Abstract
Nonmyeloablative allogeneic hematopoietic stem cell transplantation (HSCT) is increasingly being explored as therapy in patients who are not eligible for conventional myeloablative HSCT. Whether these transplants are associated with reduced risk of transplantation-related infections is unknown. We analyzed the incidence of posttransplantation cytomegalovirus (CMV) infections in 56 consecutive mycophenolate mofetil (MMF) patients with hematologic malignancies who underwent nonmyeloablative HSCT (TBI, 2Gy, day 0; MMF/cyclosporine after transplantation). In addition, 18 of 56 patients received 30 mg/m2/d fludarabine on days −4 to −2. Most donors were HLA matched and related (93%). Each case patient was matched to 2 controls who were treated by conventional HSCT during the same time period (January 1997 through April 2000). Matching criteria included CMV risk group, HSC source, donor type, age, and underlying diseases. No CMV disease occurred in the low (donor and recipient serologically negative) and intermediate (donor serologically positive and recipient negative) CMV risk groups during the first 100 days. Among cases at high risk for CMV (seropositive recipients), trends to less CMV antigenemia (P = .11), viremia (P = .16), and disease (P = .08) compared with controls were observed; all severe manifestations combined (CMV viremia and disease) were significantly reduced among cases (P = .01). However, by day 365, the overall incidence of CMV disease became similar in both groups. The onset of CMV disease was significantly delayed among case patients compared with controls (median, 130 days versus 52 days; P = .02). It was concluded that CMV disease was significantly delayed in nonmyeloablative cases, but that the overall 1-year incidence was similar to myeloablative HSCT patients. Therefore, nonmyeloablative HSCT patients should receive CMV surveillance beyond day 100 and pre-emptive ganciclovir treatment similar to that of myeloablative HSCT patients.
Introduction
Nonmyeloablative hematopoietic stem cell transplantation (HSCT) is an emerging treatment modality for malignant and nonmalignant hematologic disorders. Preclinical animal studies for the purpose of achieving allograft engraftment have shown that intensive cytotoxic and myeloablative conditioning regimens can be replaced by less intensive pretransplantation and posttransplantation immunosuppression or reduced pretransplantation conditioning therapy.1-3 On the basis of these findings, clinical nonmyeloablative SCT protocols were developed and are currently being explored in patients who are not eligible for conventional HSCT owing to age or medical contraindications.4-8 All of these protocols are highly immunosuppressive. However, the toxicity profiles, including the degrees of myeloablation, vary in the different regimens.5 9
The nonmyeloablative HSCT regimen that was developed in Seattle is based on low-dose (2 Gy) total body irradiation (TBI) with or without fludarabine for pretransplantation conditioning followed by cyclosporine (CSP) and mycophenolate mofetil (MMF) in the posttransplantation period. This regimen provides pretransplantation and posttransplantation immunosuppression that allows engraftment and initial establishment of mixed hematopoietic chimerism. Host hematopoietic cells, especially T lymphocytes, are not immediately eradicated by this HSCT regimen. Instead, it may take 3 to 6 months for host cells to disappear, as they are eradicated by the alloimmune donor cell responses over time. In contrast, conventional myeloablative conditioning regimens usually lead to early and rapid complete disappearance of host hematopoiesis. On the basis of these findings, we hypothesized that the prolonged presence of host immunity after nonmyeloablative HSCT may provide some protection against early posttransplantation infections as compared with conventional myeloablative transplantation. After conventional myeloablative allogeneic HSCT, cytomegalovirus (CMV) infections are common and contribute significantly to the morbidity and mortality in the early posttransplantation period.10 11 Data pertaining to infectious complications after nonmyeloablative conditioning regimens are missing thus far.
To test our hypothesis, we performed a matched-pair control study of results in the first 56 consecutive patients who underwent nonmyeloablative HSCT at our institutions compared with concurrently treated patients who underwent myeloablative allogeneic HSCT. Our analyses included CMV antigenemia and viremia during the first 100 days after transplantation and CMV disease during the first 365 days after transplantation.
Patients and methods
This retrospective analysis was approved by the institutional review board of the Fred Hutchinson Cancer Research Center (FHCRC) (Seattle, WA). Informed consent was provided according to the Declaration of Helsinki.
