The aorta-gonads-mesonephros (AGM) region autonomously generates the first adult repopulating hematopoietic stem cells (HSCs) in the mouse embryo. HSC activity is initially localized to the dorsal aorta and mesenchyme (AM) and vitelline and umbilical arteries. Thereafter, HSC activity is found in the urogenital ridges (UGs), yolk sac, and liver. As increasing numbers of HSCs are generated, it is thought that these sites provide supportive microenvironments in which HSCs are harbored until the bone marrow microenvironment is established. However, little is known about the supportive cells within these midgestational sites, and particularly which microenvironment is most supportive for HSC growth and maintenance. Thus, to better understand the cells and molecules involved in hematopoietic support in the midgestation embryo, more than 100 stromal cell lines and clones were established from these sites. Numerous stromal clones were found to maintain hematopoietic progenitors and HSCs to a similar degree as, or better than, previously described murine stromal lines. Both the AM and UG subregions of the AGM produced many supportive clones, with the most highly HSC-supportive clone being derived from the UGs. Interestingly, the liver at this stage yielded only few supportive stromal clones. These results strongly suggest that during midgestation, not only the AM but also the UG subregion provides a potent microenvironment for growth and maintenance of the first HSCs.
Introduction
Throughout adult life, the hematopoietic hierarchy is derived from hematopoietic stem cells (HSCs) maintained in the supportive microenvironment of the bone marrow (BM). Functional blood cells arise through a series of differentiation steps first occurring within the HSCs and proceeding through a hierarchy of progenitor cell types with increasing lineage commitment.1-3 Both maintenance and differentiation of HSCs are controlled by complex interactions with the stromal microenvironment,4 which consists of several morphologically distinct cell types including myofibroblasts and macrophages.
Efforts to examine the interactions between HSCs and the microenvironment have led to the establishment of in vitro culture systems with adherent cells from BM.5,6 Further studies have demonstrated that BM and fetal liver stromal cells maintain hematopoietic progenitors, long-term culture-initiating cells, cobblestone area–forming cells, and HSCs (cells capable of permanently repopulating the entire hematopoietic system of irradiated adult recipients).7-9 Numerous stromal cell lines of adult BM and fetal liver hematopoietic microenvironments have been cloned and characterized for the growth, maintenance, and differentiation of HSCs8-10 (see also references in Remy-Martin et al11). Generally, these studies show an initial decrease in HSC activity12 followed by an expansion phase of immature hematopoietic progenitors.13 Most notably, the AFT024 stromal clone, derived from mouse fetal liver at day 14.5 of gestation, shows the best continued maintenance of HSC activity even after 6 weeks of culture.8
During early and mid-gestation, before the fetal liver becomes the major hematopoietic site, the yolk sac and intraembryonic area surrounding the dorsal aorta serve as the main hematopoietic tissues. The intraembryonic aorta-gonads-mesonephros (AGM) region exclusively and autonomously generates the first HSCs for the adult hematopoietic system at embryonic day 10.5 (E10.5) and dramatically increases HSC numbers thereafter.14,15 These studies suggest that the unique pre–fetal liver microenvironment of the AGM region plays an important role in the generation, maintenance, and perhaps even the expansion of the first HSCs in the mouse embryo.16,17Whereas yolk sac–derived stromal cell lines have been isolated and found to support the hematopoietic proliferation and differentiation of yolk sac cells18 and BM cells,19,20 to date only 2 AGM-derived stromal clones have been well characterized. The AGM-S3 clone was found to support human cord blood progenitors,21 and the DAS 104-4 clone isolated from CD34+ sorted AGM cells was found to support murine fetal liver HSCs.22,23 However, recent studies have shown that the morphologically distinct subregions of the AGM are not equivalent in HSC activity. Whereas the first HSCs are generated in the dorsal aorta and mesenchyme (AM) of the AGM at E11, the urogenital ridges (UGs) contain HSC activity at E12 or after culture of E11 explants,24 suggesting at least a role in HSC maintenance for this subregion. Thus, with an interest in uncovering and comparing the unique hematopoietic microenvironments provided by the midgestation mouse embryo, we isolated stromal cell lines from subdissected tissues of the E11 AGM region (AM and UG) and also the embryonic liver (EL) and gastrointestinal (GI) region. We comparatively assessed the capacity of stromal clones to support hematopoietic progenitors and HSCs after extended culture periods. We report here that stromal clones isolated from both AGM subregions provide a full range of supportive capacities for hematopoietic progenitors and HSCs, with several of the clones exhibiting more potent support for such cells than adult BM stromal cells. Interestingly, the UG subregion of the AGM yielded the stromal clone most highly supportive for HSCs. Furthermore, the E11 AGM region yielded more frequent supportive stroma than the E11 liver. Taken together, these results strongly suggest that the AGM region is the most supportive microenvironment for HSCs in the midgestation mouse embryo.
