Abstract
The disruption of retinoic acid receptor (RAR) activity that characterizes human acute promyelocytic leukemia (APL) is associated with a block to granulocytic differentiation indicating that RARs are critical regulators of normal myeloid differentiation. Nevertheless, how RAR activity might be regulated in the presumably homogenous concentration of retinoids in blood and bone marrow and how these receptors might interact with specific hematopoietic cytokines to regulate normal myeloid differentiation remain unclear. Here, using several cytokine-dependent in vitro models of myeloid development, it was observed that specific hematopoietic cytokines that normally regulate myeloid lineage commitment and differentiation (interleukin-3 and granulocyte-macrophage colony-stimulating factor) trigger the enhancement of both ligand-dependent and ligand-independent transcriptional activity of both endogenous and exogenous (transiently transfected) RARs. This cytokine-mediated enhancement of RAR activity is not associated with any observed changes in expression of the RARs or their respective coactivators/corepressors. These studies define a previously unknown cytokine-RAR interaction during myelopoiesis and suggest that RAR activation might be a critical downstream event following interleukin-3 and granulocyte-macrophage colony-stimulating factor signaling during myeloid differentiation. This observation of ligand-independent activation of RARs that is mediated by certain cytokines represents a new paradigm with respect to how RAR activity might be modulated during hematopoiesis and also suggests a molecular basis for the differential sensitivity of human acute myelogenous leukemia cells to retinoids.
Introduction
Retinoic acid (RA), the natural acidic derivative of vitamin A (retinol), is an important regulator of embryonic development and also influences the growth and differentiation of a wide variety of adult cell types. The biologic effects of RA are generally mediated through specific ligand-activated nuclear transcription factors, the RA receptors (RARs). These receptors consist of 2 distinct families, the RARs and RXRs, with both receptors exhibiting modular structures harboring distinct DNA-binding and ligand-binding domains. These receptors likely mediate their biologic effects by binding as RAR-RXR heterodimers to regulatory elements (ie, retinoic acid response elements [RAREs] that are present in the promoters of their specific target genes).1 2
RARs play a critical role in regulating adult hematopoiesis, particularly myeloid differentiation. Knockout mice deficient in both RARα and RARγ display an in vitro block to granulocyte differentiation,3 and RA stimulates the growth and differentiation of granulocyte progenitors in cytokine-stimulated cultures of purified CD34+ cells.4Importantly, the 15;17 chromosome translocation in human acute promyelocytic leukemia (APL) generates the dominant negative PML-RARα fusion protein that inhibits the function of normal RARs,5-7 resulting in the block to terminal granulocytic differentiation that characterizes this leukemia.
Although the above evidence clearly portrays an important role for RARs in regulating myelopoiesis, several critical questions remain unanswered. What is the relationship of RAR activity to other important regulators of myelopoiesis, particularly those specific cytokines such as interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF) that regulate myeloid progenitor growth and differentiation? If RAR activity is ligand concentration–dependent, then how is RAR activity regulated in hematopoietic cells that are exposed to the uniform “physiologic” concentrations of retinoids that are presumably present in blood and bone marrow? Finally, and perhaps most importantly from a clinical standpoint, why do only the APLs exhibit a dramatic response to retinoids while the other 90% of acute myelogenous leukemias do not, even though these other acute myelogenous leukemias express normal RARs? Indeed, a central paradox related to this clinical response to all-transretinoic acid (ATRA) is that the ATRA-sensitive human myelogenous leukemias (ie, APL) harbor aberrant RARs exhibiting dominant negative activity (ie, the translocated PML-RARα fusion gene), while the other types of human myelogenous leukemias, which are generally ATRA-insensitive, express full-length RARs that by sequence analysis are normal.8
Here we describe observations in a number of cytokine-dependent in vitro models of myeloid differentiation that provide significant insight into these questions. We observe that in the multipotent stem cell factor (SCF)–dependent EML cells the transcriptional activity of endogenous RARs is significantly enhanced following a brief exposure to IL-3. Moreover, GM-CSF also enhances RAR transcriptional activity in the EML cell cultures to an even greater degree than IL-3. We also observe that these cytokines significantly enhance RAR activity in other in vitro models of myeloid differentiation. This cytokine-mediated enhancement of RAR activity represents a novel and unexpected point of convergence of 2 critical signal transduction pathways important for mediating myeloid cell commitment and differentiation and represents a new paradigm with respect to how RAR activity might be modulated during hematopoiesis.
Materials and methods
Cell cultures and reagents
EML cells9 were cultured in Iscoves modified Dulbecco medium supplemented with 5% horse serum (GIBCO, Grand Island, NY) and 1% l-glutamine. These cells are absolutely dependent upon SCF (50-100 ng/mL) (Peprotech, Rocky Hill, NJ) for proliferation and undergo rapid apoptosis when SCF is withdrawn. They grow best in 6-well plates (Costar 3516, Corning, Corning, NY) and generally reach saturation at 5 × 105 cells per milliliter. All experiments on EML cells were performed between passages 10 and 20.
