a small degree of basal association was observed, the inducible association between CD19 and PI3K p85 could not be detected. In contrast to ligating CD19, BCR-mediated tyrosine phosphorylation of CD19 was much enhanced, and its subsequent association with PI3K p85 was clearly observed in murine splenic B cells (Figure 2A). Therefore, these data suggest that in the CD19 ligation context, tyrosine phosphorylation of Tyr482xxM and Tyr513xxM motifs may not play a major role in PI3K activation.
Engagement of CD19 on mouse B cells induces tyrosine phosphorylation of BCAP and its association with PI3K p85.
Splenic B cells (4 × 107) were stimulated by CD19 cross-linking as in Figure 1A or by BCR cross-linking using goat anti–mouse IgM F(ab′)2 fragment (15 μg/mL) and subjected to the following analyses. (A) Immunoprecipitates with anti-CD19 Ab were separated on 7.5% SDS-PAGE and analyzed by Western blotting with antiphosphotyrosine mAb 4G10 (upper panel), anti-CD19 Ab (middle panel), or anti-p85 Ab (lower panel). (B) Immunoprecipitates with anti-BCAP Ab were separated on 7.5% SDS-PAGE and analyzed by Western blotting with antiphosphotyrosine mAb 4G10 (upper panel), anti-BCAP Ab (middle panel), or anti-p85 Ab (lower panel). The positions of the multiple mouse BCAP species, long (100 and 98 kd) and short (72 and 70 kd) isoforms, are indicated by brackets.
Engagement of CD19 on mouse B cells induces tyrosine phosphorylation of BCAP and its association with PI3K p85.
Splenic B cells (4 × 107) were stimulated by CD19 cross-linking as in Figure 1A or by BCR cross-linking using goat anti–mouse IgM F(ab′)2 fragment (15 μg/mL) and subjected to the following analyses. (A) Immunoprecipitates with anti-CD19 Ab were separated on 7.5% SDS-PAGE and analyzed by Western blotting with antiphosphotyrosine mAb 4G10 (upper panel), anti-CD19 Ab (middle panel), or anti-p85 Ab (lower panel). (B) Immunoprecipitates with anti-BCAP Ab were separated on 7.5% SDS-PAGE and analyzed by Western blotting with antiphosphotyrosine mAb 4G10 (upper panel), anti-BCAP Ab (middle panel), or anti-p85 Ab (lower panel). The positions of the multiple mouse BCAP species, long (100 and 98 kd) and short (72 and 70 kd) isoforms, are indicated by brackets.
One possibility to explain the CD19-mediated PI3K activation is the involvement of another YxxM motif–containing protein(s) because several proteins in addition to CD19 are also tyrosine phosphorylated by ligating CD19.15,25,26 In this regard, we recently cloned a novel B-cell adaptor molecule, BCAP, that contains 4 YxxM motifs. Hence, we attempted to determine whether BCAP is tyrosine phosphorylated by ligating CD19. Two transcripts are generated, presumably by alternative initiation or splicing, giving rise to 2 major protein isoforms (100- and 98-kd long isoforms and 72- and 70-kd short isoforms).18 As demonstrated in Figure 2B, tyrosine phosphorylation of both BCAP isoforms was induced upon CD19 ligation alone in murine splenic B cells. There was basal association between PI3K p85 and BCAP, and this association was further increased by ligating CD19, which suggests that the YxxM motif(s) in BCAP is phosphorylated in this context.
Phosphorylation of YxxM motifs in BCAP is critical for CD19-mediated PI3K activation
To examine the effect of BCAP on PI3K activation in CD19 signaling, we have used the BCAP-deficient DT40 B-cell line generated by gene targeting.18 Wild-type and BCAP-deficient DT40 B cells were transfected with mouse CD19 and selected in zeocin-containing media. Among various stable clones, clones expressing levels of CD19 similar to those of murine splenic B cells were selected (Figure 3A). When wild-type DT40 cells were stimulated by ligating CD19 alone, Akt activation and BCAP tyrosine phosphorylation were induced, although their peaks (10 minutes) were relatively delayed compared with those of murine splenic B cells (Figure 4A,B). In contrast to wild-type DT40 cells, the Akt response was approximately 3-fold lower in BCAP-deficient cells (Figure 4A). This inhibition extent was correlated with that of CD19-mediated PI(3,4,5)P3accumulation in mutant cells (Figure 4C). Hence, these results demonstrate an important role for BCAP in CD19-mediated PI3K activation. The observed CD19-mediated responses (Akt activation and BCAP phosphorylation) in DT40 B cells are apparently independent of the BCR expression in that these responses occurred normally even in the BCR-deficient DT40 cells expressing mouse CD19 (Figure 3A,C; Figure 4A,B).