Materials and methods
Animals
Mice were bred at Erasmus University. (C57BL/10 × CBA)F1 mice (8-16 weeks old) were used as transplant recipients and to maintain transgenic lines. Transgenic mouse lines Ln72 (humanβ-globin)25 and BL1b (Ly-6E.1 LacZ)26 were used as donors in transplantation experiments, and transgenic lines Tag5 (PGK-tsA58), Tag11(β-actin-tsA58) (Medvinsky and Oostendorp et al, submitted), and BL1b were used for stromal cell generation.
Stromal cell lines
Stromal lines were isolated from E11 embryos (3 BL1b and 3tsAg58 litters). Day 0 of gestation was the day on which the plug was found. AGM, yolk sac, EL, and GI were dissected using 27G needles, and AGM tissue was subdissected into AM and UG.24,27 Tissues were pooled and cultured on 0.1% gelatin-coated 6-well plates (Costar, Badhoevedorp, The Netherlands) as explants or trypsinized as a single-cell suspension in stroma medium (SM: 50% long-term culture (LTC) medium; M5300, StemCell Technologies), 15% fetal calf serum (FCS), 35% α-minimal essential medium (MEM), antibiotics (penicillin and streptomycin; Gibco), Glutamax-I (Gibco), and 10 μM β-mercaptoethanol (Merck). After 4 to 5 days of culture, supernatants were collected and adherent tissues or cells were trypsinized. Cells were passaged at 5 × 104 cells/cm2 in SM supplemented with 10% to 20% 0.2 μm-filtered supernatant from the previous passage. When showing consistent growth (more than 2 passages), cells were cloned in 0.1% gelatin-coated 48-well or 24-well plates in SM with 30% conditioned medium. Under these conditions, clones could be obtained from E11 AM, UG, EL, and GI, but not from yolk sacs. Control M2-10B4 (C. Miller, StemCell Technologies), S17 (R. Hendriks, Erasmus University), and FBMD-1 cells were cultured as described.28-30 All stromal cultures were maintained at 33°C, 5% CO2 in a humid atmosphere.
Enrichment of hematopoietic cells for cocultures
Adult BM cells were suspended in α-MEM plus 5% FCS and incubated twice for 15 minutes at 37°C on Nunclon (Nunc, Breda, The Netherlands) 100-mm dishes to remove adherent cells. Nonadherent cells were separated on Percoll (d = 1.077 g/mL, 20 minutes at 1000g; Biochrom KG). Low-density cells were collected and washed in LTC medium (M5300; StemCell Technologies) for LTC–colony-forming cell (CFC) assay or triple labeled with a modified procedure31 using antibodies to CD31 (ER-MP12) and Ly-6C (ER-MP20)32 (gifts from P. Leenen) and c-kit (2B8; Pharmingen).
BM cells were incubated with anti-Ly6C (4°C, 30 minutes), washed in Hanks balanced salt solution (minus Ca and Mg; Gibco) with 2% FCS (HF/2), and incubated with goat anti–rat IgPE (Caltag) (4°C, 30 minutes). Cells were washed twice in cold HF/2 and blocked with 5% normal rat serum, followed by incubation with biotinylated anti-CD31 and anti–c-kitFITC2B8 for 30 minutes in normal rat serum. This was followed by 2 washes with HF/2 and staining with TriColor-Streptavidin (Caltag) (4°C, 30 minutes). Cells were sorted on a FACSVantage SE (Becton Dickinson), and cells were counted in trypan blue on a Neubauer hemocytometer.
AGM and yolk sac cells (E10-11) were labeled with biotinylated anti-CD34 (RAM34) and anti–c-kitFITC (Pharmingen), incubated on ice for 30 minutes, washed, incubated with PE-streptavidin for 30 minutes, washed again, sorted, and counted as described above.
LTC-CFC assay
Stromal cell lines were grown to confluence and irradiated (30 Gy) in 0.1% gelatin-coated 35-mm dishes (Costar). Control normal marrow feeders were established as adherent layers from (C57BL/10 × CBA)F1 mice and irradiated with 30 Gy when subconfluent. A total of 50 000 BM cells were cultured on stroma in LTC medium with 1 μM hydrocortisone at 33°C, 5% CO2 in a humid atmosphere. Each week, half of the medium was removed and replaced with fresh medium and hydrocortisone. After 4 to 5 weeks, nonadherent and adherent cells were harvested, pooled, and tested for CFCs.
