Abstract
Allogeneic bone marrow transplantation (BMT) offers the only curative therapy for chronic myelogenous leukemia. We compared prospectively collected results of 2464 unrelated donor (URD) transplantations with 450 HLA-identical, matched sibling donor (MSD) transplantations performed at collaborating National Marrow Donor Program institutions. A total of 63% of URDs were matched at HLA-A, -B, and at -DRB1 alleles; all MSDs were genotypically identical at major histocompatibility loci. URD recipients were younger (median 36 vs 39, P = .001) than MSDs and underwent BMT later after diagnosis (median 17 [0-325 months] vs 7 [1-118 months],P = .001) and less often in chronic phase (CP) (67% vs 82%, P = .001). Multivariate analysis demonstrated a significantly increased risk of graft failure and acute graft versus host disease after URD BMT. The risk of hematologic relapse was low after either matched URD or MSD transplantations. We observed significantly though modestly poorer survival and disease-free survival (DFS) after URD transplantations. However, for those undergoing transplantation during CP within 1 year from diagnosis, 5-year DFS was similar or only slightly inferior after matched URD versus MSD transplantation (age < 30: URD 61% ± 8% vs MSD 68% ± 15%,P = .18; 30-40: URD 57% ± 9% vs MSD 67% ± 10%,P = .05; > 40: URD 46% ± 9% vs MSD 57% ± 9%,P = .02). Delay from diagnosis to BMT in CP patients led to substantially poorer 5-year DFS after matched URD than MSD BMT (CP 1-2 years: URD 39% ± 6% vs MSD 63% ± 12%; beyond 2 years: URD 33% ± 7% vs MSD 50% ± 20%). Outcome of matched URD BMT for early CP chronic myelogenous leukemia yields survival and DFS approaching that of MSD transplantation. However, delay may compromise URD outcomes to a greater extent. Improvements in URD and MSD transplantation are still needed, and results of newer, nontransplantation therapies should be evaluated against the established curative potential of URD and MSD marrow transplantation.
Introduction
Allogeneic bone marrow transplantation (BMT) is currently the only curative therapy for chronic myelogenous leukemia (CML).1-6 Both the pretransplantation chemoradiotherapy conditioning and the allogeneic immune antitumor effect are responsible for leukemic cell eradication and extended leukemia-free survival.7-10 While matched sibling donor (MSD) and unrelated donor (URD) allografts have been well recognized as suitable and potentially curative therapeutic opportunities, the distinctive clinical course, complications, and outcomes of these 2 allograft approaches have not been directly compared.11-16 We prospectively collected data on MSD transplantations performed at institutions participating with the National Marrow Donor Program (NMDP) to compare the results of MSD and URD BMT for CML. We herein report detailed results of this comparative analysis, including assessment of engraftment, graft versus host (GVH) disease, relapse, and leukemia-free survival.
Patients and methods
Patients included in this analysis were those with CML who received bone marrow transplantation from MSD (n = 450) and URD (n = 2464) with data reported to and collected by the NMDP using prospectively designed data capture methods, auditing, centralized compilation, and data error correction at the NMDP.16 17
Patients and characteristics
Characteristics of the 2914 cases are shown in Table1. The URD transplantations occurred between 1988 and 1999. Beginning in 1991, prospective data from 34 transplantation centers reporting MSD transplantations to the NMDP were collected on similar data capture forms. Additional data were collected but excluded from this analysis on 129 URD cases because of inadequate follow-up data (n = 89) or the treatment being a second or subsequent transplantation (n = 40). Similarly, data on 296 other related donor transplantations were collected but excluded from this analysis because the donor was not a matched sibling (n = 229), follow-up was inadequate (n = 44), or the transplantation was not a first transplantation (n = 23).
Unrelated marrow donors were identified through the NMDP, and marrow was collected following established procedures through NMDP-approved donor and collection centers. Transplantations were performed at 127 NMDP-affiliated transplantation centers. Specifications for donor search and identification, marrow collection and transportation, and data gathering and analysis were performed according to procedures developed by the NMDP as described.16 17
Unrelated donor/recipient HLA molecular typing for DRB118,19 was performed prior to transplantation or retrospectively using pretransplantation samples that had been cryopreserved. Serologic testing for HLA-A and -B as well as molecular testing for -DRB1 identified donor/recipient mismatching at A or B in 402 cases (16%). These included mismatch at HLA-A (n = 225) or HLA-B (n = 172) or both (n = 1); data on 4 were missing. These 402 were analyzed as partially matched. The remaining 2062 URD transplants matched at HLA-A and -B were also DRB1-matched at the allele level in 1559 cases (63% of URD transplants).18 19 Allele typing for class I loci was not available for this analysis. Sibling donor transplants were serologically matched at the HLA-A, -B and -DR regions in all 450 cases.
