Abstract
For this study, 118 children with standard-risk acute lymphoblastic leukemia (ALL) were given randomized assignments to receive native or pegylated Escherichia coli asparaginase as part of induction and 2 delayed intensification phases. Patients treated with pegaspargase had more rapid clearance of lymphoblasts from day 7 and day 14 bone marrow aspirates and more prolonged asparaginase activity than those treated with native asparaginase. In the first delayed intensification phase, 26% of native asparaginase patients had high-titer antibodies, whereas 2% of pegaspargase patients had those levels. High-titer antibodies were associated with low asparaginase activity in the native arm, but not in the pegaspargase arm. Adverse events, infections, and hospitalization were similar between arms. Event-free survival at 3 years was 82%. A population pharmacodynamic model using the nonlinear mixed effects model (NONMEM) program was developed that closely fit the measured enzyme activity and asparagine concentrations. Half-lives of asparaginase were 5.5 days and 26 hours for pegaspargase and native asparaginase, respectively. There was correlation between asparaginase enzymatic activity and depletion of asparagine or glutamine in serum. In cerebrospinal fluid asparagine, depletion was similar with both enzyme preparations. Intensive pegaspargase for newly diagnosed ALL should be tested further in a larger population.
Introduction
Combination chemotherapy for acute lymphoblastic leukemia (ALL) usually includes a bacteriall-asparaginase (ASNase) enzyme derived fromEscherichia coli or Erwiniaspecies.1 ASNase depletes circulating asparagine and selectively kills leukemic cells that require external sources of that amino acid.2-4 Foreign proteins like ASNase induce acute allergic reactions and silent immunity, characterized by circulating antibodies and rapid clearance of the enzyme from the blood. In children and adults treated with various preparations and regimens ofE coli ASNase, circulating antibodies were found in an average of 58% of patients (range 28%-96%).5-10 In reported series, severe acute allergic reactions were seen in an average of 24% of children and 29% of adults during the first time they were treated with regimens containing ASNase.
Pegaspargase is formed by covalent linking of 5000-dalton units of monomethoxypolyethylene glycol to E coli ASNase. Binding preserves the enzyme activity, but decreases immunogenicity of the protein.11,12 The elimination half-life of pegaspargase is approximately 6 days, 5 times longer than native E coli and 9 times longer than Erwinia ASNase.13 Most clinical experience with pegaspargase is in patients treated previously with native E coli ASNase.4,14-16 Acute allergic reactions were seen in very few children with relapsed ALL who were treated with pegaspargase, but more than 60% of them developed circulating antibodies.4,14,17 Children with circulating antibodies had faster clearance of ASNase, shorter periods of asparagine depletion in the blood, and poorer response rates.18 Giving pegaspargase to relapsed patients with ALL every 7 days rather than every 14 days appears to counteract the effect of rapid clearance. Patients treated with a 4-drug induction that included weekly pegaspargase had a complete remission rate of 97%, whereas those who received it every 2 weeks had a rate of only 82%.19
Pegaspargase might cause less antibody formation in patients with no prior exposure to ASNase. If fewer children develop antibodies to pegaspargase than native ASNase, they should have more sustained asparagine depletion, which is important in Children's Cancer Group (CCG) studies in which many children receive ASNase in induction and each of 2 delayed intensification (DI) phases. We conducted a clinical trial to evaluate safety, efficacy, and pharmacokinetics of a single intramuscular (IM) dose of pegaspargase instead of multiple IM doses of native E coli ASNase in each of 3 phases of therapy.
Patients, materials, and methods
Patients
Patients enrolled in CCG protocol 1962 (after IRB-approved informed consent was obtained according to the Declaration of Helsinki) had standard-risk ALL, were aged 1 through 9 years, had white blood cell (WBC) counts of less than or equal to 50 000/μL, and less than or equal to 20% surface immunoglobulin (Ig)–positive leukemic blasts. Patients were eligible with massive lymphadenopathy, massive splenomegaly, large mediastinal mass, concurrent CNS, or testicular leukemia. Between May 1997 and November 1998, 8 CCG centers enrolled 118 patients on this study, rather than the groupwide phase III trial CCG 1952. The native ASNase arm of this study was identical to A1, the standard arm of CCG 1952.
Treatment
Treatment consisted of 4 weeks of induction, 4 weeks of consolidation, 2 8-week interim maintenance phases, 2 8-week DI phases, and maintenance therapy (Table 1). The duration of therapy for girls and boys was 2 and 3 years, respectively, from the start of the first interim maintenance phase. At the start of induction, patients were randomly assigned to receive either 2500 IU/m2 of pegaspargase IM on day 3 of induction and each DI phase or 6000 IU/m2 of native ASNase IM 3 times per week, for 9 doses in induction, and 6 doses in each DI phase.
Patient and laboratory monitoring
Patients were monitored by physical examinations and complete blood cell count (CBC), creatinine, bilirubin, aspartate aminotransferase (AST) or alanine aminotransferase (ALT), and urine glucose. Bone marrow aspirates and spinal taps were done at entry and on day 7 and day 28 of induction. Patients with at least 5% lymphoblasts on day 7 had another bone marrow aspirate on day 14. Bone marrow aspirates were done at the end of DI no. 2 and the end of maintenance therapy.
