Heparin-induced thrombocytopenia/thrombosis (HIT/T) is a common complication of heparin therapy that is caused by antibodies to platelet factor 4 (PF4) complexed with heparin. The immune response is polyclonal and polyspecific, ie, more than one neoepitope on PF4 is recognized by HIT/T antibodies. One such epitope has been previously identified; it involves the domain between the third and fourth cysteine residues in PF4 (site 1). However, the binding sites for other HIT/T antibodies remain to be defined. To explore this issue, the binding site of KKO, an HIT/T-like murine monoclonal antibody, was defined. KKO shares a binding site with many HIT/T antibodies on PF4/heparin, but does not bind to site 1 or recognize mouse PF4/heparin. Therefore, the binding of KKO to a series of mouse/human PF4 chimeras complexed with heparin was examined. KKO recognizes a site that requires both the N terminus of PF4 and Pro34, which immediately precedes the third cysteine. Both regions lie on the surface of the PF4 tetramer in sufficient proximity (within 0.74 nm) to form a contiguous antigenic determinant. The 10 of 14 HIT/T sera that require the N terminus of PF4 for antigen recognition also require Pro34 to bind. This epitope, termed site 2, lies adjacent to site 1 in the crystal structure of the PF4 tetramer. Yet sites 1 and 2 can be recognized by distinct populations of antibodies. These studies further help to define a portion of the PF4 tetramer to which self-reactive antibodies develop in patients exposed to heparin.
Introduction
Heparin is the most common known cause of drug-induced immune thrombocytopenia, occurring in 1% to 3% of patients receiving unfractionated heparin.1-3 A significant fraction of these patients develop limb- or life-threatening thromboses.4,5 Heparin-induced thrombocytopenia/thrombosis (HIT/T) is mediated by antibodies directed at complexes that form between heparin or other anionic mucopolysaccharides and platelet factor 4 (PF4) in plasma and on the surface of vascular cells.6-10 Immune complexes composed of HIT/T antibodies and PF4/heparin bind to the surface of platelets and induce their activation by cross-linking FcγIIA receptors,11-13 and bind to the surface of the endothelium and monocytes,14-16 inducing procoagulant activity.11 14
PF4 is a 70–amino acid, platelet-specific CXC chemokine in which the first 2 of the 4 conserved cysteine residues are separated by 1 amino acid residue.17 PF4 has been sequenced18 and cloned,19 and its x-ray crystallographic structure has been defined (Figure1A).20-22 PF4 exists in many biologic fluids primarily in the form of a tetramer with the 3 β-sheets of each subunit facing inwards, and the N and C termini lying on the surface of the molecule. The C termini are rich in lysines, which contribute to the circumferential ring of positive charges that form the interface between the PF4 tetramer and heparin.23-25
Primary and secondary structure of human PF4.
(A) The crystal structure of the PF4 tetramer is shown with the lysine residues in the C terminus of PF4 (light blue) and other lysine and arginine residues (dark blue) indicated,20,21 forming the proposed heparin-binding domain. Pro37 of HIT site 1 is indicated in green. (B) The sequence of mature human and mouse PF4 (HHHH and MMMM, respectively) and human neutrophil-activating peptide-2 (NAP-2) (NNNN) are shown with identical amino acids (in single-letter codes) in the latter 2, with human PF4 shown by the colons. The conserved cysteine residues are in red and numbered at the top, dividing PF4 into 4 domains. Boxed in gray is HIT site 1.26 A major region of sequence difference between human and mouse PF4 found in the second domain.
Primary and secondary structure of human PF4.
(A) The crystal structure of the PF4 tetramer is shown with the lysine residues in the C terminus of PF4 (light blue) and other lysine and arginine residues (dark blue) indicated,20,21 forming the proposed heparin-binding domain. Pro37 of HIT site 1 is indicated in green. (B) The sequence of mature human and mouse PF4 (HHHH and MMMM, respectively) and human neutrophil-activating peptide-2 (NAP-2) (NNNN) are shown with identical amino acids (in single-letter codes) in the latter 2, with human PF4 shown by the colons. The conserved cysteine residues are in red and numbered at the top, dividing PF4 into 4 domains. Boxed in gray is HIT site 1.26 A major region of sequence difference between human and mouse PF4 found in the second domain.
