Mammalian erythrocytes undergo a unique maturation process in which they discard their nuclei and organelles and assume a flexible biconcave shape. We found that altered plasma lipoprotein metabolism can profoundly influence these events. Abnormal erythrocyte morphology was observed in hypercholesterolemic mice lacking the high-density lipoprotein receptor SR-BI. This was exacerbated by feeding mice a high-cholesterol diet or, more dramatically, by inactivating the apolipoprotein E gene. Erythrocytes fromSR-BI−/−/apolipoprotein E−/−mice and SR-BI−/−mice that were fed cholesterol had markedly increased membrane cholesterol. Their morphology appeared immature, with macrocytosis, irregular shape, and large autophagolysosomes. Autophagolysosomes fromSR-BI−/−/apolipoprotein E−/−erythrocytes were expelled when the erythrocytes were transfused into wild-type animals or incubated in vitro with normolipidemic serum or the cholesterol-sequestering agent methyl cyclodextrin. We propose that autophagocytosis and phagolysosome expulsion are essential steps in erythroid maturation and that expulsion is inhibited in the presence of markedly increased cellular cholesterol.
Introduction
Red blood cells are descended from multipotential hematopoietic progenitor cells in the bone marrow. Lineage commitment establishes an erythroid differentiation program in which differential gene expression leads to high-level hemoglobin production, nuclear condensation and expulsion from the cell, and the shutdown of most cellular functions. The erythroid precursors generated by the expulsion of nuclei from bone marrow erythroblasts are called reticulocytes. Early reticulocytes, which are rarely found in the peripheral blood, are irregular in shape and contain cytoplasmic organelles and polyribosomes.1 As reticulocyte maturation progresses, RNA, mitochondria, and ribosomes are degraded; protein synthesis ceases; and cell-surface transferrin receptors (Trfrs) disappear. Finally, cytoskeletal remodeling transforms reticulocytes into compact, biconcave, discoid corpuscles devoid of organelles and translational apparatus. The earliest steps in erythroid commitment and differentiation have been the subject of extensive study (reviewed in Bungert and Engel2). However, maturational events subsequent to nuclear expulsion have been only partially characterized by morphologic studies.1,3,4 Some patients with severe anemia syndromes, especially those whose spleen has been removed, have a small fraction of circulating erythrocytes containing autophagosomes.1 Because these autophagosomes contain mitochondria and ribosomes that are absent from mature erythrocytes, it has been proposed that they represent intermediates in the final stages of red cell maturation.1 Furthermore, splenic macrophages have been postulated to participate in the removal of autophagosomes from such precursors, but the mechanism of autophagosome extraction in the bone marrow was not addressed.1 We show here that hypercholesterolemic mice with abnormal lipoproteins due to inactivation of the high-density lipoprotein (HDL) receptorSR-BI gene (SR-BI−/−)5have an unexpected defect in late erythroid maturation.
SR-BI mediates cellular uptake of cholesterol and plays a key role in the transport of cholesterol from peripheral tissues to the liver by HDL (reviewed in Krieger6). During studies of the role of SR-BI in atherosclerosis,7,SR-BI−/−mice were bred to mice that were homozygous for a null mutation in the gene encoding apolipoprotein E (apoE). ApoE−/− mice also have defects in lipoprotein metabolism that result in hypercholesterolemia and the early, spontaneous development of atherosclerosis.8-10 The plasma cholesterol concentration of double-knockoutSR-BI−/−/apoE−/− mice is 2-fold higher than that of apoE−/− mice. These double-knockout mice have unusually large, HDL-like lipoprotein particles and they develop severe atherosclerosis at an accelerated pace,7 resulting in coronary artery disease and early death.29
In the current study, we observed thatSR-BI−/−/apoE−/− mice were anemic and had unusual red blood cell morphology. The erythrocytes had abnormally high-cholesterol content and resembled proposed intermediates in erythroid differentiation in that they had irregular shapes and an accumulation of large intracellular phagolysosomes. Similar but less severe morphological defects were observed in the erythrocytes from chow-fed SR-BI−/−single-knockout mice. Feeding theSR-BI−/− single-knockout mice a cholesterol-enriched diet dramatically exacerbated these abnormalities. The large intracellular phagolysosomes in the erythroid cells from the double-knockout mice were expelled both in vivo, when the abnormal cells were transfused into the circulation of normolipidemic wild-type control mice, and in vitro, when the cells were incubated with normolipidemic serum or the cholesterol-sequestering agent methyl cyclodextrin. Thus, the abnormal lipoprotein metabolism and intrareticulocyte accumulation of excess cholesterol in SR-BI−/− mice appears to induce a reversible block in the expulsion of autophagolysosomes from reticulocytes. We postulate that autophagocytosis and cholesterol-sensitive phagolysosome expulsion are important steps in normal erythropoiesis that can be interrupted when red cell lipid homeostasis is perturbed.
Methods
Animals
We housed mice in the barrier facilities at Children's Hospital (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA) and maintained them on standard mouse diet unless otherwise specified. We originally purchased homozygousapoE−/− mice on a C57BL/6 background8,9 and hemoglobin-deficit (hbd)mutant mice from the Jackson Laboratory (Bar Harbor, ME).SR-BI−/−, apoE−/−,and SR-BI−/−/apoE−/− mice were generated as previously described.5,7 We performed most experiments on 5- to 6-week-old animals. However, a second cohort of 4- to 5-week-old SR-BI−/− male mice and wild-type age- and sex-matched controls was placed on a 1% cholesterol diet for 3 months prior to analysis. We determined mouse genotypes by polymerase chain reaction analysis of tail DNA as previously described.7 For bone marrow transplantations, we irradiated mixed background (129 × C57BL/6)apoE−/− mice using a Gammacell 40 (12 Gy), and we injected them retro-orbitally with 106bone marrow cells harvested from the femurs and tibias of eitherSR-BI−/−/apoE−/− mice or littermate apoE−/− mice. Bone marrow replacement was assessed by SR-BI–genotype analysis of CD11b-expresssing cells harvested from the mice receiving transplants 3 months after transplantation. Only the mutantSR-BI allele was detected in cells prepared from theapoE−/− mice receiving bone marrow fromSR-BI−/−/apoE−/− mice, suggesting a high degree of chimerism (data not shown). Red blood cell morphology was analyzed 5 to 6 months after transplantation.