Generation of DT40 mutant cells expressing mouse CD19.
(A) Cell-surface expression levels of mouse CD19 on various mutant DT40 cells and of endogenous CD19 on purified murine splenic B cells. Flow cytometry analysis was performed by using biotin-conjugated antimouse CD19 mAb 1D3 followed by FITC-conjugated streptavidin (solid lines). Dotted lines show staining with isotype-matched control IgG2a. (B) Expression of various signaling molecules in various mutant DT40 cells was analyzed by Western blotting using indicated Abs. (C) Cell-surface expression of BCR was analyzed using FITC-conjugated anti–chicken IgM. Unstained cells were used as negative controls (dotted line).
Generation of DT40 mutant cells expressing mouse CD19.
(A) Cell-surface expression levels of mouse CD19 on various mutant DT40 cells and of endogenous CD19 on purified murine splenic B cells. Flow cytometry analysis was performed by using biotin-conjugated antimouse CD19 mAb 1D3 followed by FITC-conjugated streptavidin (solid lines). Dotted lines show staining with isotype-matched control IgG2a. (B) Expression of various signaling molecules in various mutant DT40 cells was analyzed by Western blotting using indicated Abs. (C) Cell-surface expression of BCR was analyzed using FITC-conjugated anti–chicken IgM. Unstained cells were used as negative controls (dotted line).
YxxM motifs in BCAP are required for activation of PI3K/Akt in response to CD19 stimulation.
Wild-type and its derived mutant DT40 cells expressing mouse CD19 were stimulated and subsequently analyzed as described in Figures 1 and 2. (A) CD19-induced Akt activation. (B) Tyrosine phosphorylation of BCAP and its association with p85. The positions of the multiple chicken BCAP species, long (100 and 98 kd) and short (72 and 70 kd) isoforms, are indicated by brackets. As a negative control experiment, BCAP-deficient DT40 cells were also analyzed. (C) Generation of PI(3,4,5)P3. Cells loaded with [32P]orthophosphate were incubated with or without 50 nM wortmannin for 10 minutes and subsequently stimulated. PI(3,4,5)P3 generation was analyzed as described in “Materials and methods.” Fold increases of PI(3,4,5)P3levels normalized to total phospholipids after CD19 stimulation are shown. The results are shown as mean ± SE of 3 independent experiments.
YxxM motifs in BCAP are required for activation of PI3K/Akt in response to CD19 stimulation.
Wild-type and its derived mutant DT40 cells expressing mouse CD19 were stimulated and subsequently analyzed as described in Figures 1 and 2. (A) CD19-induced Akt activation. (B) Tyrosine phosphorylation of BCAP and its association with p85. The positions of the multiple chicken BCAP species, long (100 and 98 kd) and short (72 and 70 kd) isoforms, are indicated by brackets. As a negative control experiment, BCAP-deficient DT40 cells were also analyzed. (C) Generation of PI(3,4,5)P3. Cells loaded with [32P]orthophosphate were incubated with or without 50 nM wortmannin for 10 minutes and subsequently stimulated. PI(3,4,5)P3 generation was analyzed as described in “Materials and methods.” Fold increases of PI(3,4,5)P3levels normalized to total phospholipids after CD19 stimulation are shown. The results are shown as mean ± SE of 3 independent experiments.