The mechanism by which PF4/heparin complexes become antigenic is unknown. We have previously defined an antibody-binding site (designated site 1) on PF4 that is recognized by serum antibodies from approximately one third of patients with HIT/T.26 Site 1 involves the amino acids immediately C-terminal to the third cysteine residue of the PF4 monomer (Figure 1B). This antigenic site lies on the surface of the PF4 tetramer, but does not include any of the putative heparin-binding residues.
To begin to characterize other antigenic sites on PF4/heparin recognized by HIT/T antibodies, we took advantage of KKO, a murine monoclonal antibody that has HIT/T antibody–like properties.27 KKO causes heparin-dependent platelet activation in vitro27 and in vivo,28 competes with a subset of HIT/T antibodies for binding to human PF4/heparin, and does not recognize heparin complexed with mouse PF4 or the related chemokines interleukin-8 (IL-8) and neutrophil-activating peptide 2 (NAP-2). Importantly, KKO does not recognize site 1. Therefore, we developed a series of mouse/human PF4 chimeras to examine the site recognized by KKO and to explore its relationship to the previously defined site 1 in the PF4/heparin tetramer.
Materials and methods
Recombinant proteins
The preparation and characterization of expression vectors for wildtype human and mouse PF4 and a chimeric construct with the N terminus of NAP-2 replacing the N terminus of human PF4 (NHHH) in pT7-7 (Novagen, Madison, WI) were previously described by our group.26,29 In addition, using the same overlap polymerase chain reaction technique used to make NAP-2/human PF4 chimeric expression vectors, we made chimeric constructs of human and mouse PF4.26,29 These constructs used wildtype human and mouse PF4 complementary DNAs (cDNAs) as templates and VENT polymerase enzyme (New England Biolabs, Beverly, MA). The sequences of all mutant constructs were verified by means of the Sequenase T7 DNA Polymerase Kit (BRL/Gibco, Gaithersburg, MD). All constructs were confirmed by DNA sequence analysis and were named by dividing PF4 into 4 domains: the N-terminus surface domain followed by the 2 β-sheet domains that form contact points in the PF4 tetramer and then the C-terminus lysine-rich surface alpha-helical domain. Thus, the wildtype human and mouse PF4 proteins will be referred to as HHHH and MMMM, respectively. All of the recombinant proteins were expressed inEscherichia coli as described.29 Briefly, cDNA pT7-7 constructs were introduced into E coli BL21(DE3) pLysS (Novagen), and grown in Luria broth containing 100 μg/mL ampicillin. Bacteria were grown to an OD600 of 1.0 followed by a 3-hour induction at 37°C with 1 mM isopropylthiogalactoside. Bacteria were lysed and sonicated, and the protein was purified at room temperature (RT) by affinity chromatography with the use of heparin-agarose equilibrated with 50 mM Tris HCl and 1 mM EDTA, pH 8, and eluted with a 0.2- to 2.0-M NaCl gradient. Eluted proteins were further purified by reverse-phase chromatography by means of a ProRPC (Pharmacia Biotech, Piscataway, NJ), fast protein liquid chromatography column. Protein purity was assessed as described29 by 15% (wt/vol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by Coomassie blue staining. Samples were also subjected to immunoblotting after electrotransfer to polyvinylidene difluoride membranes using commercial rabbit antihuman PF4 polyclonal antibody (Prepro Tech EC, London, England), followed by a swine antirabbit secondary antibody conjugated to horseradish peroxidase (Dako, Carpinteria, CA). Proteins were detected using an ECL chemiluminescence detection kit (Amersham Pharmacia Biotech, Piscataway, NJ) as described by the manufacturer. Protein concentrations were determined by means of a bicinchoninic acid assay with bovine serum albumin as the standard, according to the manufacturer's instructions.