The EML/GM-CSF cells were derived from the parental EML cells as follows. Following overnight exposure of the SCF-dependent EML cells to IL-3 (5 ng/mL) (Peprotech, Rocky Hill, NJ), we washed the cells and resuspended them in culture medium containing murine recombinant GM-CSF alone (2-4 ng/mL) (Peprotech). Within 24 to 48 hours following this hematopoietic growth factor switch, GM-CSF–dependent cells (referred to as EML/GM-CSF cells) were rapidly and reproducibly generated from the parental EML cells. These cultured cells proliferate indefinitely in GM-CSF and undergo rapid apoptosis within 12 to 24 hours following growth factor withdrawal.
The 32D cells10 were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and IL-3 (1-2 ng/mL). Where indicated they were washed and resuspended in the same media supplemented with GM-CSF (2-4 ng/mL) rather than IL-3.
Primary cultures of highly enriched hematopoietic precursors were initiated by isolating lin−, Sca 1+, kit+ cells from normal mouse bone marrow using fluorescence-activated cell sorting (FACS) and the appropriate antibodies as we have previously detailed.11 These cells were then cultured in 6-well plates in a combination of 4 hematopoietic growth factors including SCF, flt 3 ligand, IL-6, and IL-11 together with the immobilized notch ligand, Delta-1.12 The cells cultured under these conditions for 14 to 28 days remain relatively immature, maintain a Sca 1+, kit+ surface antigen phenotype, and harbor a subpopulation of cells that retains in vivo marrow repopulating ability (B. Varnum-Finney et al, unpublished data, June 2001).
Assay of EML CFU-GM generation
EML cells were cultured in liquid suspension at 2 × 104 to 5 × 104/mL in the presence or absence of IL-3 (5 ng/mL) with or without the RAR synthetic antagonist AGN193109 (2.5 μM) obtained from Allergan Pharmaceuticals.13 Following overnight culture the cells were harvested, washed, and 104 cells were resuspended in 0.7 mL Iscoves modified Dulbecco medium supplemented with 0.75 mL of 2.2% methylcellulose (Methocult, Stem Cell Technologies, Vancouver, BC, Canada), 5% horse serum, and 10 ng/mL GM-CSF. Cultures were incubated in 12-well plates (0.7 mL/plate), and GM-CSF–dependent colonies (> 20 cells) were counted following 5 to 7 days of incubation in a humidified incubator.
Transient transfections and reporter gene assays
All cell lines were transiently transfected by electroporation as previously detailed.14 Electroporation conditions for the EML, the EML plus IL-3 cells, and the primary Sca-1+, c-kit+ hematopoietic precursors cultured in either SCF or IL-3 were 270 V and 950 microfarads. Electroporation conditions for the EML/GM-CSF cells, the primary Sca-1+, c-kit+hematopoietic precursors cultured in GM-CSF, and the 32D cells were 450 V and 700 microfarads. The electroporated cells were cultured for 5 hours prior to harvesting for luciferase as previously described.14 The luciferase reporter is the β RARE tk-LUC that harbors sequences corresponding to the RARE present in the −55 to −33 sequence of the RARβ2 promoter (AGGGTTCACCGAAAGTTCACTCG).15 As an internal control for transfection efficiency we used the PON838 plasmid, which is a β-galactosidase reporter regulated by the β-actin promoter. A total of 25 μg of the β RARE tk-LUC reporter and 40 μg of the PON838 control plasmid were generally used for each transfection. The LRXRαSN expression vector was constructed as previously detailed.16 Relative luciferase activity was calculated as the ratio of absolute luciferase value divided by a baseline value that was arbitrarily set at 1. This baseline value varied for each set of experiments and was chosen as the lowest absolute luciferase value within a particular experimental set. As a control for variable transfection efficiency, the calculated relative luciferase activity was adjusted using the β-galactosidase activity as an internal control.
RNA isolation and Northern blot analysis
Total cell RNA was extracted with guanidine hydrochloride and electrophoresed through formaldehyde denaturing gels as previously detailed.17 The gel was blotted to nylon membranes (Nytran plus, Schleicher and Schuell, Keene, NH), and these membranes were hybridized to DNA probes radiolabeled by nick translation. The nuclear receptor corepressor (N-CoR), silencing mediator of retinoic acid and thyroid hormone receptor (SMRT), and Rac-3 (ACTR) probes were obtained from Ron Evans. The GRIP-1 probe was obtained from Michael Stallcup and the SRC-1 probe from Shaun Cowley.
Protein extraction and Western blot analysis
Whole cell protein extracts were obtained by boiling cell pellets for 5 minutes in Laemmli sample buffer (3 × 104cells/μL). Cell extracts (20 μL/lane) were separated on a SDS-PAGE gradient (7%-10%), electrophoresed under reducing conditions, and electroblotted using the Mini-Trans Blot Cell (Bio-Rad, Hercules, CA) onto Polyscreen PVDF transfer membrane (NEN Life Sciences, Boston, MA). Immunoblotting was performed using antibodies obtained from Santa Cruz Biotechnology unless otherwise indicated. Peroxidase-conjugated goat anti–rabbit antibody or peroxidase-conjugated rabbit anti–mouse was used as secondary antibody (Santa Cruz Biotechnology) followed by detection with enhanced chemiluminescence (ECL kit, Amersham/Pharmacia).