To further substantiate the importance of BCAP, we determined the requirement for the YxxM motifs of BCAP in CD19-mediated Akt activation. For this purpose, BCAP-deficient DT40 cells expressing CD19 were transfected with the wild-type BCAP cDNA encoding 100- and 98-kd long isoforms and its mutant cDNA (Y4F).18 This mutant cDNA was designed to change the 4 potential YxxM motifs to FxxM sequences. Clones expressing similar levels of exogenous BCAP as the endogenous BCAP were selected and analyzed (Figure 3B). In contrast to wild-type BCAP, CD19-mediated tyrosine phosphorylation of Y4FBCAP mutant was significantly decreased (Figure 4B), suggesting that these sites are indeed phosphorylated by ligating CD19 alone. The mutant BCAP failed to associate with PI3K p85 subunit (Figure 4B) or to restore the defective Akt activation in CD19 signaling (Figure 4A). Hence, these data further strengthen our conclusion that BCAP, after being phosphorylated, provides the docking site(s) for the p85 SH2 domains, thereby participating in CD19-mediated PI3K activation. However, the residual Akt activation in BCAP-deficient DT40 B cells suggests the existence of an alternative Akt activation mode(s) in CD19 signaling.
Lyn participates in BCAP phosphorylation
To identify the protein tyrosine kinases (PTKs) responsible for BCAP tyrosine phosphorylation upon CD19 cross-linking, we transfected DT40 cell lines lacking Lyn, Syk, or Btk with mouse CD19. After selecting the clones expressing similar levels of CD19 (Figure 3A,B), we examined the tyrosine phosphorylation status of BCAP. In contrast to wild-type DT40 cells, Syk-deficient B cells exhibited the high level of BCAP phosphorylation before stimulation, whereas the CD19-mediated phosphorylation on BCAP short isoforms was diminished in Btk-deficient cells (Figure 5A). Nevertheless, both mutant cells elicited BCAP phosphorylation upon CD19 ligation. However, this phosphorylation could not be detected in Lyn-deficient cells. Because DT40 B cells dominantly express Lyn among Src-family PTKs,19 we sought to determine whether the defect in Lyn-deficient cells can be compensated for by expression of other Src PTKs such as Fyn. For this purpose, Lyn-deficient cells expressing either wild-type or kinase-negative Fyn19 21 were transfected with mouse CD19 cDNA (Figure 3A,B; Figure 5B, right). As demonstrated in Figure 5B, wild-type Fyn, but not kinase-negative Fyn, restored the defect in BCAP phosphorylation in Lyn-deficient DT40 B cells, implicating functional redundancy between Lyn and Fyn. Furthermore, our data demonstrate the requirement for kinase activity of Src PTKs in CD19-mediated BCAP phosphorylation. Consistent with our conclusion that tyrosine phosphorylation of BCAP is important for CD19-mediated Akt activation, Lyn-deficient DT40 cells failed to induce Akt activation (Figure 5C).
BCAP tyrosine phosphorylation in DT40 cells deficient in Lyn, Syk, or Btk.
Wild-type and its derived mutant DT40 cells expressing mouse CD19 were stimulated with CD19 and subsequently subjected to the following analyses. (A,B, left panels) Immunoprecipitation was performed with anti-BCAP Abs. The blots were stained with 4G10 mAb (upper panels) and anti-BCAP Abs (lower panels). Expression of wild-type (wt) Fyn or kinase-negative (kn) Fyn is shown by Western blot analysis using anti-Fyn Abs (B, right panel). (C) CD19-induced Akt activation in Lyn-deficient DT40 B cells.
BCAP tyrosine phosphorylation in DT40 cells deficient in Lyn, Syk, or Btk.
Wild-type and its derived mutant DT40 cells expressing mouse CD19 were stimulated with CD19 and subsequently subjected to the following analyses. (A,B, left panels) Immunoprecipitation was performed with anti-BCAP Abs. The blots were stained with 4G10 mAb (upper panels) and anti-BCAP Abs (lower panels). Expression of wild-type (wt) Fyn or kinase-negative (kn) Fyn is shown by Western blot analysis using anti-Fyn Abs (B, right panel). (C) CD19-induced Akt activation in Lyn-deficient DT40 B cells.
Mutation of 2 YxxM motifs in CD19 does not impair CD19-mediated Akt response in DT40 B cells
To formally demonstrate the dispensability for phosphorylation of Tyr482xxM and Tyr513xxM motifs of CD19 in Akt activation, we transfected DT40 B cells with the mutant CD19 in which 2 YxxM motifs were mutated to FxxM sequences (Y2FCD19). As shown in Figure 6A, this mutant CD19 was still able to elicit the same level of Akt activation as wild-type CD19. Furthermore, the Y2FCD19 mutant showed a similar level of tyrosine phosphorylation as wild-type CD19 in DT40 cells, judged by antiphosphotyrosine mAb blotting (Figure 6B), which suggests that these 2 sites may not be phosphorylated by CD19 ligation alone. Like exogenously expressed human CD19,17 2 protein isoforms of mouse CD19 were detected in DT40 B cells (Figure 6B), and the upper one was expressed on the cell surface as assessed by cell-surface labeling experiments (data not shown).