Human and murine antibodies
Sera were obtained from patients who developed thrombocytopenia (with or without thrombosis) while receiving heparin and who were referred to the Coagulation Laboratory at the Hospital of the University of Pennsylvania (Philadelphia) for the detection of heparin-dependent antiplatelet antibodies. All samples contained heparin-dependent antiplatelet antibodies determined by14C-serotonin release assay (14C-SRA),10 and anti-PF4/heparin antibodies detected by means of the GTI (Brookfield, WI) enzyme-linked-immunosorbent assay (ELISA) kit.6-10Research studies on discarded sera from the clinically indicated studies were used with the approval of the Institutional Ethics Committee of the University of Pennsylvania School of Medicine. Sera obtained from patients with no known hematological disturbances and negative 14C-SRA and PF4/heparin ELISA tests were used as normal controls. All samples were stored at −70°C until used. The HIT-like murine monoclonal immunoglobulin (Ig)–G2bκ antibody KKO was previously described.25 KKO was isolated from ascites fluid by means of Hi Trap affinity columns (Amersham Pharmacia Biotech) according to the manufacturer's instructions. An isotype control for KKO was also isolated and characterized in the same manner, as previously described.27
Direct ELISAs
Binding of antibody to PF4, PF4 variants, or other chemokines complexed to heparin was measured with an ELISA-based method as previously described.10 Briefly, 96-well microtiter plates were coated overnight at RT with 50 μL phosphate-buffered saline (PBS) (0.01M sodium phosphate, 0.138 M NaCl, 0.0027M KCl [pH 7.4]) per well containing recombinant wildtype or mutated PF4 (final concentration 10 μg/mL) in the presence or absence of heparin (0.2 U/mL) (Elkins-Sinn, Cherry Hill, NJ). The plates were washed 3 times with PBS containing 0.01% (vol/vol) Tween-20 (BioRad, Richmond, VA) (PBS-T), blocked with 10% fetal calf serum (FCS) (200 μL per well) (Hyclone, Logan, UT) in PBS for 2 hours at RT and washed one additional time. Binding of KKO and HIT/T sera to immobilized PF4/heparin complexes was measured by means of a similar approach. Then, 100 μL KKO diluted in 10% FCS/PBS (an amount that caused maximal binding) was added to duplicate wells for 1 hour at RT and then washed with PBS-T. Goat antimouse IgG (100 μL per well) labeled with alkaline phosphatase (ICN, Costa Mesa, CA) and diluted 1:1000 in 10% FCS/PBS was then added for 1 hour at RT. After washing, 100 μL per well Sigma Fast p-Nitrophenyl phosphate substrate (Sigma Chemical, St Louis, MO) was added, and the absorbance at 405 nm was measured 1 hour later. Binding of HIT/T or control serum antibody diluted 1:200 in PBS/10%FCS (100 μL per well) was measured in the same way with the use of alkaline phosphatase–conjugated goat antihuman IgG, IgA, and IgM (Cappel, Organon Teknika, Westchester, PA) diluted 1:2000. The capacity of HIT/T serum to compete with KKO for binding to human PF4/heparin–coated wells was assessed with the use of a concentration of KKO (diluted in 10% FCS/PBS) that produced 75% of maximal binding (0.5 to 0.1 μg KKO). Binding of KKO in the presence of a 1:100 dilution of HIT/T sera was measured as described above.
PF4 structural analysis
The coordinates of the crystal structure for human PF421 were obtained from the Brookhaven Protein Database (http://www.rcsb.org/pdb/ [accessed November 18, 2001]) and modeled by means of the Swiss-PdbViewer (version 3.6) (http://www.expasy.ch/spdbv/ [accessed November 18, 2001]).30
Results
KKO/HIT sera competition binding
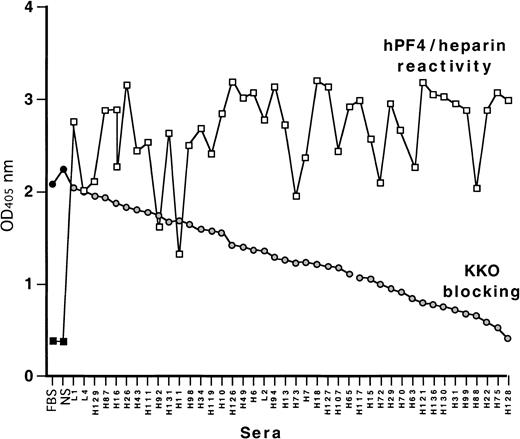
We have previously reported that the HIT/T-like murine monoclonal antibody KKO does not bind to site 1 on PF4/heparin, which is recognized by many HIT/T sera.27 In view of this, we asked whether KKO could be used to identify the site recognized by site-1–independent HIT/T antibodies.26 To do so, we first identified the proportion of HIT/T sera that blocked KKO binding determined by competition-inhibition studies using human PF4/heparin complexes as the target. Each of the 41 HIT/T sera containing anti-PF4/heparin antibodies studied inhibited the binding of KKO to a greater extent than normal sera (Figure2). Approximately one third of the HIT/T sera inhibited KKO binding more than 50%. Thus, the site recognized by KKO appears to be identical to, or overlapping with, an epitope recognized by a large fraction of HIT/T antibodies. Therefore, studies were performed to identify the KKO binding site(s) in greater detail.
