Recent evidence suggests that dendritic cells (DCs) can regulate and amplify immune responses. Flt3 ligand (FL)–derived DC function was tested as a stimulator of allogeneic lymphocytes in vitro and in vivo. Treatment of mice with FL dramatically expanded DC number, but DCs isolated from FL-treated mice (FL DCs) were poor stimulators of allogeneic T-cell responses in vitro. Further activation of FL DCs did not restore their stimulatory ability, and FL DCs did not suppress the stimulation of the allogeneic T cells by normal DCs. FL treatment significantly increased the CD8α+ DC subset, which appeared to be the reason for their poor stimulatory capacity. These observations were confirmed in vivo using a mouse model of acute graft-versus-host disease (GVHD) wherein host DCs play a critical role. FL treatment of recipients before allogeneic bone marrow transplantation dramatically suppressed donor T-cell responses to host antigens, thereby reducing GVHD mortality (P < .01). These data represent a novel strategy that alters host DCs and reduces acute GVHD.
Introduction
Flt3 ligand (FL) stimulates the proliferation of hematopoietic progenitor cells through binding to the FL receptor, which is restricted to cells of hematopoietic origin.1-3Tissues in FL-treated mice display dramatic increases in DCs of multiple organs, including spleen, lymph nodes, blood, thymus, liver, lungs, Peyer patches, and bone marrow.4,5 Therefore, FL has been proposed as a means to boost protective immunity against several diseases including cancer and infectious agents. FL can augment immunologic responses,6-14 but it can also induce tolerance.15
Dendritic cells (DCs) are centrally involved in the initiation of T-cell–dependent immune responses. In mouse spleens, at least 2 major subpopulations of DCs have been described, CD8α+ and CD8α−, which differ in their phenotype and in certain regulatory features. CD8α+ DCs stimulate allogeneic CD4+ and CD8+ T cells less efficiently than CD8α− DCs in mixed lymphocyte reaction (MLR) in vitro,16-18 and studies suggest that CD8α+DCs may regulate the alloresponses of CD4+ T cells by Fas/Fas ligand (FasL)–induced apoptosis16 and of CD8+ T cells by limiting their interleukin-2 (IL-2) production.17 The regulatory capacity of CD8α+ DCs is further supported by a recent report that CD8α+ DCs may play a critical role in the maintenance of peripheral tolerance to self-reactive T cells.19 However, such regulatory functions of CD8α+ DCs are controversial and are yet to be established in vivo.
Given the controversy regarding FL effects on DCs and their control of immune responses, we evaluated FL for its effects on DCs of allogeneic T-cell responses as stimulators. We show that FL DCs, though increased in number, only weakly stimulate allogeneic T-cell responses in vitro, and this reduction is correlated with large increases in the CD8α+ DC subset. We confirmed the physiologic importance of these observations using a mouse model of acute graft-versus-host disease (GVHD), in which systemic administration of FL to recipients before bone marrow transplantation (BMT) dramatically expanded DCs but suppressed donor T-cell responses to host antigens, resulting in significantly less GVHD.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b, CD45.2+), B6D2F1 (H-2b/d, CD45.2+), B6.Ly-5a (H-2b, CD45.1+), and B6.129P2-Il10tm1Cgn (IL-10−/−, H-2b) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). The age range of mice used as BMT recipients was between 11 and 16 weeks.
Flt3 ligand treatment
Mice were injected subcutaneously once daily with 10 μg recombinant human FL (kindly provided by Immunex, Seattle, WA) for 8 consecutive days.
Isolation of DCs
Spleens were injected with 0.5 mL collagenase D (1 mg/mL; Boehringer Mannheim, Indianapolis, IN) per spleen, cut into small pieces, and incubated for 45 minutes at 37°C. The resultant digested tissue suspension was teased through a 70-μm filter, centrifuged, and washed twice. In some experiments, cells were cultured overnight at 37°C and 5% CO2 in plastic tissue culture dishes to remove adherent macrophages. Next, the nonadherent cells were collected, resuspended in 1.035 g/mL Percoll (Pharmacia Biotech, Uppsala, Sweden), and underlain with an equivalent volume of 1.075 g/mL Percoll.20 After centrifugation, the resultant band was harvested and washed twice, and DCs were isolated by using CD11c (N418) MicroBeads and the autoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). In some experiments, these DCs were further separated according to their CD8α+ expression, and 2 populations (CD11c+ CD8α+ and CD11c+CD8α−) were sorted on a FACS Vantage SE cell sorter (Becton Dickinson, San Jose, CA).
Flow cytometric analysis
Monoclonal antibodies (mAbs) used were fluorescein isothiocyanate (FITC)–, phycoerythrin-, or allophycocyanin (APC)–conjugated anti–mouse CD45.1, CD3ε, CD8α, CD11b, CD11c (HL3), CD25, CD40, CD49, CD80, CD86, and I-Ab (BD PharMingen, San Diego, CA). Cells were preincubated with mAbs 2.4G2 (rat antimouse FcγR mAbs) for 15 minutes at 4°C to block nonspecific FcγR binding of labeled antibodies and then incubated with the relevant mAbs for 30 minutes at 4°C. Finally, cells were washed twice with 0.2% bovine serum albumin in phosphate-buffered saline, fixed with 1% paraformaldehyde in phosphate-buffered saline, and analyzed by EPICS Elite ESP cell sorter (Beckman-Coulter, Miami, FL). Irrelevant IgG2a/b mAbs were used as a negative control. Ten thousand live events were acquired for analysis.
Cell culture
Splenic T cells were purified by passage through nylon wool columns (Polysciences, Warrington, PA) and then used as responders at 1 × 105 cells/well, together with graded numbers of irradiated (20 Gy) freshly isolated DCs. Percentages of CD11+ cells in the DC fraction were determined by flow cytometric analysis, and CD11c+ DC number was adjusted before placement in culture. Cultures were maintained in 10% heat-inactivated fetal calf serum in complete Dulbecco modified essential medium at 37°C in 7.5% CO2. After 1 to 5 days of culture, supernatants were harvested from the culture for cytokine measurement, and cells were pulsed with 3H-thymidine (1 μCi [0.037 MBq] per well) for an additional 16 hours. Proliferation was determined on a TopCount NTX (Packard Instrument, Meriden, CT).
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) for interferon (IFN)–γ, IL-2, IL-4, IL-10, tumor necrosis factor-α (BD PharMingen), and IL-12 p70 (R&D Systems, Minneapolis, MN) were performed according to the manufacturer's protocol. Briefly, samples were diluted 1:1 to 1:4, and each cytokine was captured by the specific primary mAbs and detected by biotinylated secondary mAbs. Assays were developed with avidin–horseradish peroxidase and substrate. Plates were read at 450 nm using a MAXLine microplate reader (Molecular Devices, Sunnyvale, CA). Samples and standards were run in duplicate, and the sensitivity of the assays was 31.3 pg/mL for IFN-γ, 3.1 pg/mL for IL-2, 7.8 pg/mL for IL-4, 31.3 pg/mL for IL-10, 15.6 pg/mL for tumor necrosis factor-α, and 7.8 pg/mL for IL-12 p70.