Patients
Fifty-six consecutive patients who had undergone nonmyeloablative allogeneic HSCT (cases patients) between December 1997 and April 2000 were analyzed as a case group (Table1). Fifty-five case patients had hematologic malignancies and one had renal cell carcinoma. Patients were treated either at the FHCRC or at the Veterans Affairs Medical Center, both in Seattle, WA. These 2 institutions had similar surveillance and prevention strategies for infections. Each case patient was matched to 2 control patients.
The controls were patients who received transplants at the FHCRC during January 1997 and April 2000 with the use of myeloablative conditioning regimens. The controls were identified among 854 patients who underwent myeloablative HSCT during the study period. Case and control patients received comparable antiviral and antifungal prophylactic regimens. Matching criteria included the following: CMV risk group (low, intermediate, high); donor type (HLA-matched related donor; HLA-matched unrelated donor); HSC source (peripheral blood stem cells versus bone marrow); age at transplantation (younger than 20 years, from 20 to 40 years, older than 40 years); and diagnoses (good risk versus poor risk).
The CMV risk groups were defined on the basis of previous results from myeloablative transplantations12: low risk (donor and recipient serologically negative); intermediate risk (donor serologically positive and recipient negative); and high risk (recipient positive and donor either negative or positive). The stratification based on underlying disease was as follows: poor prognosis was defined as active de novo or relapsed acute nonlymphocytic leukemia; myelodysplastic syndrome (MDS); refractory anemia [RA] with excess of blasts or excess of blasts in transformation; acute lymphocytic leukemia; chronic lymphocytic leukemia; non-Hodgkin lymphoma; Hodgkin disease; multiple myeloma (MM) regardless of status; renal cell carcinoma; or accelerated chronic myeloid leukemia (CML) or blast crisis of CML. Good prognosis was defined as aplastic anemia or any of the above named diseases with unknown disease status or in remission except for MM, CML chronic phase, and MDS (RA or RA with ringed sideroblasts).
Matching was achieved at 100% for the CMV risk group, donor match, and stem cell source; at 86% for age (older than 40 years, 89% of cases versus 78% of controls; P = .07); and at 63% for underlying diseases (high risk, 68% of cases versus 44% of controls; P = .003). Most patients in both groups were white (48 of 56 cases [86%] and 97 of 112 controls [87%]).
Preparative regimens
Patients in the case group received low-dose TBI (2 Gy, day 0) followed by CSP and MMF after transplantation.5 This regimen was modified after the first 38 patients by adding fludarabine, 30 mg/m2 body surface area, on days −4 to −2. Patients in the control group received different types of conditioning regimens as shown in Table 1, most commonly cyclophosphamide (60 mg/kg/d for 2 consecutive days) followed by TBI (12 Gy), or busulfan (4 mg/kg/d for 4 consecutive days) followed by cyclophosphamide (60 mg/kg/d for 2 consecutive days). One control patient (with aplastic anemia) received antihuman thymozyte globulin and cyclophosphamide for conditioning.
Chimerism analyses
At days +28, +56, +84, and +180 after transplantation, the degree of hematopoietic chimerism in the peripheral blood was evaluated in the nonmyeloablative transplantation patients by means of fluorescent in situ hybridization to detect X and Y chromosomes for sex-mismatched transplants and by polymerase chain reaction–based analysis for polymorphic microsatellite regions for sex-matched transplants.13 Chimerism analyses were performed for peripheral blood T lymphocytes (CD3+ cells) as well as for granulocytes.
Prophylaxis against graft-versus-host disease
Case patients were assigned to receive 6.25 mg/kg CSP by mouth (PO) twice a day (BID) from day −1 to day +35 for related-donor transplants and from day −1 to day +100 for unrelated-donor transplants. CSP was then tapered, so that the last dose was given on days +56 (for related donors) and +180 (for unrelated donors), respectively. Tapering schedules were modified at the discretion of the attending physician if active graft-versus-host disease (GVHD) was present. MMF was given at a dose of 15 mg/kg PO BID from days 0 to +27 for related-donor transplants and through day 40 with subsequent taper to day 96 for unrelated-donor transplants.14 Controls received one of several different GVHD prophylaxis regimens during the study period, most commonly the combination of CSP and methotrexate (MTX). CSP was given at a dose of 1.5 mg/kg intravenously (IV) BID or 6.25 mg/kg PO BID, days −1 to +60 and then tapered until day +180. MTX was scheduled to be administered IV at a dose of 15 mg/m2of body surface area on day +1, and 10 mg/m2 on days +3, +6, and +11.