Asparaginase, antibody, and amino acid analyses
Blood samples were collected on days 0, 7, 14, 21, and 28 of induction and each DI phase. CSF samples were collected on induction days 0, 7, and 28. At least 4 specimens were obtained from 57 and 45 patients in the pegaspargase and native ASNase arms, respectively. Some serum and CSF specimens were collected within 2 days of each of these induction days. The actual day of sampling was used in all calculations. Blood and CSF samples were placed immediately in an ice-water bath. Blood was allowed to coagulate under these conditions. No inhibitors of ASNase enzymatic activity were added to the tubes. We found that asparagine and glutamine were not deaminated when added to serum that contained 1 IU/mL ASNase activity and the tubes were placed rapidly in an ice bath. A specimen transmittal form was used to record time and ASNase dose and specimen collection times.
ASNase activity was measured by ammonia produced from asparagine with a Nessler reaction. Reacted enzymatic activity solutions were placed in an enzyme-linked immunosorbent assay (ELISA) plate and the ELISA plate reader was used to read optical density, calculate calibration line, and quantify specimens. ASNase protein was measured by ELISA similar to the one for anti-ASNase antibody.13
ASNase antibody assays were done by a modified indirect solid-phase ELISA.13 The assay was done with a computer-controlled instrument from Dynatech Laboratories (Chantilly, VA). An antibody against native E coli ASNase was used initially to create a titration curve for both native E coli ASNase and pegaspargase. Later, the sera from patients who had high-titer antibodies to pegaspargase were used for that enzyme preparation. The titers were compared with the same patient's pretreatment control serum and negative control serum from a healthy volunteer. The assay had excellent linearity, reproducibility, and low detection limits, but the absorbance of control varied between assays. Day-to-day variation was corrected by expression of antibody titers as the ratio of sample over negative control for each assay. In the protocol, high-titer antibody was defined as a ratio of serum antibody to the average control value of 2.5.20 21 For statistical analyses, we used the highest ratio of 4 posttreatment samples collected from each patient during each ASNase-containing phase.
Asparagine, aspartic acid, glutamic acid, and glutamine were assayed by modification of a reported high-performance liquid chromatography (HPLC) method22 in which amino acids are derivatized with phenylisothiocyanate (PITC). The derivatized samples were analyzed using a μC18 column by 2-step gradient elution with a flow rate of 0.8 mL/min. The first step comprised a 25-minute linear gradient from 100% buffer A (0.05 M ammonium acetate, pH 6.8) to 90% A and 10% of buffer B (0.1 M ammonium acetate, pH 6.8-acetonitrile, 50:50). The second step consisted of a 10-minute gradient from 90% A and 10% buffer B to 0% A and 100% B. Absorbance was followed at 254 nm. Calibration curves were used to quantify amino acid concentrations. The lowest limit of detection from the linear portion of the calibration lines was 0.01 μM for asparagine and 1 μM for glutamine.
Pharmacokinetic and pharmacodynamic studies
One-compartment and noncompartmental pharmacokinetic analyses were done of the pegaspargase serum levels. A one-compartment open model was used to fit the serum concentrations of ASNase enzymatic activity. A population model for the one-compartment open model using the NONMEM computer program was designed as described.23 Nonlinear Mixed Effects Model (NONMEM), is a computer program licensed by the NONMEM Project Group, UCSF (San Francisco, CA), written in FORTRAN 77 and designed to fit general statistical (nonlinear) regression-type models to pharmacokinetic data. The program allows for the estimation of average (population) values of PK parameters as well as estimation of inter- and intra-individual variabilities. The Michaelis-Menten equation was programmed into a separate ADVAN/TRANS subroutine of this program so we could model serum ASNase enzymatic activity and its substrate asparagine simultaneously. Noncompartmental pharmacokinetic model analyses also were done using the method based on the statistical moment theory.
Toxicity
We used version 1 of the National Cancer Institute's toxicity and complications criteria. The site, measure, and grade of all grade 3 and 4 nonhematologic toxicities were reported on data collection forms. Specific forms captured grade 3 or 4 coagulopathy, clinical allergy and pancreatitis, hyperglycemia that required insulin, grade 4 neurologic dysfunction, days of hospitalization, and infections.
Response criteria
Bone marrow status was defined as follows: M1 was less than 5% lymphoblasts regardless of proportion of mature lymphocytes. Remission also required normal marrow cellularity and elements. M2 and M3 were defined as 5% to 25% lymphoblasts and more than 25% blasts, respectively.