Bone marrow transplantation
Before transplantation, recipient mice were pretreated with either FL (10 μg/d) or diluent for 8 consecutive days (days −9 to −2). Mice then received transplants according to a standard protocol as described previously.21 Briefly, on day 0, mice received 12 to 13 Gy total body irradiation (TBI) (cesium Cs 137 source) split into 2 doses, separated by 3 hours to minimize gastrointestinal toxicity. Then 5 × 106 BM cells and 2.5 × 106 nylon wool purified splenic T cells resuspended in 0.25 mL were injected intravenously into recipients. For analysis of cell division, nylon wool–purified splenic T cells were labeled with carboxy fluorescein diacetate-succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) and were transferred into irradiated recipients. In some experiments, BMT recipients were treated with 200 μg NK1.1 mAbs (clone PK134) on days −2 and −1 of BMT to eliminate natural killer (NK) cells.22 Mice were housed in sterilized micro-isolator cages; they received autoclaved hyper-chlorinated drinking water for the first 3 weeks after BMT and filtered water thereafter.
Systemic and histopathologic analysis of acute GVHD
Survival after BMT was monitored daily, and the degree of clinical GVHD was assessed weekly by a scoring system that sums changes in 5 clinical parameters: weight loss, posture, activity, fur texture, and skin integrity (maximum index, 10) as previously described.23 This index is a more sensitive index of acute GVHD severity than weight loss alone, a parameter that has been found to be reliable indicator of systemic GVHD in multiple murine models. Acute GVHD was also assessed by detailed histopathologic analysis of liver, a primary GVHD target organ. Sections of liver (right lobe) were fixed in 10% buffered formalin. Specimens were then embedded in paraffin, cut into 5-μm–thick sections, and stained with hematoxylin and eosin for histologic examination. Slides were coded without reference to prior treatment and were examined systematically by a single pathologist using a semiquantitative scoring system as previously described.24
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates. The Mann-Whitney U test was used for the statistical analysis of in vitro data and clinical GVHD scores, whereas the Mantel-Cox log rank-test was used to analyze survival data. P < .05 was considered statistically significant.
Results
FL administration preferentially expands resting DCs
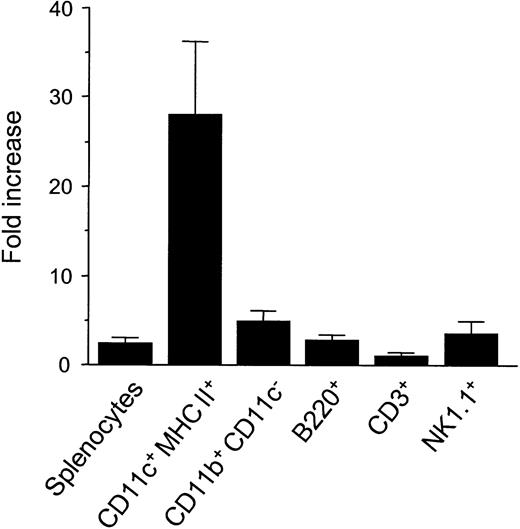
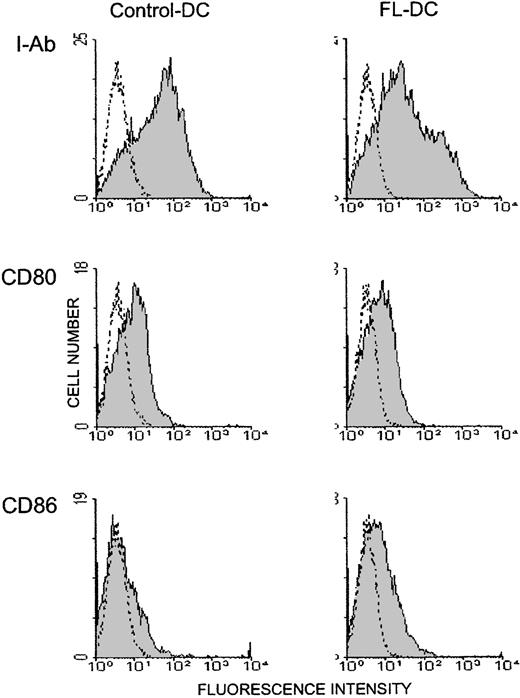
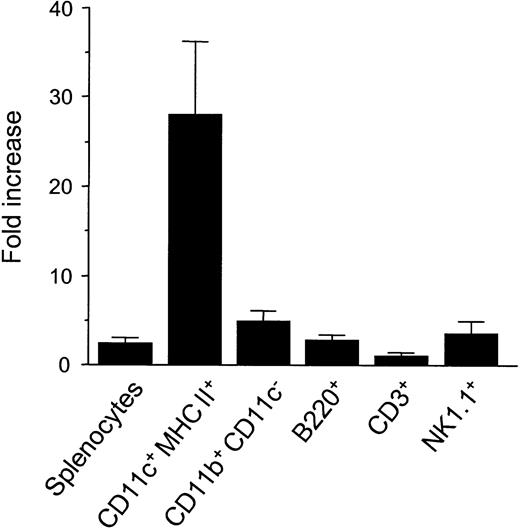
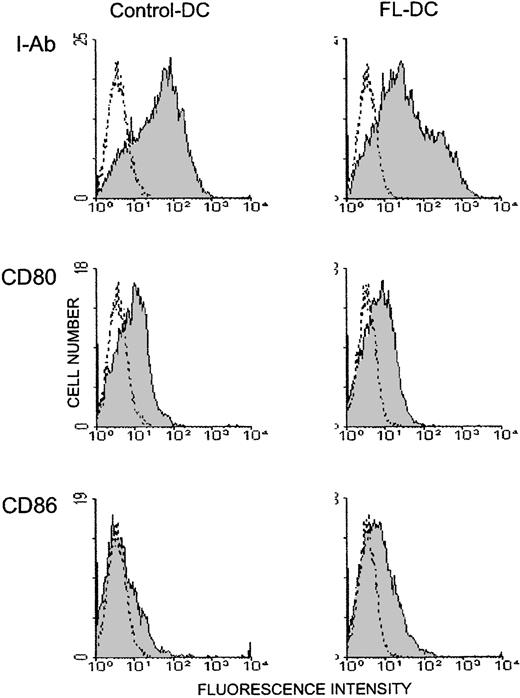
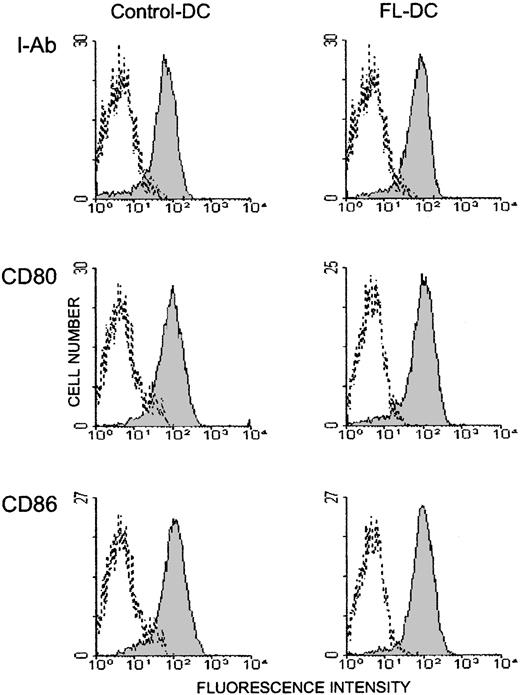
Mice were injected with 10μg FL or diluent for 8 days, and their splenocytes were analyzed by a flow cytometry. Mice treated with FL showed a 2.4-fold increase in overall cellularity in spleen with a dramatic 28.2-fold increase in the absolute number of CD11c+ major histocompatibility complex (MHC) class II+ cells (75.6 ± 15.5 × 106/spleen) (Figure 1). In addition, numbers of CD11b+ CD11c− macrophages, B220+ B cells, and NK1.1+ cells were increased by 5.0-, 2.8-, and 3.9-fold, respectively, as previously shown,4 though the number of CD3+ cells was unaffected. To determine the effects of FL on DC activation, splenic DCs were gated according to forward versus side scatter and CD11c positivity, and their surface antigen expression was analyzed by 2-color immunofluorescence. Freshly isolated control DCs and FL DCs displayed a resting, nonactivated phenotype, as evidenced by low levels of surface MHC class II, CD80, and CD86 (Figure 2).
