Mast cells are thought to participate in a variety of immune responses, such as parasite resistance and the allergic reaction. Mast cell development depends on stem cell factor (Kit ligand) and its receptor, c-Kit. Gab2 is an adaptor molecule containing a pleckstrin homology domain and potential binding sites for SH2 and SH3 domains. Gab2 is phosphorylated on tyrosine after stimulation with cytokines and growth factors, including KitL. Gab2-deficient mice were created to define the physiological requirement for Gab2 in KitL/c-Kit signaling and mast cell development. In Gab2-deficient mice, the number of mast cells was reduced markedly in the stomach and less severely in the skin. Bone marrow–derived mast cells (BMMCs) from the Gab2-deficient mice grew poorly in response to KitL. KitL-induced ERK MAP kinase and Akt activation were impaired in Gab2-deficient BMMCs. These data indicate that Gab2 is required for mast cell development and KitL/c-Kit signaling.
Introduction
Mast cells are hematopoietic-lineage cells that participate in immunoglobulin (Ig)E-associated immune responses, including allergic reactions and parasite resistance (see Galli1 for a review). It was recently shown that mast cells also participate in the innate immunity to bacterial infection, in which IgE may not be involved.2 Genetic evidence indicates that Kit ligand (KitL) and its receptor, c-Kit, play essential roles in mast cell development. Mutations in the mouseKit ligand and c-Kit genes (Steel andWhite spotting) lead to defects in the development of melanocytes, germ cells, erythroid cells, basophils, and mast cells.3-5 c-Kit is a receptor-type tyrosine kinase that displays some homology with platelet-derived growth factor receptors. The binding of KitL to c-Kit induces the dimerization and transphosphorylation of c-Kit. Tyrosyl-phosphorylated c-Kit recruits signaling molecules containing the Src homology 2 (SH2) domain, such as phosphatidyl inositol (PI)-3 kinase,6phospholipase Cγ1,7,8 Grb2, and the Src kinase,9 to c-Kit and initiates cytoplasmic signaling. In addition to KitL/c-Kit signaling, interleukin (IL)-3 is also involved in mast cell development. IL-3–deficient mice maintain a basal level of mast cells, whereas mast cells fail to expand in response to infection by the nematode Stronglyoides venezuelensis.10 Double-mutantKitW/KitW-v, IL-3−/−mice display a more severe reduction in mast cell and basophil expansion elicited by the nematode infection than do single-mutant mice.10 The result suggests that IL-3 is not essential for the generation of mast cells in a resting state but that it is required for the increase in mast cells in the immune response elicited by parasites. On the other hand, KitL-mediated signals are required for the development of the basal level of mast cells.
Gab2 is a member of the Gab/DOS family of adapter molecules, which contain a pleckstrin homology (PH) domain and potential binding sites for the SH2 and SH3 domains.11-14 Gab2 is tyrosine phosphorylated on stimulation by growth factors, cytokines, and T- and B-cell antigen receptors, including KitL and IL-3, and phosphorylated Gab2 binds SHP-2 and p85 PI-3 kinase.11,15,16 Overexpression of Gab2 enhances the activation of cytokine-dependent ERK mitogen-activated protein kinase (MAPK) and gene expression.11,12 Expression of a mutant Gab2 inhibits IL-3–dependent transcription.12These reports suggest that Gab2 is involved in the signaling of growth factors and cytokines. To investigate the roles of Gab2 in vivo, we generated mice lacking Gab2 by gene targeting.
Study design
Generation of mutant mice
A NotI-linearized targeting vector was electroporated into R1 embryonic stem (ES; 129X1x12953) cells. G418 (400 ng/mL)- and ganciclovir (2 μg/mL)-resistant clones were selected, and homologous recombination events were detected by polymerase chain reaction (PCR) and Southern blot analysis with a probe located on the 5′ side of the exon. To create chimeric male founders, we used 2 independent Gab2 gene-targeted ES clones and injected them into C57BL/6 blastocysts. Chimeric offspring were mated to C57BL/6 mice to generate F1 heterozygous progeny. The F1 progeny were intercrossed to generate F2 progeny. All the mice used in our experiments have a mixed genetic background of 129 and C57BL/6. Genotypes of the mice were identified by PCR and Southern blot analysis. Precise information about the primers used for PCR is available on request.