Statistical methods
The primary endpoint of the study was incidence of high-titer ASNase antibodies in DI no. 1. Based on the literature, we assumed that 50% of patients treated with native ASNase would develop these antibodies during the first DI phase. The study was designed to detect a change from 50% to 25% or less in incidence of antibodies, with a power of 80% for one-sided hypothesis test. This led to a sample requirement of approximately 118 patients, assuming that 10% of patients might not have samples available for testing (because of early relapse or noncompliance). Secondary endpoints were comparison of the 2 treatment arms for incidence of antibodies in DI no. 2; ASNase activity, ASNase protein, and ASN levels in serum during induction and DI phases; and in the CSF during induction. Analysis of clinical outcome included response rates during induction at day 7, day 14, and end of induction marrow exams. Analysis of disease outcome also examined event-free survival (EFS), but it was recognized that only large differences in this index would be detectable. EFS events included induction death, no induction response, relapse at any site, and second malignant neoplasm. Kaplan-Meier estimates24were used for life-table estimation, and the log-rank test25 was used to compare EFS outcomes.
Comparisons of induction response rates and some categorical analyses of antibody ratio levels and ASNase activity groupings used exact χ2 tests26 that involved global tests of differences and tests for trend (ordering) when appropriate. For comparisons of actual values for ASNase antibodies and antibody ratio, the Wilcoxon nonparametric rank test was used.27 Analysis of asparaginase-related pharmacokinetic indices used only patients who were started appropriately on their asparaginase treatment regimens (2 patients were excluded). Life-table comparisons of EFS outcomes for treatment regimens used intent-to-treat analyses that included all randomly assigned patients.
Results
Patient characteristics
For this study, 118 children were entered and randomly assigned, 59 to each of the ASNase formulations. Table2 shows patient characteristics. At diagnosis, a higher percentage of patients in the native ASNase arm were aged 1 to 2 years, had platelet counts of less than 50 000/μL, and had CNS 2, defined as 0 to 4 cells/μL and at least 1% blasts. None of these or other risk factors were significantly different in the 2 arms. Institutional immunophenotypes found that among 116 patients tested, 103 were CD10+, 107 were CD19+, and 2 were classified as T lineage (both in the native ASNase arm). No one had B-cell leukemia. In each arm, 2 to 4 children had massive splenomegaly or hepatomegaly. One patient in each arm had massive lymphadenopathy. No patient had concurrent CNS or testicular leukemia. Of the participants, 3 children had Down syndrome, 2 of whom were treated with pegaspargase. Also, 2 patients were excluded from pharmacokinetic and pharmacodynamic analyses: one had a Philadelphia chromosome and was taken off the study at the end of induction and treated with more intensive therapy including a bone marrow transplantation; the other was mistakenly given both forms of ASNase during induction.
On day 14 of induction, 4 children treated with native ASNase had M3 bone marrow; on day 28 of induction, one child on pegaspargase had M2 bone marrow. As stipulated in the protocol, those patients were taken off the study at the end of induction and treated with a more intensive regimen.28 One patient left the country during maintenance phase and was lost to follow-up. There were 10 children (8 in the pegaspargase arm) who did not receive all required doses of ASNase in DI no. 1 or no. 2 because of toxicity, protocol violation, or parental choice.
Antibodies to ASNase
The primary endpoint of this study was to find whether incidence of high-titer anti-ASNase antibodies in children treated with pegaspargase was decreased by a least 50% in DI no. 1 compared with those treated with native ASNase. A secondary endpoint was to show whether the same decrease occurred in DI no. 2. The mean ± SEM antibody ratio in DI no. 1 was 1.9 ± 0.8 (n = 47) for children treated with pegaspargase and 3.0 ± 0.7 (n = 43) for those treated with native ASNase (P = .001 by Wilcoxon 2-sample test). The respective mean ± SEM ratios for pegaspargase and native were 1.3 ± 0.2 (n = 41) and 2.3 + 0.9 (n = 47) for induction (NS) and 2.1 + 0.8 (n = 45) and 2.1 + 0.6 (n = 45) for DI no. 2 (NS).
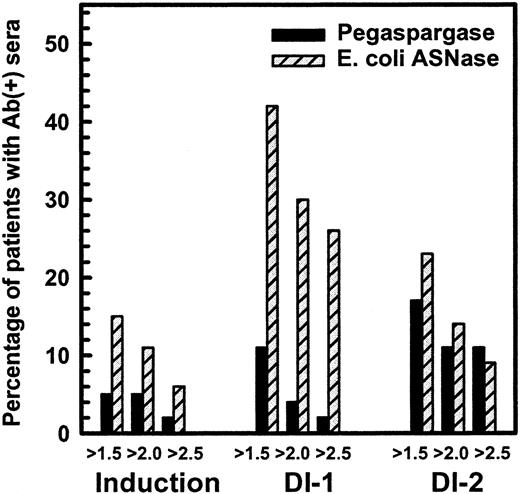
Figure 1 shows the percentage of children in induction, DI no. 1, and DI no. 2 with a maximal ratio of antibody over negative control of at least 1.5, 2.0, and 2.5. The difference in high-titer antibodies was especially evident in DI no. 1, in which 11 of 43 children in the native ASNase arm had ratios of more than or equal to 2.5, whereas 1 of 47 in the pegaspargase arm had that level (P = .001, Wilcoxon test). The differences were less apparent in DI no. 2 (P = .09, Wilcoxon test) and not significant during induction. Comparison of the maximum antibody ratio of each patient, irrespective of cycle, showed higher titers in the native ASNase patients (P = .0009, Wilcoxon test). We anticipated that 50% of children treated with native ASNase would have antibody ratios of at least 2.5 at some time during their therapy. Only 26% of children in the native ASNase arm had ratios of more than or equal to 2.5, but over 40% of them had ratios of more than or equal to 1.5. The antibody levels tended to decrease between days 7 and 28 of each ASNase-containing phase and were lower in DI no. 2 than DI no. 1.