Pretransplantion conditioning included fractionated or single-dose total body irradiation along with cyclophosphamide, either alone or in combination with other chemotherapeutic agents in 2340 cases (287 MSD and 2053 URD transplantations). Only 574 patients received chemotherapy-only conditioning (without radiation), most often busulfan plus cyclophosphamide. In vivo GVH disease prophylaxis using cyclosporine or tacrolimus along with methotrexate or prednisone was used in 2271 patients (373 MSD and 1898 URD), while 555 received ex vivo T-cell–depleted marrow. T-cell depletion was more frequently employed for URD transplantation (P = .02).
Statistical analysis
As previously described, the NMDP uses prospectively designed forms and methods for data collection on all transplantations facilitated through the network.16 17 Baseline information and follow-up reporting were submitted at scheduled posttransplantation intervals. Similar forms, adapted for matched sibling transplantation, were used for the prospective analysis of MSD transplantations.
Patient outcome was analyzed to the date of last reported follow-up or the date of death. Engraftment (neutrophil recovery to 0.5 × 109/L [500/μL]) required survival to day +22, and patients without engraftment were considered graft failures.20 Death before day +22 (100 URD, 8 MSD) and incomplete data (14 URD) excluded patients from analyses of engraftment. Relapse was analyzed as hematologic relapse. Cytogenetic sampling or reverse transcriptase–polymerase chain reaction analyses of the BCR-ABL molecular abnormality was not routinely performed at defined, scheduled posttransplantation intervals, resulting in potential reporting bias as well as imprecision in the assessment of the clinical outcome following such observations. Survival was calculated by the method of Kaplan and Meier.21 In the calculation of time-to-event for analysis of relapse, engraftment, or GVH disease where early death could alter the assessment frequency, the cumulative incidence method was used.22 In univariate analyses, the log-rank test statistic was employed to compare differences in outcome among subgroups, and the 95% confidence intervals were calculated from the SEs.
In multiple regression analyses for engraftment, acute and chronic GVH disease, hematologic relapse, survival, and disease-free survival (DFS), the logistic regression (engraftment) or proportional hazards model23 was employed including the following covariates: donor and recipient age, gender, cytomegalovirus (CMV) serology, disease stage, interval from diagnosis to transplantation, donor/recipient HLA matching [class I matched or mismatched; DRB1 matched or mismatched; or number of loci mismatched HLA-A, -B, -DRB1 (0-3)], total body irradiation containing conditioning (yes/no), T-lymphocyte depletion (yes/no), and year of transplantation. The impact of transplantation center was assessed by a fixed effects variable. Statistical interactions of donor type (MSD or URD) with several risk factors were examined, testing if the effect of donor type varies among subgroups of patients identified by these risk factors. No such interactions were detected.
The data collection period for MSD BMT (1991-1995) differed from the extended period of URD data collection (1988-1999). For the 233 URD transplantations that preceded and 1396 URD transplantations that followed the MSD cohort, we analyzed the era of URD BMT as an added variable in Cox regression models but observed no effect of URD transplantations that preceded (P = .41) or followed (P = .32) the 1991 to 1995 MSD era on DFS following BMT (not shown).
Results
Timing of BMT
As shown in Table 1, BMT using MSD occurred sooner after diagnosis than URD (median 7 vs 17 months, P = .001). This may be attributable to delays in decision making and donor availability that complicate URD transplantation. The average time for donor searching until transplantation was shorter for transplantations performed in first chronic phase (CP1) versus those in accelerated phase or after blast phase (mean search time 322, 419, and 418 days, respectively,P = .004). Even in the CP transplantations, search time was shorter to identify matched URD donors (mean 267 days) than partially matched URD (mean 377 days, P = .0001) though there was no statistically significant interaction between disease stage at transplantation and HLA match (P = .14).
Engraftment
Posttransplantation recovery to neutrophil level 0.5 × 109/L (500/μL) was quicker and more likely after MSD versus URD transplantation. As shown in Table2, significantly more patients undergoing URD matched or partially matched transplantations failed to achieve engraftment. Additionally, transplantations for CML beyond CP1 were associated with a lower likelihood of successful engraftment. As shown in Figure 1, for CP1 patients, graft failure occurred by day +100 in 1% ± 1% (95% confidence limits) of MSD, 4% ± 1% of matched URD, and 8% ± 2% of partially matched URD transplantations (P < .0001).
Engraftment.
Engraftment (recovery to neutrophils > 0.5 × 109/L [500/μL]) following BMT for CP CML using MSD (n = 364), matched URD (n = 1209), or partially matched URD (n = 386) BMT. Graft failure occurred in 1% ± 1% MSD, 4% ± 1% matched URD, and 8% ± 2% partially matched URD recipients (P < .0001).
Engraftment.
Engraftment (recovery to neutrophils > 0.5 × 109/L [500/μL]) following BMT for CP CML using MSD (n = 364), matched URD (n = 1209), or partially matched URD (n = 386) BMT. Graft failure occurred in 1% ± 1% MSD, 4% ± 1% matched URD, and 8% ± 2% partially matched URD recipients (P < .0001).