Cross-competition of KKO binding to human PF4/heparin with HIT sera by ELISA.
Binding of 41 HIT sera tested against human PF4 heparin complexes is shown in squares as well as binding by fetal bovine serum (FBS) and normal sera (NS) controls. Binding of KKO in the presence of competing FBS, NS, and HIT sera is shown as circles. The HIT samples are ordered from the least to the greatest degree of block.
Cross-competition of KKO binding to human PF4/heparin with HIT sera by ELISA.
Binding of 41 HIT sera tested against human PF4 heparin complexes is shown in squares as well as binding by fetal bovine serum (FBS) and normal sera (NS) controls. Binding of KKO in the presence of competing FBS, NS, and HIT sera is shown as circles. The HIT samples are ordered from the least to the greatest degree of block.
KKO binding to variant PF4/heparin complexes
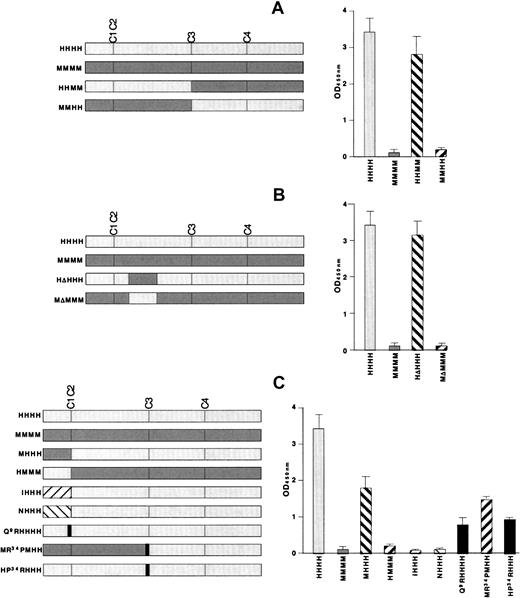
We took advantage of the fact that KKO does not bind to mouse PF4 complexed with heparin.27 The first set of studies was performed with the use of 2 chimeras made by switching the N terminal–2 domains between human and mouse PF4 to create HHMM and MMHH. Binding of KKO tracked with the origin of the N-terminal half of the protein (Figure 3A, right side) as binding occurred to HHMM, but not to MMHH. This result is consistent with our previous demonstration that KKO does not bind to site 1, which is located in the third domain of PF4.
KKO antigenicity of recombinant PF4 mutant constructs by ELISA.
On the left of each figure are the constructs studied and their names. The right of each figure shows their antigenicity when complexed to heparin in an ELISA study with KKO. (A) The initial N-terminal switches are shown. (B) ELISA studies involving the switching of the major sequence difference between human and mouse PF4 in the second domain (Figure 1B, underlined) are shown. (C) Studies of switches between human and mouse PF4 and with NAP-2 and IL-8 are shown. Also shown are studies of point mutations at the N terminus of PF4 and at the Pro34 position. The names of the recombinant appear in gray for portions derived from human PF4 and in black for substitutions from other proteins.
KKO antigenicity of recombinant PF4 mutant constructs by ELISA.