Cell expansion in spleens from FL-treated mice.
Mice were treated with 10 μg FL or diluent for 8 days. Spleens were harvested, and splenocytes were phenotyped by flow cytometry as DCs (CD11c+ MHC class II+), macrophages (CD11b+ CD11c−), B cells (B220+), T cells (CD3+), and NK cells (NK1.1+). Fold-increase is the ratio of the number of cells from FL-treated mice to control mice, and mean ± SD of fold increase was shown from 4 experiments.
Cell expansion in spleens from FL-treated mice.
Mice were treated with 10 μg FL or diluent for 8 days. Spleens were harvested, and splenocytes were phenotyped by flow cytometry as DCs (CD11c+ MHC class II+), macrophages (CD11b+ CD11c−), B cells (B220+), T cells (CD3+), and NK cells (NK1.1+). Fold-increase is the ratio of the number of cells from FL-treated mice to control mice, and mean ± SD of fold increase was shown from 4 experiments.
Freshly isolated DCs in spleens from FL-treated mice show resting phenotype.
DCs were freshly isolated from spleens as in Figure 1, and 2-color flow cytometric analysis was performed. Gray histograms indicate Ag-specific antibodies; transparent histograms, isotype controls. Data are representative of 3 separate experiments.
Freshly isolated DCs in spleens from FL-treated mice show resting phenotype.
DCs were freshly isolated from spleens as in Figure 1, and 2-color flow cytometric analysis was performed. Gray histograms indicate Ag-specific antibodies; transparent histograms, isotype controls. Data are representative of 3 separate experiments.
FL DCs are poor stimulators of allogeneic T cells
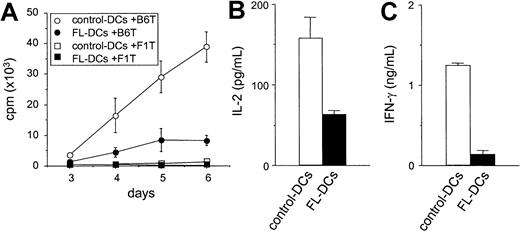
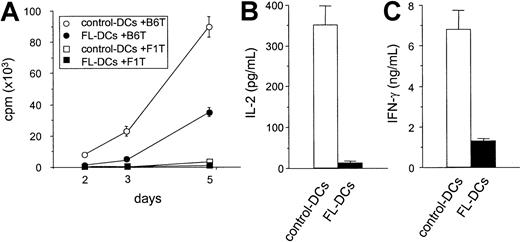
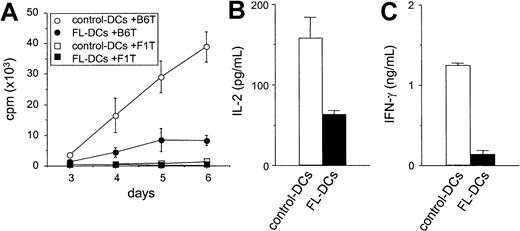
We next compared FL DCs to control DCs as stimulators of T-cell responses in an allogeneic MLR. As shown in Figure3A, FL DCs were less effective stimulators than control DCs throughout the culture period. Similar results were obtained at different T/DC ratios and in other allogeneic responder–stimulator combinations (data not shown). Levels of IL-2 and IFN-γ, critical cytokines secreted during allogeneic T-cell responses, were also decreased in the supernatants of MLR cultures stimulated with FL DCs (Figure 3B-C). Neither IL-4 nor IL-10 was detected in any of the supernatants. Immature DCs can induce tolerance by generating IL-10–producing regulatory T (Tr1) cells in an IL-10–dependent fashion.25 26 Therefore, T cells from IL-10−/− mice were used as responders in MLR. However, T-cell proliferation from IL-10−/− mice and wild-type mice were equally impaired in culture with FL DCs (data not shown), ruling out IL-10 production by Tr1 cells as a mechanism for the lack of response.
Impaired allostimulatory activity of FL DCs in MLR.
(A) Five thousand irradiated DCs isolated from B6D2F1 mice were cultured with 105 T cells from either B6 (allogeneic) or B6D2F1 (syngeneic) mice as described in “Materials and methods.” T-cell proliferation was determined by 3H-thymidine incorporation. Allogeneic T-cell responses to control DCs (open circle) and to FL DCs (closed circle) and syngeneic T-cell responses to control DCs (open square) and to FL DCs (closed square) are shown. Data are the mean ± SD of quadruplicate cultures. (B, C) Supernatants of cultures with control DCs (open bar) or with FL DCs (closed bar) were collected at 24 hours for IL-2 (B) and at 72 hours for IFN-γ (C) and measured by ELISA as described in “Materials and methods.”
Impaired allostimulatory activity of FL DCs in MLR.
(A) Five thousand irradiated DCs isolated from B6D2F1 mice were cultured with 105 T cells from either B6 (allogeneic) or B6D2F1 (syngeneic) mice as described in “Materials and methods.” T-cell proliferation was determined by 3H-thymidine incorporation. Allogeneic T-cell responses to control DCs (open circle) and to FL DCs (closed circle) and syngeneic T-cell responses to control DCs (open square) and to FL DCs (closed square) are shown. Data are the mean ± SD of quadruplicate cultures. (B, C) Supernatants of cultures with control DCs (open bar) or with FL DCs (closed bar) were collected at 24 hours for IL-2 (B) and at 72 hours for IFN-γ (C) and measured by ELISA as described in “Materials and methods.”