Acute and chronic GVH disease
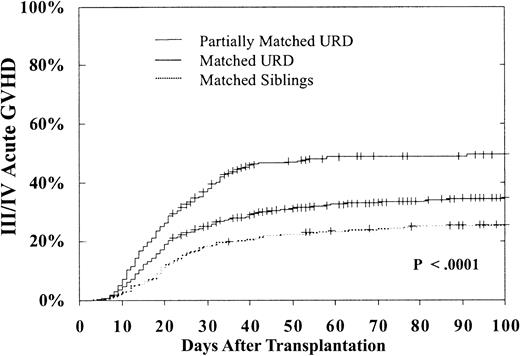
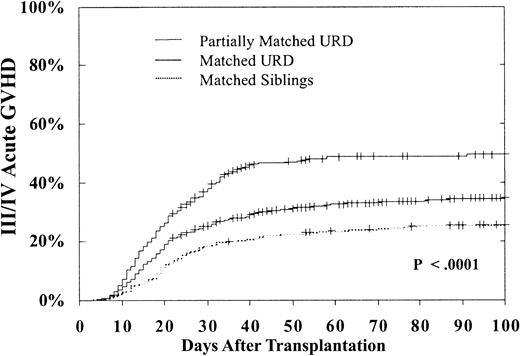
Advanced acute GVH disease (grade III-IV) occurred significantly more frequently after URD transplantation. As shown in Table 2, for BMT during CP, compared with MSD transplantations, matched URD transplantations were 1.31 times more likely (P = .01) to result in acute GVH disease and partially matched URD had a 1.83 times greater likelihood of acute GVH disease (P = .0001). Transplantations beyond CP1 (especially in blast phase) were associated with more frequent acute GVH disease. In addition, GVH disease was significantly more frequent in transplantations from older donors and in transplantations performed beyond 1 year from diagnosis. Interferon therapy prior to BMT was associated with a lower risk of acute GVH disease. As shown in Figure2, in CP patients the 100-day cumulative incidence of grade III-IV GVH disease was only 26% ± 5% in MSD, 35% ± 3% in URD matched, and 49% ± 5% in URD partially matched transplantations (P < .0001).
Cumulative 100-day incidence of grade III/IV acute GVH disease.
Incidence after MSD (26% ± 5%), matched (35% ± 3%), or partially matched (49% ± 5%) URD BMT for CP CML (P < .0001).
Cumulative 100-day incidence of grade III/IV acute GVH disease.
Incidence after MSD (26% ± 5%), matched (35% ± 3%), or partially matched (49% ± 5%) URD BMT for CP CML (P < .0001).
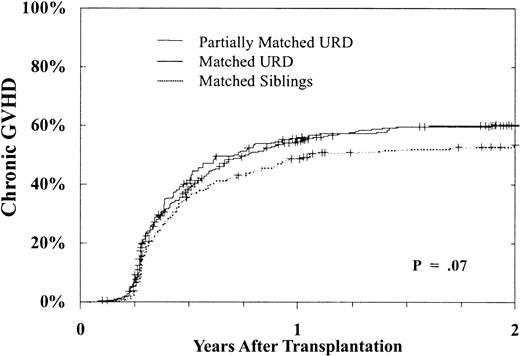
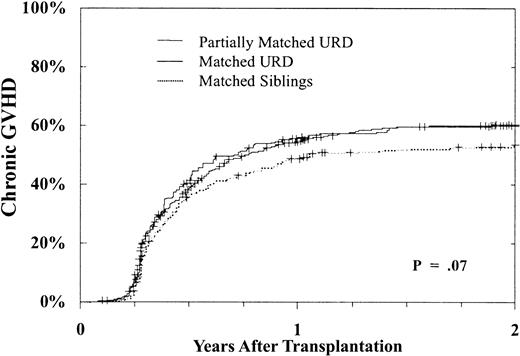
Chronic GVH disease occurred more frequently after URD transplantation as well. Table 2 shows that there was a nearly 1.5-fold greater risk of chronic GVH disease after URD transplantation when compared with MSD BMT. This increased risk was similar following matched and partially matched URD BMT. In addition, chronic GVH disease was more frequent in transplantations performed beyond the CP and using a female donor. Figure 3 shows the cumulative incidence of chronic GVH disease in patients undergoing transplantation in CP1. As shown, at 2 years 53% ± 5% of MSD recipients, 60% ± 3% of URD matched recipients, and 60% ± 6% of URD partially matched recipients developed chronic GVH disease (P = .07).
Cumulative incidence of chronic GVH disease.
Incidence in day +80 survivors after MSD (n = 343), matched (n = 992), or partially matched (n = 241) URD BMT for CP CML. By 2 years, MSD 53% ± 5%, 60% ± 3% matched, and 60% ± 6% partially matched URD developed chronic GVH disease,P = .07.