Erythrocyte analysis
Blood was obtained by retro-orbital bleeding or direct cardiac puncture. We determined complete blood counts and erythrocyte measurements (mean corpuscular volume [MCV] and mean corpuscular hemoglobin) using an automated ADVIA 120 analyzer (Bayer Diagnostics, Tarrytown, NY). We analyzed at least 4 animals to determine average values for every measurement. We fixed peripheral blood smears in 100% methanol for 7 minutes, stained them with Wright-Giemsa (Fisher Diagnostics, Pittsburgh. PA) for 7 minutes, and washed them 4 times with fresh distilled water for 1 minute each before visualizing by means of an Olympus BX50 microscope (Lake Success, NY). We visualized fixed, unstained smears with Differential Interference Contrast (DIC) optics (Zeiss Axioplan microscope [Thornwood, NY] and Chroma Optical [Chroma Technology, Brattleboro, VT] custom filter sets). We captured images digitally and analyzed them using Adobe PhotoShop (San Jose, CA). We prepared 200 μL peripheral blood for transmission electron microscopy as described previously.11
Detection of lysosomes and RNA in reticulocytes
We visualized lysosomes by washing fresh red blood cells and diluting them 1:1000 in Eagle modified minimum essential medium with low calcium and without L-glutamine (BioWhittaker, Walkersville, MD) and then incubating them with Lysotracker (Molecular Probes, Eugene, OR), a pH-specific marker for lysosomes, or with fluorescein di-(β-D-galactopyranoside) (FDG) (Sigma, St Louis, MO), a substrate of β-galactosidase that yields a fluorescent cleavage product. We visualized fluorescence by confocal imaging on a Kr/Ar Zeiss Axio Vert s100 microscope.
Flow cytometry
Trfr was detected with a phycoerythrin-conjugated monoclonal anti-Trfr antibody (Pharmingen, San Jose, CA), and RNA was detected with thiazole orange (Becton Dickinson, San Jose, CA) as previously described.12 SR-BI was detected with a polyclonal rabbit anti–SR-BI antibody13 and a 488 fluorophore–labeled antirabbit antibody (Molecular Probes). We detected circulating erythroid cells at all stages of maturity with a phycoerythrin-conjugated antimouse TER-119 antibody (Pharmingen).
Induced reticulocytosis
Reticulocytosis was induced in wild-type mice by daily removal of 0.3 to 0.5 mL blood for a period of 7 days, maintaining the hematocrit above 20%. Iron dextran (5 mg) (Sigma) was administered intraperitoneally on day 3. Final reticulocyte counts in these animals ranged from 45% to 55%.
In vitro incubation of blood cells
We washed red blood cells once with phosphate-buffered saline (PBS) and once with minimum essential medium (BioWhittaker) augmented with 1 g/L glucose (5.5 mM), 25 mM Hepes, 20% fetal calf serum, 2 mM L-glutamine, 200 U/mL penicillin, and 200 μg/mL streptomycin, (GibcoBRL, Grand Island, NY), pH 7.4. We then counted cells and diluted them to 2 × 107/mL in this medium. We immediately harvested an aliquot of cells by centrifugation as the zero time sample. We monitored the medium pH regularly and maintained the medium at pH 7.4 with small additions of NaOH as previously described.14 We took samples at 24-hour intervals and prepared them for electron microscopy and flow cytometric analysis. In some experiments, we treated duplicate samples of 2 × 107 red cells per milliliter for 5 minutes prior to the in vitro incubation with 0.1% methyl B cyclodextrin (Sigma) in the incubation medium at 37°C.
In vivo biotin labeling and reinfusion of red blood cells
We labeled red blood cells with biotin in vivo as previously described.15 We prepared 16 μg NHS-Biotin (Calbiochem, La Jolla, CA) per gram of body weight dissolved in 30 μL dimethylacetamide (Calbiochem) and diluted to 0.5 mL in PBS, and injected it through the tail vein of donor mice. At 2 hours after infusion, we collected blood in tubes containing EDTA as an anticoagulant and then transfused 0.5 mL into each recipient mouse. At 24 and 48 hours after transfusion, we bled the mice and analyzed cells by live confocal microscopy to detect biotin (with phycoerythrin-streptavidin; Molecular Probes) and autophagolysosomes (with Lysotracker as described above). We further analyzed the samples by flow cytometry as described above. We determined biotin-labeling efficiency by diluting cells and incubating 1 × 106 red cells with 15 micrograms of 1 mg/mL phycoerythrin-streptavidin.
Filipin detection of cholesterol
We preserved blood in vitro in Karnovsky fixative (2.5% paraformaldehyde, 2% glutaraldehyde, 0.2 M cacodylate buffer). The cells were then incubated for 2 hours in 50 μg/mL (from stock solution of 12 mg/mL in dimethylsulfoxide) of filipin (Sigma) in PBS to label cholesterol and generate a fluorescent signal. Fluorescence was observed by means of 4′6–diamidino–2-phenylindole–2 HCl filters for confocal microscopy and UV filters for flow cytometry.
Quantitative cholesterol analysis
We prepared erythrocytes for cholesterol quantitation by first centrifuging whole blood and removing plasma and buffy coat. The cells were then washed 3 times with PBS and diluted 1:1 in deionized water to cause cell lysis. Total cholesterol was extracted by adding 100 μL the internal standard epicoprostamol (1 mg/mL in methanol) (EPIC, Sigma Chemicals) either to 100 μL red blood cell lysate or to the positive control, human plasma. Human plasma was used as the positive control instead of pure cholesterol to compensate for any extraction effect. The samples were then saponified by adding 1 mL 10% potassium hydroxide/90% ethanol (Fisher Scientific, Fair Lawn, NJ) to each sample and incubating for 60 minutes at 60°C. The samples were cooled to room temperature and extracted with 2 mL hexane (Fisher Scientific) by mixing for 1 minute and then aspirating off the hexane layer. We repeated this 3 times and then combined the hexane extracts and evaporated them to dryness under nitrogen at 40°C. Finally, we resuspended the samples in 100 μL pyridine and 100 μL N, O-Bis(trimethylsilyl)-trifluoroacetamide/trimethylchlorosilane (Alltech Associates, Folsom, CA) and heated them for 60 minutes at 60°C.16 17 After cooling the extract, we injected 1 μL into a gas chromatograph mass spectrometer and measured the cholesterol levels (Hewlett Packard Model 5890/5972 [Atlanta, GA] equipped with a DB-1 column of 30 cm length, 0.25 mm inner diameter, and 0.25 μm film thickness [Alltech Associates]). Unesterified cholesterol was determined as above except that saponification was omitted.