Percentage of patients with antiasparaginase antibody ratio over negative control more than 1.5, 2.0, and 2.5 in CCG-1962 study.
Percentage of patients with antiasparaginase antibody ratio over negative control more than 1.5, 2.0, and 2.5 in CCG-1962 study.
Studies have shown that ASNase activity is often low when the antibody titer is high.4 18 Table 3shows the fraction of samples collected 3 to 14 days after the start of ASNase with ASNase activity more than 0.1 IU/mL, a level usually considered adequate to deplete asparagine. During that time, we expected all samples to have ASNase activity more than 0.1 IU/mL. There were usually 2 samples per patient during that interval. Table 3 shows that more than 89% of native ASNase samples and more than 95% of pegaspargase samples had those levels when the antibody ratio was low (<1.5). In contrast, during DI no. 1, only 50% of samples from native ASNase patients with antibody ratios of more than or equal to 1.5 had ASNase activity more than 0.1 U/mL (P = <.001 by trend test) (Table 3). The association between increased antibody ratio and low ASNase activity also was seen in DI no. 2 (P = .01 by trend test). Patients treated with pegaspargase showed 2 major differences (Table 3): fewer samples had elevated antibody ratios and all pegaspargase samples with antibody ratios of more than or equal to 1.5 had adequate ASNase activity.
Day 7 and day 14 bone marrow and outcomes
Table 4 shows the bone marrow results during induction. There was more rapid clearance of blasts at day 7 and day 14 in the pegaspargase arm than in the native ASNase arm (P = .05 and .015, respectively); χ2 test with ordering. Twice as many patients in the native ASNase arm had M3 bone marrow on day 7 than in the pegaspargase arm. All 4 patients with M3 bone marrow on day 14 were in the native ASNase arm. One patient in the pegaspargase arm had M2 bone marrow on day 28. Those 5 patients were treated with more intensive therapy and remain in first remission.
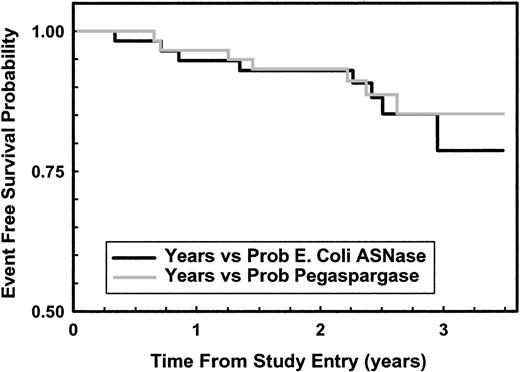
As of February 2001, 7 patients had relapsed in the pegaspargase arm (2 BM, 3 CNS, one combined BM and CNS, and one death after BM relapse) and 8 in the native ASNase arm (4 BM, 4 CNS). Figure2 shows that 3-year EFS rates for pegaspargase and native ASNase were 85% and 78%, respectively (NS). Of the 15 relapses, 3 were in patients taken off the study because of Philadelphia and ALL, parental refusal to have a second DI phase, and pancreatitis preventing ASNase treatment. Excluding those 3 patients, the 3-year EFS rates for pegaspargase and native ASNase were 88% and 85%, respectively (NS).
Kaplan-Meier plot of EFS for all randomly assigned patients.
Solid line (black) shows data for 59 native ASNase patients; gray line for 59 pegaspargase patients, P = .773 log-rank.
Kaplan-Meier plot of EFS for all randomly assigned patients.
Solid line (black) shows data for 59 native ASNase patients; gray line for 59 pegaspargase patients, P = .773 log-rank.
Safety
Table 5 lists the grade 3 and grade 4 toxic events during the ASNase-containing phases of chemotherapy. There were no toxicity-related deaths in either group. Incidence and type of toxic events were very similar between pegaspargase and native ASNase arms and were similar to other low- and standard-risk acute lymphoblastic leukemia protocols.1,29 30 Central nervous system (CNS) thrombosis occurred in 2 patients in each arm: 3 on days 14 to 16 of induction and one on day 22 of DI no. 1. Patients who developed CNS thrombosis received no further ASNase. Other central nervous system complications included seizures (3 patients), tremors after cytarabine therapy (one patient), hemiparesis (2 patients), mood disorder requiring psychiatric intervention (one patient), motor weakness after intrathecal methotrexate (one patient), and moderate sensory nerve dysfunction (one patient). One patient in each arm had pancreatitis during induction therapy. In each arm, 3 patients had hyperglycemia. In the pegaspargase arm, 2 acute allergic reactions to ASNase occurred during DI no. 1. One patient had a grade 1 allergic reaction and another grade 3 hives.