GVHD and treatment
Diagnosis and clinical grading of acute GVHD (aGVHD) were performed according to established criteria.15 Data on aGVHD were available for 33 case patients and 77 controls. The distribution within cases and controls was similar (P = .57): grade 0 (36% and 26%); grade 1 (0% and 5%); grade 2 (58% and 58%); grade 3 (3% and 8%); and grade 4 (3% and 3%). Grouping aGVHD grades 0-2 versus grades 3-4 did not show a significant difference between case patients (94% versus 6%) and controls (90% versus 10%; P = .72). Grouping aGVHD grades 0-1 versus grades 2-4 also did not show a significant difference between case (36% versus 64%) and control patients (31% versus 69%, respectively; P = .66). The median day of onset of any aGVHD was delayed in case patients (day 43; range, days 8-109) compared with controls (day 21; range, days 7-52; P < .0001). GVHD was usually treated with prednisolone and/or restart of CSP if it was already tapered.
Anti-infectious prophylaxis
All patients received prophylactic antibiotics (ceftazidime or ciprofloxacin) when absolute neutrophil counts were less than 0.5 × 109/L. Patients who were serologically positive for herpes simplex virus received prophylactic low-dose acyclovir from day −5 until day +30 or until resolution of mucositis, whichever occurred earlier.11 No antiviral prophylaxis directed against CMV was given in any of the patients. Immunoglobulins were substituted according to the standard guidelines in our center: patients with immunoglobulin levels below 400 mg/dL received substitutions of intravenous immunoglobulins until posttransfusion levels exceeding 400 mg/dL were reached. All other patients did not receive immunoglobulins. Fluconazole (400 mg/d) was given to all patients from the start of conditioning to day +75 after transplantation.16 Prophylaxis against Pneumocystis carinii was performed with the use of trimethoprim-sulfamethoxazole as first-line treatment and dapsone (50 mg BID daily) as second-line treatment until day 120 after transplantation.17
Infection surveillance
Patients were monitored through day 365 after transplantation for the development of CMV infections and diseases. Surveillance from blood samples for CMV (pp65 antigenemia, blood culture) was performed on a weekly to biweekly basis.11 After day 100, biweekly surveillance was recommended for both groups; however, adherence could not be assessed after discharge from Seattle. Patients with suspected pneumonia were evaluated by bronchoalveolar lavages (BALs) and/or lung biopsies. Viral direct antigen detecting fluorescent assay (DFA) and shell vial (SV) centrifugation cultures were performed on all BAL, lung biopsy, and autopsy specimens throughout the study period; specimens were submitted for routine bacterial, fungal, and acid-fast bacilli cultures.
Pre-emptive CMV therapy
In general, during the first 100 days after transplantation, all patients with CMV antigenemia at any level or CMV viremia received ganciclovir (GCV) induction therapy (5 mg/kg IV twice daily) for 7 to 14 days followed by maintenance therapy (GCV 5 mg/kg IV daily) until day 100. After day 100, pre-emptive therapy (7 to 14 days of induction followed by 14 days of maintenance) was recommended when antigenemia was at least 5 positive cells per slide or when CMV viremia by culture was present.
Three case patients did not receive pre-emptive therapy at the discretion of the treating physicians owing to low-grade antigenemia at their first positive test. Four control patients were enrolled in a protocol of adoptive ex vivo–expanded CMV-specific T-cell therapy. In these patients, pre-emptive GCV therapy was given only for culture-proven viremia.
Definitions
Previously described definitions for infections were used in this study.11 The day of onset of an infection was defined as the day when the diagnostic test was performed. CMV antigenemia was diagnosed on the basis of positive blood pp65 testing (150 000 cells counted per slide), and CMV viremia on the basis of a positive blood culture or SV centrifugation culture.18 CMV pneumonia was diagnosed on the basis of signs and symptoms compatible with a diagnosis of pneumonia (hypoxemia, x-ray) and a BAL or lung biopsy specimen positive for CMV by DFA, culture, or immunohistology. CMV gastrointestinal (GI) disease was diagnosed when GI signs or symptoms occurred, and evidence of CMV in the GI tract was diagnosed by culture, immunohistochemistry, or in situ hybridization from biopsy specimens.