Surface antigen phenotyping
The cultured cells were stained with antibodies directly conjugated with fluorescein isothiocyanate or phycoerythrin and analyzed on a FACScan (Becton Dickinson) as previously detailed.11 The antibodies used included anti-Gr-1, anti-CD11b, anti–c-kit, and anti–Sca-1 (Pharmingen).
Results
IL-3 induction of the multipotent EML cells to granulocyte/monocyte progenitors is associated with enhanced RA receptor activity
The SCF-dependent EML cell line is multipotent, exhibiting the potential to differentiate along the erythroid, lymphoid, myeloid, or mast cell lineages.9 The addition of IL-3 to these SCF-dependent EML cells induces their rapid commitment to GM-CSF–responsive granulocyte/monocyte progenitors (colony-forming units [CFU]–GM)9,14 (Figure1). Curiously, we observe that the synthetic retinoid, AGN193109, a potent competitive inhibitor of RAR activity,13 does not inhibit CFU-GM production in the SCF-dependent EML cells but significantly inhibits this IL-3–mediated enhancement of CFU-GM production in these cultures (Figure 1, compare columns 3 and 4), suggesting that this activity of IL-3 might be mediated through RARs. To test this hypothesis we used transient transfection of a luciferase reporter driven by a RARE to compare RAR transcriptional activity in the parental EML cells versus the same EML cells treated overnight with IL-3. We noted that this relatively brief (15- to 20-hour) exposure of the EML cells to IL-3 enhanced both basal and ATRA-induced reporter activity at least 3- to 5-fold (Figure2A). This enhanced reporter activity is not secondary to differences in transfection efficiency of the IL-3–treated cells because all luciferase values were corrected using a cotransfected β-galactosidase reporter as an internal control. Moreover, this increase in RAR activity is not a generalized phenomenon, because other transfected reporter constructs, including an RSV-luciferase reporter, do not display enhanced activity in the IL-3–treated EML cells (Figure 2C). This IL-3–mediated enhancement of RAR functional activity was not associated with any obvious increase in RXR or RAR protein levels in the IL-3–treated cells. Indeed, these IL-3–treated cells exhibited somewhat decreased RXR expression (Figure 2B).
A RAR antagonist inhibits IL-3 enhancement of CFU-GM production in EML cells.
CFU-GM production was assessed in EML liquid suspension cell cultures treated overnight with IL-3 with or without the RAR antagonist AGN193109 (2.5 μM) as indicated.
A RAR antagonist inhibits IL-3 enhancement of CFU-GM production in EML cells.
CFU-GM production was assessed in EML liquid suspension cell cultures treated overnight with IL-3 with or without the RAR antagonist AGN193109 (2.5 μM) as indicated.
IL-3 enhances the transcriptional activity of endogenous RARs in EML cells.
(A) EML cells with or without overnight (15- to 18-hour) treatment with IL-3 were transfected with the RXR-RAR–responsive RARE tk-LUC reporter, and relative luciferase activity was determined in cell extracts after 5 hours of culture in the indicated concentration of ATRA. Calculated luciferase activity was normalized for transfection efficiency using a β-galactosidase reporter regulated by the β-actin promoter as described in “Materials and methods.” Results are the average of at least 3 independent experiments. (B) Western blot analysis of RXRα and RARα expression in the EML cells with or without overnight IL-3 and in EML/GM-CSF cells. (C) Relative luciferase activity of an RSV-luciferase reporter transfected into EML cells with or without overnight IL-3 and incubated for 5 hours in the presence or absence of ATRA (10−6 M). Results are the mean of at least 3 independent experiments.
IL-3 enhances the transcriptional activity of endogenous RARs in EML cells.
(A) EML cells with or without overnight (15- to 18-hour) treatment with IL-3 were transfected with the RXR-RAR–responsive RARE tk-LUC reporter, and relative luciferase activity was determined in cell extracts after 5 hours of culture in the indicated concentration of ATRA. Calculated luciferase activity was normalized for transfection efficiency using a β-galactosidase reporter regulated by the β-actin promoter as described in “Materials and methods.” Results are the average of at least 3 independent experiments. (B) Western blot analysis of RXRα and RARα expression in the EML cells with or without overnight IL-3 and in EML/GM-CSF cells. (C) Relative luciferase activity of an RSV-luciferase reporter transfected into EML cells with or without overnight IL-3 and incubated for 5 hours in the presence or absence of ATRA (10−6 M). Results are the mean of at least 3 independent experiments.
IL-3 enhances the activity of transfected chimeric RARs in EML cells
The EML cells were initially generated by transducing a truncated dominant negative RAR construct (RARαΔ403) into normal mouse bone marrow.9 This dominant negative RARαΔ403, which lacks the RARα COOH terminal activation domain, likely competes with normal RARs for binding to the RXR heterodimeric partner,18 and the cultured EML cells express relatively high levels of this truncated, dominant negative RAR.14 The presence of this dominant negative RARαΔ403 in the EML cells complicates the interpretation of the above studies because it is unclear whether IL-3 might be enhancing the activity of the endogenous RXR/RAR versus the truncated RXR/RARαΔ403 heterodimer. Moreover, the presence of the dominant negative RARαΔ403 might potentially mask or dilute the stimulation of any endogenous RAR activity in the IL-3–treated EML cells. We therefore performed transient transfection studies in the cultured EML cells using chimeric RAR expression vectors that harbor an intact RA ligand binding domain but which recognize and stimulate reporters driven by glucocorticoid response elements rather than RAREs19 20 (Figure 3A). These chimeric receptors are activated by RA, but their activity on the glucocorticoid response element–driven reporter should not be inhibited by the dominant negative RARαΔ403, because the latter recognizes RAREs rather than glucocorticoid response elements.