Isolation of primary bone marrow-derived mast cells and cell proliferation assay
Mouse bone marrow cells were collected by flushing the marrow cavity of femurs, and bone marrow–derived mast cells (BMMCs) were selectively grown in RPMI 1640 supplemented with IL-3 (supernatant of an mIL-3–producing cell line, Chinese hamster ovary mIL-3-3-12M; a kind gift from T. Sudo, Toray Industry, Japan) and 10% fetal bovine serum for 4 weeks. During culture, the medium was changed every 3 to 4 days, and the cells were transferred to new dishes to remove adherent cells.17 BMMCs cultured for 4 weeks were used in all experiments in vitro. For the proliferation assay, BMMCs were starved for 12 hours in medium without IL-3, and the cells were cultured at 5 × 104 cells/well in presence of various concentrations of mKitL or mIL-3 (Pepro Tech, London, England) for 52 hours. The cells were pulsed for the last 16 hours of the 52-hour culture period with 0.5 μCi/well of 3H-labeled thymidine, followed by scintillation counting.
Antibodies and Western blotting
The anti–Gab2 antibody, which recognizes amino acid 380-563 of human Gab2, was described previously.11 Immunoblotting was performed with anti–diphospho ERKs (Promega, Madison, WI), anti-ERK2 (C-14; Santa Cruz Biotechnology, Santa Cruz, CA), anti–phospho Akt (Ser473), and Akt (New England Biolabs, Beverly, MA) antibodies. Rat anti–c-Kit mAb was purified from the supernatant of hybridoma ACK2 (a kind gift from S. I. Nishikawa). The immunoblotting method was described previously.11,18 19
Staining and counting of mast cells
Stomachs and pieces of dorsal skin were removed from 5-week-old mice and were embedded in paraffin. Sections were stained with Alcian blue and nuclear Fast red, or with berberine sulfate.20Two points of the section were marked in ink, and the number of all mast cells between these 2 points was counted. The number of mast cells thus obtained in the stomach and skin was divided by the length of the portion in which mast cells were counted, and the value was expressed as the number of mast cells per centimeter of stomach or skin, as described previously.21
Results and discussion
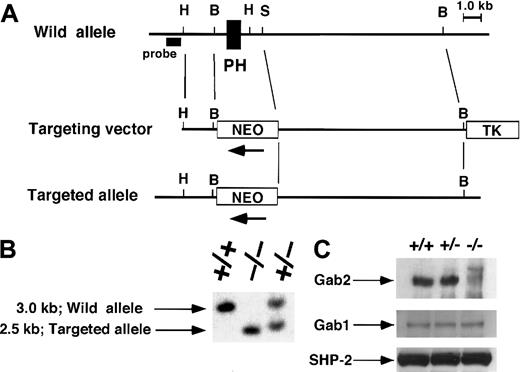
We generated a Gab2 mutation by homologous recombination in ES cells. In the targeting vector, a neomycin-resistance gene was inserted into the exon that encodes the major part of the PH domain (amino acids 28-128) of Gab2 (Figure 1A). Homologous recombination was identified by PCR and Southern blot analysis (Figure1B). The Gab2 protein was detected in the testis of the wild-type mice, in which Gab2 mRNA is strongly expressed,11 by immunoblotting with an antibody that recognizes the carboxy terminal region of Gab2. However, Gab2 was not detected in the testis of Gab2-deficient mice, which expressed normal levels of Gab1 and SHP-2 (Figure 1C). Gab2-deficient mice were born according to Mendelian inheritance and appeared normal. Although Gab2 is highly expressed in the testis and ovary, Gab2-deficient mice were fertile, indicating its dispensable role in these organs.
Targeted disruption of the Gab2 locus.
(A) Restriction map of the Gab2 locus and targeting vector. The deleted region contains an exon encoding part of the PH domain. This region was replaced by the neomycin-resistance gene (neo). H, HindIII; B, BamHI; S, SpeI. A λFixII 129/Sv mouse strain genomic library (Stratagene) was screened by hybridization with the mouse Gab2 cDNA fragment containing an exon encoding the PH domain (amino acids 1-128). A targeting vector was designed to replace theBamHI-SalI fragment containing the PH domain with the neomycin-resistance gene (neo). To construct the targeting vector, the 1.5-kb HindIII-BamHI fragment of mouse Gab2 genomic DNA, the 2.0-kb BamHI-XbaI fragment of pgk-neo, the 9.0-kb SpeI-BamHI fragment of mouse Gab2 genomic DNA, and the 2.0-kb XhoI fragment of the thymidine kinase gene were subcloned into the HindIII andXhoI sites of pBluescript SK+. Further details are available upon request. (B) Southern blot analysis for genotyping.EcoRI and BglII-digested DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice was hybridized with the 5′ probe shown in (A). (C) Immunoblotting analysis of Gab2. Gab2 immunoprecipitated from the testis of Gab2+/+, +/−, and −/−mice was analyzed by immunoblotting with anti–Gab2 antibody. Lysate from each of the same samples were immunoblotted with anti–Gab1 or anti–SHP-2 antibodies for loading control.