Statistical analysis
To compare characteristics of case patients with controls, summary statistics, including frequency counts and percentages for categorial variables, as well as medians and ranges for ages at transplantation, were calculated. Comparisons from 2 × 2 tables were made by means of chi-squared and Fisher exact tests. The median times until the onset of events were compared by means of the Wilcoxon rank sum test. Cumulative incidence curves up to 100 days after transplantation were produced for CMV antigenemia and viremia, and up to 365 days for CMV disease.
Univariate and multivariate Cox regression models were used to analyze the influence of selected variables on the risks of CMV disease up to day 100 and for the time period from days 101 to 365 after transplantation. Times to CMV disease were the outcomes for the Cox regression models with censoring at death, subsequent transplantation, or the end of follow-up. Positive antigenemia and acute GVHD were entered as time-dependent covariates. Follow-up times in the model examining early CMV disease were censored at day 100. Variables for the multivariate models were selected with backward stepwise elimination with significance exceeding .05 as the criterion for removal from the models. A variable indicating whether the patients were in the case or control group was included in the models regardless of its significance. Confidence limits were calculated assuming normality of parameter estimates, and P values were calculated using the likelihood ration test.
One-year survivals after CMV disease diagnosis among case patients were compared with those of controls by means of a Kaplan-Meier curve and the log-rank test.
Results
The median follow-up of case patients was 12.7 months (range, 1.1-28.4 months) and 12.7 months (range, 0.1-48.1 months) for controls.
Posttransplantation host chimerism in nonmyeloablative HSCT patients
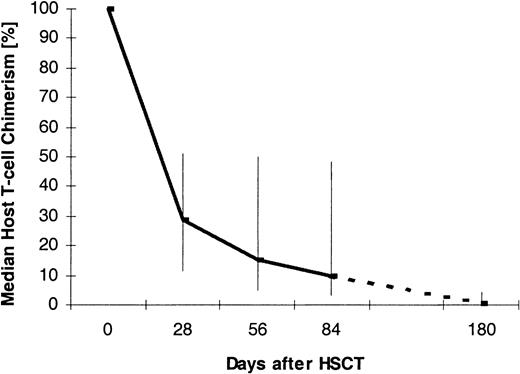
Figure 1 displays the percentage of host T lymphocytes in the peripheral blood of case patients at different time points after transplantation. The medians as well as the 25th and 75th percentiles are shown. For the analysis, data from 28 ± 8 days (n = 56), 56 ± 8 days (n = 55), 84 ± 8 days (n = 30), and 180 ± 8 days (n = 12) after transplantation were used. The graph shows that host T cells survived the HSCT and then decreased over time.
Median T-cell chimerism after nonmyeloablative HSCT.
Vertical bars indicate 25th and 75th percentiles. The numbers of patients per analysis time point were 56 (day +28), 55 (day +56), 30 (day +84), and 12 (day +180).
Median T-cell chimerism after nonmyeloablative HSCT.
Vertical bars indicate 25th and 75th percentiles. The numbers of patients per analysis time point were 56 (day +28), 55 (day +56), 30 (day +84), and 12 (day +180).
CMV antigenemia, viremia, and disease during the first 100 days after transplantation
CMV antigenemia.