IL-3 enhances exogenous RAR activity in transfected EML cells.
(A) Mutating a 5–amino acid region within the DNA-binding domain (P-box) of RARα (EGCKG) to mimic the glucocorticoid receptor P-box (GSCKV) creates a mutated RAR (designated RARα-PGR) that now, together with its RXR partner, activates a novel DR-5 harboring a glucocorticoid response element (designated DR-5 R5G). In contrast, the wild-type RXR-RAR heterodimer neither binds to nor activates this novel DR5-R5G response element. (B) EML cells were transfected with expression vectors for both the normal RXRα and the engineered RARα-PGR together with the luciferase reporter harboring the corresponding DR-5 R5G response element. Cells were then cultured in the indicated concentration of ATRA for 5 hours and cell extract luciferase activity determined. Results are the mean of at least 3 independent experiments.
IL-3 enhances exogenous RAR activity in transfected EML cells.
(A) Mutating a 5–amino acid region within the DNA-binding domain (P-box) of RARα (EGCKG) to mimic the glucocorticoid receptor P-box (GSCKV) creates a mutated RAR (designated RARα-PGR) that now, together with its RXR partner, activates a novel DR-5 harboring a glucocorticoid response element (designated DR-5 R5G). In contrast, the wild-type RXR-RAR heterodimer neither binds to nor activates this novel DR5-R5G response element. (B) EML cells were transfected with expression vectors for both the normal RXRα and the engineered RARα-PGR together with the luciferase reporter harboring the corresponding DR-5 R5G response element. Cells were then cultured in the indicated concentration of ATRA for 5 hours and cell extract luciferase activity determined. Results are the mean of at least 3 independent experiments.
Using this approach we observed that overnight treatment of the EML cells with IL-3 markedly enhanced the activity of the transiently transfected chimeric receptor on the corresponding R5G reporter (Figure3B). This enhanced receptor activity was observed at all concentrations of ATRA, including the lower “physiologic” concentrations of 10−8 to 10−9M (Figure 3B). Thus, the addition of IL-3 to the SCF-dependent EML cells enhances the activity of both endogenous (Figure 2A) and exogenous (Figure 3B) RARs.
Derivation and characterization of GM-CSF–dependent cells from the SCF-dependent EML cells
Because the increased RAR activity that we observed in the IL-3–treated EML cells (Figures 2A, 3B) was associated with the enhanced production of GM-CSF–dependent granulocyte/monocyte progenitors (Figure 1), we wished to determine whether such committed progenitors derived from the EML cells also displayed enhanced RAR functional activity. To approach this question, as detailed in “Materials and methods,” we used the parental SCF-dependent EML cells to derive GM-CSF–dependent cell lines (designated EML/GM-CSF) that were enriched in these granulocyte/monocyte precursors.
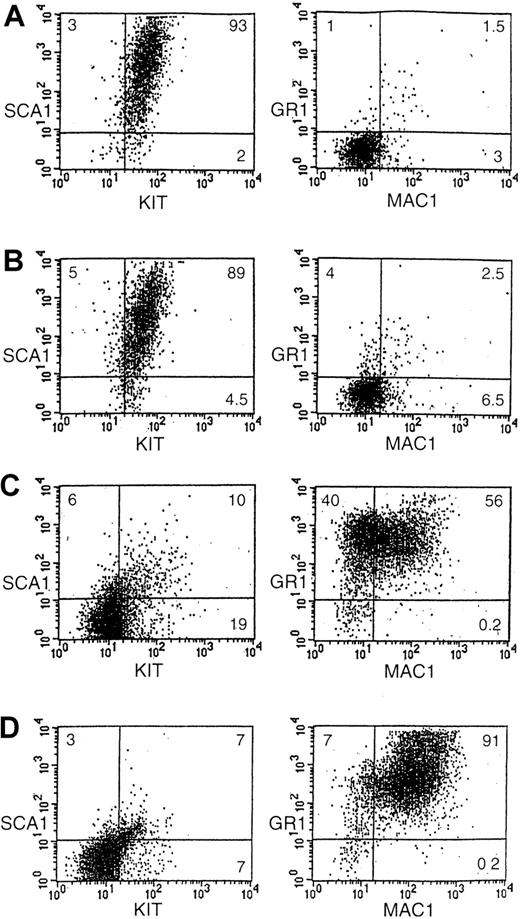
The characterization of the EML/GM-CSF cells and their comparison with the parental SCF-dependent EML cells is summarized in Figures 4 and 5. Morphologically the parental EML cells as well as the EML cells treated overnight with IL-3 appear relatively undifferentiated (Figure4A,B) and display an immature surface antigen phenotype that is predominantly Sca 1+, kit+ with few Gr1+, Cd11b mature myeloid cells present (Figure 5A,B). In contrast, the EML/GM-CSF cells display a more differentiated phenotype, morphologically resembling myeloblasts and promyelocytes (Figure 4C) with loss of Sca-1 and kit expression and a predominance in the culture of mature Gr1+, Cd11b+ myeloid cells (Figure5C). Of note is the marked difference in the response of these cells to ATRA. The addition of ATRA (10−5M) to the parental, SCF-dependent EML cells does not significantly alter the morphology or surface antigen phenotype of the cells (not shown). In contrast, ATRA enhances the generation of morphologically mature Gr-1+, Cd11b+ granulocytes in the EML/GM-CSF cultures (Figures 4D,5D). Thus, in contrast with the immature, multipotent SCF-dependent EML cultures, the EML/GM-CSF cells represent a relatively mature population of committed myeloid progenitors that displays terminal granulocytic differentiation in response to ATRA.