The most common toxic events were infectious (Table6). There were 17 episodes of bacteremia reported in each arm during induction and the DI courses. There were 2 life-threatening infections in the pegaspargase arm, and one in the native arm. Life-threatening infection was defined as septic shock with hypotension or requiring intubation. No patients had invasive fungal diseases.
The median number of hospital days was 17 in each arm. During induction, patients in the native ASNase arm spent 1 more day in the hospital (mean 9.5 ± 0.7 SEM, median 8 days) than those in the pegaspargase arm (8.2 ± 0.7 SEM, median 6 days). The maximum number of hospital days in the native arm was 62; for the pegaspargase arm it was 69. The median number of days to complete the 3 phases of therapy was 161 and 165 in the native ASNase and pegaspargase arms, respectively.
Pharmacokinetics of pegaspargase
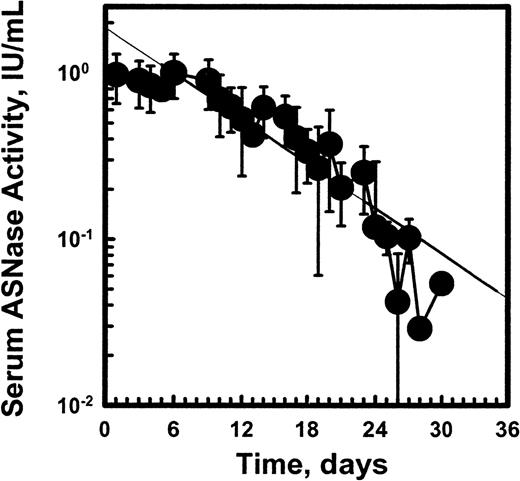
Figure 3 shows the mean ASNase activity over time after the first 2500 IU/m2 dose of pegaspargase. The mean pegaspargase activity in serum peaked on day 5 after the IM dose and averaged 1 IU/mL. The absorption from the IM site and elimination of pegaspargase from the serum were best described by single exponential functions. The mean half-life of absorption from the IM site was 1.7 days and the elimination half-life of pegaspargase was 5.5 days. The one-compartment population analysis showed an apparent volume of distribution for the central compartment (Vdc) of 1.5 L/m.2 Noncompartmental analysis showed that area under the curve (AUC) was 14.7 IU/mL*d and area under the moment curve (AUMC) was 161 IU/mL*d2 with a mean residence time (MRT) of 10.95 days and moment half-life, el of 7.5 days. Clearance by noncompartmental and one-compartment models was 0.169 and 0.18 L/m2 per day, respectively. The volume of distribution at “steady state” (VDSS) estimated from the relationship of MRT*clearance (CL) ranged from 1.86 L/m2 to 1.97 L/m2. The Vss estimated from the relationship of Vss = (Dose*AUMC)/(AUC)2 was 1.86 L/m2, calculated by noncompartmental method based on the statistical moment theory. The systemic volume of distribution (Vd) estimated from the one-compartmental analysis from the relationship of CL = Vd*Kel was 1.34 L/m2. The average volume of distribution from different analyses was 1.5 L/m2.
Pharmacokinetic profile of pegaspargase enzymatic activity in sera of pediatric patients with standard-risk ALL at induction.
Multiple specimens were obtained during the induction phase from 57 patients. (Symbols: mean ± SD of n = 45 to 52.)
Pharmacokinetic profile of pegaspargase enzymatic activity in sera of pediatric patients with standard-risk ALL at induction.
Multiple specimens were obtained during the induction phase from 57 patients. (Symbols: mean ± SD of n = 45 to 52.)
Elimination of native ASNase after first induction dose was determined in one patient who had multiple samples drawn. A peak activity of 2 IU/mL was seen 4 hours after IM administration. The elimination half-life was 1.1 days. The mean serum ASNase activity versus the days after the last ASNase dose were plotted for all the patients who received native ASNase. Those plots showed an elimination half-life for induction, DI no. 1, and DI no. 2 of 1.8, 1.5, and 1.5 days, respectively. However, the sample time after last native L-ASNase was not specified accurately enough for pharmacokinetic modeling.
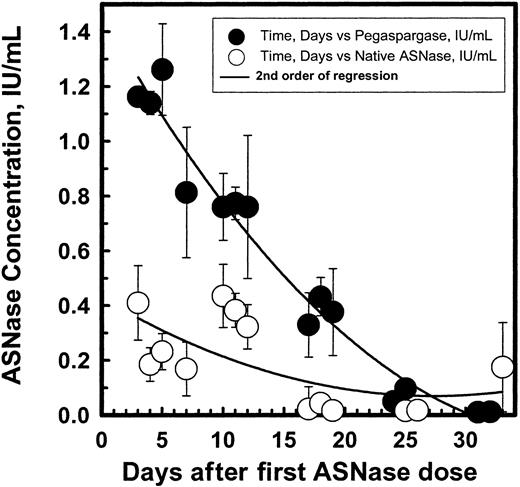
Figure 4 shows the mean ASNase activity ± SEM for native ASNase and pegaspargase during DI no. 1. Plots of those values during induction and DI no. 2 were similar. The values for pegaspargase were greater than those for native ASNase but the points for native ASNase represent nadir values 1 to 3 days after the last dose, since doses were given 3 times per week for 2 weeks beginning on day 3. The native ASNase data does not fit a second order equation because of repeated dosing.