Results
Mice lacking SR-BI and apoE develop an unusual anemia
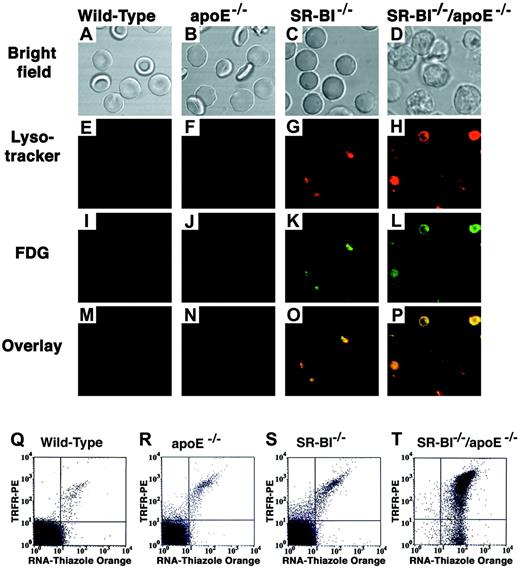
SR-BI−/−/apoE−/−mice fed a standard chow diet had low hematocrits and hemoglobin values, which differed significantly from wild-type controls (Table1) (P < .0001). TheSR-BI−/−/apoE−/− red blood cells were abnormally large as compared with wild-type cells (MCV, P < .0001), but had normal or near-normal cellular hemoglobin content. All circulatingSR-BI−/−/apoE−/− red blood cells were reticulocytes. Unlike wild-type andapoE−/− mice, whose red blood cells appeared normal in blood smears (Figure 1A, B), the SR-BI−/−/apoE−/− mice had a nonuniform population of macrocytic red blood cells (Figure 1D) with a targetlike appearance. However, these targetlike cells sometimes appeared to have multiple intracellular inclusions and were often spiculated. They were more irregular than classical target forms (eg, the targetlike erythrocytes from homozygous hbdmice18 in Figure 1E). Spiculated cells could be seen in smears from the SR-BI−/− mice fed a standard chow diet (Figure 1C), but their abnormalities were not as striking as those from the SR-BI−/−/apoE−/−mice. We also observed morphologic abnormalities inSR-BI−/− andSR-BI−/−/apoE−/− mice using DIC microscopy. Unlike the normal morphologies of erythrocytes from wild-type and apoE−/− mice (Figure1F,G), many erythrocytes from SR-BI−/−mice had irregularities within their cytoplasms, suggesting the presence of small, membrane-enclosed intracellular inclusions (Figure1H). Virtually all the erythroid cells fromSR-BI−/−/apoE−/− mice (Figure1I) had one or more large, membrane-enclosed intracellular inclusions that were considerably larger than those seen inSR-BI−/− animals. Their appearance was distinctly different from that of the target cells of hbdmice (Figure 1J). Furthermore, these cells fromSR-BI−/−/apoE−/− mice stained strongly with both acridine orange and thiazole orange,19 20 indicating that they retained a high RNA content, a characteristic of reticulocytes but not mature erythrocytes (Figure 4T and data not shown).
Morphology of red blood cells from wild-type and mutant mice.
Red blood cell preparations from wild-type,apoE−/−, SR-BI−/−, SR-BI−/−/apoE−/−, andhbd mice were examined by means of standard blood-smear Wright-Giemsa light microscopy photographed at × 100 magnification (panels A-E); DIC microscopy photographed at × 100 magnification (panels F-J) (arrow shows inclusions [panels H, I] and hemoglobin in target cell [panel J]; transmission electron microscopy (panels K-P) (panel P is a higher-magnification image of anSR-BI−/−/apoE−/−erythrocyte that shows mitochondria and ribosomes within the membrane-enclosed inclusion (arrows).
Morphology of red blood cells from wild-type and mutant mice.
Red blood cell preparations from wild-type,apoE−/−, SR-BI−/−, SR-BI−/−/apoE−/−, andhbd mice were examined by means of standard blood-smear Wright-Giemsa light microscopy photographed at × 100 magnification (panels A-E); DIC microscopy photographed at × 100 magnification (panels F-J) (arrow shows inclusions [panels H, I] and hemoglobin in target cell [panel J]; transmission electron microscopy (panels K-P) (panel P is a higher-magnification image of anSR-BI−/−/apoE−/−erythrocyte that shows mitochondria and ribosomes within the membrane-enclosed inclusion (arrows).
We further characterized the intracellular inclusions using electron microscopy. Wild-type and apoE−/− cells were indistinguishable (Figure 1K,L), exhibiting the biconcave disc morphology typical of mature red cells. In contrast, someSR-BI−/− cells (approximately 75%, Figure 1M) and all SR-BI−/−/apoE−/− cells (Figure 1N) were irregularly shaped. We rarely saw mitochondria and ribosomes in wild-type and apoE−/−erythrocytes, but they were present in approximately 15% ofSR-BI−/− erythrocytes and almost allSR-BI−/−/apoE−/− cells. These features are characteristic of immature (stage I) reticulocytes, which are not usually found in the peripheral circulation. In addition, many of the SR-BI−/− cells had small, membrane-enclosed inclusions that occasionally appeared to contain organelles. All SR-BI−/−/apoE−/−cells had complex inclusions that resembled those of theSR-BI−/− erythrocytes, but were larger. Their contents (mitochondria, ribosomes) and overall optical density appeared similar to the surrounding cytoplasm (Figure 1P).
Membrane-enclosed inclusions within erythrocytes increase with cholesterol feeding and are correlated with cellular cholesterol content
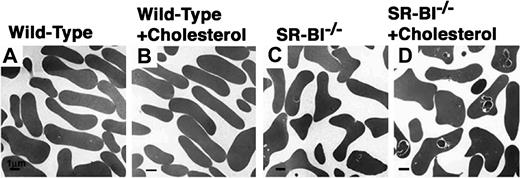
Because of the possibility that increased hypercholesterolemia accounted for the increased severity of defects inSR-BI−/−/apoE−/− mice relative to SR-BI−/− mice, we compared the red blood cells from wild-type and SR-BI−/− mice maintained for 3 months on diets consisting either of normal, low-cholesterol chow or chow supplemented with 1% cholesterol. In wild-type mice, the high-cholesterol diet did not appear to influence the hematocrit, the MCV (Table 1; P not significant), or the morphology of the cells (Figure 2A,B). In contrast, SR-BI−/− mice fed cholesterol had exacerbation of a mild anemia (Table 1) (change inSR-BI−/− hematocrit significant;P = .0007) and marked macrocytosis (Table 1) (change inSR-BI−/− MCV significant;P = .0004). Electron microscopy showed thatSR-BI−/− mice fed cholesterol had larger and more frequent intracellular inclusions than the controls fed normal chow (Figure 2C,D). However, the cholesterol-feeding–induced abnormalities in hematocrit, MCV, and erythroid cell morphology inSR-BI−/− mice were not as severe as those seen in SR-BI−/−/apoE−/− mice.