Assays for CFCs
Numbers of committed progenitors erythroid-burst-forming unit (BFU-E), colony-forming unit-granulocyte, erythrocyte, macrophage, megakaryocyte (CFU-GEMM), and colony-forming unit-granulocyte, macrophage (CFU-GM) were determined by plating test cells in methylcellulose medium supplemented with pokeweed mitogen–spleen-conditioned medium (M3430; StemCell Technologies). Colonies containing more than 30 cells were scored after 12 days of incubation at 37°C.
Assays for long-term repopulating ability
CD31+Ly6C−c-kit+ transgenic BM cells were injected at limiting numbers into adult recipients given a split dose of 9.5 Gy from a 137Cs source.24,27,33 Normal syngeneic spleen cells (2 × 105) were coinjected to ensure their short-term survival. At more than 4 months after transplantation, peripheral blood samples (100-200 μL) were taken and genomic DNA was analyzed by polymerase chain reaction (PCR) for the presence of humanβ-globin or LacZtransgenes.14,24,26,27 Recipients were considered positive only if more than 10% of the DNA content was of donor genotype. In some cases, confirmation of percentage donor contribution was established by Southern blot analysis.14,24 27
Multilineage analysis
Blood-derived T, B, and granulopoietic cells; thymic cells; splenic T and B cells; and marrow-derived myeloid, lymphoid, and erythroid cells were sorted14,27 using directly labeled antibodies anti-CD4 (H126.19), anti-CD8a (53-6.7), anti-CD11b (M1/70), anti-CD31 (Mac-1, MEC13.3), anti-CD45R (B220, RA3-6B2) and anti-Ly-6G (Grl, RB6-8C5) from Pharmingen and anti-CD31 (ER-MP12) and anti-Ly-6C (ER-MP20) from P. Leenen. Purity of sorted cells was between 95% and 98%. Detection of the donor transgene in genomic DNA was by PCR analysis.14,24,26 27
Phenotypic analysis of stromal cells
Confluent stromal cells were irradiated (30 Gy) and grown for 2 weeks in culture flasks or overnight on glass coverslips (Nunc) in LTC medium. For FACScan (Becton Dickinson) analysis, stromal cells were trypsinized, washed, and stained with antibodies against CD31 and c-kit (Immunotech) and anti-CD34, anti–flk-1, and anti-Sca1 (Pharmingen). For immunostaining, cells were fixed (cold 35% methanol, 35% acetone, and 5% acetic acid), dried, washed with phosphate-buffered saline (PBS), and blocked (10% FCS, 0.05% Tween 20 in PBS) for 1.5 hours at room temperature (RT) while shaking. A further wash (0.5% Tween 20 in PBS) was performed and followed by staining overnight at RT with the primary antibody to smooth-muscle actin (SMA; from Dr C. Meijers, Erasmus University, Rotterdam, The Netherlands), Pax-2 (Zymed), or endoglin (Pharmingen) at the appropriate dilution in 1% FCS, 0.05% Tween 20 in PBS. After more washing, secondary antibody was added and incubated for 1 hour at 37°C. A series of washing steps was performed, followed by a wash in distilled water, before mounting and fluorescence microscope analysis (Leica DMRBE).
Matrigel cultures were performed as described by the manufacturer (Becton Dickinson) and grown in serum-free medium.
Results
Establishment of stromal cell lines and clones from embryonic subregions
Stromal cell lines and clones were isolated from the hematopoietic tissues of E11 mouse embryos transgenic for the temperature-sensitive form of the SV40 large T antigen (tsA58) immortalizing gene or a control LacZ marker gene (Medvinsky and Oostendorp et al, submitted). From the AM and UG subregions of the AGM, EL, and GI, a total of 25 primary cell lines was established. Usually a growth crisis occurred between 2 and 10 weeks of culture, followed by increases in cell number. Thereafter, the cell lines (which were assumed to be heterogeneous) were cloned. More than 100 clones from the different E11 subregional tissues were obtained. A detailed description of these cell lines and clones is provided by Medvinsky and Oostendorp et al (submitted).
Embryonic stromal cell lines and clones support CFC production in murine LTC
To determine whether the stromal lines and clones from different embryonic tissues and AGM subregions could support the sustained generation of CFCs, we performed long-term in vitro cultures (LTC-CFC). Others have shown that under such conditions, the maintenance of hematopoietic progenitor production reflects the ability of the culture to also maintain HSCs.34 Because prohibitive numbers of adult recipients would be required for an initial screen by in vivo HSC transplantation assays, the LTC-CFC assay provided the most reasonable method to begin characterizing the numerous stromal cell lines and clones for HSC supportive capacity. Adult murine BM cells were cultured on confluent, irradiated stromal cell layers for 4 to 5 weeks and assayed for clonogenic cell content. In this manner, we tested all 25 primary cell lines and 53 stromal clones. Published stromal cell lines M2-10B428 and S1735 and normal BM feeders were used as controls.