Allostimulatory activity of FL DCs remains impaired after maturation in culture
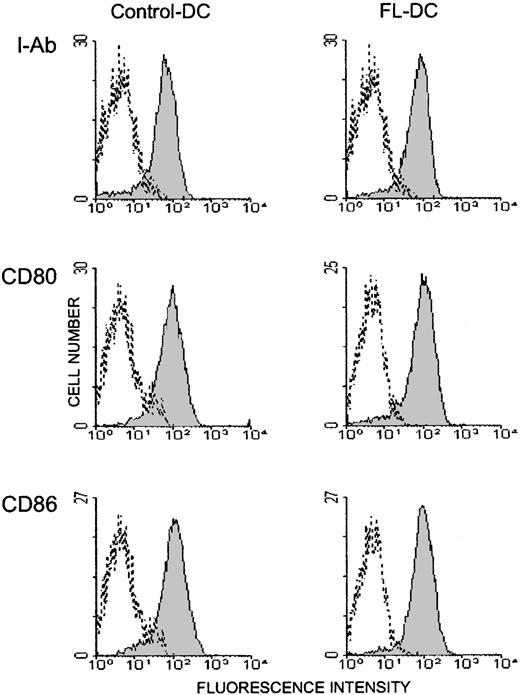
To determine whether the impaired allostimulatory function of FL DCs was caused by the expansion of immature DCs, we examined the ability of overnight culture, which can drive maturation,27 28 to restore the allostimulatory capacity of FL DCs. Overnight culture induced comparable phenotypic maturation in FL DCs and control DCs as characterized by increased expression of MHC class II, CD80, and CD86 (Figure 4). This phenotypic maturation correlated with an increased ability of cultured control DCs to stimulate proliferation and cytokine secretion of allogeneic T cells in MLR (Figure 5, Figure 3). By contrast, cultured FL DCs remained poor stimulators of all allogeneic T-cell responses. Thus, the impaired allostimulatory function of FL DCs could not be explained by reduced expression of costimulatory molecules on their surfaces.
Overnight culture activates DCs.
DCs were isolated from spleens after overnight culture, and 2-color flow cytometric analysis was performed. Data are representative of 2 experiments. Histogram shades are as in Figure 2.
Overnight culture activates DCs.
DCs were isolated from spleens after overnight culture, and 2-color flow cytometric analysis was performed. Data are representative of 2 experiments. Histogram shades are as in Figure 2.
Phenotypically mature FL DCs retain impaired allostimulatory activity.
DCs from FL or control mice were cultured overnight as in Figure 4 and used in MLR as in Figure 3. (A) Five thousand DCs isolated from B6D2F1 mice were cultured with 105 T cells isolated from either B6 (allogeneic) or B6D2F1 (syngeneic) mice. T-cell proliferation was determined by 3H-thymidine incorporation. Allogeneic T-cell responses to control DCs (open circle) and to FL DCs (closed circle) and syngeneic T-cell responses to control DCs (open square) and to FL DCs (closed square) are shown. Data are the mean ± SD of quadruplicate cultures. Supernatants were collected at 24 hours for IL-2 (B) and at 72 hours for IFN-γ (C) and were measured by ELISA, as described in “Materials and methods.”
Phenotypically mature FL DCs retain impaired allostimulatory activity.
DCs from FL or control mice were cultured overnight as in Figure 4 and used in MLR as in Figure 3. (A) Five thousand DCs isolated from B6D2F1 mice were cultured with 105 T cells isolated from either B6 (allogeneic) or B6D2F1 (syngeneic) mice. T-cell proliferation was determined by 3H-thymidine incorporation. Allogeneic T-cell responses to control DCs (open circle) and to FL DCs (closed circle) and syngeneic T-cell responses to control DCs (open square) and to FL DCs (closed square) are shown. Data are the mean ± SD of quadruplicate cultures. Supernatants were collected at 24 hours for IL-2 (B) and at 72 hours for IFN-γ (C) and were measured by ELISA, as described in “Materials and methods.”
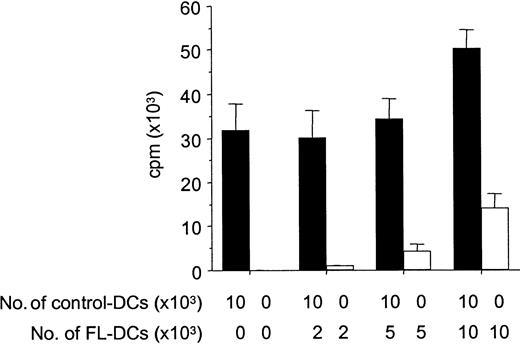
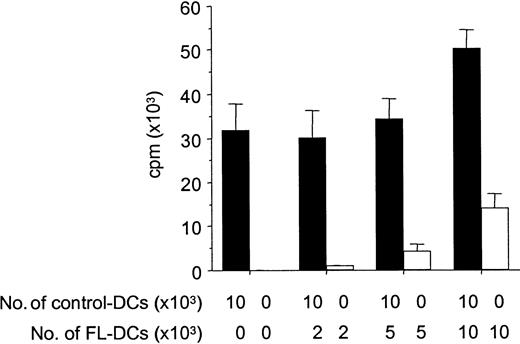
Because APCs can inactivate T cells with which they interact through a veto function,29 we next tested FL DCs for such inhibitory activity by adding graded numbers of FL DCs to control DCs in an allogeneic MLR. FL DCs did not inhibit the stimulation of allogeneic T cells by control DCs, but they showed an additive stimulatory capacity, ruling out possible veto activity (Figure6).
FL DCs do not inhibit alloresponses of control DCs.
B6 T cells (1 × 105) were stimulated with DCs from B6D2F1 in MLR. Graded numbers of FL DCs were added to constant numbers of control DCs at the initiation of culture. Allogeneic T-cell proliferation with control DCs admixed with graded numbers of FL DCs (solid bar) or with graded numbers of FL DCs only (open bar) was determined by 3H-thymidine incorporation. Data represent mean ± SD of quadruplicate cultures.
FL DCs do not inhibit alloresponses of control DCs.
B6 T cells (1 × 105) were stimulated with DCs from B6D2F1 in MLR. Graded numbers of FL DCs were added to constant numbers of control DCs at the initiation of culture. Allogeneic T-cell proliferation with control DCs admixed with graded numbers of FL DCs (solid bar) or with graded numbers of FL DCs only (open bar) was determined by 3H-thymidine incorporation. Data represent mean ± SD of quadruplicate cultures.