Cumulative incidence of chronic GVH disease.
Incidence in day +80 survivors after MSD (n = 343), matched (n = 992), or partially matched (n = 241) URD BMT for CP CML. By 2 years, MSD 53% ± 5%, 60% ± 3% matched, and 60% ± 6% partially matched URD developed chronic GVH disease,P = .07.
Hematologic relapse
Hematologic relapse was uncommon and occurred with similar frequency in recipients of MSD (n = 42, 9.3%) or URD (n = 199, 8.1%) transplants (P = .39). As shown (Table 2), the donor type had only modest impact on hematologic relapse after transplantation. However, relapse was significantly more frequent in transplantations beyond the CP1, in older patients, and in patients receiving marrow from a male donor. Hematologic relapse was infrequent when performed in CP1: 5% to 8% for all 3 donor types (Figure4A) (P = .24). As shown in Figure 4B, while the risk of relapse was higher for those undergoing transplantation beyond the CP (17%-20%), it was still similar after MSD or URD transplantation (P = .54).
Hematologic relapse.
Hematologic relapse of CML after BMT using MSD, matched, or partially matched URD in CP1 ([A] n = 369, 1244, 401, respectively;P = .24) and beyond CP (accelerated phase/blast phase; [B] n = 80, 536, 262, respectively; P = .54).
Hematologic relapse.
Hematologic relapse of CML after BMT using MSD, matched, or partially matched URD in CP1 ([A] n = 369, 1244, 401, respectively;P = .24) and beyond CP (accelerated phase/blast phase; [B] n = 80, 536, 262, respectively; P = .54).
Donor lymphocyte infusions
Donor lymphocyte infusions (DLIs) were given to only 68 (2.8%) patients after URD BMT. DLIs were given to 32 URD patients following CML relapse; of the 32, 17 had received a T-depleted graft. Two received DLIs for prevention of hematologic relapse (1 had received T-depleted BM). Fourteen others, 12 with T-depleted BM, received DLIs for management of B-cell lymphoproliferative disease, 4 for graft failure, 3 for management of viral infection, and 13 for unspecified reasons. DLIs were given to 17 (3.8%) MSD recipients, 16 for treatment of relapse (3 recipients of T-depleted marrow). Thus, of 555 recipients of T-depleted BM, 52 received DLI treatments compared with 33 DLIs in 2359 recipients of unmanipulated grafts (P = .001).
Survival and DFS
Patients in this series had a median follow-up of 2.1 years (range 0-11) in the URD recipients and 4.8 years (range 0.3-6.1) in the MSD recipients, although 233 (24%) of URD survivors have been followed for more than 5 years after BMT. A total of 988 (40.1%) URD and 268 (59.6%) MSD recipients are alive. Multivariate analysis showed better survival after MSD versus URD transplantations and significantly better survival for transplantations performed in CP (Table3). Superior survival was seen in younger patients, in CMV-seronegative recipients, following transplantation (in CP) within 1 year of diagnosis, and in transplantations using younger donors. Even for transplantations performed during CP1, 5-year survival was superior for MSD (63% ± 5%) compared with matched URD (45% ± 3%) or partially matched URD (31% ± 5%) transplantation (Figure 5A,P = .0001). These differences were still apparent, with substantially poorer 5-year survival for transplantations performed beyond CP1 (Figure 5B, MSD 31% ± 11%; matched URD 20% ± 4%; partially matched URD 16% ± 6%, P = .002).
Survival.
Survival following BMT during CP1 ([A] P = .0001) and beyond CP ([B] P = .002).
Survival.
Survival following BMT during CP1 ([A] P = .0001) and beyond CP ([B] P = .002).