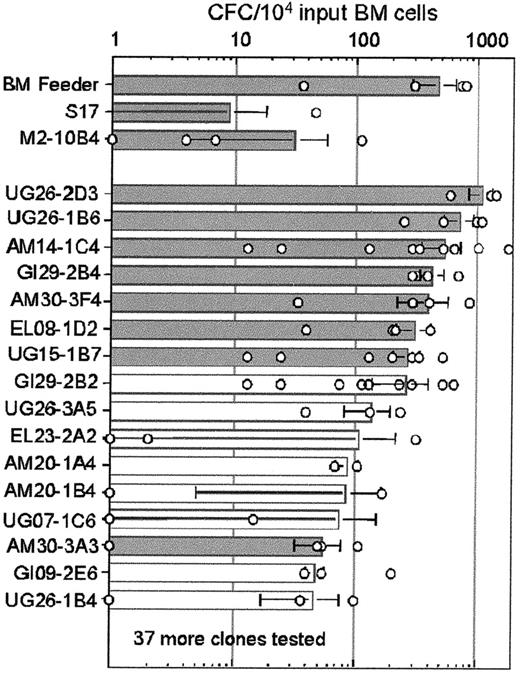
In the absence of pre-established stromal cell feeders, the light-density BM cells failed to produce any CFCs, whereas cocultures with normal BM feeders yielded a mean of 474 CFCs per 104input BM cells (range, 40-800 CFCs in 4 experiments; Figure1). The control stromal clones M2-10B4 and S17 produced a mean of 30 and 10 CFCs/104 input BM cells, respectively. Analysis of 53 embryonic stromal clones revealed a wide range of CFC supporting activity, with one clone (UG26-2D3, derived from tsA58 transgenic embryos) showing as many as 1100 CFCs/104 input cells. Interestingly, 4 other stromal cell clones were found to support the growth of more than 500 CFCs/104 input BM cells, exceeding the CFC numbers observed for the normal BM feeders. At least another 20 clones supported the growth of CFCs better than the control stromal lines. Thus, embryonic stromal cell clones are robust in their support of CFC growth from long-term cultured low-density BM cells.
Long-term support of hematopoietic progenitors by embryonic stromal cells.
Results of LTC-CFC assays are shown. All cultures were started with 5 × 104 plastic nonadherent, light-density BM cells, and the mean number of CFCs after 4 to 5 weeks of culture is shown by columns with the SD indicated. Gray columns indicate the 8 stromal clones chosen for further study. Results of individual experiments are represented by open circles. Fifty-three stromal clones and 2 control cell lines were tested. The results of only 16 clones are shown. The stromal clones not shown supported the production of fewer than 50 CFCs/104 input cells. In the absence of pre-established stromal cell feeders, no CFCs were produced. Sites of origin of clones are defined in the text. Clones derived from the tsA58transgenic embryos include UG26-2D3, UG26-1B6, GI29-2B4, AM30-3F4, GI29-2B2, UG26-3A5, EL23-2A2, AM20-1A4, AM20-1B4, AM30-3A3, and UG26-1B4. Clones derived from the LacZ transgenic embryos include AM14-1C4, EL08-1D2, UG15-1B7, UG07-1C6, and GI09-2E6.
Long-term support of hematopoietic progenitors by embryonic stromal cells.
Results of LTC-CFC assays are shown. All cultures were started with 5 × 104 plastic nonadherent, light-density BM cells, and the mean number of CFCs after 4 to 5 weeks of culture is shown by columns with the SD indicated. Gray columns indicate the 8 stromal clones chosen for further study. Results of individual experiments are represented by open circles. Fifty-three stromal clones and 2 control cell lines were tested. The results of only 16 clones are shown. The stromal clones not shown supported the production of fewer than 50 CFCs/104 input cells. In the absence of pre-established stromal cell feeders, no CFCs were produced. Sites of origin of clones are defined in the text. Clones derived from the tsA58transgenic embryos include UG26-2D3, UG26-1B6, GI29-2B4, AM30-3F4, GI29-2B2, UG26-3A5, EL23-2A2, AM20-1A4, AM20-1B4, AM30-3A3, and UG26-1B4. Clones derived from the LacZ transgenic embryos include AM14-1C4, EL08-1D2, UG15-1B7, UG07-1C6, and GI09-2E6.