FL preferentially expands CD8α+ DCs with less capacity of allostimulation
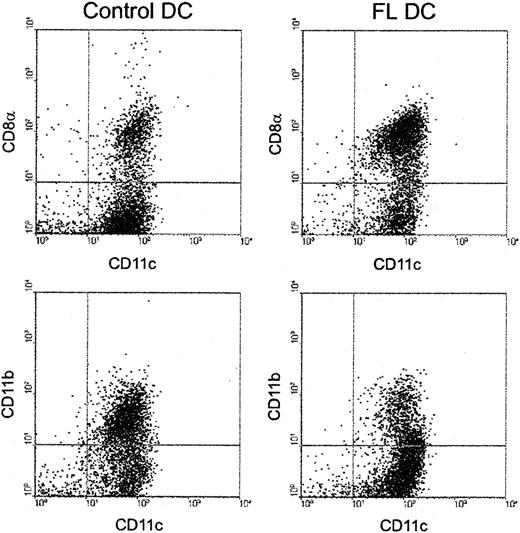
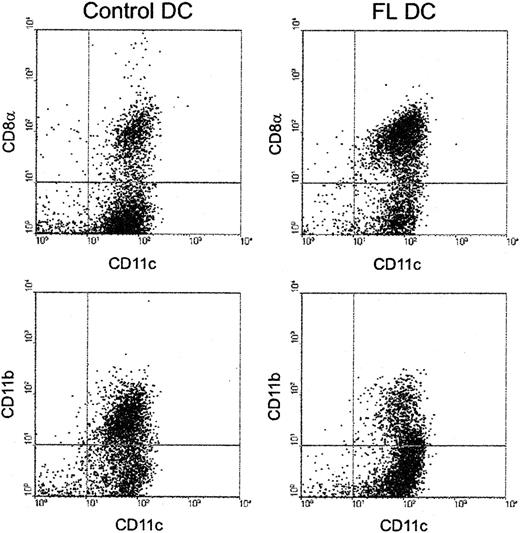
Because the CD8α+ DC subset stimulates allogeneic T cells less efficiently than CD8α− DCs in MLR in vitro,16-18 we next investigated the effect of FL administration on these DC subsets. As shown in Figure7, FL preferentially expanded CD8α+ DCs. The number of CD8α+ DCs was 50.9 ± 7.4 × 106/spleen (46-fold higher than control mice), and the number of CD8α− DCs was 33.7 ± 19.3 × 106/spleen (19-fold higher than control mice). The ratio of CD8α+ to CD8α− DCs increased 3.0-fold (1.51 ± 0.74 vs 0.47 ± 0.06;P < .01). CD8α+ DCs express low levels of CD11b on their surfaces in contrast to the high levels detected on CD8α− DCs.4,30 FL treatment also increased the ratio of CD11b− to CD11b+ DCs by 3.3-fold (Figure 7), showing that the increase in CD8α+ DC number was not attributed to an isolated up-regulation of CD8α on the cell surface but rather to an expansion of the CD8α+ DC subset. When stimulated with a combination of Staphylococcus aureus Cowan I and granulocyte macrophage–colony-stimulating factor (as well as various combinations of cytokines including IFN-γ and IL-4), CD8α+ DCs consistently produced 10-fold more IL-12 p70 than CD8α− DCs (data not shown), in agreement with previously published reports.30-32
Preferential expansion of CD8α+ DCs by FL treatment.
DCs were isolated from spleens and were stained with anti-CD11c–phycoerythrin and anti-CD8α-FITC or anti-CD11b-FITC, as described in “Materials and methods.” Results are representative of 5 replicate experiments.
Preferential expansion of CD8α+ DCs by FL treatment.
DCs were isolated from spleens and were stained with anti-CD11c–phycoerythrin and anti-CD8α-FITC or anti-CD11b-FITC, as described in “Materials and methods.” Results are representative of 5 replicate experiments.
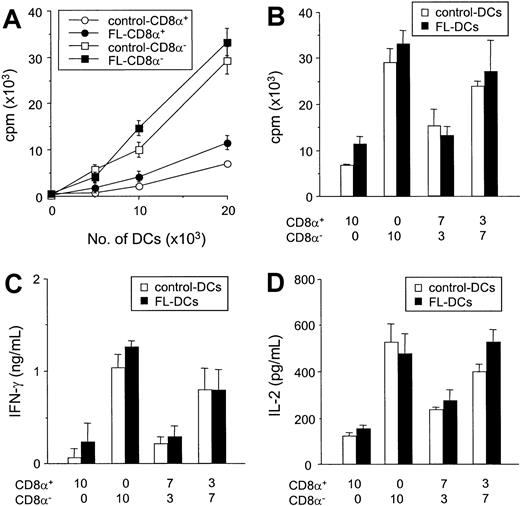
Comparative allostimulatory activity of sorted CD8α+ and CD8α− DCs
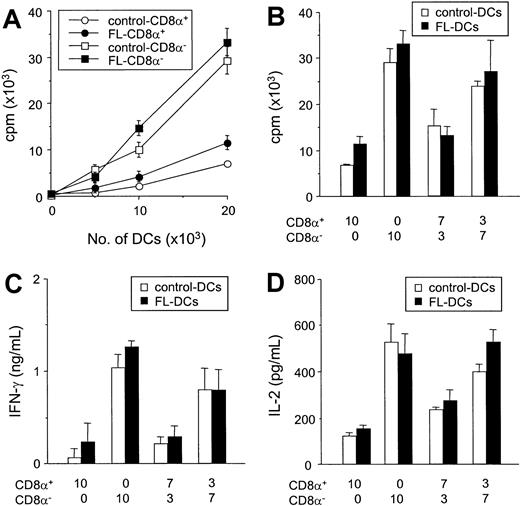
We next compared the ability of isolated CD8α+ DCs to CD8α− DC subsets to stimulate the allogeneic T cells in vitro. Freshly isolated control DCs and FL DCs were sorted according to their CD8α+ expression, and reanalysis of the sorted cell populations confirmed greater than 98% purity of each subset. In culture, freshly isolated CD8α− DCs were more efficient stimulators of allogeneic T cells than CD8α+ DCs in MLR (Figure 8A), as previously described.16,17,33 34 Of importance, each subclass of FL DCs and control DCs stimulated T-cell proliferation to an identical degree (Figure 8A). Furthermore, when CD8α+ and CD8α− fractions sorted from control DCs were mixed at the same ratio as that found in splenocytes from mice treated with FL (CD8α+/CD8α−, 7:3), the allostimulatory capacity of the control DC mixture was equivalent to the FL DC mixture (Figure 8B). Similarly, when a ratio of CD8α+ to CD8α− DCs in spleens from FL-treated mice was changed to that found in control spleens, (CD8α+/CD8a−, 3:7), the allostimulatory capacity of the mixture was equivalent in FL-treated DCs and controls. Similarly, IFN-γ and IL-2 production from MLRs using CD8α− DCs was greater than that in cultures using CD8α+ DCs (Figure 8C-D). For all ratios at which the CD8α+ and CD8α− fractions from FL DCs or control DCs were compared, IFN-γ and IL-2 production were identical in each treatment group. Neither IL-4 nor IL-10 was detected in any supernatant. Thus, the impaired allostimulatory activity of FL DCs in MLR was caused primarily by the increased numbers of CD8α+ DCs produced by FL administration.