Asparaginase enzymatic activity in sera over time profiles in pediatric patients with ALL after native asparaginase or pegaspargase administration during DI no. 1.
(Symbols: mean ± SEM.)
Asparaginase enzymatic activity in sera over time profiles in pediatric patients with ALL after native asparaginase or pegaspargase administration during DI no. 1.
(Symbols: mean ± SEM.)
More important than those mean values was the percentage of patients with ASNase activity considered adequate to deplete asparagine. Table7 shows that a higher percentage of samples from children in the pegaspargase group had ASNase activity of more than 0.03 IU/mL or 0.1 IU/mL on day 21 of therapy in DI no. 1 and DI no. 2. In induction, native ASNase was given for 3 weeks, so very few patients had those low levels of ASNase activity on day 21. However, a similar trend was seen on day 28 of induction, ASNase activity was more than 0.03 IU/mL in 48% of the pegaspargase patients and in only 15% of native ASNase patients.
Asparagine, glutamine, aspartic acid, and glutamic acid concentrations
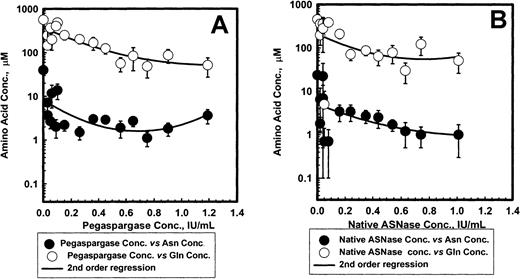
Figure 5 shows the mean ± SEM serum asparagine and glutamine concentrations for pegaspargase (A) and native ASNase (B) during induction. The ASNase concentrations versus asparagine or glutamine data fit a second order regression for both enzyme preparations. Asparagine levels fell rapidly by 4 days after the first ASNase dose and remained low for about 3 weeks. The mean levels appeared slightly higher for pegaspargase (Figure 5A) than for native ASNase (Figure 5B). Plots of asparagine concentrations in DI no. 1 and DI no. 2 were very similar. Serum glutamine concentrations also declined during the first 2 weeks of induction therapy. Glutamine concentrations fell to even lower levels during the first 2 weeks of DI no. 1 and DI no. 2.
Asparagine and glutamine in serum after pegaspargase or native asparaginase treatment during induction.
Specimens were collected during the induction phase from 57 and 45 patients in the pegaspargase (A) and native ASNase (B) arms, respectively. Specimens were collected from 45 and 45, and 41 and 45 for the DI no. 1 and DI no. 2 phases in those arms. (Symbols: mean ± SEM, n = 21 to 50 for the pegaspargase and 18 to 45 for the native ASNase arms, respectively. Asn indicates asparagine; Gln, glutamine.)
Asparagine and glutamine in serum after pegaspargase or native asparaginase treatment during induction.
Specimens were collected during the induction phase from 57 and 45 patients in the pegaspargase (A) and native ASNase (B) arms, respectively. Specimens were collected from 45 and 45, and 41 and 45 for the DI no. 1 and DI no. 2 phases in those arms. (Symbols: mean ± SEM, n = 21 to 50 for the pegaspargase and 18 to 45 for the native ASNase arms, respectively. Asn indicates asparagine; Gln, glutamine.)
Figure 6 shows the relationship between ASNase activity and serum asparagine and glutamine concentrations during induction. Pretreatment asparagine concentrations were 41 ± 4 and 55 ± 5 μM for native ASNase and pegaspargase, respectively. Asparagine fell to less than 3 μM in most patients when ASNase activity was more than 0.1 IU/mL. With each enzyme preparation there was a small decrease in asparagine concentration with increased ASNase activity. At each ASNase activity, there appeared to be a trend toward lower asparagine concentrations with native ASNase than with pegaspargase. Plots for DI no. 1 and DI no. 2 showed similar trends.
Relation between asparaginase and asparagine or glutamine in serum after pegaspargase (A) or native asparaginase (B) administration during induction.
(Symbols: mean ± SEM.)
Relation between asparaginase and asparagine or glutamine in serum after pegaspargase (A) or native asparaginase (B) administration during induction.
(Symbols: mean ± SEM.)
There was strong correlation between ASNase activity and glutamine concentrations in serum after administration of preparation (Figure6A,B). Glutamine concentrations were less than 100 μM when ASNase was more than 0.4 IU/mL with either form of ASNase. Aspartic acid levels fell about 50% whereas glutamic acid levels increased 2 to 3 times during ASNase treatment.