To test which embryonic microenvironment best supports the production of hematopoietic progenitors, we pooled the results of all LTC-CFC experiments performed with the initial cell lines and the clones isolated from the subregions of E11 embryos (summarized in Table1). We considered cocultures that yielded more than 200 LTC-CFCs/104 input BM cells after 4 weeks as highly supportive. Even though the number of primary embryonic cell lines tested was low, they generally appeared to be poor supporters of in vitro hematopoiesis. In contrast, the clones showed a high degree of support, with 31% of AM-derived clones and 41% of UG-derived clones being highly supportive for long-term in vitro hematopoiesis. Unlike the AGM-derived stromal clones, only 6% of the EL-derived stromal clones demonstrated this high supportive capacity. Thus, stromal clones supportive for LTC-CFCs are more frequently derived from the AGM region than from the liver at E11.
Embryonic stromal cell clones support the long-term maintenance of adult repopulating HSCs
On the basis of the LTC-CFC data and site of origin, we chose 7 highly supportive clones and 1 intermediate supportive clone for further study: 3 AM-derived clones, 3 UG-derived clones, 1 EL-derived clone, and 1 GI-derived clone. These stromal clones were examined for the ability to support transplantable HSCs in LTC. Genetically marked adult BM was obtained from humanβ-globin transgenic mice,25 and HSCs were enriched by flow cytometric sorting31 of the CD31+c-kit+Ly6C− fraction. This fraction was found to contain HSCs at a frequency of 1 HSC per 300 cells (Table 1). Individual cocultures containing irradiated monolayers of stromal clones and 2000 CD31+c-kit+Ly6C− cells were established. After a 1-week or 4-week culture, the individual cocultures were harvested and injected into 4 irradiated adult recipients (equivalent to 1.6 HSCs plated at the start of the culture per recipient). Peripheral blood cells were obtained from recipients at more than 4 months after transplantation, and DNA was isolated and tested by PCR for the presence of the donor genetic marker (Table2).
After 1 week of coculture, all the stromal clones maintained HSCs. In total, 33 of 73 mice (45%) undergoing transplantation were found to be high-level-repopulated (more than 10% hematopoietic engraftment) by the progeny of donor marked HSCs. Support of HSCs ranged from 10% of recipients repopulated by coculture with the UG15-1B7 clone to 80% of recipients repopulated by coculture with the UG26-2D3 clone. Multilineage analysis of these mice demonstrated that donor cells contributed to all tested hematopoietic lineages and tissues (data not shown).
After 4 weeks of coculture with embryonic stromal clones, HSC repopulating ability was decreased (Table 2) such that only 11 of 82 recipients (15%) showed high-level engraftment. This is an overall 66% decrease in the ability of the stromal clones to support HSCs as compared with the 1-week coculture data. However, the UG26-1B6 stromal clone showed the same maintenance of HSCs (46% of recipients repopulated after both 1 and 4 weeks of coculture) compared with a closely related clone, UG26-2D3. Multilineage analysis of recipient mice (Figure 2 and data not shown) revealed that all hematopoietic cell lineages, myeloid, B cell, T cell, and erythroid obtained from peripheral blood, thymus, spleen, or BM, were highly repopulated (range, 9% to 100%; average 79%) by the progeny of the donor HSCs cultured for 4 weeks on the stromal clones. No difference in the multilineage repopulating ability of HSCs was found between the cocultures of stromal clones derived from the different subregions of the AGM; nor did the multilineage analyses show a detectable difference in HSC quality between the 1-week and 4-week cocultures (data not shown). Thus, several stromal clones from the distinct subregions of the E11 embryo can maintain HSCs for 1 to 4 weeks in coculture, with the most supportive clone, UG26-1B6, derived from urogenital ridges.
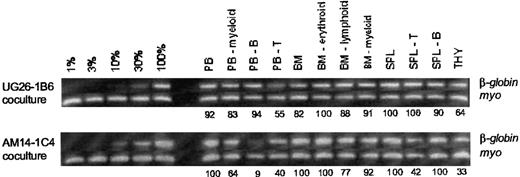
Representative long-term engraftment analysis of 2 radiation chimeric mice.
This analysis demonstrates high-level multilineage contribution in hematopoietic tissues. CD31+c-kit+Ly6C− BM-enriched HSCs were cocultured on the UG26-1B6 or the AM14-1C4 stromal clone for 4 weeks and transplanted into irradiated adult recipient mice. At 1 year after transplantation, hematopoietic tissues were obtained and several lineages were sorted. DNA from each population was tested by PCR for the presence of the donor-cell transgenic marker (humanβ-globin). The myogenin (myo) gene served as a control for DNA quantity. Percentage donor contribution to each population is indicated beneath each lane. Contribution controls of 1%, 3%, 10%, 30%, and 100% were made by mixing humanβ-globin transgenic DNA with nontransgenic DNA. Quantitation was performed by densitometry using the myosignal for DNA normalization. PB indicates peripheral blood; B, B lymphocyte; T, T lymphocyte; BM, bone marrow; SPL, spleen; and THY, thymus.