On the left of each figure are the constructs studied and their names. The right of each figure shows their antigenicity when complexed to heparin in an ELISA study with KKO. (A) The initial N-terminal switches are shown. (B) ELISA studies involving the switching of the major sequence difference between human and mouse PF4 in the second domain (Figure 1B, underlined) are shown. (C) Studies of switches between human and mouse PF4 and with NAP-2 and IL-8 are shown. Also shown are studies of point mutations at the N terminus of PF4 and at the Pro34 position. The names of the recombinant appear in gray for portions derived from human PF4 and in black for substitutions from other proteins.
The most extensive difference between the N-terminal halves of human and mouse PF4 lies in a single long sequence in the second domain (Figure 1B, underlined). To examine the contribution of this region, we switched this region in human and mouse PF4 to make HΔHHH and MΔMMM (Figure 3B). To our surprise, exchange of this region alone had no impact on KKO binding to either PF4 species (Figure 3B, right side).
We then examined the role of the N-terminal domain itself using several approaches. First, we exchanged the entire first N-terminal domain between the 2 PF4 species, creating MHHH and HMMM (Figure 3C). MHHH lost approximately 50% of the antigenicity of HHHH, although HMMM did not bind KKO better than MMMM (Figure 3C, right side). Second, substitution of the N-terminal GluLeuArg sequence from IL-8 or Ala GluLeuArg from NAP-2 to make IHHH and NHHH eliminated KKO binding (Figure 3C, right side). Third, replacement of Gln9 in human PF4 with the arginine located at that position in IL-8 and NAP-2 (designated Q9RHHHH), decreased the binding of KKO by approximately 60% (Figure 3C, right side). Taken together, these studies indicate that the N terminus of human PF4 is required, but not sufficient for full recognition by KKO.
In Figure 3A, we showed that MMHH was nonantigenic. We also studied an MMHH variant that contained a proline at position 34 from the human sequence instead of the arginine that occurs at this position in the mouse protein (designated MR34PMHH). KKO retained approximately 30% of its binding to MR34PMHH compared with HHHH (Figure 3C). Conversely, introducing this single amino acid substitution from mouse into human PF4 (HP34RHHH) caused a comparable decrease in KKO binding to that seen with Q9RHHHH, suggesting that both the N terminus and the region around Pro34 are part of the antigenic site.
Localization of the antigenic site for KKO in the crystal structure of PF4
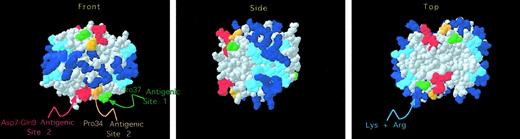
We then examined the location of the 2 regions proposed to be part of the KKO antigenic site in the crystal structure of the PF4 tetramer. Figure 4 shows that the 2 involved portions of the N terminus (shown in red) and Pro34 (shown in orange) are immediately adjacent to each other. Pro34 was measured to be 0.74 nm from Ile8 (from Cα to Cα), which is consistent with these 2 regions forming a single antigenic locus. We then examined the physical relationship between the KKO binding site (designated site 2) and the previously described site 1. As shown in Figure 4, site 1 (shown in green) is adjacent to the KKO antigenic site (shown in red and orange) in 3-dimensional space.
Structural localization of the KKO antigenic site.
As in Figure 1, the crystal structure of the human PF4 tetramer is shown. In addition, HIT site 2 is shown with Asp7 through Gln9 in red and Pro34 in orange. In addition, Pro37 of HIT site 1 is shown in green.
Structural localization of the KKO antigenic site.
As in Figure 1, the crystal structure of the human PF4 tetramer is shown. In addition, HIT site 2 is shown with Asp7 through Gln9 in red and Pro34 in orange. In addition, Pro37 of HIT site 1 is shown in green.