Effects of cholesterol feeding on the morphology of red blood cells from wild-type and
SR-BI−/− mice. Wild-type (panels A-B) and SR-BI−/−(panels C,D) mice were fed normal, low-cholesterol chow (panels A,C) or 1% cholesterol–supplemented chow (panels B,D) for 3 months. Then, red blood cells were prepared and examined by transmission electron microscopy. Bar = 1 μm.
Effects of cholesterol feeding on the morphology of red blood cells from wild-type and
SR-BI−/− mice. Wild-type (panels A-B) and SR-BI−/−(panels C,D) mice were fed normal, low-cholesterol chow (panels A,C) or 1% cholesterol–supplemented chow (panels B,D) for 3 months. Then, red blood cells were prepared and examined by transmission electron microscopy. Bar = 1 μm.
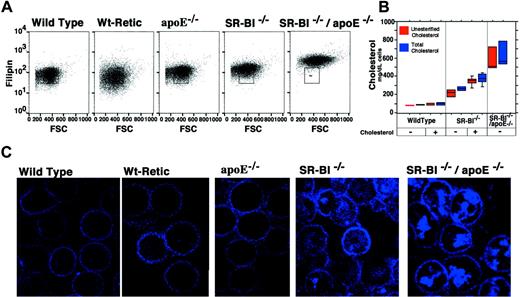
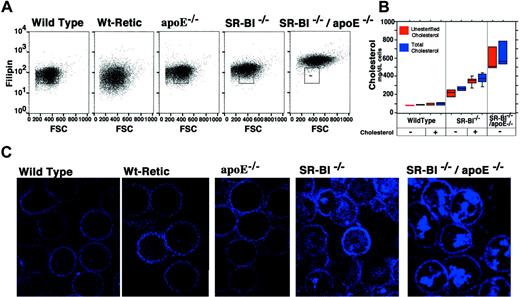
To determine if the morphological abnormalities of the erythrocytes correlated with their cholesterol compositions, we stained red blood cells from the different mouse strains with the cholesterol-binding fluorescent dye filipin and measured the filipin staining. Flow cytometric analysis showed that wild-type erythrocytes and reticulocytes contained less cholesterol (filipin stain) than erythrocytes from animals with mutations in apoE, SR-BI, or both genes (Figure 3A; the box in each panel indicates the approximate distribution of wild-type erythrocytes). Cholesterol levels were highest inSR-BI−/−/apoE−/− erythrocytes. The filipin staining was also visualized by confocal microscopy (Figure3B,C). Although the plasma membranes of the cells fromSR-BI−/− andSR-BI−/−/apoE−/− mice appeared to stain more intensely with filipin than those of the controls (erythrocytes from normal wild-type mice; reticulocytes isolated from phlebotomized wild-type mice; and erythrocytes fromapoE−/− mice), the most striking differences were due to intense staining of intracellular structures inSR-BI−/− andSR-BI−/−/apoE−/− erythrocytes. These intensely stained intracellular structures resemble the inclusions visualized by other methods (Figure 1) and probably represent the same vesicles. The levels of unesterified and total (unesterified plus esterified) cholesterol in the red blood cells, measured directly with an enzymatic assay (Figure 3B,C), correlated with the intensity of filipin staining.
Quantitation and localization of erythrocyte cholesterol in wild-type and mutant mice.
The cholesterol content of erythrocytes from wild-type, reticulocyte-enriched wild-type, apoE−/−, SR-BI−/−, andSR-BI−/−/apoE−/−mice was examined by 2 methods. First, cells were incubated with filipin, to allow fluorescent detection of cholesterol by flow cytometry (panel A) and confocal microscopy (panel C). (Note that in panel A the y-axis is logarithmic.) Second, cholesterol in erythrocyte lysates was measured directly by a biochemical method (panel B). The boxes show the range for 80% of the data points; the lines within the boxes show the median values; and the bars show outlying data points (if any). At the bottom of the plot, − indicates normal mouse chow diet, and + indicates 3 months on the high-cholesterol diet.
Quantitation and localization of erythrocyte cholesterol in wild-type and mutant mice.
The cholesterol content of erythrocytes from wild-type, reticulocyte-enriched wild-type, apoE−/−, SR-BI−/−, andSR-BI−/−/apoE−/−mice was examined by 2 methods. First, cells were incubated with filipin, to allow fluorescent detection of cholesterol by flow cytometry (panel A) and confocal microscopy (panel C). (Note that in panel A the y-axis is logarithmic.) Second, cholesterol in erythrocyte lysates was measured directly by a biochemical method (panel B). The boxes show the range for 80% of the data points; the lines within the boxes show the median values; and the bars show outlying data points (if any). At the bottom of the plot, − indicates normal mouse chow diet, and + indicates 3 months on the high-cholesterol diet.
The abnormal inclusions are autophagolysosomes
The inclusions inSR-BI−/−/apoE−/− red blood cells had the appearance of autophagolysosomes, vesicles that result from sequestration of cytoplasmic contents in a membrane-enclosed compartment that has low internal pH and contains lysosomal enzymes.21 22 To explore this possibility, we incubated red blood cells with Lysotracker, a fluorescent dye that accumulates in low pH compartments, and with FDG, a substrate for lysosomal β-galactosidase. Wild-type and apoE−/−erythrocytes (Figure 4A,B) rarely contained fluorescent inclusions (Figure 4E-F,I-J,M-N). In contrast, erythrocytes from SR-BI−/− andSR-BI−/−/apoE−/− mice (Figure4C,D) were stained with these phagolysosomal dyes in overlapping patterns (Figure 4G,H,K,L,O,P) that were similar to those of the inclusions described above. Thus, the inclusions are probably autophagolysosomes.
Fluorescence microscopic and flow cytometric analysis of the characteristics of red blood cells from wild-type and mutant mice.
(A-P) Red blood cell preparations from wild-type,apoE−/−, SR-BI−/−, andSR-BI−/−/apoE−/−mice were simultaneously stained with 2 lysosomal markers: Lysotracker (low-pH indicator, red) and FDG (lysosomal beta-galactosidase substrate, green), and were examined by phase contrast (panels A-D) and fluorescence (panels E-P) microscopy. Panels E-H show Lysotracker staining; panels I-L, FDG staining; and panels M-P, merged red and green fluorescence images. Yellow indicates spatial overlap of the signals. (Q-T) Dual-color fluorescence flow cytometric analysis of red blood cells from the indicated mice stained with thiazole orange to detect RNA (x-axis) and a phycoerythrin-conjugated monoclonal anti-Trfr antibody to detect cell-surface Trfr (y-axis). For each cell analyzed, the relative intensities of thiazole orange fluorescence and phycoerythrin fluorescence are indicated by a dot and presented on log scales. Each panel represents analysis of 10 000 cells. Dots in the lower left quadrant of the cytogram represent double-negative, mature erythrocytes.