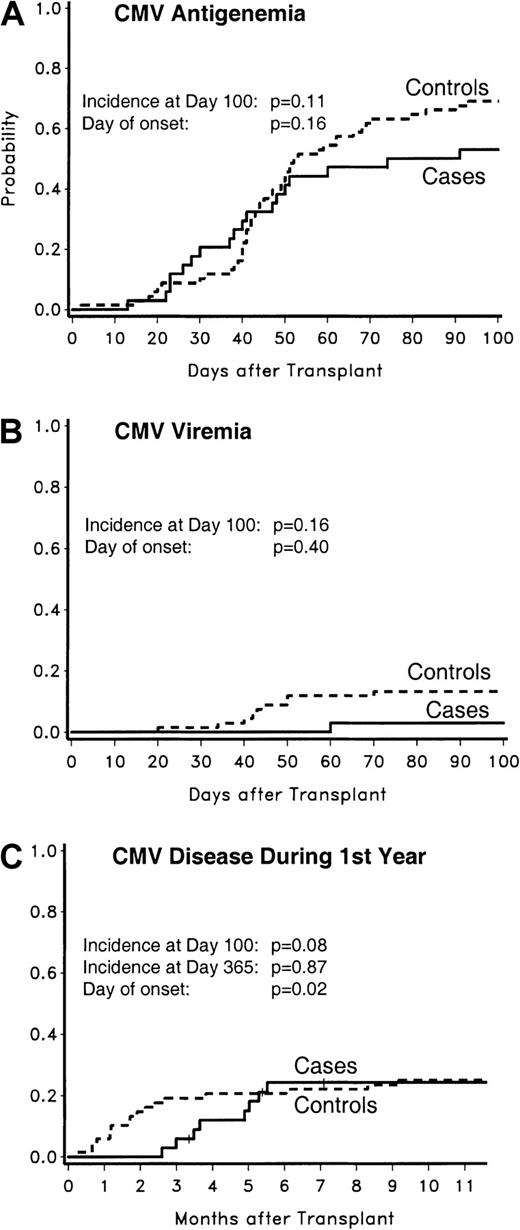
None of the CMV low-risk cases (0 of 12; 0%) developed antigenemia, whereas 2 CMV low-risk controls did (2 of 24; 8%;P = .54). Two of the CMV intermediate-risk cases (2 of 10; 20%) developed antigenemia, compared with 3 controls (3 of 20; 15%;P = 1.00). CMV antigenemia tended to be less frequent among CMV high-risk cases compared with controls (18 of 34 [53%] versus 47 of 68 [69%]; P = .11) (Figure2).
CMV antigenemia, viremia, and disease in CMV high-risk patients.
The probabilities of CMV antigenemia (panel A), CMV viremia (panel B), and CMV disease (panel C) are displayed. The probability for CMV disease was analyzed for the first year after transplantation; for other events, the analyses were done for the first 100 days after transplantation.
CMV antigenemia, viremia, and disease in CMV high-risk patients.
The probabilities of CMV antigenemia (panel A), CMV viremia (panel B), and CMV disease (panel C) are displayed. The probability for CMV disease was analyzed for the first year after transplantation; for other events, the analyses were done for the first 100 days after transplantation.
For CMV antigenemia among CMV high-risk patients, there was no significant difference in median times to onset between cases and controls (39 days; range, 13-91 days; versus 44 days; range, 2-93 days;P = .16).
CMV viremia.
CMV viremia occurred in neither CMV low-risk cases nor controls. No CMV viremia occurred in CMV intermediate-risk cases (0 of 10; 0%), and one occurred in controls (1 of 20; 5%; P = 1.00). There was a trend to less CMV viremia in CMV high-risk cases compared with controls (1 of 34 [3%] versus 9 of 68 [13%]; P = .16) (Figure2). In this risk group, the median time to onset of CMV viremia was 60 days in the one case patient compared with 43 days (range, 20-70 days) in controls (P = .40).
CMV disease.
CMV disease occurred in neither low- and intermediate-risk CMV cases nor controls. There was a trend to less CMV disease in CMV high-risk cases compared with their controls (2 of 34 [6%] versus 13 of 68 [19%]; P = .08) (Figure 2).
CMV disease was significantly delayed in cases of the CMV high-risk group compared with controls (median, 85 days; range, 79-91 days; versus median, 36 days; range, 6-81 days; P = .04).
Combined CMV manifestations.
In the CMV high-risk group, a combined analysis of the more severe manifestations of CMV, ie, CMV viremia and CMV disease, showed a significant difference between the percentage of cases with severe manifestations by day 100 compared with controls (3 of 34 [9%] versus 21 of 68 [31%]; P = .01). The median time to event was significantly delayed in cases compared with controls (median, 79 days [range, 60-91 days] versus 42 days [range, 6-81 days]; P = .02). When CMV antigenemia, viremia, and disease during the first 100 days were combined in the CMV high-risk group, the percentage of case patients with events was significantly lower compared with controls with events (53% versus 78%;P = .01).