Representative long-term engraftment analysis of 2 radiation chimeric mice.
This analysis demonstrates high-level multilineage contribution in hematopoietic tissues. CD31+c-kit+Ly6C− BM-enriched HSCs were cocultured on the UG26-1B6 or the AM14-1C4 stromal clone for 4 weeks and transplanted into irradiated adult recipient mice. At 1 year after transplantation, hematopoietic tissues were obtained and several lineages were sorted. DNA from each population was tested by PCR for the presence of the donor-cell transgenic marker (humanβ-globin). The myogenin (myo) gene served as a control for DNA quantity. Percentage donor contribution to each population is indicated beneath each lane. Contribution controls of 1%, 3%, 10%, 30%, and 100% were made by mixing humanβ-globin transgenic DNA with nontransgenic DNA. Quantitation was performed by densitometry using the myosignal for DNA normalization. PB indicates peripheral blood; B, B lymphocyte; T, T lymphocyte; BM, bone marrow; SPL, spleen; and THY, thymus.
Embryonic stromal clones support the maintenance of E11 ckit+CD34+ AGM HSCs
Because some embryonic stromal clones supported the long-term maintenance of adult BM HSCs, we tested whether they would also support HSCs from the AGM region. Previously, we showed that all E11 AGM HSCs capable of repopulating irradiated adult recipient mice are CD34+c-kit+.33 Others have shown that before E10.5, CD34+c-kit+ cells from yolk sac and AGM can repopulate neonatal but not adult recipient mice.36 Thus, we sorted for early E10 and E11 CD34+c-kit+ cells from AGM or yolk sac and cocultured them on the stromal cell clones for 4 to 5 days to test for the induction and/or maintenance of adult repopulating HSCs (Table3). Cocultures initiated with CD34+c-kit+ cells from either early E10 AGM or yolk sac failed to yield any donor-derived repopulation after transplantation into adult recipients, suggesting that HSCs were not induced, were not present, or were present only in small, undetectable numbers in these cultures. Conversely, when E11 CD34+c-kit+ AGM cells were cocultured on the UG26-1B6 or the EL08-1D2 clone, in both cases 2 of 4 adult recipient mice were long-term, high-level repopulated. Multilineage analysis revealed that all the hematopoietic lineages and tissues were more than 10% donor-cell derived (ranging from 36% to 100%; data not shown). Furthermore, the cocultures allowed the repopulation of adult recipients with the equivalent of only 1000 to 2000 input E11 CD34+c-kit+ AGM cells, whereas 4000 input control cells were necessary for repopulation in the absence of the coculture step. Thus, 2 of 5 of the embryonic stromal clones tested provided potent support for E11 AGM-derived HSCs.
Characterization of stromal cell lines for subregion markers
Similar to the phenotypic heterogeneity observed in adult BM and fetal liver stroma, the isolation of mouse EL, GI, and AGM subregion stroma yielded clones of several different morphologies. It could be expected that the AM subregion yields clones of endothelial, smooth muscle, or mesenchymal phenotype, whereas those clones derived from the UG are of a pro/mesonephros, mesenchymal, or primordial germ cell phenotype.37 To discriminate the cell types represented by the highly supportive stromal cell clones, we performed immunostaining and MatriGel cultures.
Flow cytometric and immunohistochemical analysis of CD31, CD34, Flk1, and endoglin/CD105 expression revealed that most stromal clones lacked cell-surface expression of these endothelial markers (data not shown). The GI29-2B4 clone showed intermediate levels of CD34. Similarly, Flk1 was expressed at marginal levels on EL08-1D2 cells and GI29-2B4 cells, whereas endoglin was expressed at intermediate levels on AM30-3F4 cells. To examine the clones further for endothelial characteristics, we investigated tubule formation in MatriGel cultures. The AM30-3F4 and AM20-1B4 clones formed long tubules, whereas the UG26-1B6 and EL08-1D2 clones did not. However, the tubules did not express CD31 (data not shown). Thus, it is unlikely that the AM-derived stromal clones are endothelial.