Targeted disruption of the Gab2 locus.
(A) Restriction map of the Gab2 locus and targeting vector. The deleted region contains an exon encoding part of the PH domain. This region was replaced by the neomycin-resistance gene (neo). H, HindIII; B, BamHI; S, SpeI. A λFixII 129/Sv mouse strain genomic library (Stratagene) was screened by hybridization with the mouse Gab2 cDNA fragment containing an exon encoding the PH domain (amino acids 1-128). A targeting vector was designed to replace theBamHI-SalI fragment containing the PH domain with the neomycin-resistance gene (neo). To construct the targeting vector, the 1.5-kb HindIII-BamHI fragment of mouse Gab2 genomic DNA, the 2.0-kb BamHI-XbaI fragment of pgk-neo, the 9.0-kb SpeI-BamHI fragment of mouse Gab2 genomic DNA, and the 2.0-kb XhoI fragment of the thymidine kinase gene were subcloned into the HindIII andXhoI sites of pBluescript SK+. Further details are available upon request. (B) Southern blot analysis for genotyping.EcoRI and BglII-digested DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice was hybridized with the 5′ probe shown in (A). (C) Immunoblotting analysis of Gab2. Gab2 immunoprecipitated from the testis of Gab2+/+, +/−, and −/−mice was analyzed by immunoblotting with anti–Gab2 antibody. Lysate from each of the same samples were immunoblotted with anti–Gab1 or anti–SHP-2 antibodies for loading control.
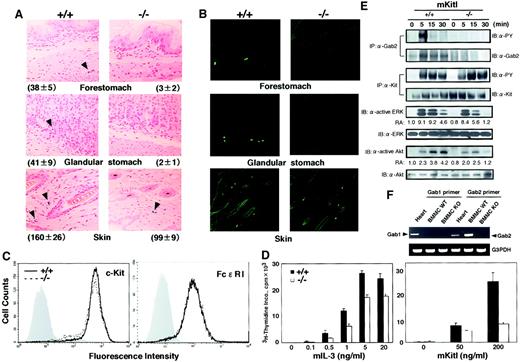
The numbers of red blood cells, white blood cells, and platelets in the peripheral blood were normal in Gab2-deficient mice. Flow cytometry analysis revealed normal numbers of macrophages and mature lymphocytes in the spleens of Gab2-deficient mice (data not shown). These results indicate that Gab2 is not required for hematopoiesis in general. However, we found that the number of mast cells was severely reduced in the stomach (Alcian blue-positive cells; Figure2A, Table1) and in the peritoneum of Gab2-deficient mice compared with that of wild-type mice (Table 1). The number of mast cells was also reduced in the skin of Gab2-deficient mice, but less severely so than in the stomach (Figure 2A, Table 1). The reduction in mast cells was further confirmed by staining with berberine sulfate (Figure 2B). Results indicated that Gab2 is required for mast cell development.
Reduced mast cell numbers in Gab2-deficient mice.