CSF asparagine fell from a median pretreatment level of 2.3 μM to 1.1 μM on day 7 and 0.6 μM on day 28 of induction in the pegaspargase patients. Similarly, median CSF asparagine fell from 2.8 μM to 1.0 μM and 0.3 μM at those time points in the native ASNase patients. (Figure 7A,B). Best-fit lines through those points showed no significant differences in elimination kinetics. CSF glutamine and aspartic acid did not change during therapy.
Asparagine concentrations in CSF of pediatric patients after pegaspargase (A) or native asparaginase (B) administration in CCG-1962 during induction.
(Symbols: mean ± SEM.)
Asparagine concentrations in CSF of pediatric patients after pegaspargase (A) or native asparaginase (B) administration in CCG-1962 during induction.
(Symbols: mean ± SEM.)
Pharmacodynamic model of pegaspargase
Serum pegaspargase activity and asparagine values for pegaspargase patients during induction were analyzed using a combined population pharmacokinetic-pharmacodynamic model implemented with the NONMEM program. Pharmacokinetic parameters were fixed to estimates obtained in a separate analysis of measured pegaspargase data and only pharmacodynamic parameters were estimated. Decrease of asparagine levels from baseline values in the presence of pegaspargase in serum used the Michaelis-Menten equation. Steady-state asparagine concentration was assumed to be 50 μM, even though that may vary during the day, depending on type of food intake and input from tissues.
The NONMEM population model achieved an excellent fit of the predicted to the observed values. The mean ± SEM serum asparagine concentration was 52.4 ± 4.5 μM (n = 46) before treatment. The asparagine Vd in the central compartment was estimated at 59.3 mL/m2. The population model predicted that average serum asparagine 4 days after pegaspargase would be 0.7 ± 1.0 μM. The measured mean ± SEM and median concentrations were 1.3 ± 0.3 and 0.5 μM (n = 50), respectively. At that time, mean ± SEM of estimated ASNase concentration was 0.83 ± 0.04 IU/mL. The peak ASNase activity was higher than that concentration, so serum asparagine might have been lower than that determined on day 5 after IM injection. The population model estimated an apparent Km (Michaelis-Menten rate constant) for asparagine deamination of 29 μM, which is approximately 3-fold higher than the literature value of 12 μM. The relationship between ASNase activity and percentage of asparagine deamination was best fit to a sigmoid curve. That curve indicated that serum asparagine would be decreased 50% at an ASNase activity of 0.05 IU/mL. A concentration of 1.8 IU/mL of ASNase activity was estimated to cause a decrease of 96% in serum asparagine. There was no correlation between the volume of distribution, the half-lives of absorption or elimination of pegaspargase, and surface area or age of patients.
Discussion
Native and pegylated E coli ASNase have major differences in pharmacokinetic parameters and immunogenicity.4,12,13,15,17,31 Comparative studies of their pharmacokinetics after IM administrations have not been done in newly diagnosed pediatric patients assigned randomly to one of those preparations. Only a few untreated pediatric patients have been studied.13 Development of anti-ASNase antibody can cause severe, life-threatening clinical reactions that prevent further use of ASNase, or can silently alter the clearance and clinical effectiveness of this treatment.4,9 10
Only 2 of our 118 patients had clinical allergic reactions, both during their second exposures to pegaspargase. This incidence appears low but reported frequencies of hypersensitivity reactions vary from 0% to 45%.5-10 Our patients received 3 days of high-dose prednisone before the start of ASNase. In contrast, most of the 18% of children with allergic reactions in a recent trial had these events during a consolidation phase when they may have been less immunosuppressed.10 Patients on the current Children's Cancer Group ALL trial (CCG 1991) are given pegaspargase only after 2 to 4 days of high-dose dexamethasone. That much larger trial should show whether this schedule of ASNase decreases the frequency of clinical hypersensitivity.
Compared with clinical allergy, the incidence of silent antibodies increased significantly in DI no. 1, the second course of enzyme treatment (Figure 1). Between 26% and 42% of the native ASNase group had anti-ASNase antibodies in DI no. 1, depending on whether we defined positive antibody as a ratio of ELISA reactivity of sample/control of more than or equal to 2.5, 2.0, or 1.5. A similar percentage of children developed antibodies to native ASNase in a recent trial of newly diagnosed children.10 A higher percentage was reported in older series and in studies of children with relapsed ALL who had been treated with ASNase.4
We initially defined high-titer antibody as a sample-control ratio of more than 2.5 because that would correspond to the ELISA absorbance reported.18 As expected for the native ASNase arm, many samples with antibody ratios of more than or equal to 2.5 had ASNase activity in an ineffective range of less than 0.1 U/mL. As shown in Table 2, antibody ratios of 1.5 and 2.0 also were associated with low ASNase activity. Therefore, an antibody ratio of more than or equal to 1.5 might be considered clinically significant for native ASNase.