None of the tested clones expressed the c-kit receptor tyrosine kinase or Pax-2 transcription factor,38 normally expressed by hematopoietic and primordial germ cells or the pro/mesonephros, respectively. However, all tested clones were positive for SMA expression, which is indicative of myofibroblasts. The UG26-1B6 and UG26-2B3 clones showed patched SMA expression, whereas the AM30-3F4 and AM20-1B4 clones showed a regular pattern of expression. Similar to SMA, all stromal clones tested expressed the Sca-1 marker, which has been found by others to be expressed on supportive stromal cells.39 Taken together, these data suggest that the stromal clones never expressed lineage markers, or lost the marker expression, indicative of their embryonic site of origin.
Discussion
Although it is well known that beginning at E10.5, the whole AGM explant contains an environment supportive of the first HSCs, there has been no clear demonstration concerning the quality of the individual cells within the AGM or its component parts to provide a microenvironment capable of maintaining HSCs or immature progenitors. In the studies presented here, we have isolated and compared more than 100 stromal cell lines and clones from the subregions of the AGM (AM and UG), the EL, and GI. Our results demonstrate that these 4 subregions from E11 mouse embryos contain potent stromal cells that can be isolated, propagated, and used to support the ex vivo long-term maintenance of hematopoietic progenitors (LTC-CFCs) and HSCs, and, in many cases, these stromal clones provide better maintenance of hematopoietic progenitors than stromal cells from adult BM.
Rare support by the E11 fetal liver microenvironment
Beginning at E9, the murine liver is actively functioning as a hematopoietic tissue,40 containing multipotential and committed hematopoietic progenitors but not adult repopulating HSCs.14,15,36 It is generally accepted that the liver is colonized by hematopoietic cells generated within the yolk sac and/or AGM region and thus, the liver is expected to be an important supportive and differentiative microenvironment for hematopoietic cells during midgestational stages.16,33 Indeed, we and others have found at late E11 to early E12 the appearance of, and thereafter a large increase in, HSC numbers in the liver, supporting this notion.33,41,42 However, until the studies presented here, no comparisons have been made among hematopoietic stroma from different midgestational embryonic sites to determine which provide the best hematopoietic support. Our comparisons of AGM-derived and liver-derived stromal clones reveal that the main microenvironment for the support of immature hematopoietic progenitors at E11 is the AGM region. Indeed, constraints on the differentiation, maintenance, and emergence of hematopoietic progenitors and HSCs may exist within the several hematopoietic microenvironments of the embryo (ie, AGM, liver, and yolk sac). In this way, our finding that only 1 of 18 isolated E11 liver stromal clones (6%) supported CFCs after 4 weeks suggests that it is not yet a highly supportive environment for the maintenance of hematopoietic progenitors. Our finding is in line with the poor ability of E11/12 livers to support HSCs in organ culture15(Kumaravelu et al, submitted). It may be that the primary function of E11/12 liver is to generate mature hematopoietic cells from HSCs immigrating from the AGM and yolk sac to fill the needs of the rapidly growing embryo (Kumaravelu et al, submitted). In contrast, Moore et al8 found at E14 that 16% of 225 isolated liver stromal clones were able to maintain cobblestone areas at 4 weeks. Although we have isolated many fewer stromal lines from the liver, these data together suggest that with time, the liver greatly increases its capacity to maintain immature progenitors by increasing supportive stromal cell frequency. With this in mind, it would be interesting to examine the embryonic origins of the liver hematopoietic supportive microenvironment and to determine whether such stroma colonize and/or expand in the liver simultaneously with or before progenitor and HSC colonization.
In addition, the GI provides more frequent hematopoietic progenitor supportive clones than the EL. This finding corresponds well to the previous observation of CFU-S8 and other progenitors in this region43 and suggests that these cells may also provide supportive function in vivo.
The UGs contain highly supportive stroma cells
The AGM was subdissected to compare the HSC-supportive capacity of the major arterial compartment of the embryo body (dorsal aorta and surrounding mesenchyme) and the developing urogenital organs (pro/mesonephros and gonads). HSC-supportive stromal clones were found in both the AM and UGs. The most highly supportive stromal clone, UG26-1B6, which maintained adult BM HSCs for at least 4 weeks in cocultures, was derived from the E11 urogenital ridges. Although we expected to find supportive stromal cells in the AM because the first HSCs emerge in this subregion, the finding of UG-derived supportive stromal clones was somewhat surprising but is consistent with the presence of HSCs in E11 UG organ-explant cultures and in vivo in E12 UGs.24 For many years, it has been known that the pronephroi provide a potent hematopoietic microenvironment in nonmammalian vertebrates, particularly amphibians.44Hematopoietic transcription factors GATA-2 and SCL have been found to be expressed in the pronephroi of amphibians and zebra fish.45,46 Similarly, the expression of several hematopoietic factors (AML-1, GATA-2, GATA-3, SCL, Sca-1) has been demonstrated in the mesonephroi of mammalian embryos (mouse) during mid- and early gestation.26 47-50 Thus, the UGs may represent a center for maintenance of stem cells per se because it is well known that the gonads are the supportive microenvironment for primordial germ cells, and our data suggest that the pro/mesonephros may play an important in vivo role in HSC maintenance before the establishment of the liver microenvironment.