CMV antigenemia, viremia, and disease during the first year after transplantation
The results of CMV antigenemia and viremia testing beyond day 100 were too few for statistical analysis. This was in part due to the fact that the patients were discharged, testing was performed locally, and testing adherence decreased.
No case patients, but one control, in the intermediate-risk CMV group developed CMV disease (day 130). The probabilities of CMV disease in the CMV high-risk population during the first 365 days after transplantation are graphically shown in Figure 2. The percentage of patients with CMV disease during this time period was similar between CMV high-risk cases and their controls (8 of 34 [24%] versus 17 of 68 [25%]; P = .87). The difference in the median times of onset remained statistically significant (130 days [range, 79-168 days] versus 52 days [range, 6-279 days]; P = .02).
Differences in quantitative CMV antigenemia
To determine if there were differences in the magnitude of CMV antigenemia, we analyzed the quantified test results (CMV-positive cells per slide), comparing cases with controls in the CMV high-risk group. The first and the maximal CMV antigenemia results were grouped into 5 or fewer, more than 5, more than 10, more than 50, and more than 100 positive cells per slide, and the percentages of patients within each group were compared between cases and controls. The denominator for the analyses of percentages and the P values of maximal and first positive antigenemia included all patients with antigenemia. There were no significant differences in the degree of maximal CMV antigenemia between cases and controls in any of these groups. Examining the numeric quantity of the first positive CMV test of each patient also revealed no significant differences between cases and controls in any of the groups.
Time to CMV antigenemia clearance
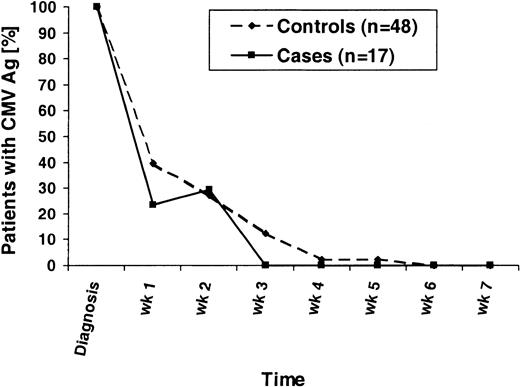
To determine if there were any differences in the kinetics of the CMV antigenemia clearance, we analyzed the quantified test results following the first positive result of each patient. Included were results from all patients who developed CMV antigenemia and who received similar ganciclovir induction and maintenance therapy. Four controls were excluded in this analysis because they had initially received ex vivo–expanded CMV-specific T cells for treatment; 3 cases were excluded because they had low-grade antigenemia at their first positive test and had not received ganciclovir induction at that point. Patients who had negative CMV antigenemia results for longer than 14 days were considered to have cleared the CMV. The results of the analysis are shown in Figure 3. Of note, one case patient initially had low-grade antigenemia (0.5 positive cells per slide), received pre-emptive GCV therapy, was then CMV Ag-negative on week 1, became low-grade positive on week 2 again (0.5 positive cells per slide), and then cleared his CMV antigenemia. There was a trend toward a shorter time to CMV clearance in the cases (P = .15, Mantel-Haenszel test).
Time to CMV antigenemia clearance.
All patients who developed CMV antigenemia during the first 100 days after transplantation and who received ganciclovir induction and maintenance therapy are displayed. For weekly intervals, the percentages of CMV Ag-positive patients are shown following the first positive CMV Ag test (diagnosis). The denominators are all patients initially CMV Ag-positive.
Time to CMV antigenemia clearance.
All patients who developed CMV antigenemia during the first 100 days after transplantation and who received ganciclovir induction and maintenance therapy are displayed. For weekly intervals, the percentages of CMV Ag-positive patients are shown following the first positive CMV Ag test (diagnosis). The denominators are all patients initially CMV Ag-positive.