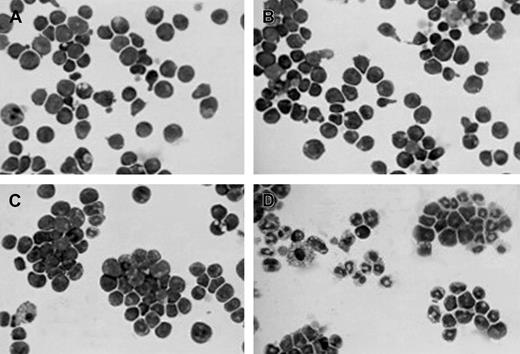
Morphology of the different cytokine-dependent EML cells.
Cytospin preparations of the indicated cells stained with Wright-Giemsa. Cells include (A) SCF-dependent EML cells, (B) EML cells treated overnight with IL-3, (C) EML/GM-CSF cells, and (D) EML/GM-CSF cells treated for 5 days with ATRA (10−6 M). Magnification is × 400.
Morphology of the different cytokine-dependent EML cells.
Cytospin preparations of the indicated cells stained with Wright-Giemsa. Cells include (A) SCF-dependent EML cells, (B) EML cells treated overnight with IL-3, (C) EML/GM-CSF cells, and (D) EML/GM-CSF cells treated for 5 days with ATRA (10−6 M). Magnification is × 400.
Surface antigen phenotype of the different cytokine-dependent EML cells.
Shown are FACS-generated histograms of Sca-1 and c-kit expression as well as Mac-1 (Cd11b) and Gr-1 expression in (A) EML cells, (B) EML cells treated overnight with IL-3, (C) EML/GM-CSF cells, and (D) EML/GM-CSF cells treated for 5 days with ATRA (10−5 M). The percent of cells in each quadrant is indicated.
Surface antigen phenotype of the different cytokine-dependent EML cells.
Shown are FACS-generated histograms of Sca-1 and c-kit expression as well as Mac-1 (Cd11b) and Gr-1 expression in (A) EML cells, (B) EML cells treated overnight with IL-3, (C) EML/GM-CSF cells, and (D) EML/GM-CSF cells treated for 5 days with ATRA (10−5 M). The percent of cells in each quadrant is indicated.
Differential activity of the RARs in EML/GM-CSF versus the parental EML cells
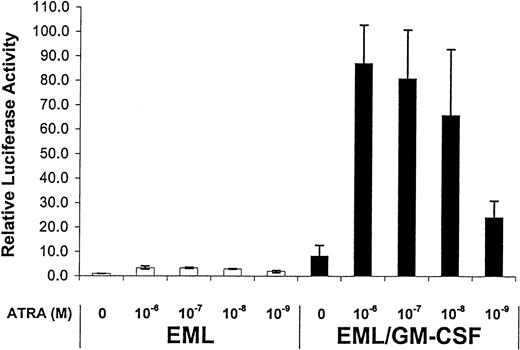
Similar to the experiments described in Figure 2, we compared the functional activity of RARs in the EML/GM-CSF cultures with the parental, SCF-dependent EML cells. We observed a relatively large (8- to 10-fold) increase in the activity of the endogenous RARs in the EML/GM-CSF cells compared with the parental EML cells (Figure6). This GM-CSF–mediated enhancement was observed for both basal as well as ATRA-induced receptor activity (Figure 6). As with the IL-3–treated EML cells, we observed no increase in expression of either RXR or RAR in the EML/GM-CSF cells compared with the parental EML cells, and indeed RXR expression appeared somewhat diminished in the EML/GM-CSF cells (Figure2B).
Enhanced RAR transcriptional activity in EML/GM-CSF cells.
The indicated cells were transfected with the RXR/RAR-responsive RARE tk-LUC reporter and then cultured for 5 hours in the indicated concentrations of ATRA. Relative luciferase activity was then determined on harvested cell extracts using a β-galactosidase reporter as an internal control as detailed in “Materials and methods.” The results represent the mean of at least 3 independent experiments.
Enhanced RAR transcriptional activity in EML/GM-CSF cells.