Fluorescence microscopic and flow cytometric analysis of the characteristics of red blood cells from wild-type and mutant mice.
(A-P) Red blood cell preparations from wild-type,apoE−/−, SR-BI−/−, andSR-BI−/−/apoE−/−mice were simultaneously stained with 2 lysosomal markers: Lysotracker (low-pH indicator, red) and FDG (lysosomal beta-galactosidase substrate, green), and were examined by phase contrast (panels A-D) and fluorescence (panels E-P) microscopy. Panels E-H show Lysotracker staining; panels I-L, FDG staining; and panels M-P, merged red and green fluorescence images. Yellow indicates spatial overlap of the signals. (Q-T) Dual-color fluorescence flow cytometric analysis of red blood cells from the indicated mice stained with thiazole orange to detect RNA (x-axis) and a phycoerythrin-conjugated monoclonal anti-Trfr antibody to detect cell-surface Trfr (y-axis). For each cell analyzed, the relative intensities of thiazole orange fluorescence and phycoerythrin fluorescence are indicated by a dot and presented on log scales. Each panel represents analysis of 10 000 cells. Dots in the lower left quadrant of the cytogram represent double-negative, mature erythrocytes.
Erythroid differentiation is reversibly disrupted inSR-BI−/−/apoE−/−mice
During normal reticulocyte maturation, there is a coordinate decrease in both cellular RNA content and Trfr expression.23 Both can be readily measured by means of fluorescent stains and flow cytometry.12 As expected from the morphologic studies, we could detect neither RNA nor Trfr in most of the fully differentiated erythrocytes circulating in wild-type andapoE−/− mice (lower left quadrant of cytograms in Figure 4Q,R). In keeping with the morphologic studies,SR-BI−/− mice had a larger fraction of immature cells (RNA+ and Trfr+, upper right quadrant, Figure 4S) than the wild-type andapoE−/− mice, but most of the cells were double negative. Strikingly, virtually all of theSR-BI−/−/apoE−/− cells retained a high RNA content, and most, but not all, expressed substantial levels of Trfr (Figure 4T). This suggests that inSR-BI−/−/apoE−/− mice, some steps in reticulocyte maturation are completely blocked (normal cytoskeletal remodeling, clearance of organelles, and RNA) while others can proceed at least partially (loss of cell-surface Trfr).
Erythrocytes from SR-BI−/− animals contained abnormal autophagolysosomes, which were more prominent when the mice were fed a high-cholesterol diet or when plasma cholesterol levels were elevated as a consequence of inactivation of theapoE gene (double-knockout mice). The severity of the morphologic phenotype correlated with cellular cholesterol content. It seemed possible that disruption in erythroid differentiation might have been due to loss of SR-BI expression in the reticulocytes themselves (cell autonomous effects), in other tissues (eg, the liver, which has a major role in lipoprotein metabolism), or both. Using immunofluorescence methods, we have detected SR-BI protein expression in a punctate pattern in Trfr+ reticulocytes from phlebotomized wild-type mice (data not shown). However, expression of SR-BI in reticulocytes does not necessarily mean that SR-BI in these cells is essential for maturation. To address this issue, we examined the morphologies of erythrocytes isolated from lethally irradiatedapoE−/− mice that had subsequently received bone marrow transplantation from apoE−/− orSR-BI−/−/apoE−/−donor mice. All cells in the recipients of bone marrow fromapoE−/− mice were SR-BI+, while all cells except the bone marrow–derived cells (eg, erythroid cells) in the recipients of marrow fromSR-BI−/−/apoE−/−donors were SR-BI+. The apoE−/−donor marrow generated morphologically normal erythrocytes, as expected (data not shown). The erythrocytes generated from theSR-BI−/−/apoE−/− marrow in theapoE−/− recipient mice did not contain autophagolysosomes (data not shown). This suggested that accumulation of the autophagolysosomes in the erythrocytes ofSR-BI−/−/apoE−/− mice was not simply a consequence of the absence of SR-BI expression in the erythroid precursors. These results also suggested that the dyslipidemic environment in the plasma ofSR-BI−/−/apoE−/− mice may have played a critical role in the disruption of reticulocyte maturation. If that is the case, it might be possible to reverse the block in development by transferring the abnormal erythrocytes fromSR-BI−/−/apoE−/− mice into normolipidemic wild-type mice.
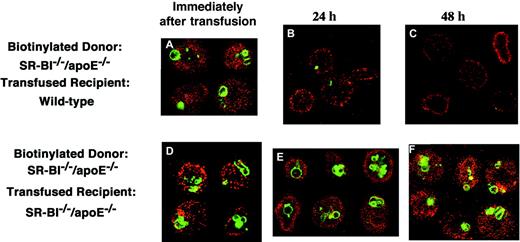
To examine the role of the extracellular environment in erythrocyte maturation in vivo, we transfused biotin-labeled erythrocytes fromSR-BI−/−/apoE−/− mice into either wild-type or SR-BI−/−/apoE−/−mice and determined if residence in the circulation of the recipient animal affected the retention of cytoplasmic autophagolysosomes (Figure 5). We examined a sample of biotinylated donor cells at transfusion and samples of blood from the recipient animals collected 24 or 48 hours after transfusion by staining with fluorescent streptavidin (red signal). We visualized autophagolysosomes by staining with Lysotracker (green signal). Prior to transfusion, all biotin-labeled erythrocytes contained autophagolysosomes (Figure 5A,D). When the labeledSR-BI−/−/apoE−/− cells were transfused into SR-BI−/−/apoE−/−recipient mice, circulation for up to 48 hours did not appear to affect the autophagolysosomes in the cells (Figure 5D-F). In contrast, autophagolysosomes were efficiently removed from the labeled cells when they circulated in wild-type mice, with most autophagolysosomes lost by 24 hours (Figure 5A-C). It seems likely that the loss of autophagolysosomes was a consequence of maturation, because another marker of immature cells, surface Trfr, was present on transfusedSR-BI−/−/apoE−/− erythrocytes and was substantially decreased after cells circulated in wild-type recipients. Immediately after transfusion, 82% of the cells with detectable surface biotin were strongly positive for Trfr, when analyzed by flow cytometry. By 24 hours, only 66% of the biotin-labeled cells had detectable Trfr. By 48 hours, only 50% of the biotin-labeled cells had detectable Trfr, and the amount of Trfr on the Trfr+ cells was significantly less than on the first day. Taken together, the bone marrow transplantation and biotin-labeling/transfusion experiments indicate that abnormal retention of autophagolysosomes inSR-BI−/−/apoE−/− erythrocytes is reversible and that these cells appear to undergo normal maturation when placed in a permissive environment in vivo.