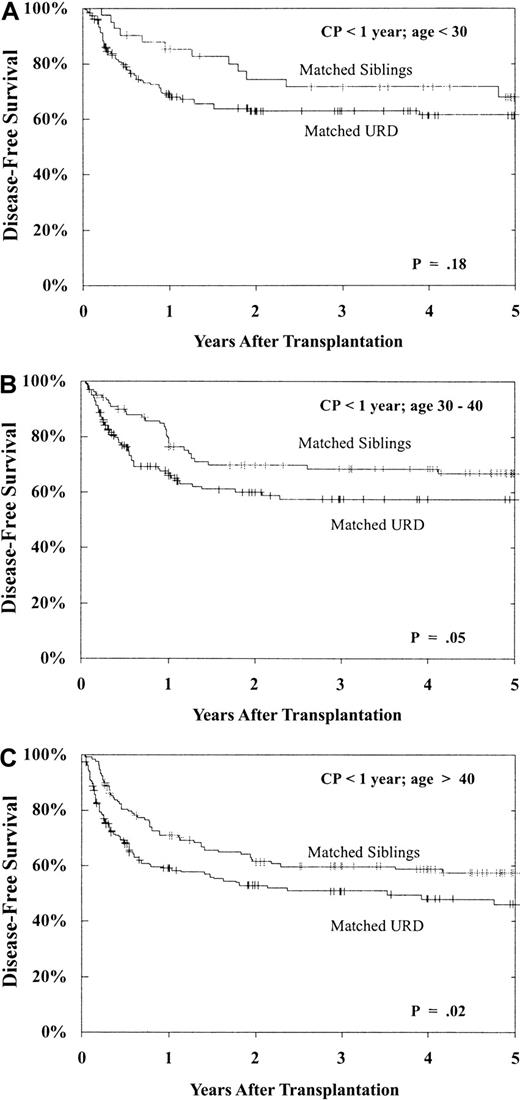
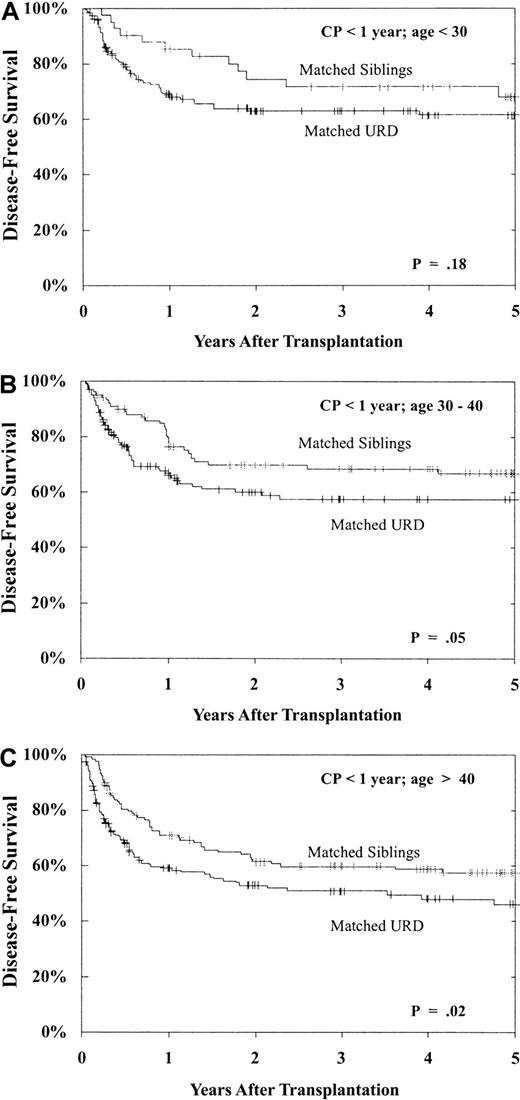
Prolonged survival without leukemia relapse is, of course, the desired outcome following allogeneic transplantation. As shown in Table 3, DFS was best after MSD versus URD transplantation, best when performed in CP, and significantly better for younger patients (and a trend with younger donors), for CMV-seronegative recipients and, importantly, for patients undergoing transplantation within 1 year of diagnosis. These prognostic indicators (donor type, disease phase, recipient age, and time from diagnosis to transplantation) had a major impact on both individual cohort outcome and on the comparison between URD and MSD BMT. As shown in Figure 6, for patients undergoing transplantation within 1 year of diagnosis, still within the CP1, patients aged less than 30 years, 30 to 40 years, and more than 40 years have superior 5-year DFS with MSD versus matched URD BMT, though the differences are modest (age < 30: MSD 68% ± 15% vs matched URD 61% ± 8%, P = .18; 30-40: MSD 67% ± 10% vs matched URD 57% ± 9%, P = .05; > 40: MSD 57% ± 9% vs matched URD 46% ± 9%, P = .02).
DFS following BMT during CP within 1 year from diagnosis: effect of patient age.