The indicated cells were transfected with the RXR/RAR-responsive RARE tk-LUC reporter and then cultured for 5 hours in the indicated concentrations of ATRA. Relative luciferase activity was then determined on harvested cell extracts using a β-galactosidase reporter as an internal control as detailed in “Materials and methods.” The results represent the mean of at least 3 independent experiments.
IL-3– and GM-CSF–mediated activation of RAR activity in other cultured hematopoietic cells
To determine whether the IL-3– and GM-CSF–mediated activation of RAR transcriptional activity that we observed in the EML cells extended to other in vitro models of myeloid differentiation, we assessed RAR transcriptional activity in normal mouse hematopoietic precursors (lin− c-kit+ Sca-1+) cultured, as detailed in “Materials and methods,” in liquid suspension in a mixture of hematopoietic growth factors together with the immobilized notch ligand, Delta-1.12 Similar to the EML cells, virtually all of these cultured normal hematopoietic precursors exhibit a relatively immature Sca-1+, c-kit+ surface antigen phenotype (not shown). We washed and then cultured these Sca-1+, c-kit+ cells in either SCF alone, SCF plus IL-3, or GM-CSF alone and then used transient transfection of the RARE luciferase reporter to assess the activity of endogenous RARs in these different cytokine-stimulated, immature hematopoietic precursors. Similar to our observation in EML cells, the stimulation of these SCF-dependent cells with IL-3 enhanced the transcriptional activity of the endogenous RARs, and this was evident at both relatively high (10−6 M) as well as physiologic (10−9 M) concentrations of ATRA (Figure 7). Moreover, as in EML cells, switching the cytokine from SCF alone to GM-CSF alone also resulted in a marked enhancement of RAR functional activity (Figure 7).
Cytokines regulate endogenous RAR activity in cultured hematopoietic precursors.
The lin− c-kit+ Sca-1+hematopoietic precursors were isolated from normal mouse bone marrow and cultured in liquid suspension as detailed in “Materials and methods.” After 21 days these cells were washed and then cultured for an additional 2 days in either SCF alone, SCF plus IL-3, or GM-CSF alone as indicated. These different cytokine-stimulated cells were then transfected with the RXR/RAR-responsive RARE tk-LUC reporter and cultured for an additional 5 hours in the presence or absence of the indicated concentration of ATRA. Relative luciferase activity was then determined on harvested cell extracts using a β-galactosidase reporter as an internal control.
Cytokines regulate endogenous RAR activity in cultured hematopoietic precursors.
The lin− c-kit+ Sca-1+hematopoietic precursors were isolated from normal mouse bone marrow and cultured in liquid suspension as detailed in “Materials and methods.” After 21 days these cells were washed and then cultured for an additional 2 days in either SCF alone, SCF plus IL-3, or GM-CSF alone as indicated. These different cytokine-stimulated cells were then transfected with the RXR/RAR-responsive RARE tk-LUC reporter and cultured for an additional 5 hours in the presence or absence of the indicated concentration of ATRA. Relative luciferase activity was then determined on harvested cell extracts using a β-galactosidase reporter as an internal control.
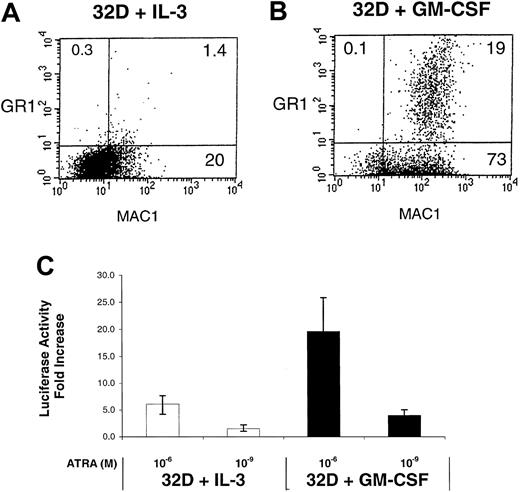
We also assessed the activity of RARs in the murine 32D myeloid cell line exposed to different cytokines. The growth of these cells is IL-3–dependent, but their differentiation to granulocytes/monocytes is induced by switching the growth factor to GM-CSF.10 We observe that the 32D cells cultured in GM-CSF alone exhibit an increase in myeloid surface antigen expression (Gr1 and CD11b) compared with the IL-3–dependent 32D cells (Figure 8A,B). This enhanced GM-CSF–mediated myeloid differentiation is associated with enhanced ATRA-induced RAR activity in these cells (Figure 8C). Thus, the cytokine-mediated enhancement of RAR activity is not a phenomenon unique to EML cells but is also observed in other cytokine-responsive in vitro models of myeloid differentiation.
Enhanced RAR activation in GM-CSF–treated 32D cells.
Shown are FACS-generated histograms of Mac-1 (Cd11b) and Gr-1 expression in (A) 32D cells cultured in IL-3 alone and (B) 32D cells cultured in GM-CSF alone (6 days). (C) The 32D cells cultured in either IL-3 or GM-CSF were transfected with the RXR/RAR-responsive RARE tk-LUC reporter, cultured for 5 hours in the presence or absence of the indicated concentration of ATRA, followed by determination of luciferase activity. The fold activation represents the ratio of uninduced versus induced luciferase activity for the different concentrations of ATRA.