(A) Mast cells in the forestomach, glandular stomach, and skin of Gab2+/+ and Gab2−/− mice. Sections were stained with Alcian blue and nuclear Fast red. Granules of mast cells were stained with Alcian blue. We observed at least 10 fields per each sample, and the representative field was shown. Average numbers of mast cells in 1-cm strip sections are shown. (B) Mast cells stained with berberine sulfate in the forestomach, glandular stomach, and skin of Gab2+/+ and Gab2−/− mice. Berberine sulfate recognizes heparin proteoglycan, which is expressed on mast cells in connective tissues. Berberine sulfate-positive cells were observed under a confocal laser microscope (LSM510; Carl Zeiss, Jena, Germany). (C) Expression of c-Kit and FcεRI. c-Kit expression was detected by staining with biotin–anti–c-Kit (2BP) mAb. To evaluate the FcεRI expression, BMMCs (cultured for 4 weeks) were treated with anti–DNP IgE mAb, and the bound IgE was detected with biotinylated anti–mouse IgE mAb and fluorescein isothiocyanate–streptavidin. Gray histograms indicate unstained negative controls. (D) IL-3 or KitL-mediated proliferation of Gab2+/+ and Gab2−/− BMMCs. Mast cells were expanded by culturing bone marrow cells with IL-3 for 4 weeks. After IL-3 starvation, the cells were cultured in the presence of the indicated concentrations of mIL-3 (left panel) or mKitL (right panel) and were pulsed with 0.5 μCi/well of3H-labeled thymidine for the last 16 hours of the 52-hour culture. Cells were collected by an automated cell harvester, and radioactivity of the incorporated 3H-thymidine was determined by a liquid scintillation counter. (E) Biochemical analysis of signal transduction pathways in BMMCs from Gab2+/+ and Gab2−/− mice. BMMCs were stimulated with mKitL (100 ng/mL) for the indicated periods. Cell lysates were immunoprecipitated with anti–Gab2 or anti–Kit antibodies (ACK2) and were subjected to immunoblotting with anti–phosphotyrosine (4G10), anti–Kit, and anti–Gab2 antibodies. Whole lysates were immunoblotted with anti–diphospho ERKs, anti–phospho Akt (S473), anti-ERK2, and anti–Akt antibodies. Results from the immunoblot analysis were quantified by the densitometric scanning of Western blot bands and indicated as relative activity (RA, activity versus the activity in unstimulated Gab2+/+ BMMCs). (F) Reverse transcription–PCR analysis of Gab1 and Gab2 expression. Total RNAs were isolated from Gab2+/+ and Gab2−/− BMMCs and then reverse transcribed. The cDNA was used for PCR with the Gab1- and Gab2-specific primers.
Reduced mast cell numbers in Gab2-deficient mice.
(A) Mast cells in the forestomach, glandular stomach, and skin of Gab2+/+ and Gab2−/− mice. Sections were stained with Alcian blue and nuclear Fast red. Granules of mast cells were stained with Alcian blue. We observed at least 10 fields per each sample, and the representative field was shown. Average numbers of mast cells in 1-cm strip sections are shown. (B) Mast cells stained with berberine sulfate in the forestomach, glandular stomach, and skin of Gab2+/+ and Gab2−/− mice. Berberine sulfate recognizes heparin proteoglycan, which is expressed on mast cells in connective tissues. Berberine sulfate-positive cells were observed under a confocal laser microscope (LSM510; Carl Zeiss, Jena, Germany). (C) Expression of c-Kit and FcεRI. c-Kit expression was detected by staining with biotin–anti–c-Kit (2BP) mAb. To evaluate the FcεRI expression, BMMCs (cultured for 4 weeks) were treated with anti–DNP IgE mAb, and the bound IgE was detected with biotinylated anti–mouse IgE mAb and fluorescein isothiocyanate–streptavidin. Gray histograms indicate unstained negative controls. (D) IL-3 or KitL-mediated proliferation of Gab2+/+ and Gab2−/− BMMCs. Mast cells were expanded by culturing bone marrow cells with IL-3 for 4 weeks. After IL-3 starvation, the cells were cultured in the presence of the indicated concentrations of mIL-3 (left panel) or mKitL (right panel) and were pulsed with 0.5 μCi/well of3H-labeled thymidine for the last 16 hours of the 52-hour culture. Cells were collected by an automated cell harvester, and radioactivity of the incorporated 3H-thymidine was determined by a liquid scintillation counter. (E) Biochemical analysis of signal transduction pathways in BMMCs from Gab2+/+ and Gab2−/− mice. BMMCs were stimulated with mKitL (100 ng/mL) for the indicated periods. Cell lysates were immunoprecipitated with anti–Gab2 or anti–Kit antibodies (ACK2) and were subjected to immunoblotting with anti–phosphotyrosine (4G10), anti–Kit, and anti–Gab2 antibodies. Whole lysates were immunoblotted with anti–diphospho ERKs, anti–phospho Akt (S473), anti-ERK2, and anti–Akt antibodies. Results from the immunoblot analysis were quantified by the densitometric scanning of Western blot bands and indicated as relative activity (RA, activity versus the activity in unstimulated Gab2+/+ BMMCs). (F) Reverse transcription–PCR analysis of Gab1 and Gab2 expression. Total RNAs were isolated from Gab2+/+ and Gab2−/− BMMCs and then reverse transcribed. The cDNA was used for PCR with the Gab1- and Gab2-specific primers.