Embryonic stromal clones are robust supporters of HSCs but do not appear to induce HSCs
At this time, the direct precursor cells to HSCs are unknown, but are suggested to be hemangioblasts, hemogenic endothelial cells, or mesenchymal cells residing within the environs of the major vessels of the mid-gestation embryo,16,17 or the c-kit+CD34+ neonatal repopulating cells described by Yoder et al.36 If the neonatal repopulating cells are the precursors to HSCs, it is possible that they require an inducing signal from either the AGM or liver to become HSCs. However, when we tested for the ability of 5 of our stromal clones to induce HSC activity from a candidate c-kit+CD34+ pre-HSC population from early E10 AGM and yolk sac, at a time before HSC activity is detected, no HSC activity was found after coculture with any of the stromal clones (AGM or liver derived). These results suggest either that this panel of stromal clones is unable to induce HSCs or, alternatively, that the c-kit+CD34+ fraction of E10 cells is not the precursor to HSCs. This is in contrast to the recent results with the AGM-S3 stromal clone, which is claimed to support the induction of HSCs from E8/E9 yolk sac and para-aortic splanchnopleural cells.51 Thus, in further stromal cell coculture experiments, we will examine a larger panel of stromal clones with other putative pre-HSC populations.
Is there a correlation among stromal phenotype, anatomic localization, and supportive function?
The phenotypic characteristics of our AGM stromal clones do not conform to general expectations for anatomic origins from the endothelium or mesonephros. Although AM20-1B4 and AM30-3F4 formed elongated tubules in MatriGel cultures, they did not express CD31, suggesting either that they are not endothelial or that culture conditions down-regulated the hallmark characteristics of this cell lineage. The ability to form tubules in matrigel has been found in the previously described UG-derived cell lines37 and AGM-derived stromal clone DAS104.4, which also expresses CD34.22 Interestingly, all the stromal clones tested, whether from the AM, UG, liver, or GI, expressed Sca-1, a marker found to be expressed on BM-derived hematopoietic supportive stromal cells.39 Within the E11 mouse embryo, Sca-1 is expressed at high levels in the cells lining the pro/mesonephric tubules and surrounding mesenchymal cells.26 However, none of the Sca-1+ UG-derived clones expressed the Pax-2 marker, indicative of mesonephric cells from this stage of development. Thus, Sca-1 expression by the stromal cells may be indicative of mesenchymal origins within the AGM region.
Finally, with the isolation of more than 100 E11 stromal clones, it is of long-term interest to compare the overall gene and protein expression profiles of supportive stroma and nonsupportive stroma to determine what molecules may play a role in HSC growth and maintenance. At present, a comparison of vascular smooth-muscle differentiation-related markers has been made with more than 58 known, functionally characterized stromal lines.11 A continuous acquisition of markers along this pathway suggests that most HSC-supportive lines are midway in the pathway, between a true mesenchymal phenotype and a fully differentiated vascular smooth-muscle phenotype. Others have used subtractive cDNA cloning approaches23 52 to identify genes important in stromal cell function. Perhaps, by comparing embryonic versus fetal liver versus adult BM-derived stromal clones for the specific gene and protein profiles, we may uncover the specific panel of molecules involved in the generation, expansion, and/or maintenance of HSCs. Such studies should give insights into the molecular mechanisms by which HSCs emerge in the embryo and provide important breakthroughs in HSC in vitro growth and manipulation for clinical purposes.
We thank all laboratory members for their helpful comments and assistance with various aspects of this work. We thank Dr K. Moore (Princeton University) for helpful comments concerning these studies and for critical evaluation of the manuscript. We also thank Corné Snoijs for the excellent flow cytometric sorting and L. Braam, B. Dortland, and the Erasmus Dierexperimenteel Centrum (EDC) for animal care.
Supported by research grants of the Netherlands Scientific Research Organization 901-08-090 (E.A.D.), National Institutes of Health grant R01 DK51077 (E.A.D.), Netherlands Cancer Society EUR 1999-1965 (E.A.D.), Erasmus U. Breedtestrategie Program (K.N.H.), and AMGEN, Thousand Oaks, CA (C.S. and E.A.D.). A.L.M. is a senior MRC fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elaine A. Dzierzak, Department of Cell Biology and Genetics, Erasmus University Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands; e-mail: dzierzak@ch1.fgg.eur.nl.