Reaction of HIT/T antibodies with the KKO antigenic site
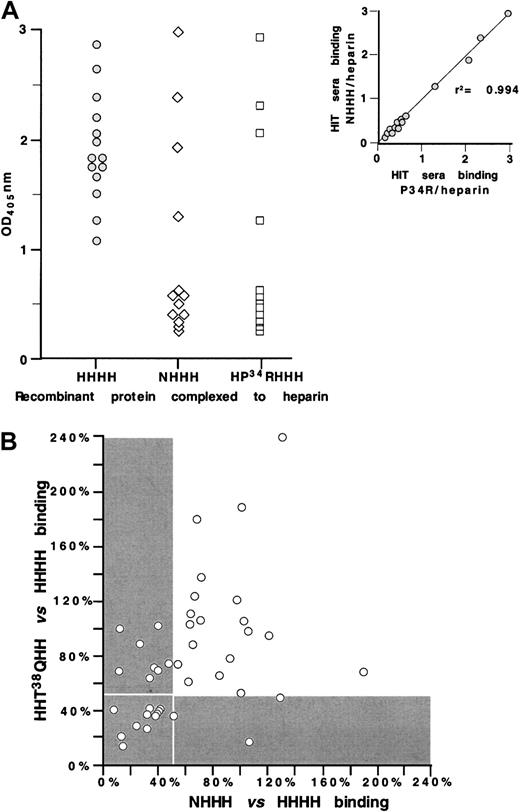
We then examined the fine specificity of HIT/T sera that react with the KKO antigenic site. To do so, we tested 14 HIT/T serum samples for antibody binding to wildtype PF4, NHHH, and HP34RHHH, each complexed with heparin (Figure 5A). Ten (approximately 70%) of the samples lost reactivity to both NHHH and HP34RHHH, while 4 bound similarly to the variants as to wildtype complexes. For each HIT/T serum tested, the loss of reactivity to NHHH and HP34RHHH was similar (r2 = 0.994), consistent with the behavior of KKO, again suggesting that the N terminus and Pro34 both contribute to the formation of a single antigenic site.
Characterizing sites 1 and 2 using HIT sera.
(A) ELISA studies with wildtype PF4 ( ), NHHH (⋄), and HP34RHHH (■) complexed to heparin for 14 HIT serum samples. Insert shows the comparative binding of the 14 HIT sera to the 2 mutant proteins demonstrating high correlation. (B) ELISA studies with wildtype PF4 NHHH and HHT38QHH complexed to heparin of 41 HIT sera. For each HIT serum, reactivity with NHHH and with HHThr38GlnHH was normalized for binding to wildtype PF4 complexed to heparin. Reactivity of less than 50% compared with wildtype PF4 is shown in the gray area; the white boxed area shows the samples with less than 50% antigenicity for both the site 1 and site 2 mutations.
), NHHH (⋄), and HP34RHHH (■) complexed to heparin for 14 HIT serum samples. Insert shows the comparative binding of the 14 HIT sera to the 2 mutant proteins demonstrating high correlation. (B) ELISA studies with wildtype PF4 NHHH and HHT38QHH complexed to heparin of 41 HIT sera. For each HIT serum, reactivity with NHHH and with HHThr38GlnHH was normalized for binding to wildtype PF4 complexed to heparin. Reactivity of less than 50% compared with wildtype PF4 is shown in the gray area; the white boxed area shows the samples with less than 50% antigenicity for both the site 1 and site 2 mutations.
Characterizing sites 1 and 2 using HIT sera.
(A) ELISA studies with wildtype PF4 ( ), NHHH (⋄), and HP34RHHH (■) complexed to heparin for 14 HIT serum samples. Insert shows the comparative binding of the 14 HIT sera to the 2 mutant proteins demonstrating high correlation. (B) ELISA studies with wildtype PF4 NHHH and HHT38QHH complexed to heparin of 41 HIT sera. For each HIT serum, reactivity with NHHH and with HHThr38GlnHH was normalized for binding to wildtype PF4 complexed to heparin. Reactivity of less than 50% compared with wildtype PF4 is shown in the gray area; the white boxed area shows the samples with less than 50% antigenicity for both the site 1 and site 2 mutations.
), NHHH (⋄), and HP34RHHH (■) complexed to heparin for 14 HIT serum samples. Insert shows the comparative binding of the 14 HIT sera to the 2 mutant proteins demonstrating high correlation. (B) ELISA studies with wildtype PF4 NHHH and HHT38QHH complexed to heparin of 41 HIT sera. For each HIT serum, reactivity with NHHH and with HHThr38GlnHH was normalized for binding to wildtype PF4 complexed to heparin. Reactivity of less than 50% compared with wildtype PF4 is shown in the gray area; the white boxed area shows the samples with less than 50% antigenicity for both the site 1 and site 2 mutations.