Fate of biotinylated erythrocytes from
SR-BI−/−/apoE−/− mice transfused into wild-type andSR-BI−/−/apoE−/−recipients. A donorSR-BI−/−/apoE−/−mouse was injected with biotin to label erythrocytes in vivo. Samples of this blood were then injected into either wild-type (panels A-C) orSR-BI−/−/apoE−/−(panels D-F) recipient mice. Samples of blood from these recipient mice were taken immediately after infusion (panels A,D), at 24 hours (panels B,E), and at 48 hours (panels C,F), and the cells were labeled with Lysotracker. Blood cells were analyzed by confocal microscopy showing biotin in red and Lysotracker in green. Immediately following transfusion, 9.4% of the cells in the wild-type recipient were labeled with biotin. This decreased to 9.1% at 24 hours and to 6.0% at 48 hours, for a 48-hour survival rate of 64%.
Fate of biotinylated erythrocytes from
SR-BI−/−/apoE−/− mice transfused into wild-type andSR-BI−/−/apoE−/−recipients. A donorSR-BI−/−/apoE−/−mouse was injected with biotin to label erythrocytes in vivo. Samples of this blood were then injected into either wild-type (panels A-C) orSR-BI−/−/apoE−/−(panels D-F) recipient mice. Samples of blood from these recipient mice were taken immediately after infusion (panels A,D), at 24 hours (panels B,E), and at 48 hours (panels C,F), and the cells were labeled with Lysotracker. Blood cells were analyzed by confocal microscopy showing biotin in red and Lysotracker in green. Immediately following transfusion, 9.4% of the cells in the wild-type recipient were labeled with biotin. This decreased to 9.1% at 24 hours and to 6.0% at 48 hours, for a 48-hour survival rate of 64%.
Expulsion of autophagolysosomes fromSR-BI−/−/apoE−/−reticulocytes in vitro
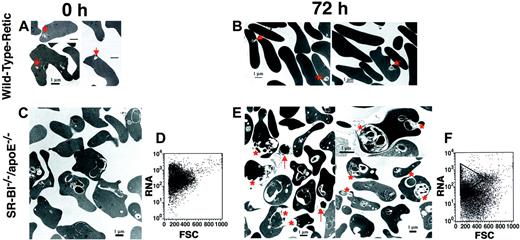
To study the expulsion of autophagolysosomes fromSR-BI−/−/apoE−/− reticulocytes in vitro, we harvested red blood cells fromSR-BI−/−/apoE−/− mice and incubated them in medium containing normolipidemic fetal calf serum under conditions that support cell metabolism and cell survival for several days. For positive controls, we harvested reticulocyte-rich red blood cells from wild-type mice that had been repeatedly phlebotomized. Expulsion of autophagolysosomes was assessed by transmission electron microscopy before (0 hours) and after 72 hours of incubation in vitro (Figure 6).
Effects of in vitro incubation in normolipidemic serum on reticulocytes from phlebotomized wild-type mice and erythrocytes from
SR-BI−/−/apoE−/−mice. Erythrocytes from wild-type mice with phlebotomy-induced reticulocytosis (panels A-B) and fromSR-BI−/−/apoE−/−mice (panels C,E) were incubated in vitro in medium containing 20% normolipidemic fetal calf serum for 0 hours (panels A,C) or 72 hours (panels B,E); the cells were then analyzed by transmission electron microscopy. The red asterisks show membrane-enclosed, cytoplasmic vesicles that appeared to be undergoing expulsion from theSR-BI−/−/apoE−/−erythrocytes (panel E). Arrows denote cell-free vesicles found in the incubation medium (panel E). Arrowheads show structures in reticulocytes from phlebotomized wild-type mice that appear similar to the cytoplasmic vesicles seen inSR-BI−/−/apoE−/−erythrocytes (panels A-B). Panels D and F show flow cytometry analysis of RNA content (thiazole-orange staining) versus forward scatter of the cells (FSC) forSR-BI−/−/apoE−/−samples at 0 hours (panel D) and 72 hours (panel F).
Effects of in vitro incubation in normolipidemic serum on reticulocytes from phlebotomized wild-type mice and erythrocytes from
SR-BI−/−/apoE−/−mice. Erythrocytes from wild-type mice with phlebotomy-induced reticulocytosis (panels A-B) and fromSR-BI−/−/apoE−/−mice (panels C,E) were incubated in vitro in medium containing 20% normolipidemic fetal calf serum for 0 hours (panels A,C) or 72 hours (panels B,E); the cells were then analyzed by transmission electron microscopy. The red asterisks show membrane-enclosed, cytoplasmic vesicles that appeared to be undergoing expulsion from theSR-BI−/−/apoE−/−erythrocytes (panel E). Arrows denote cell-free vesicles found in the incubation medium (panel E). Arrowheads show structures in reticulocytes from phlebotomized wild-type mice that appear similar to the cytoplasmic vesicles seen inSR-BI−/−/apoE−/−erythrocytes (panels A-B). Panels D and F show flow cytometry analysis of RNA content (thiazole-orange staining) versus forward scatter of the cells (FSC) forSR-BI−/−/apoE−/−samples at 0 hours (panel D) and 72 hours (panel F).
At 0 hours, many of the erythrocytes from the phlebotomized wild-type mice contained small vesicles (Figure 6A, arrowheads). We interpret this to be the result of marked erythropoietic stress, due to phlebotomy, that led to the release of immature cells into the circulation. This observation is consistent with an earlier report postulating autophagocytosis in patients with severe anemia syndromes.1 At 0 hours, the inclusions in theSR-BI−/−/apoE−/− erythrocytes were larger and more complex than those in the erythrocytes from the phlebotomized wild-type mice (Figure 6C). At 72 hours, both wild-type and SR-BI−/−/apoE−/− cells appeared to be extruding their membrane-enclosed cytoplasmic vesicles (Figure 6B,E). The cytoplasm of most wild-type cells and manySR-BI−/−/apoE−/− cells appeared darker and more homogeneous than at 0 hours, consistent with maturation. Strikingly, the RNA content of theSR-BI−/−/apoE−/− erythrocytes decreased over the course of the incubation (Figure 6D,F). Free phagolysosomal vesicles could easily be identified in the medium surrounding the SR-BI−/−/apoE−/−cells (Figure 6E, arrows). Thus, it appears that incubation in vitro with normolipidemic fetal calf serum was able, at least in part, to relieve the in vivo block in erythroid differentiation inSR-BI−/−/apoE−/−mice.