KitL/c-Kit signaling is required for the development of mast cells, and it is thought that Gab2 is involved in KitL/c-Kit signaling. Therefore, we examined the KitL-induced proliferation of BMMCs from 8-week-old Gab2-deficient and wild-type mice. Mast cells were expanded in vitro from bone marrow cells in the presence of IL-3 for 4 weeks. The expression of c-Kit and FcεRI was not affected in Gab2-deficient BMMCs (Figure 2C). We detected the expression of Gab2 but not Gab1 in wild-type BMMCs (Figure 2E,F). However, we did not detect Gab2 expression in Gab2-deficient BMMCs (Figure 2E). After 4-week culture of bone marrow cells with IL-3, the number of BMMCs recovered from Gab2-deficient mice was 65% of that obtained from the wild-type littermates (1.44 ± 0.88 × 108 cells vs 2.20 ± 0.79 × 108 cells, n = 6). Consistent with this, the level of proliferative response of Gab2-deficient BMMCs restimulated with IL-3 (5 ng/mL) was 65% of wild-type BMMCs (Figure2D). These results suggest that IL-3 signaling is partly perturbed in the Gab2-deficient mast cells. It is also possible that Gab2-mediated signaling, which cooperatively acts with IL-3 signaling, is impaired. On the other hand, the level of proliferative response of Gab2-deficient BMMCs stimulated with KitL (200 ng/mL) was 30% of wild-type BMMCs (Figure 2D), indicating the involvement of Gab2 in KitL/c-Kit signaling. In fact, KitL-induced the tyrosine phosphorylation of Gab2 in wild-type BMMCs but not in Gab2-deficient BMMCs (Figure 2E). These results indicate that IL-3 and KitL/c-Kit signaling are affected by Gab2 disruption.
The Ras/MAPK and PI-3 kinase pathways play important roles in the signal transduction of KitL-mediated cell proliferation and survival.22 23 We examined whether Gab2 deficiency affected the KitL-induced activation of ERK MAP kinase and Akt, which act downstream of Ras and PI-3 kinase, respectively. When Gab2-deficient BMMCs were stimulated with KitL, the phosphorylation level of ERK at 5 minutes after stimulation was almost the same as that of wild-type BMMCs. It decreased more rapidly 15 minutes after stimulation in Gab2-deficient BMMCs than in wild-type BMMCs, although we did not observe any difference between the time course of the KitL-induced tyrosine phosphorylation of c-Kit between Gab2-deficient and wild-type BMMCs (Figure 2E). Furthermore, the KitL-induced Akt phosphorylation was lower in Gab2-deficient than in wild-type BMMCs (Figure 2E). These results indicate that Gab2 is an indispensable adapter molecule linking the c-Kit receptor to ERKs and Akt in mast cells.
Although KitL and IL-3 are involved in mast cell development, IL-3 is not essential for the generation of the basal level of mast cells, but it is involved in the expansion of mast cells in response to parasite infection.10 We found that the number of mast cells was lower in the stomach and skin of Gab2-deficient mice under physiological conditions than it was in wild-type mice, and we showed that Gab2 is indispensable for KitL/c-Kit signaling in mast cells. Future studies will clarify whether the Gab2-mediated signal is involved in the expansion and activation of mast cells in the immune response to parasites and bacteria.
Mutations in KitL and c-Kit lead to a drastic reduction in the numbers of mast cells in the stomach and skin. In the Gab2-deficient mice, stomach mast cells were more severely diminished than skin mast cells. This finding can be explained if Gab1 is expressed in the skin mast cells during their development, and it compensates for the lack of Gab2 in vivo. Alternatively, PI-3 kinase and Shc may interact directly with c-Kit in the skin mast cells and provide redundant signaling pathways for the signals normally generated by Gab proteins. In summary, this report provides genetic evidence that Gab2 is required for mast cell development and the KitL-mediated ERK and Akt activation in mast cells.
We thank R. Masuda and A. Kubota for secretarial assistance.
Supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan and by the Osaka Foundation for Promotion of Clinical Immunology. K.N. is a Research Fellow of the Japan Society for the Promotion of Science.
K.N. and L.W. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
After submission of this manuscript, Gu et al24 reported that Gab2 is essential for allergic reaction in vivo.
Author notes
Toshio Hirano, Dept of Molecular Oncology (C-7), Osaka University Graduate School of Medicine, 2-2, Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail: hirano@molonc.med.osaka-u.ac.jp.