We also compared 41 HIT/T serum samples for antibody binding to site 1 (using HHT38QH)26 and site 2 (using NHHH) (Figure 5B). The results for each sample were normalized for binding to wildtype PF4 complexed to heparin. In contrast to the parallelism seen between antibody binding to NHHH and HP34RHHH (both of which are involved in the formation of site 2), little correlation was evident between antibody binding to NHHH (site 2) and HHT38QH (site 1) (r2 = 0.424). This outcome suggests that despite the physical proximity of the 2 sites on the PF4 crystal structure, antibodies with only one or the other specificity are present in many HIT/T patients. The gray region in Figure 5B represent the situation in which antibody binding to the mutant protein was less than 50% of the reactivity seen with wildtype PF4. Our data show that only approximately 25% of the HIT/T samples (those within the boxed gray area) lack reactivity to both site 1 and site 2 mutants, implying that these 2 sites comprise the immunodominant epitopes. On the other hand, approximately 50% of the HIT/T sera reacted strongly with both mutant proteins (data lie outside both gray areas), consistent either with these patients' having antibodies to both sites simultaneously or with the existence of HIT/T antibodies that recognize at least one additional epitope that is independent of site 1 and 2.
Discussion
It is currently believed that HIT/T is caused by antibodies that recognize the complex between PF4 and heparin. Anti-PF4/heparin antibodies are found in the sera of more than 90% of patients with HIT/T.31 Mice immunized with HIT antibodies develop anti-PF4/heparin antibodies as part of the anti-idiotypic response and develop thrombocytopenia when exposed to heparin.32Passive transfer of a murine monoclonal anti-PF4/heparin antibody into mice transgenic for human PF4 and platelet FcγRIIA leads to severe thrombocytopenia and disseminated thrombosis, the salient clinical features of the human disease.28
These findings indicate the need to understand how such self-reactive antibodies arise once a complex is formed between PF4 and heparin. The fact that such antibodies also develop in the majority of individuals with persistent platelet activation as a result of atherosclerosis after repetitive stimulation with heparin33-36 suggests there is something unique about the complex between PF4 and heparin that is not shared by other heparin-binding proteins. Our approach to understanding the pathogenesis of autoantibodies in this disease has been, in part, to ask whether there is a single or limited number of immunodominant epitopes recognized by HIT/T antibodies and whether serologic specificity of the antibodies provides insight into the risk for clinical sequelae.
The fact that HIT antibodies cross-react with low-molecular-weight heparin and with heparinoids to some extent37-39 suggests that there is considerable promiscuity/tolerance in the glycosaminoglycan (GAG) that can bind PF4 and induce HIT/T antigenic sites. This was elegantly pointed out by the studies of Greinacher et al,40 who help define the determinants in the GAGs required for antigenicity. These studies suggest that HIT/T antibodies recognize neoepitopes induced in PF4 to a variable extent by a range of GAGs and other large negatively charged molecules.41 The low prevalence of antibodies to other heparin-binding proteins, including the related heparin-binding chemokines NAP-2 and IL-8 in patients with HIT/T,31 provides additional evidence that analysis of the PF4 molecule itself will provide insight into the initial steps of the autoimmune response. Indeed, nuclear magnetic resonance (NMR) analysis of human PF4 variants has suggested that binding by heparin leads to significant relaxation and unfolding of the PF4 structure.42
Progress in elucidating the specificity of anti-PF4/heparin antibodies has been slow and largely indirect. Horsewood et al43 showed that most HIT/T antibodies recognized noncontiguous epitopes. On the basis of cross-competition studies, Suh et al44 proposed the existence of 2 or 3 discrete antigenic sites within the PF4/heparin complex. Using recombinant PF4 and chimeras with NAP-2, we partially defined one antigenic site that involved the third PF4 domain (Pro37 to Leu41).26 This site, which we term HIT site 1, is near the surface of the tetramer, but does not approximate the lysine/arginine–rich ring implicated in binding heparin.