Incidence of elevated antibody ratios was lower in DI no. 2 than DI no. 1. Samples collected at the start of each DI cycle often showed higher antibody ratios than those later in that cycle. We believe that dexamethasone and chemotherapy during those cycles might have caused progressive immunosuppression. Only 2%, 4%, and 11% of children treated with pegaspargase had antibody ratios of more than or equal to 2.5, 2.0, and 1.5, respectively, during DI no. 1 (Figure 1). None of the samples with antibody ratios of more than or equal to 1.5 had low ASNase activity. Thus, the antibody does not appear to neutralize or speed the clearance of pegaspargase, perhaps because of less interaction between the antibody and pegaspargase in serum than under denaturing conditions of the ELISA assay. The antibody also might be binding to regions far from the active site.
A higher incidence of antibodies has been reported in children with relapsed ALL after treatment with pegaspargase4 and children with newly diagnosed high-risk ALL treated with native ASNase during induction, followed by pegaspargase during consolidation and interim maintenance.32 Those children show a rapid clearance of pegaspargase. There are 2 factors that might explain the difference between those studies and our current findings. The relapsed and the newly diagnosed high-risk patients were probably sensitized to native ASNase and merely expanded antibodies that cross-reacted with pegaspargase. Also, in the current study patients might have been immunosuppressed by 3 days of high-dose steroid before the first ASNase dose.
Children treated with pegaspargase had more rapid clearance of lymphoblasts from day 7 and day 14 bone marrow aspirates than native ASNase patients (Table 4). The reason for the difference is unknown but could be from more persistent, higher ASNase activity in the pegaspargase patients. The proportion of patients with M1, M2, and M3 bone marrows on day 7 and day 14 in the native ASNase arm was similar to that reported in concurrent and prior CCG studies of standard-risk patients treated with the same induction therapy. The current standard-risk study, CCG 1991, treats all children with pegaspargase on day 3 to day 5 of induction and delayed intensification. Hopefully that study will also find more rapid clearance of BM lymphoblasts. The study was not designed to have sufficient power to detect a difference in EFS. To date, EFS is similar in the 2 arms.
Absorption of pegaspargase from the IM site was slower (peak about 5 days) than for native ASNase (peak 1-2 days).13 The differences in rates of absorption from IM injection site and elimination from blood could be from the larger molecular weight of pegaspargase. The elimination half-life of pegaspargase activity and protein was 4 days longer than native ASNase. The volume of distribution appeared to be equal to plasma volume. Asparagine was depleted rapidly in serum and CSF. Glutamine concentration decreased in serum but at a slower rate than asparagine. Glutamine depletion was seen before with high doses of native ASNase.33 Glutamine depletion might augment the asparagine depletion because glutamine is required for synthesis of asparagine in tissues. There was strong second-order correlation between ASNase activity and glutamine concentrations and a weaker correlation between enzymatic activity and asparagine in serum after administration of either formulation (Figure 6A,B). Similar patterns were seen between ASNase activity and asparagine and glutamine concentrations during DI no. 1 and DI no. 2.
Reports have estimated that ASNase activity of 0.03 IU/mL was sufficient for depletion of asparagine to below 0.1 μM.3Our data and pharmacodynamic modeling suggested that at least 0.1 IU/mL ASNase activity might be needed to appreciably deplete asparagine. Recently, other investigators also have suggested that 0.1 IU/mL be the minimum target ASNase activity.34
The estimated population pharmacokinetic parameters for pegaspargase enzymatic activity in this group were similar to those reported in a smaller cohort of pediatric patients.13 The population pharmacodynamic model for pegaspargase showed that elimination characteristics of CSF asparagine were superimposable after administration of the 2 preparations (Figure 7A,B).
These observations of asparagine elimination are important because CSF specimens lack ASNase enzymatic activity, so are not subject to ex vivo deamination while specimens are collected and processed. Our studies also showed that the earliest time when serum had the lowest asparagine concentrations, the serum and CSF specimens were both 0 in 70 patient specimens (from patients in both arms), and in 6 patients asparagine was detectable only in serum and not CSF (data not shown). These data suggest that ex vivo deamination was minimal under ice-cold conditions of collecting and storing blood specimens and an ASNase inhibitor was not needed.35
We recommend that pegaspargase replace native asparaginase for treatment of pediatric ALL because of its prolonged effect, lower incidence of silent antibodies, absence of antibodies that cause rapid clearance, similar safety profile, and convenience. A companion pharmacoeconomics component to our trial showed that total patient costs of pegaspargase were comparable to native ASNase when we considered the decreased number of doses and clinic visits. The major patient concern is pain from 1 to 2 large IM injections.36Pegaspargase is given by intravenous infusion in Europe without a higher incidence of adverse events. Theoretically, intravenous dosing should further decrease antibody formation. We believe that there should be further evaluation of intravenous pegaspargase.
The authors thank Shaun Mason for editorial assistance.
Supported in part by an unrestricted grant from Rhone-Poulenc Rorer, Collegeville, PA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vassilios I. Avramis, Children's Cancer Group, PO Box 60012, Arcadia, CA; e-mail: vavramis@chla.usc.edu.