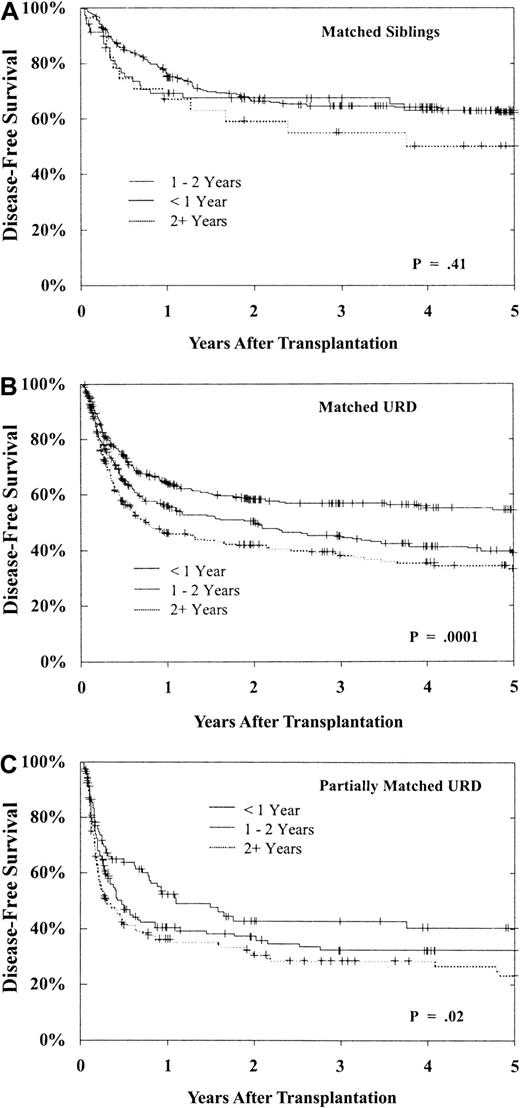
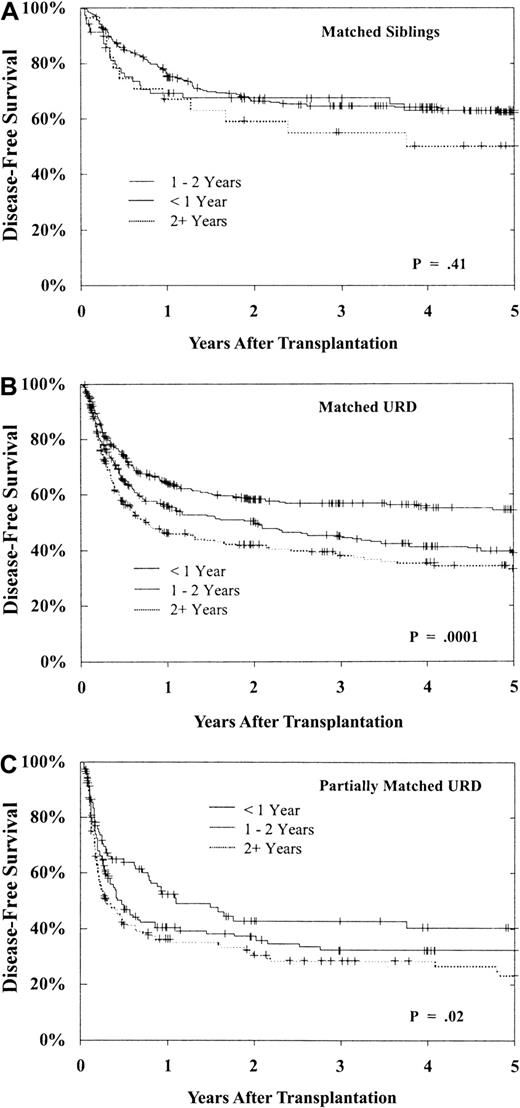
As shown in Figure 7, 5-year DFS for patients in CP is best with a less than 1-year interval from diagnosis to BMT. Delay until BMT had a greater adverse impact on URD transplantation. There is little decrement in DFS for MSD recipients undergoing transplantation more than 1 year from diagnosis when compared with those undergoing transplantation within the first year (P = .41). However, while DFS after matched URD BMT is similar to MSD in the early (< 1 year) CP patients, DFS in the URD cohort is substantially worse after a delay of more than 1 year from diagnosis (Figure 7B, P = .0001). Similar falloff in DFS with longer diagnosis to BMT interval is observed with partially matched URD as well (Figure 7C, P = .02). Therefore, the outcome for transplantation in CP within 1 year from diagnosis is comparable in matched URD and MSD recipients, but for BMT delayed beyond 1 and 2 years the outcome for the URD recipients is notably worse.
DFS after BMT during CP1 of CML based upon time from diagnosis to BMT.
(A) Results of MSD BMT within 1 year (n = 270), 1 to 2 years (n = 70), and beyond 2 years (n = 29) from diagnosis (P = .41). (B) Results of matched URD BMT within 1 year (n = 556), 1 to 2 years (n = 394), and beyond 2 years (n = 294) from diagnosis (P = .0001). (C) Results of partially matched URD BMT within 1 year (n = 104), 1 to 2 years (n = 143), and beyond 2 years (n = 154) from diagnosis (P = .02).
DFS after BMT during CP1 of CML based upon time from diagnosis to BMT.
(A) Results of MSD BMT within 1 year (n = 270), 1 to 2 years (n = 70), and beyond 2 years (n = 29) from diagnosis (P = .41). (B) Results of matched URD BMT within 1 year (n = 556), 1 to 2 years (n = 394), and beyond 2 years (n = 294) from diagnosis (P = .0001). (C) Results of partially matched URD BMT within 1 year (n = 104), 1 to 2 years (n = 143), and beyond 2 years (n = 154) from diagnosis (P = .02).
Discussion
While only 20 years ago no curative therapy existed for CML, now allotransplantation can cure a sizable fraction of patients.1-6,11-16 Other advances have shown that α-interferon, with or without cytarabine, can extend time in CP and prolong survival.24-26 More recently, tyrosine kinase inhibitors can reverse the clinical manifestations of Philadelphia-positive CML with even less toxicity, although their impact on time to blast crisis and survival is as yet unknown.27 28 These promising alternatives, however, must be judged in the context of the defined value of allogeneic marrow transplantation with its well-established curative potential despite its initial toxicity and mortality.