FL treatment does not change allostimulatory functions of CD8α+ and CD8α− DC subclasses.
(A) Allogeneic B6 T cells were cultured with various numbers of each DC subset isolated from FL- and control-treated mice. T-cell responses to CD8α+ (open circle) and CD8α− (open square) DCs from control-treated mice and responses to CD8α+ (closed circle) and CD8α− (closed square) DCs from control-treated mice were determined after 3-day MLR by 3H-thymidine incorporation. Data shown are the mean ± SD of quadruplicate cultures. (B-D) Sorted CD8α+ and CD8α− fractions from control DCs (open bar) or FL DCs (closed bar) were mixed at various ratios and cultured with allogeneic T cells from B6 mice for 3 days. Cells were then pulsed with3H-thymidine for the last 16 hours of culture, and proliferation was determined (B). Supernatant was collected 48 hours after culture for IFN-γ measurement (C) and IL-2 measurement (D) by ELISA.
FL treatment does not change allostimulatory functions of CD8α+ and CD8α− DC subclasses.
(A) Allogeneic B6 T cells were cultured with various numbers of each DC subset isolated from FL- and control-treated mice. T-cell responses to CD8α+ (open circle) and CD8α− (open square) DCs from control-treated mice and responses to CD8α+ (closed circle) and CD8α− (closed square) DCs from control-treated mice were determined after 3-day MLR by 3H-thymidine incorporation. Data shown are the mean ± SD of quadruplicate cultures. (B-D) Sorted CD8α+ and CD8α− fractions from control DCs (open bar) or FL DCs (closed bar) were mixed at various ratios and cultured with allogeneic T cells from B6 mice for 3 days. Cells were then pulsed with3H-thymidine for the last 16 hours of culture, and proliferation was determined (B). Supernatant was collected 48 hours after culture for IFN-γ measurement (C) and IL-2 measurement (D) by ELISA.
Expansion of host-type DCs by the administration of FL inhibits early donor T cell expansion and activation during a GVH reaction
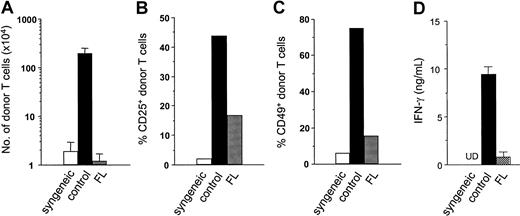
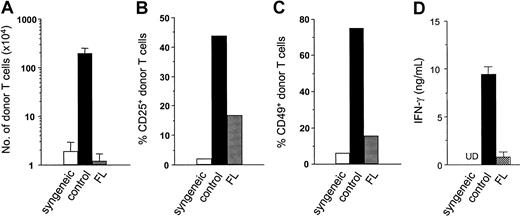
In a final set of experiments, we investigated the in vivo relevance of these in vitro findings using a mouse model of GVHD, wherein host-derived DCs play a critical role. B6D2F1 (H-2b/d, CD45.2+) mice were pretreated with either FL (10 μg/d) or diluent for 8 consecutive days before allogeneic BMT (days −9 to −2). On day 0, mice received 2.5 × 106 nylon wool–purified splenic T cells from allogeneic B6.Ly-5a (H-2b, CD45.1+) donor mice after 13 Gy TBI. Four days after TBI, host-derived DCs (CD45.2+ CD11c+) were undetectable in spleens of mice receiving control diluent, whereas 6.8 × 104 DCs were detected in FL-treated recipients; by day 6, these DCs had disappeared from the spleen. Donor T-cell (CD45.1+CD3+) expansion was evaluated in spleens on day 3 after transfer, when they responded to differences in MHC class I and MHC class II and multiple minor histocompatibility antigens of the host. Despite the marked increase of DCs in recipients by the administration of FL before BMT, allogeneic donor T-cell expansion was almost completely ablated in FL-pretreated recipients on day 4 (Figure9A). Analysis of cell division among these cells using carboxy fluorescein diacetate-succinimidyl ester dye showed lack of cell division correlating with this impaired donor T-cell expansion (3.1 × 104 FL vs 50.9 × 104 control). In control recipients of allogeneic T cells, T cell activation markers CD25 and CD49 appeared on donor CD45.1+ CD3+ T cells on day 4 but were dramatically suppressed in FL-pretreated mice (Figure 9B-C). As an additional index of systemic T-cell responses in vivo, serum levels of IFN-γ were measured on day 4, the time of peak concentration, and were also significantly decreased in FL-pretreated mice (Figure 9D). However, donor T-cell expansion in FL-treated mice did increase and equaled that of control mice on day 7 after BMT (data not shown). Thus, FL pretreatment of recipients inhibits early donor T-cell expansion and activation to host alloantigens in vivo, which is critical for the entire clinical course of acute GVHD.35 36
Pretreatment of recipient mice with FL inhibits donor T-cell activation and expansion to host antigens.
B6D2F1 (CD45.2+) mice were pretreated with either 10 μg/d FL or control diluent from day −8 to day −2, followed by 13 Gy TBI and adoptive transfer of T cells from B6Ly5.2 (CD45.1+) donor mice. Syngeneic control B6 (CD45.2+) mice received B6Ly5.2 (CD45.1+) T cells. Spleens were harvested from recipients after transfer of allogeneic T cells in control-treated (dark bar) and FL-treated (hatched bar) animals or transfer of syngeneic T cells (open bar). (A) Number of donor T cells (CD45.1+ CD3+) was determined by 2-color flow cytometric analysis of splenocytes on day 4. (B, C) Expression of activation markers was determined by the percentage of CD25+ (B) and CD49+ (C) on donor T cells (CD3+ CD45.1+) in spleens on day 4. (d) Serum IFN-γ levels were determined in peripheral blood obtained from animals 4 days after adoptive transfer. (A-D) Data represent mean value ± SD of 3 mice. UD indicates undetected.
Pretreatment of recipient mice with FL inhibits donor T-cell activation and expansion to host antigens.
B6D2F1 (CD45.2+) mice were pretreated with either 10 μg/d FL or control diluent from day −8 to day −2, followed by 13 Gy TBI and adoptive transfer of T cells from B6Ly5.2 (CD45.1+) donor mice. Syngeneic control B6 (CD45.2+) mice received B6Ly5.2 (CD45.1+) T cells. Spleens were harvested from recipients after transfer of allogeneic T cells in control-treated (dark bar) and FL-treated (hatched bar) animals or transfer of syngeneic T cells (open bar). (A) Number of donor T cells (CD45.1+ CD3+) was determined by 2-color flow cytometric analysis of splenocytes on day 4. (B, C) Expression of activation markers was determined by the percentage of CD25+ (B) and CD49+ (C) on donor T cells (CD3+ CD45.1+) in spleens on day 4. (d) Serum IFN-γ levels were determined in peripheral blood obtained from animals 4 days after adoptive transfer. (A-D) Data represent mean value ± SD of 3 mice. UD indicates undetected.