We considered the possibility that excess cellular cholesterol directly interfered with expulsion of the autophagolysosomes. To investigate this, we briefly treatedSR-BI−/−/apoE−/− cells with or without the cholesterol-sequestering agent methyl cyclodextrin (MCD, 0.1% solution, 5 minutes)24 prior to incubating them in vitro as described above (Figure 7). Filipin-staining analysis showed that the MCD treatment reduced the cholesterol content ofSR-BI−/−/apoE−/− cells to the level observed in erythrocytes from normal wild-type mice (lower panels). MCD treatment dramatically accelerated the expulsion of autophagolysosomes from the cells compared with control incubations without MCD treatment (eg, see Figure 6). After 24 hours of in vitro incubation (Figure 7B), the intracellular inclusion bodies were substantially smaller than before incubation (Figure 7A), and most had disappeared after 48 hours of incubation (Figure 7C). At the same time, the cytoplasm of the SR-BI−/−/apoE−/−erythrocytes became darker and more homogeneous, suggesting that ribosomes are no longer present and that hemoglobin is more concentrated in the cytoplasm. In addition, the size and shape of the cells were altered by MCD treatment, as is apparent from the decrease in FSC (Figure 7, bottom panels). These results suggest that, by decreasing cellular cholesterol, we have relieved a block preventing autophagolysosome expulsion. This supports the possibility that elevated cellular cholesterol is the primary cause of defective reticulocyte maturation inSR-BI−/−/apoE−/− mice.
Effects of MCD treatment on in vitro vesicle expulsion from erythrocytes from
SR-BI−/−/apoE−/−mice. Erythrocytes fromSR-BI−/−/apoE−/−mice were preincubated for 5 minutes in medium with (this Figure) or without (not shown; however, see Figure 6) 0.1% MCD; cells were then incubated in vitro without MCD as described in Figure 6. Samples were taken before MCD treatment (panel A) and at 24 (panel B) and 48 (panel C) hours of incubation and analyzed by electron microscopy. At 24 hours (panel B) and 48 hours (panel C), the membrane-enclosed, cytoplasmic vesicles appear to be undergoing expulsion from theSR-BI−/−/apoE−/−erythrocytes (red arrows). The plots below each electron micrograph correspond to the photos above and show a decrease in filipin staining as plotted against FSC. The rectangles on the 24- and 48-hour plots show the position of the bulk population of cells at 0 hours. Preincubation without MCD (data not shown) gave results similar to those shown in Figure 6.
Effects of MCD treatment on in vitro vesicle expulsion from erythrocytes from
SR-BI−/−/apoE−/−mice. Erythrocytes fromSR-BI−/−/apoE−/−mice were preincubated for 5 minutes in medium with (this Figure) or without (not shown; however, see Figure 6) 0.1% MCD; cells were then incubated in vitro without MCD as described in Figure 6. Samples were taken before MCD treatment (panel A) and at 24 (panel B) and 48 (panel C) hours of incubation and analyzed by electron microscopy. At 24 hours (panel B) and 48 hours (panel C), the membrane-enclosed, cytoplasmic vesicles appear to be undergoing expulsion from theSR-BI−/−/apoE−/−erythrocytes (red arrows). The plots below each electron micrograph correspond to the photos above and show a decrease in filipin staining as plotted against FSC. The rectangles on the 24- and 48-hour plots show the position of the bulk population of cells at 0 hours. Preincubation without MCD (data not shown) gave results similar to those shown in Figure 6.
Discussion
Investigation of an unusual anemia in mice with defective lipoprotein metabolism has provided a unique window into late erythroid maturation. Abnormal erythrocyte morphology was observed in mice lacking the HDL receptor SR-BI. This was exacerbated by feedingSR-BI−/− mice a high-cholesterol diet or, more dramatically, by inactivating the apoE gene along with theSR-BI gene(SR-BI−/−/apoE−/−). These erythrocytes had immature features, including macrocytosis, irregular shape, high content of RNA and Trfrs, and large intracellular lysosomelike vesicles that appear to have arisen by autophagocytosis of organelles, polyribosomes, and cytoplasm. The size and number of autophagolysosomes were correlated with the cholesterol content of the erythrocytes. The autophagolysosomes inSR-BI−/−/apoE−/− cells were expelled when the mutant erythrocytes were transfused into wild-type animals, or incubated in vitro with normolipidemic serum. Although SR-BI was expressed in reticulocytes from wild-type mice, bone marrow transplantation experiments suggested that the accumulation of autophagolysosomes in the SR-BI−/− cells was not a simple cell-autonomous phenomenon but, rather, depended on the extracellular environment. Indeed, pretreating the cells with the cholesterol-sequestering agent MCD dramatically accelerated the in vitro expulsion of the autophagolysosomes. Thus, it appears that the novel dyslipidemic lipoprotein profile of SR-BI−/−mice causes abnormal cholesterol accumulation in erythrocyte precursors, which in turn interferes with maturation and results in the accumulation of autophagolysosomes, RNA, and Trfrs. The appearance of the erythrocytes fromSR-BI−/−/apoE−/− mice suggests a mechanism by which immature reticulocytes can discard unneeded cellular structures, reduce cell volume, and complete their maturation.
Do the autophagolysosome-containing, Trfr+, and RNA-rich erythrocytes in SR-BI−/−/apoE−/−mice represent arrested intermediates in the normal red blood cell differentiation pathway, or are they irrelevant off-pathway, dead-end artifacts induced by the novel dyslipidemia inSR-BI−/− mice? Transfusion experiments clearly established that the accumulation of autophagolysosomes and the persistence of RNA and Trfrs inSR-BI−/−/apoE−/− erythrocytes are reversed by circulation in normolipidemic, wild-type animals. Thus, these cells apparently retain the capacity to progress through the latter stages of erythrocyte development if an environmental block is released. The observation of autophagosomes in the erythrocytes of diverse human patients with severe anemia1 and in repeatedly phlebotomized mice (this study) indicates that such structures can be observed independently of SR-BI deficiency when erythropoiesis is markedly stressed. Although other explanations are possible, it appears that autophagolysosomes play an evanescent, yet important, role in normal erythroid differentiation. We propose that they are essential for discarding cytoplasmic contents and initiating shape, volume, and biochemical changes that ultimately produce organelle-free, biconcave, mature erythrocytes.