In this report, we show the comparative value of MSD and URD allografts for treatment of CML in the largest cohort of allotransplantation recipients to be studied. Although the outcome overall is superior in the MSD cohort, certain populations of patients have remarkably similar outcomes after matched URD and MSD transplantations. Patients in CP receiving transplants within 1 year from diagnosis enjoy more than 60% DFS after MSD transplantations and 55% DFS at 5 years following matched URD transplantation. Both delay beyond 1 year and delay until clinical acceleration of the leukemia markedly compromises outcome, especially for URD recipients. Therefore, the clinical choice of transplantation versus other therapies in the early CP becomes even more vexing for those lacking an available related donor. Delay from diagnosis until MSD or URD transplantation has been previously reported to compromise clinical outcome.1-3 14-16 Although subclinical disease acceleration (even without basophilia, fibrosis, rising blast percentage, or cytogenetic evolution) may contribute to the poorer results accompanying later transplantations, other mechanisms may be operative. These may include immune compromise due to replacement of normal dendritic cells or natural killer cells with BCR-Abl–positive effectors. Inconsistent findings have been reported regarding the adverse effect of prolonged (> 6 or 12 months) interferon therapy preceding allogeneic URD transplantation.
Few earlier reports have contrasted the outcomes of MSD versus URD transplantations for leukemia, primarily in children.29-31 Similar, though more complex, clinical courses followed the URD allografts. The immediate or early hazards of graft failure, infection, GVH disease, and death would certainly give pause to all patients facing the choice of pharmacologic versus transplantation therapy soon after diagnosis. However, formal decision analyses (prepared before the era of tyrosine kinase inhibitors) that have weighed these results support the choice of early transplantation for patients with an available donor. However, the post-BMT survival advantage is not seen until 4 or more years following transplantation.32 Even with the encouraging results reported herein, this survival advantage might not be realized until even later following URD transplantation. Recent reports suggest that outcomes after URD BMT may be further improved by allele matching of class I and II histocompatibility between donors and recipients.33,34 Despite these data, however, treatment guidelines have weighed heavily against early transplantation in favor of hoped-for improvements in medical therapy without loss of curative potential of early allotransplantation.35 36 In view of the current results showing quite good outcomes for younger, early CP transplantations using either MSD or matched URD donors and the substantive survival penalty for delay, the strategies of deferred BMT may be questioned.
Our results strongly document the importance of early URD transplantation. We recognize need for improvements in survival following URD transplantation, especially when performed beyond CP and beyond the first year after diagnosis. For the most favorable group, however, including early CP patients up to age 40, new nontransplantation approaches must be tested as rigorously in prospective fashion to best help patients and their physicians make the optimal clinical choice.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Daniel J. Weisdorf, University of Minnesota—Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail:weisd001@tc.umn.edu.