Pretreatment of hosts with FL reduces death from acute GVHD
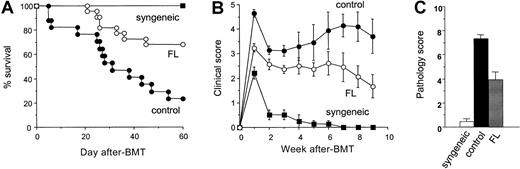
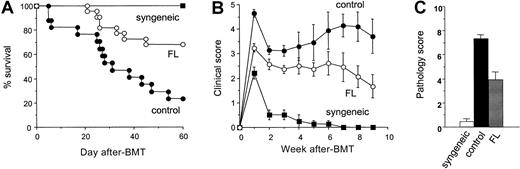
Donor T-cell expansion and activation after BMT normally result in severe systemic GVHD and death in this model system. B6D2F1 recipient mice were treated with either 8-day FL (10 μg/d, day −9 to day −2) or diluent and then were given 12 Gy TBI on day 0 and received transplants of 5 × 106 BM cells and 2.5 × 106 nylon wool–purified splenic T cells from B6.Ly-5a donors. GVHD was severe in controls, with 24% recipient survival at day 60, whereas 68% of FL-pretreated recipients survived (Figure 10A, P < .01). Allogeneic control mice developed severe clinical GVHD compared with syngeneic controls, as assessed by alterations in fur texture, skin integrity, posture, mobility, and weight loss.23 These signs were significantly less severe in FL-treated mice at all time points compared to allogeneic controls (P < .05), though scores in remained significantly higher than in syngeneic controls (Figure 10B). Weight loss as a single parameter of acute GVHD was also less pronounced in FL-treated mice than in control mice at week 9 (20% ± 2% vs 32% ± 1%; P < .05). Histopathologic examination of the liver on day 14 showed significantly lower GVHD pathology scores in FL-treated animals (Figure 10C;P < 0.05). Analysis of donor engraftment at day 60 after BMT in peripheral blood showed complete donor engraftment in FL-pretreated recipients (99.3% ± 0.7%), ruling out rejection or mixed chimerism as a potential cause of reduced GVHD. FL treatment before BMT thus, significantly reduced the ability of host APCs to activate donor T cells and significantly attenuated, but did not eliminate, acute GVHD.
Pretreatment of BMT recipients with FL reduces acute GVHD lethality.
B6D2F1 mice were injected subcutaneously with 10 μg FL for 8 consecutive days (days −9 to −2) and received transplants of BM and splenic T cells from B6 donor mice after 12 Gy TBI on day 0. (A) Survival in control-treated (n = 17, closed circle) and FL-treated (n = 22, open circle; P < .01) animals after allogeneic BMT and syngeneic BMT (n = 4, closed square). (B) GVHD clinical score was significantly lower in FL-treated animals (n = 22, open circle) than in controls (n = 17, closed circle) at all time points (P < .05) and was significantly higher than in syngeneic BMT recipients (n = 4, closed square; P < .01). Data represent mean ± SE from 2 similar experiments. (C) Liver disease 2 weeks after BMT. Liver GVHD was significantly less in FL-treated animals (P < .05) but was higher than in syngeneic controls (P < .05). Randomized coded slides were scored semiquantitatively, as described in “Materials and methods,” and results compared to allogeneic controls represent the mean ± SE of 3 mice per group.
Pretreatment of BMT recipients with FL reduces acute GVHD lethality.
B6D2F1 mice were injected subcutaneously with 10 μg FL for 8 consecutive days (days −9 to −2) and received transplants of BM and splenic T cells from B6 donor mice after 12 Gy TBI on day 0. (A) Survival in control-treated (n = 17, closed circle) and FL-treated (n = 22, open circle; P < .01) animals after allogeneic BMT and syngeneic BMT (n = 4, closed square). (B) GVHD clinical score was significantly lower in FL-treated animals (n = 22, open circle) than in controls (n = 17, closed circle) at all time points (P < .05) and was significantly higher than in syngeneic BMT recipients (n = 4, closed square; P < .01). Data represent mean ± SE from 2 similar experiments. (C) Liver disease 2 weeks after BMT. Liver GVHD was significantly less in FL-treated animals (P < .05) but was higher than in syngeneic controls (P < .05). Randomized coded slides were scored semiquantitatively, as described in “Materials and methods,” and results compared to allogeneic controls represent the mean ± SE of 3 mice per group.
FL treatment expands NK cells,37 and host NK cells play a role in hybrid resistance in this strain combination.22 To examine the in vivo effects of FL on host NK cells and GVHD, B6D2F1 mice received FL (10 μg/d, day−9 to day −2) and were treated with either 200 μg anti-NK1.1 mAbs22 or diluent on days −2 and −1; then they underwent BMT as described above. Elimination of host NK cells before BMT significantly augmented donor T-cell expansion in control-treated and FL-treated recipients on day 4 after BMT (Table1). Nevertheless, in NK-depleted recipients donor T-cell expansion was still impaired in FL-treated mice compared with control-treated mice. Death from GVHD in FL-treated, NK-depleted mice was again significantly lower than in control-treated, NK-depleted mice, ruling out the possibility that host NK cells expanded by FL treatment were primarily responsible for the reduction of acute GVHD.
Discussion
FL treatment of mice reduced their ability to stimulate allogeneic T cells in MLR despite the fact that DC numbers increased and that freshly isolated FL DCs and control DCs from spleens were in similarly inactivated and resting states, as characterized by the low expression of MHC class II and costimulatory molecules.11,38 This observation is consistent with recent findings that demonstrate the impaired allostimulatory capacity of DCs isolated from bone marrow of FL-treated mice, though that study suggested that the impaired function of FL DCs was probably a result of their immaturity.11 In our experiments, however, FL DCs and control DCs displayed similar phenotypes. Freshly isolated splenic DCs express little MHC class II, CD80, or CD86, but these molecules are up-regulated in culture even without any additional stimuli.27,28 Impaired functions of FL DCs were not restored by the activation of FL DCs by overnight culture in vitro. Thus, the allostimulatory function of FL DCs is impaired regardless of their maturational status. Immature DCs can induce tolerance by inducing Tr1 cells,25,26 but IL-10, a critical cytokine secreted by Tr1 cells,25 26 was not detected in the supernatant of MLR or in the serum of mice treated with FL. FL DCs were also poor stimulators of IL-10−/−allogeneic T cells. Furthermore, multiple mixing experiments of FL DCs and control DCs suggest that the impaired allostimulation by FL DCs does not involve an active inhibition. Immune deviation from Th1 to Th2 is also an unlikely mechanism for these altered responses because IL-4 was also not detected in these cultures.