Enhanced RAR activation in GM-CSF–treated 32D cells.
Shown are FACS-generated histograms of Mac-1 (Cd11b) and Gr-1 expression in (A) 32D cells cultured in IL-3 alone and (B) 32D cells cultured in GM-CSF alone (6 days). (C) The 32D cells cultured in either IL-3 or GM-CSF were transfected with the RXR/RAR-responsive RARE tk-LUC reporter, cultured for 5 hours in the presence or absence of the indicated concentration of ATRA, followed by determination of luciferase activity. The fold activation represents the ratio of uninduced versus induced luciferase activity for the different concentrations of ATRA.
Expression of nuclear hormone receptor corepressors and coactivators in the different cytokine-stimulated cells
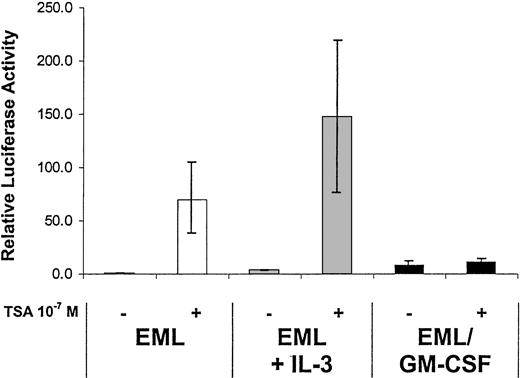
The current model of RAR activation suggests that in the absence of ligand the RXR-RAR heterodimer interacts with repressor complexes that harbor histone deacetylase (HDAC) activity and whose critical components include the corepressors N-CoR 21 and/or SMRT.22 The addition of ligand (RA) results in a distinct conformational change in the RAR, leading to the release of such corepressors and recruitment of transcriptional coactivators including SRC-1, ACTR, and GRIP-1.23 We have noted at least a 5- to 10-fold enhancement of the RAR basal activity (ie, activity in the absence of exogenous ligand) in the EML/GM-CSF cells compared with the parental EML cells (Figure 6), suggesting that the functional activity of the N-CoR/SMRT repressor complexes harboring HDAC activity may be diminished in the EML/GM-CSF cells. Interestingly, we observe that the HDAC inhibitor trichostatin A (TSA) readily activates the RA-responsive reporter in the parental EML cells and in the EML cells treated overnight with IL-3 but induces very little if any RAR activation in the EML/GM-CSF cells (Figure 9). This observation suggests that the functional activity of HDAC-containing repressor complexes may be greater in the parental EML cells compared with the more mature EML/GM-CSF cells. However, we observe no significant differences in messenger RNA or protein expression of the corepressors N-CoR or SMRT in the different cytokine-treated cells (Figure 10). Similarly, we observe no significant differences in expression of the nuclear hormone receptor coactivators SRC-1, ACTR, or GRIP-1 that might account for the differences in the functional activity of the RARs that we have observed in the different cytokine-treated EML cells (Figure10).
The HDAC inhibitor TSA activates endogenous RAR transcriptional activity in EML but not EML/GM-CSF cells.
The indicated cells were transfected with the RXR/RAR-responsive RARE tk-LUC reporter and then cultured for 5 hours in the presence or absence of TSA (10−7 M). Relative luciferase activity was then determined on harvested cell extracts using a β-galactosidase reporter as an internal control. The results represent the mean of at least 3 independent experiments.
The HDAC inhibitor TSA activates endogenous RAR transcriptional activity in EML but not EML/GM-CSF cells.
The indicated cells were transfected with the RXR/RAR-responsive RARE tk-LUC reporter and then cultured for 5 hours in the presence or absence of TSA (10−7 M). Relative luciferase activity was then determined on harvested cell extracts using a β-galactosidase reporter as an internal control. The results represent the mean of at least 3 independent experiments.
Nuclear hormone receptor corepressor/coactivator expression in the different cytokine-treated EML cells.
Northern and Western blots were performed as detailed in “Materials and methods” on RNA and protein extracts from the indicated cell types using the indicated molecular probes and antibodies.
Nuclear hormone receptor corepressor/coactivator expression in the different cytokine-treated EML cells.
Northern and Western blots were performed as detailed in “Materials and methods” on RNA and protein extracts from the indicated cell types using the indicated molecular probes and antibodies.
Discussion
The role of specific hematopoietic cytokines such as IL-3 and GM-CSF in regulating myeloid cell proliferation and differentiation is well established. Similarly, RARs, particularly RARα, must also be important regulators of granulocyte differentiation because dominant negative forms of RARα, including the well-studied PML-RARα fusion protein that characterizes human APL, generate a block to granulocytic differentiation at the promyelocyte stage. Our present observations provide a direct functional link between cytokine stimulation and RAR activation. We note an IL- or GM-CSF–mediated enhancement of RAR activity in the SCF-dependent EML cell line, in primary cultures of highly-enriched Sca-1+, c-kit+ hematopoietic precursors, and in the cytokine-dependent 32D myeloid cell line. Our observations connect 2 distinct signal transduction pathways that are known to be critical regulators of myeloid differentiation and indicate the presence of a novel and unexpected functional crosstalk linking cytokine signaling and RAR activation that occurs at different stages of myeloid cell commitment and differentiation. Given the known activity of RARs in regulating terminal granulocyte differentiation, it is likely that such RAR activation might be a critical downstream event following IL-3 and/or GM-CSF signaling during normal myeloid differentiation. Moreover, this cytokine-mediated activation of RARs may also regulate earlier events during myelopoiesis because we have observed that synthetic retinoids that inhibit RAR activity will inhibit the IL-3–mediated commitment of the multipotent EML cells to the granulocyte/monocyte lineage (Figure 1).