![Fig. 1. Engraftment. / Engraftment (recovery to neutrophils > 0.5 × 109/L [500/μL]) following BMT for CP CML using MSD (n = 364), matched URD (n = 1209), or partially matched URD (n = 386) BMT. Graft failure occurred in 1% ± 1% MSD, 4% ± 1% matched URD, and 8% ± 2% partially matched URD recipients (P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.1971/6/m_h80622296001.jpeg?Expires=1769118575&Signature=WR7q-O0EYx6nJBFs9Q-2sRZO5DgQVt--EBONMmk8rQ~2fCQ3~0FFOC26BqfnTnpiO5uvvNoqujNJB9nHi-E6bBW7r0TkOimJsUktys0Mv-k1ct2eTPENhjm5NRyPlWvrg6qIwZDONRNWuQoqkxdZJ-w1eYIyGOFeOYVAXs7PqPcigZtOhzkohpuIQiUnbWUxEdrlx34njV254Jg3QQbbsEu9W0AL7gpYNJkhU4zeKi5nuntEtfpA3CJ1rZ4nRynriynvTLRHir~EYyME2yrSS9Q2hRe-PHKT8cOvNCPES1t7-xyQwhPP7ANon~LAvjOIVDa-SR3sXMPhlcoz10Q6eA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Hematologic relapse. / Hematologic relapse of CML after BMT using MSD, matched, or partially matched URD in CP1 ([A] n = 369, 1244, 401, respectively;P = .24) and beyond CP (accelerated phase/blast phase; [B] n = 80, 536, 262, respectively; P = .54).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.1971/6/m_h80622296004.jpeg?Expires=1769118575&Signature=W4z22pBye1UMI5xebZoqplPlgRUB-yI~i~J0ek0UW1lL~4k4VPMZM9iMuF33A-Ik5aUjyScJglR6~OAamH8ThHpOar2HXfau5AmchuLs4OTbF3E7vL3N-8hO22gwO7hKI4udLEp7asEouPIoTAKoKYa-eH9k5J6MmYGza~oHB12RJ~x9vcDRIC~NPScTP5jMOnzhaatElHFHDOYyG40U5Epsic~38SjMMyPkA~08u5gKwlqiXH--0YObePX3vmZr7bcdODQLefVUxm7clFqvYINJtqqZqs0v2miBcHZbYCcOloN3B0GFw833hNCUikfPiEVjEPeEdKwyyzKaPfqExw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Survival. / Survival following BMT during CP1 ([A] P = .0001) and beyond CP ([B] P = .002).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.1971/6/m_h80622296005.jpeg?Expires=1769118575&Signature=KAeDbXc9EOJWeNWfOcboihB8V0VsVjKWilg8BUB1PBIeG1s4Lk~jl5w61EQuFJR8BBPb6xYPopBfxbyyLinziLwE59sA0PwrhonIDPjm31PtWawG7f-OC~VxOmcy33Hno1a1DR6DarlsoHaLzr~hL0vDZ-U5-ECErmiB5aAuTk6AunaTQJqjubdrem0jXrunQrAVwOfu34Ml2i8BWtegrv6AUgL8qkGCNqjGLkrxP4dsyyuVb-x70Yz~sP4E4787Nt71QI0eG-EKKKXdTuYjkoj1tPxdjfRfxTXsoHRv2A0TOQt3t38IFusr6-vGymB7nRTJK3tRWB~-1y3SmNTwSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Engraftment. / Engraftment (recovery to neutrophils > 0.5 × 109/L [500/μL]) following BMT for CP CML using MSD (n = 364), matched URD (n = 1209), or partially matched URD (n = 386) BMT. Graft failure occurred in 1% ± 1% MSD, 4% ± 1% matched URD, and 8% ± 2% partially matched URD recipients (P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.1971/6/m_h80622296001.jpeg?Expires=1769118576&Signature=YndQiYAt32VrQn3x8JXQY-k6BHutXY3vUhygZ6KIxzKZA7Tc0B2QuhU6ecACOCklCUHf2zwtJ-YfuuNyPKcJaoPW-0w0AEOqXwTOHdkOPhThFC5iMhdNhMpNDFbQBWWeyIBEp2HOm9pdlNh8uBkqz7C0GJcuzOPhKvWycgU3cva67KOt-UId9E5NSMrJg6v8A32YI3EsQIwyKSSuSCrJsLS5DIObEAxqCZOMlvXih7YhFbwldu5q2YkEiqXCUsk3SSvRiQDhDwMCXmkFK~WNRgbn0HVV0AthZI84XABRM-I73Fz3pzVomMG2K0IZTrBM3rgXNwqn60g6sj49QgtYoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Hematologic relapse. / Hematologic relapse of CML after BMT using MSD, matched, or partially matched URD in CP1 ([A] n = 369, 1244, 401, respectively;P = .24) and beyond CP (accelerated phase/blast phase; [B] n = 80, 536, 262, respectively; P = .54).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.1971/6/m_h80622296004.jpeg?Expires=1769118576&Signature=Iw49PYFdNxPDOE1esxl3xf1zfAtRLYbSS0PWAx4fI9wR7tKoaJuW8iZb224hvJrMyCzjiPZJTACnQE8-YeGueuHEZI3N8nlwz3HxcQ99JxOruplCkSN7HO2GyJh1S3D3cJUBx01bNXlqKajTTEF6PS7Jipx8Mik4wf6SMR5yEfHAXoMtZRAjRh1mibvRsI3o8r573g4Pdg43lHKguD09zqNIDLOvpYZLvMdsNwADNPgSwXJY3Zj41GA0XfxvSNY042R4Lw5XZxFct5quwMcex63JYuHPLlZuRoG0ksXxpiIo28rC1XNFZ5enXS7g1XaN2JQAUFVfTNCzG42BZEKSkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Survival. / Survival following BMT during CP1 ([A] P = .0001) and beyond CP ([B] P = .002).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.1971/6/m_h80622296005.jpeg?Expires=1769118576&Signature=3PS9Hq7-oiuo07lYy6dgudX8efJkOh6hNifqnyomzOT2Aoc9XF4gomuTW5tbqvbHirWbNh6-gS3p3jf6rW4rt5SB1vZfHmCNUk4wtkLMcjQ2Dpi~RXJtRtm5GMGAPS1sFyHUHwMjPhpvHaSRNd8LR2NNFr8VSLF5BLRCbc5ILGZWb0MCYzSNWFxzCRxbLzcSvYXLAXWJy9xhgcif-J1N-dGwBtV9O44C779wc6oijjopgeSXxXaiezDk1w28H8O9ZDz0oiObmDV9tBM2AwqEspuptHYiV59suJ~oFIfqzEgidA3WHLyfHZvyenvFcNElVmngSsX35EbObDn~G7nyvg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)