FL expanded splenic DCs primarily by increasing proliferation of CD8α+ DCs, as previously described;39 these CD8α+ DCs were poor stimulators of allogeneic T cells in MLR, also as described.16,17,33 Given the equivalent phenotypic maturity of FL DCs and control DCs, we hypothesized that an increased ratio of CD8α+ to CD8α− DCs was responsible for the impaired allostimulatory function of FL DCs. We confirmed that CD8α+ DCs were poor stimulators of allogeneic T cells in MLR and found identical stimulation of allogeneic responses in a comparison of FL DCs and control DCs by subclass. Thus, the impaired allostimulatory function of splenic FL DCs in MLR appeared to be caused by a proportional increase in the CD8α+ DC subset induced by FL. This mechanism is consistent with the reduced allostimulatory capacity of splenic DCs in RelB mutant mice, which have only CD8α+ DCs in spleen because the transcription factor RelB is essential for the development of CD8α−, but not of CD8α+, DCs.40
In vitro studies have suggested that CD8α+ DCs play a regulatory role in T-cell interactions that ultimately result in diminished allogeneic T-cell responses.16-18,33 However, mechanisms by which CD8α+ DCs stimulate allogeneic T-cell responses less efficiently than CD8α− DCs are still controversial. For example, CD8α+ DCs produce large amounts of IL-12,30-32 and IL-12 administration can prevent acute GVHD by increasing activation-induced cell death.35,36 It has been proposed that CD8α+DCs inhibit proliferation of allogeneic CD4+ T cells by Fas-mediated killing.16 However, a recent study demonstrated that both subsets of DCs express FasL equally and that the proliferation of lpr T cells (lacking Fas) was still inferior to CD8α+ DCs.34 In that study, the production of IL-2 was elevated after stimulation with CD8α+ DCs, suggesting that IL-2–induced, activation-induced cell death might be a mechanism for the diminished T-cell response. This result is inconsistent with previous studies showing that CD8α+ DCs limit IL-2 production from CD8+ T cells in MLR.17,33 In our hands, CD8α+ DCs caused diminished IL-2 production from T cells in vitro, and the increased ratio of CD8α+ to CD8α− DCs in vivo after FL treatment also inhibited IL-2 production and the activation of allogeneic donor T cells. Furthermore, mixing experiments of CD8α+ and CD8α− DCs demonstrated that CD8α+ DCs did not suppress the ability of CD8α− DCs to stimulate allogeneic T cells, even though CD8α+ DCs can inhibit overall T-cell reactivity to peptide-loaded CD8α− DCs.41 Thus, these results suggest that activation-induced cell death is an unlikely mechanism for the reduced capacity to stimulate T cells.
We confirmed the importance of the decrease in allostimulation caused by FL in vivo using a mouse model of GVHD in which the interaction of donor-derived T cells with allogeneic host APCs plays a fundamental role.42 Systemic administration of FL does not change the distinct localization pattern of DC subsets: CD8α+ DCs are present in T-cell areas of secondary lymphoid organs, whereas CD8α− DCs remain in marginal zones.30Despite the increased number of host DCs after FL treatment, early donor T-cell responses to host antigens during the GVH reaction were dramatically inhibited, including their activation, expansion, and differentiation to Th1 cells. Because these donor T-cell responses early after BMT determine the entire clinical outcome of acute GVHD,35,36 their inhibition in FL-pretreated hosts resulted in an overall reduction in GVHD morbidity and mortality. FL treatment also expands NK cells,37 and depletion of NK cells expanded donor T cells in our study. Nevertheless, donor T-cell expansion and GVHD mortality were reduced in FL-treated, NK-depleted hosts compared with control-treated, NK-depleted hosts. These results rule out the possibility that the expansion of host NK cells by FL treatment is primarily responsible for reduced numbers of donor T cells early after BMT and the subsequent reduction of acute GVHD.
GVHD is a major cause of morbidity in BMT, and new approaches to this difficult problem are required. Efforts to prevent GVHD have long focused on the suppression of donor T-cell functions.43 Given the important role of host-derived APCs in GVHD induction, strategies to inactivate host DCs in BMT appear to be promising, though such novel selective targeting of APCs in vivo has not yet been clinically tested. We believe that these data represent the first demonstration of reduced GVHD by the modulation of host-derived DCs through the prophylactic administration of FL. This approach is unique because GVHD is ameliorated by the expansion rather than the elimination of host-derived DCs. The timing of FL administration within this strategy is critical, and our results stand in stark contrast to a recent report of the exacerbation of GVHD when recipients were given FL after BMT.44 One explanation for the difference between these studies is the lack of the effects of FL on host DCs in that study because host DCs essentially disappeared by day 6 after BMT. FL treatment did not expand DCs in that report, but it did up-regulate inflammatory cytokine expression. It is possible that macrophages–monocytes that were activated by TBI may be further stimulated by FL, and that could also increase the severity of GVHD.
Recent evidence suggests that immature DCs and genetically engineered DCs that constitutively express immunosuppressive molecules have the potential to down-regulate immune responses.45 Our data suggest a novel mechanism to modulate immune responses by the administration of FL. This concept is also supported by a recent report that donor-derived CD8α+ DCs blunt host T-cell responses to allogeneic donor BM cells and that they are critical for successful engraftment of hematopoietic stem cells across a full MHC barrier.46 Additional support for this notion derived from vaccination studies, in which strategies that expand CD8α− DCs alone elicit more efficient antitumor immunity than those that preferentially expand CD8α+ DCs in association with the secretion of FL.47
Our study showed that although FL treatment to recipients before BMT significantly attenuated GVHD, it did not eliminate it. Perhaps the impaired allostimulation by FL DCs is short lived because of an extremely rapid turnover of CD8α+ DCs compared to CD8α− DCs.48 In fact, the retention of some antihost reactivity by donor T cells may be beneficial if the important graft-versus-leukemia effect is preserved.49 It is also possible that the biologic effects of FL in humans will differ from those observed in mice. For example, FL administration in humans increases the ratio of CD11c+ DC1 (equivalent to CD8α− DCs in mice) to CD11c− IL-3 receptor+ DC2 (equivalent to CD8α+ DCs in mice) in peripheral blood. A recent analysis of the outcome of BMT in humans suggested that the increased number of DC2 in the graft was associated with less chronic GVHD.50 Studies are in progress to examine this issue and to determine whether the new balance of DC subsets produced by FL administration can be exploited in clinical transplantation protocols.
Supported by National Institutes of Health grants CA39542 (J.L.M.F.) and HL03565-05 (K.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James L. M. Ferrara, Department of Internal Medicine, University of Michigan Cancer Center, 1500 E Medical Center Dr, Ann Arbor, MI 48109-0942; e-mail; ferrara@umich.edu.