CD19-mediated Akt activation is not affected by mutation of 2 YxxM motifs (Tyr482Phe, Tyr513Phe) in CD19.
DT40 cells expressing mouse wild-type CD19 or Y2FCD19 mutant (Tyr482, Tyr513 changed to Phe482, Phe513) were stimulated and subsequently subjected to the following analyses. (A) CD19-induced Akt activation. (B) Tyrosine phosphorylation of CD19.
CD19-mediated Akt activation is not affected by mutation of 2 YxxM motifs (Tyr482Phe, Tyr513Phe) in CD19.
DT40 cells expressing mouse wild-type CD19 or Y2FCD19 mutant (Tyr482, Tyr513 changed to Phe482, Phe513) were stimulated and subsequently subjected to the following analyses. (A) CD19-induced Akt activation. (B) Tyrosine phosphorylation of CD19.
Discussion
Expression of the CD19/CD21 complex provides not only an enhancing signal for lowering the threshold of B-cell activation, but also mediates a survival signal for B cells.2,3,13 Here, we demonstrate that ligating CD19 alone activates PI3K, leading to subsequent Akt activation in B cells. The molecular mechanism underlying the CD19-mediated PI3K activation was thought to involve the phosphorylation of Tyr482xxM and Tyr513xxM motifs in CD19, which, through its association with the PI3K p85 SH2 domains, results in the activation of PI3K.14
Several observations, however, lead us to conclude that this mechanism is not dominantly operating. First, tyrosine phosphorylation of CD19 by its ligation in murine splenic B cells was barely detectable (Figure2A). Second, although the BCR-induced association between PI3K p85 and CD19 was clearly observed in primary mouse B cells, we were unable to detect this inducible association in the CD19 signaling context (Figure2A), which implies that ligating CD19 alone does not induce phosphorylation on tyrosines Tyr482xxM and Tyr513xxM in CD19. However, because antibodies toward phospho-Tyr482 and phospho-Tyr513 are currently not available, we cannot completely exclude the alternative possibility that the low level of phosphorylation on Tyr482xxM and Tyr513xxM indeed occurs, but that this level is not sufficient to recruit the SH2 domains of PI3K p85. Finally, the Y2FCD19 mutant (Tyr482Phe, Tyr513Phe) was still able to activate Akt to the same extent as wild-type CD19 in DT40 B cells (Figure 6A).
Thus, these results prompted us to reexamine the CD19-mediated PI3K activation mechanism(s) in B cells. In this regard, we recently purified a novel B-cell adaptor forPI3K, termed BCAP, by using the p85 SH2 domain.18 Two major isoforms, presumably generated by alternative initiation or splicing, contain 4 YxxM motifs that, when tyrosine phosphorylated upon BCR cross-linking, form binding sites for the p85 SH2 domains. The results presented here show that not only BCR, but also CD19 ligation alone, induces tyrosine phosphorylation of both BCAP isoforms in mouse and chicken B cells (Figures 2B and 4B). Moreover, the above-mentioned CD19-mediated responses, namely BCAP phosphorylation and Akt activation, occur independently of BCR expression because the BCR-deficient DT40 B cells were still able to induce these responses (Figure 4A,B).