The RARs were originally defined by their concentration-dependent activation by RA,24 and investigators studying the role of retinoids in hematopoiesis routinely use relatively high “pharmacologic” concentrations of ATRA (10−6-10−8 M) to trigger RAR activation. However, an unresolved question relates to how RAR activity in hematopoietic cells might be differentially regulated in the presence of the invariant, homogenous “physiologic” concentration of retinoids that are presumably present in blood and bone marrow. Our studies provide insight into this question because in our different in vitro model systems we observe that specific cytokines significantly up-regulate both the baseline activity of RARs (ie, their activity in the absence of exogenous ligand) as well as the RAR activity triggered by the relatively low “physiologic” concentrations of ligand (10−8-10−9 M) that are normally present in serum (Figures 2, 6, 7, 8). These observations offer a new paradigm with respect to cytokine/RAR activity suggesting that an important role of certain hematopoietic cytokines might be to modulate the transcriptional activity of RARs at the homogenous “physiologic” ligand concentrations that are normally present in vivo.
What is the molecular basis for this cytokine-mediated enhanced transcriptional activity of the RARs that we have observed in these different in vitro models of myeloid differentiation? In the EML cells enhanced expression of the components of the RXR/RAR heterodimer cannot be responsible for this augmented receptor activity because we observe no increase in the expression levels of either RXR or RAR in the IL-3– or GM-CSF–treated cells (Figure 2B). Our observation that the HDAC inhibitor trichostatin A markedly activates receptor activity in the immature, SCF-dependent EML cells but not in the more mature EML/GM-CSF cells (Figure 9) suggests that there might be differences in the repressor complexes harboring HDAC activity that interact with the RARs in these different cell types. However, with the different cytokine exposure, we can discern no significant changes in expression of the RAR corepressors (N-CoR or SMRT) that likely mediate the interaction of HDAC-containing complexes to the RARs23 (Figure 10). Similarly, the expression of the RAR coactivators (SRC-1, GRIP-1, ACTR) appears similar in the different cytokine-treated cells (Figure 10). These observations suggest that the cytokine-mediated enhancement of RAR activity may involve posttranslational modification of either the RARs themselves or of their associated coactivators/corepressors. Site-specific phosphorylation has been previously observed to alter the activity of certain nuclear hormone receptors25,26 as well as their associated corepressors27 and coactivators.28 29 It is possible that exposure to different cytokines might trigger certain posttranslational events that modulate RAR activity at different stages of myeloid development, but such molecular events remain to be defined.
In these studies we have noted and emphasized the role of specific cytokines in modulating RAR activity in different in vitro models of myeloid differentiation. However, an alternative interpretation to consider is that the observed differences in RAR activity primarily reflect differences in the level of myeloid commitment/differentiation of these different cytokine-dependent cells. For example, the EML/GM-CSF cells are more differentiated than the parental, SCF-dependent EML cells both by morphology (Figure 4) and surface antigen expression (Figure 5), and these more differentiated EML/GM-CSF cells display considerably greater RAR transcriptional activity than the parental EML cultures (Figure 6). This differential activity of RARs at different stages of myeloid differentiation may be related to the clinically relevant differential sensitivity exhibited by human myelogenous leukemia cells to ATRA therapy. We hypothesize that this differential response of myeloid leukemia cells to ATRA reflects the underlying retinoid sensitivity of the normal hematopoietic cell lineages corresponding to these different leukemias. Thus, in myeloid leukemias corresponding to relatively immature myeloid precursors (such as the SCF-dependent EML cells), RAR activity in response to retinoids is relatively blunted. In contrast, in myeloid leukemias corresponding to a more mature myeloid progenitor (such as the more mature EML/GM-CSF cells), RAR activity in response to retinoids is enhanced. Determining the molecular basis for these differences in RAR activity that is observed at different stages of myeloid differentiation may have direct relevance to the question of why some leukemias (ie, APL) respond dramatically to retinoids while most others (the non-APL leukemias) do not.
We thank Jutta Fero for excellent technical assistance; Ron Evans and Michael Stallcup for gifts of molecular probes; Elliot Klein (Allergan Pharmaceuticals) for the gift of the RAR-PGR expression vector; Dave Flowers for aid in the surface antigen phenotyping of the cultured cells; and Carrie Stein and Barbara Varnum-Finney for the normal mouse hematopoietic precursor (lin−c-kit+ Sca-1+) cell cultures.
Supported by National Institutes of Health grant CA58292 (S.J.C.) and by National Institutes of Health grant HL54881.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven J. Collins, Human Biology Div, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle WA 98109; e-mail: scollins@fhcrc.org.