Clinical course of patients with early and late CMV disease
Clinical characteristics of CMV high-risk case and control patients who developed early or late CMV disease are shown in Table2. CMV disease of the lung occurred in 6 of 8 cases (75%). One case (12%) had CMV disease in the bone marrow, and one case had the disease in the GI tract. At the time of CMV disease diagnosis, copathogens were present at the site of infection or systemically in 5 of 8 (63%) case patients. Copathogens wereAspergillus species as well as other fungi. Within 90 days of CMV disease diagnosis, 3 of 8 (38%) case patients died; copathogens were present in all. Two cases with concomitant Aspergillusand CMV infections of the lung survived.
CMV disease of the lung occurred in 11 of 17 controls (65%). In one control patient, the lungs were involved as well as the bone marrow, and 5 of 17 controls (29%) had CMV disease of the GI tract. At the time of CMV disease diagnosis, copathogens were present at the site of infection or systemically in 6 of 17 patients (35%). Twelve of 17 controls (71%) with CMV disease died.
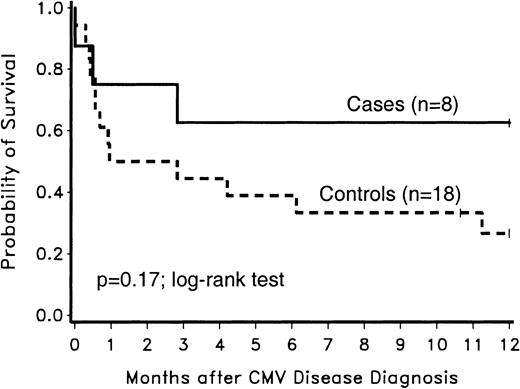
The 1-year survival after CMV disease diagnosis was not significantly different between case and control patients (P = .17; log-rank test) (Figure 4). The intermediate-risk control patient who developed CMV disease was included in the survival analysis.
CMV disease risk-factor analysis
Univariate and multivariable analyses were performed to identify risk factors for CMV disease. Initial analyses were restricted to the first 100 days after transplantation to examine early CMV disease; analyses were then extended to the time from days 101 to 365 for late CMV disease (Table 3).
The multivariable analyses with adjustment for other factors showed that, during the first 100 days after transplantation, patients who received a nonmyeloablative transplant had a reduced risk for early CMV disease (P = .01). Patients who were in the poor prognosis group had an increased risk for early CMV disease (P = .04); this was true as well for CMV high-risk patients (P < .001). In contrast, from day 101 to day 365 after transplantation, patients who received a nonmyeloablative transplant had an increased risk for late CMV disease (P = .03). Also, having had CMV antigenemia during the first 100 days increased the risk for late CMV disease (P < .001).
Discussion
We hypothesized that defenses against viral infections among nonmyeloablative HSCT patients consisted of contributions from both residual host memory immune responses and emerging donor graft–derived immunity. This would be reflected in better early immune responsiveness and, consequently, lower early infection rates. Consistent with this hypothesis, the following observations were made in this study.
Following nonmyeloablative HSCT, host T cells were present in the peripheral blood for up to 6 months. CMV-positive recipients of a nonmyeloablative allogeneic HSC transplant showed trends toward a lower incidence of CMV antigenemia, CMV viremia, and CMV disease during the first 100 days after transplantation compared with controls. When observations on severe manifestations of CMV, such as viremia and disease, were pooled, this difference reached statistical significance. CMV disease occurred significantly later among case patients than among controls. This was a key finding of the study. It demonstrated that, although the time of CMV antigenemia onset was similar in case and control patients, fewer case patients with CMV antigenemia continued to develop viremia or early disease. Therefore, it supported our hypothesis that the prolonged persistence of host immune-competent cells among patients in the case group resulted in some protection against viral infections.
The other important finding of this study was that the incidence of late CMV disease was increased in nonmyeloablative transplant recipients compared with myeloablative transplant recipients. We identified several risk factors for late CMV disease. The strongest association was observed with CMV antigenemia before day 100. This was consistent with earlier results of Zaia et al,19 who found early CMV infection (as determined by CMV DNA in plasma) to be associated with late CMV disease. Additional factors associated with late CMV disease in univariate models were presence of a positive CMV serostatus of the recipient before transplantation, acute GVHD higher than grade 1, MMF-containing posttransplantation immunosuppression, and TBI/fludarabine-containing conditioning regimen, although not all associations reached statistical significance.