The importance of BCAP in CD19-mediated PI3K activation was highlighted by the inhibition of both PI(3,4,5)P3generation (Figure 4C) and the subsequent Akt activation (Figure 4A) in BCAP-deficient DT40 B cells. In addition, the BCAP mutant, in which the 4 potential YxxM motifs were changed to FxxM sequences, showed a decreased level of tyrosine phosphorylation relative to wild-type BCAP (Figure 4B), thereby being unable to restore the defect of Akt activation in BCAP-deficient cells (Figure 4A). Given the recent evidence that the CD19/CD21 complex is translocated into glycosphingolipid-enriched microdomains (GEMs) by its ligation alone,27 GEM-associated Lyn could be activated upon translocation of CD19 into GEMs,16,28,29 thereby mediating tyrosine phosphorylation of BCAP. Indeed, Lyn was apparently required for CD19-mediated BCAP phosphorylation in DT40 B cells (Figure 5A). Two mechanisms by which BCAP mediates PI3K activation have been suggested by previous studies.30-32 First, binding of PI3K with BCAP per se could up-regulate the PI3K enzymatic activity, as previously reported for other p85-binding proteins.30,31 Second, assuming that redistribution of PI3K to GEMs is a prerequisite for its activation,32 BCAP could be involved in CD19-mediated translocation of PI3K to GEMs. In this regard, we have been unable to demonstrate a significant decrease of the PI3K recruitment to GEMs in BCAP-deficient DT40 cells compared with wild-type DT40 cells (data not shown), suggesting that an as yet unidentified molecule plays a major role in translocation of PI3K to GEMs in the CD19 signaling context. Thus, the concerted actions of this candidate molecule and BCAP are likely required for full PI3K activation.
PH domain–containing PI3K targets, including Akt, Btk, and Vav, are thought to mediate PI3K actions. For instance, because the selective binding of the Vav PH domain with PI(3,4,5)P3, a product of PI3K activity, is important for the subsequent Rac/Cdc42-dependent JNK/p38 activation,33 the CD19-mediated PI3K activation likely lies upstream of Vav-mediated activation of the JNK/p38 pathway. In addition, the CD19-mediated activation of Btk and Akt via PI3K, similar to the BCR signaling context,34 could participate in calcium mobilization and NF-κB activation, respectively. Hence, given the importance of these downstream signaling events in B-cell activation and survival, our data imply that BCAP serves as one of the key components in regulating the biologic functions of the CD19/CD21 complex.
We thank Drs T. Okada, T. Yamazaki, M. Ishiai, and A. Hashimoto for helpful discussion and M. Kurosaki and N. Narumai for expert technical assistance.
Supported by grants from the Ministry of Education, Sciences, Sports, and Culture of Japan (K.I., T.K.); the Uehara Foundation (T.K.); and the Toray Science Foundation (T.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tomohiro Kurosaki, Dept of Molecular Genetics, Institute for Liver Research, Kansai Medical University, Moriguchi 570-8506, Japan; e-mail: kurosaki@mxr.mesh.ne.jp.



![Fig. 4. YxxM motifs in BCAP are required for activation of PI3K/Akt in response to CD19 stimulation. / Wild-type and its derived mutant DT40 cells expressing mouse CD19 were stimulated and subsequently analyzed as described in Figures 1 and 2. (A) CD19-induced Akt activation. (B) Tyrosine phosphorylation of BCAP and its association with p85. The positions of the multiple chicken BCAP species, long (100 and 98 kd) and short (72 and 70 kd) isoforms, are indicated by brackets. As a negative control experiment, BCAP-deficient DT40 cells were also analyzed. (C) Generation of PI(3,4,5)P3. Cells loaded with [32P]orthophosphate were incubated with or without 50 nM wortmannin for 10 minutes and subsequently stimulated. PI(3,4,5)P3 generation was analyzed as described in “Materials and methods.” Fold increases of PI(3,4,5)P3levels normalized to total phospholipids after CD19 stimulation are shown. The results are shown as mean ± SE of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.584/6/m_h80221990004.jpeg?Expires=1769329874&Signature=3Rz01SXshyuA2unliqRP15OP-61IL3OvDhg8f-6SUr8N1eoUfp5Gu4FyUBx94--tcGnNq6B8ZR5NUuRCiXJvEsA0Gnl38wa9ZgBdvgdifa6cSgOfOMfl3zc~vF~WiBF3SFlbwz-msgriVCoFLKpfqpkP8AzftEiXMY3gQtkYvitO8BHf893kTAk~x9-TnXp2kRGVJJBam5N61XUTswIUQN0u3t1f789aHhL4EJCr0Fsu7NH9fiXNafdq2qtJkB-8nAvePm99cA9nH53zJay4vwj~5ZPRSAuqTHhfJUhIjlqDIavCCUIyCHJWpo82TQ1J-znczt4ljWQfmXDklbRtyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