Although our results are perhaps not surprising given the intriguing role that cholesterol is thought to play in regulated trafficking and sorting (reviewed in Hoekstra and van IJzendoorn25), the details of the mechanisms by which erythrocytes expel autophagolysosomes and by which dyslipidemia and excess cholesterol arrest this expulsion remain to be determined. We do not yet know if the mechanisms for nuclear expulsion from early erythrocyte precursors26 and phagolysosomal expulsion from reticulocytes are similar. Nuclei were no longer present in theSR-BI−/−/apoE−/− erythrocytes. This suggests that nuclear expulsion does not depend on the loss of autophagolysosomes and is not sensitive to the environmental factors (eg, dyslipidemia) that lead to autophagolysosome accumulation inSR-BI−/−/apoE−/− mice. Acceleration of in vitro autophagolysosome expulsion by the cholesterol-sequestering agent MCD may provide a useful tool for the future analysis of the mechanism of expulsion.
In vivo, reticuloendothelial macrophages of the liver and spleen play an important role in removing particulate matter (eg, nuclear remnants, precipitated hemoglobin) from circulating erythroid cells.27 28 As the erythrocytes pass through narrow vascular spaces, macrophages are thought to aid in cellular remodeling by actively removing membrane-enclosed vesicles containing cellular debris. Although autophagolysosome expulsion fromSR-BI−/−/apoE−/− erythrocytes can occur in the absence of phagocytic cells in vitro, it seems likely that reticuloendothelial macrophages participate in the removal of residual autophagosomes from circulating immature reticulocytes in living animals, either subsequent to or concurrently with their expulsion from cells. Thus, it is possible that insufficient macrophage-mediated organelle clearance activity or macrophage dysfunction in SR-BI−/−/apoE−/−mice might contribute to autophagolysosome accumulation in their erythrocytes.
In conclusion, the novel abnormalities ofSR-BI−/−/apoE−/− mice have permitted the identification and characterization of transient intermediates in normal erythroid maturation that were proposed to exist previously, but were difficult to capture in morphological studies. The arrest ofSR-BI−/−/apoE−/− erythroid development, apparently dependent upon cholesterol, is reversible both in vivo and in vitro. Thus, further analysis of these mice may provide additional insights into the mechanisms underlying erythroid development.
We thank Helena Miettinen for critical suggestions, advice, and help with cholesterol-feeding experiments; Mark Fleming and Antonio Perez-Atayde for assisting us in the interpretation of pathology slides; and Mohandas Narla, Ellis Neufeld, David Clapham, Sam Lux, Joanne Levy, and Robert Levy for helpful discussions. We thank Jeff Macklis and Lisa Catapano for assisting us with differential interference contrast microscopy. Howard Mulhern in the Department of Pathology carried out electron microscopy at Children's Hospital, Boston. Michelle Lowe in the Confocal and Multiphoton Core Facility at the Brigham and Women's Hospital performed confocal microscopy. Lynne Montross kindly performed all mouse tail vein injections.
Supported by grants from the National Institutes of Health (M.K. and N.C.A.). A.B. was a European Molecular Biology Organization and Human Frontiers Science Program postdoctoral fellow. B.L.T. was a Medical Research Council of Canada postdoctoral fellow. N.C.A. is an Associate Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nancy C. Andrews, HHMI/Hematology, Enders 720, Children's Hospital, 300 Longwood Ave, Boston, MA 02115; e-mail:nandrews@enders.tch.harvard.edu.

![Fig. 1. Morphology of red blood cells from wild-type and mutant mice. / Red blood cell preparations from wild-type,apoE−/−, SR-BI−/−, SR-BI−/−/apoE−/−, andhbd mice were examined by means of standard blood-smear Wright-Giemsa light microscopy photographed at × 100 magnification (panels A-E); DIC microscopy photographed at × 100 magnification (panels F-J) (arrow shows inclusions [panels H, I] and hemoglobin in target cell [panel J]; transmission electron microscopy (panels K-P) (panel P is a higher-magnification image of anSR-BI−/−/apoE−/− erythrocyte that shows mitochondria and ribosomes within the membrane-enclosed inclusion (arrows).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1817.h8001817_1817_1824/5/m_h80522205001.jpeg?Expires=1765273271&Signature=nj1x7n5ZMmUvMA0NzdY3YSaS9Pyhi6krLRYL2P4pL8U2AdV3rMGoJ-MQ55gxXNOMp~dmcyanCUXNazrHwa3zA-pa-iOC7nduGkjpEySa7MPhXFKsb0pfn-lvodqxuchfSR9yB305HUPtCOOiL4VSJahQoMZ6kaEtL7VHlNlyPwVIcMaZbedgNo7Z9N8sWHd3VKDbLUbohRITuuRQP35GOUECz-MulcdexlRTedaVv3aATF-PFL9SgNr0jmuSsrwxRJ23-beuKHzfCdzKuPcz4xF9hTGGq-rk5tHjpA5MK~x9MjupKSeP4pH2WwCi~kIKHwc4HOwO9NpzDt96QpulSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 1. Morphology of red blood cells from wild-type and mutant mice. / Red blood cell preparations from wild-type,apoE−/−, SR-BI−/−, SR-BI−/−/apoE−/−, andhbd mice were examined by means of standard blood-smear Wright-Giemsa light microscopy photographed at × 100 magnification (panels A-E); DIC microscopy photographed at × 100 magnification (panels F-J) (arrow shows inclusions [panels H, I] and hemoglobin in target cell [panel J]; transmission electron microscopy (panels K-P) (panel P is a higher-magnification image of anSR-BI−/−/apoE−/− erythrocyte that shows mitochondria and ribosomes within the membrane-enclosed inclusion (arrows).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1817.h8001817_1817_1824/5/m_h80522205001.jpeg?Expires=1765416444&Signature=mGYWMYqqVDMI92jsjHEyb8XhqWg0MGzcy9He0wWpNgTOBDVb~YvhBg4peYMd~FfpSXyNNHDAQt2-7PQSZ2-oVpK5YwcbzYWPLKGgcMBLf9l78c2le2FhzZ8hM8XZaDgrFkry~i3~tw6OirkySKBiZSEmpgCS59PKTwXJeNIoI4Y7PGvrFTTpbUj9F90HB~t-mKU4KQGAQ98GoOpNRgs4v2TzM5HF2y-~OmuPGyP-AsHZs4zeZ37H7X65ivEfOPxm3Nsn~Sk6nTWz6G0BRp7NZ1PM7udArRE4uCIeJwwqEL4izJeuS2Ndr7JKWEQ8lIOMDMmBTt0q7OZZxf7o6cf26Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)