It is well established that delayed CMV-specific immune reconstitution leads to an increase in late CMV disease after HSCT20 and that use of ganciclovir, presence of GVHD, or use of corticosteroids may delay recovery of CMV-specific T-cell responses.12 21-24
In our study, the use of pre-emptive ganciclovir therapy before day 100 was similar for cases and controls; this factor was thus unlikely to explain the difference in late CMV disease seen in this study. Although continued biweekly surveillance and preemptive therapy was recommended after day 100 for both myeloablative and nonmyeloablative patients, assessing whether surveillance was actually performed was difficult. Thus administration of preemptive strategies may have been different between the groups. Another factor that was clearly different in the 2 groups and that might explain, at least in part, the higher risk for late CMV disease in the nonmyeloablative patients was the GVHD prophylaxis regimen. Most patients in the myeloablative group received MTX and CSP for GVHD prophylaxis, whereas in the nonmyeloablative patients MMF and CSP were given for immunosuppression. We have shown in earlier canine studies that the combination of MMF and CSP provided synergistic immunosuppressive effects on T cells that seemed to be stronger than those achieved with the combination of MTX and CSP, although the differences were not statistically significant.25 It is possible that the differences in the applied immunosuppression, ie, MMF/CSP in the nonmyeloablative patients versus mostly MTX/CSP in the myeloablative controls, caused delayed immune reconstitution and led to the differences seen in CMV disease onset by direct interference with CMV-specific immune reconstitution similar to corticosteroids. Since MMF/CSF was almost exclusively given to nonmyeloablative patients, we were unable to separate the effect the GVHD prophylaxis in our statistical models.
It is also conceivable that the strong immunosuppression provided in the nonmyeloablative patients led to a suppression of allogeneic effects earlier after transplantation and therefore decreased the early allogeneic activation mechanisms that have been described as risk factors for CMV disease.11,26 This would be consistent with the rather low rate of early CMV disease seen in this study. Later, when the immunosuppression was tapered off, allogeneic effects became operative. This might lead to both direct reactivation of CMV27 and the development of GVHD, which was then treated with high-dose corticosteroids. Both effects might have contributed to the observed effect of late-onset CMV disease. Definitive explanations of the immunologic mechanisms will come from ongoing studies in our laboratory aimed at defining CMV-specific immune-reconstitution patterns in patients undergoing nonmyeloablative compared with myeloablative transplantation.
Other studies of early CMV disease in patients who received myeloablative conditioning regimens and pre-emptive ganciclovir therapy reported incidences of early CMV disease of 3.5% to 10.5%.10-12,28-32 Interestingly, our control group had a somewhat higher incidence of CMV disease than previously reported. This might be due to the fact that our control group had a median age of 46 years, which was higher than in prior study populations, and age has been described as a risk factor for CMV disease.11 31 The observed incidence of early CMV disease in the nonmyeloablative CMV-seropositive recipients in our study was on the low end of the reported results in myeloablative patients. Although the numbers were small, the outcome of CMV disease in the nonmyeloablative patients seemed to be favorable compared with the control population. Especially intriguing are the 2 case patients who had concomitant aspergillus disease and who survived.
In conclusion, our data showed that, within the first year after transplantation, recipients of nonmyeloablative HSC transplants had an incidence of CMV disease similar to that of their matched controls who underwent myeloablative HSCT. The important difference was that the time of CMV disease onset was delayed in nonmyeloablative compared with myeloablative HSC transplant recipients, with most cases occurring after day 100. We therefore conclude that screening for CMV should be continued in patients at risk for the first year after transplantation, especially in those who had CMV infection before day 100. Pre-emptive antiviral therapy should be initiated according to the guidelines used in myeloablative allogeneic transplantations. Since several nonmyeloablative conditioning regimens with differing immunosuppressive potential are used at different centers, it seems advisable to determine the risk for early and late viral infections for each regimen.
We thank the medical, nursing, data processing, and clinical staffs at the listed institutions for their important contributions to this study through their dedicated care of the patients.
Supported by National Institutes of Health grants HL36444, HL03701, CA18221, CA18029, CA78902, and CA15704; by the German Research Council grant DFG JU 417/1-1 (C.J.); and by a research grant from the Lady Tata Trust, London, United Kingdom (M-T.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Boeckh, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D3-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: mboeckh@fhcrc.org.