The polyspecific response in patients with HIT/T has limited our ability to map other antigenic sites. The development of KKO, an HIT/T-like monoclonal antibody that does not react with mouse PF4/heparin or site-1 mutants27 has now enabled us to extend these studies. Using this monoclonal antibody and a series of human/mouse PF4 chimeras and point mutations in human PF4, we have defined a second antigenic site, which we have termed site 2. This antigenic site appears to involve 2 nonlinear regions on PF4, the N terminus and Pro34 near the third cysteine residue (Figure 4).
Of interest, sites 1 and 2 appear spatially close to one another in the crystal structure of the PF4 tetramer (Figure 4B). Yet antibodies to the 2 sites appear to develop in an independent fashion, with the result that sera containing antibodies to site 1 may or may not have antibodies to site 2, and vice versa. This is in direct contrast to the 2 regions we identified as making up site 2. Although site 2 is composed of discontinuous regions based on linear structure, sera that require the N terminus to bind PF4/heparin also require Pro34 (Figure4A). This suggests to us that in at least some of the patients, sites 1 and 2 may correspond to separate epitopes recognized by separate populations of B cells.
On the basis of these findings, we propose that when heparin binds to PF4, a region that includes sites 1 and 2 undergoes a significant structural change, exposing at least 2 neoepitopes. The distance between sites 1 and 2 may increase, but the 2 parts of site 2 remain in close physical proximity. It will require structural analysis of PF4 complexed to heparin or mutant versions of PF4 binding HIT/T antibodies in the absence of heparin to verify these predictions. Certainly, such a model is consistent with recently described NMR studies of PF4 complexed to heparin, which suggest a partial unfolding of the protein.42
Defining the antigenic sites on the PF4/heparin complex still leaves many issues concerning the development of HIT/T unresolved. For example, there remains no explanation of the capacity of unfractionated heparin to reveal cryptic epitopes in PF4 far more efficiently than low-molecular-weight heparin. Second, the distribution of cellular proteoglycans capable of mimicking the effect of heparin and the regulation of GAG expression in healthy and diseased tissue become important scientific issues. Third, although anti-PF4/heparin antibodies can be demonstrated in a significant proportion (15% to 70%) of asymptomatic patients repetitively exposed to heparin,32-35 the reason only a subset of immunized patients develop symptomatic disease remains unknown. Clinical variables such as atherosclerosis, surgery, and vascular trauma may contribute to the risk,45-47 while some researchers have implicated differences in antibody titer,48affinity,49 isotype,50subclass,51,52 and platelet Fc receptor polymorphism (FcγRIIA-H/R131).50-52 However, it is clear that such serologic or clinical differences do not permit clear separation of asymptomatic patients with anti-PF4/heparin antibodies from those who develop thrombocytopenia and those who develop thrombocytopenia and thrombosis. Fourth, the potential relationship between the fine antigen specificity of HIT/T antibodies and the clinical expression of disease has not been addressed. In vitro studies support both a prothrombotic and an antithrombotic function for PF4. PF4 may be prothrombotic by modulating heparin-dependent anti–thrombin-III activity.53 PF4 may be antithrombotic by binding to thrombomodulin and/or protein C and enhancing activated protein C activity.54 Whether epitope specificity alters the function of PF4 and predisposes to thrombosis is as yet unknown. Future studies using serum from patients treated with heparin who do or do not develop HIT or HIT/T will be required to see if antibody specificity correlates with clinical outcome. If such a correlation is demonstrated, then testing for antibodies against specific determinants in PF4 may help to identify those patients who are at highest risk of developing clinical complications of this immune disease.
The recent development of a murine model for HIT28 and the availability of an enlarging repertoire of recombinant PF4 proteins and monoclonal HIT-like antibodies offer new tools that may lead to new insights into the pathogenesis of this disease and, perhaps, to developing new strategies to prevent or halt the progression to life-threatening thrombotic complications.
We are indebted to Dr Kevin H. Mayo at University of Minnesota Health Science Center for providing recombinant IHHH.
Supported in part by NIH research grants HL54749 (M.P., D.B.C.) and KO8 HL04009 (G.M.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mortimer Poncz, The Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, ARC; Room 316H, Philadelphia, PA 19104; e-mail: poncz@emailchop.edu.