Abstract
Primary T cells expressing chimeric receptors specific for tumor or viral antigens have considerable therapeutic potential. Unfortunately, their clinical value is limited by their rapid loss of function and failure to expand in vivo, presumably due to the lack of costimulator molecules on tumor cells and the inherent limitations of signaling exclusively through the chimeric receptor. Epstein-Barr virus (EBV) infection of B lymphocytes is near universal in humans and stimulates high levels of EBV-specific helper and cytotoxic T cells, which persist indefinitely. Our clinical studies have shown that EBV-specific T cells generated in vitro will expand, persist, and function for more than 6 years in vivo. We now report that EBV-specific (but not primary) T cells transduced with tumor-specific chimeric receptor genes can be expanded and maintained long-term in the presence of EBV-infected B cells. They recognize EBV-infected targets through their conventional T-cell receptor and tumor targets through their chimeric receptors. They efficiently lyse both. EBV-specific T cells expressing chimeric antitumor receptors may represent a new source of effector cells that would persist and function long-term after their transfer to cancer patients.
Introduction
The genetic modification of human T cells to express tumor antigen-specific chimeric receptors is an attractive means of providing large numbers of effector cells for adoptive immunotherapy. The primary mechanisms by which tumor cells escape from immune recognition, such as down-regulation of major histocompatibility complex (MHC) molecules, are efficiently bypassed through use of this strategy. T lymphocytes engineered to express the recombinant receptor genes are capable of both specific lysis and cytokine secretion on exposure to tumor cells expressing the requisite target antigen.1 2
Although adoptive transfer of chimeric receptor-expressing peripheral blood-derived T lymphocytes has produced some antitumor activity in mice,3-5 clinical results have been disappointing.6,7 The most pertinent issue is that chimeric T cells fail to expand and rapidly lose their function in vivo. Activation studies performed in transgenic mice suggest that the function of chimeric receptor proteins depends on the activation status of the T cell.8,9 Signaling through chimeric T-cell receptors alone was shown to be insufficient to induce proliferation and effector function in primary T lymphocytes, unless they had been prestimulated through their native receptor.8,9 Even under these conditions, responsiveness was soon lost. This problem is accentuated by the general lack of tumor cell costimulatory molecules essential for the induction and maintenance of a T-cell response.10 The development of strategies to prevent functional inactivation of chimeric receptor-modified cells in vivo would greatly enhance their therapeutic value.
Immune regulation of latent EBV infection is one of the best-studied examples of persistent T cell-mediated immune control. More than 90% of adults are seropositive for this virus, and their B cells expressing EBV-encoded latency-associated transforming proteins are tightly controlled by high levels of EBV-specific HLA-restricted cytotoxic T lymphocytes (CTLs), which persist indefinitely.11 The interaction of specific T lymphocytes with the target cells of latent EBV infection in immunocompetent hosts is characterized by a complex self-modulating network of cellular immune-mediated interactions, resulting in potent target cell lysis. These EBV-specific immune responses can be reconstituted by transfusion of in vitro–generated EBV-specific CTL lines into patients with EBV-associated infections and malignancies.12-15 The transfused T lymphocytes show a high initial degree of in vivo expansion and contain all necessary subpopulations to produce regression of even bulky EBV+tumors. Gene marking studies have demonstrated their persistence for more than 6 years with retained ability to respond to viral stimulation in vivo.12-15
The rapid expansion of EBV-specific T cells in vivo and their persistence in a functional state, lifelong without further immunization, make them attractive candidates for tumor cell targeting via chimeric T-cell receptors (TCRs). We show here that EBV-specific CTLs can be engineered to recognize and lyse tumor cell targets via chimeric receptors while maintaining their ability to proliferate in response to EBV target antigens and to destroy virus-infected cells.
Materials and methods
Cell lines and antibodies
The neuroblastoma cell line LAN-1 was provided by Dr Seeger's laboratory at the University of California Los Angeles, and the JF line was established in our laboratory. The ecotropic packaging cell line Phoenix16 was provided by Gary P. Nolan (Stanford, CA). A-204, HSB-2, Jurkat, and PG-13 cells were obtained from the American Type Culture Collection (Rockville, MD). The hybridoma cell line 14.G2a (mouse IgG2a-κ)17 was provided by Ralph A. Reisfeld (La Jolla, CA), and anti-14.G2a idiotypic antibody 1A718(TriGem) by Titan Pharmaceuticals (South San Francisco, CA). The LMP-2/HLA-A2 tetramer (HLA-A-0201/CLGGLLTMV) (single letter amino acid codes) was obtained from the MHC Tetramer Core Facility (Atlanta, GA).
Construction of chimeric receptor genes
The variable domains of monoclonal antibody 14.G2a were cloned as single-chain Fv (scFv) molecules into the replicative form of fUSE5 vector phage DNA.19 GD2-binding phages were selected by immunoscreening with enzyme-linked immunosorbent assay (ELISA). Chimeric γ chain receptor genes were assembled using pRSV-γ.1 The human ζ chain transmembrane and cytoplasmic portions were amplified from pGEM3zζ.20 A truncated receptor was engineered by polymerase chain reaction (PCR) by inserting a stop codon after the first 3 cytoplasmic amino acids. The chimeric genes were subcloned into the BamHI andNcoI sites of the retroviral vector SFG21(provided by R. C. Mulligan, Cambridge, MA).
Production of recombinant retrovirus
Fresh retroviral supernatants collected from transiently transfected Phoenix-eco cells were used to infect the packaging cell line PG13 in the presence of polybrene (8 μg/mL) twice for 48 hours at 32°C. Viral supernatants were generated on the resulting bulk producer cell lines for 24 hours at 32°C.
Generation of EBV-transformed B cell lines
Peripheral blood-derived mononuclear cells (5 × 106) were incubated with 10 μL concentrated supernatant from the EBV producer cell line B95-8 in a total of 200 μL medium for 30 minutes. The cells were then plated at 106 cells/well in a flat-bottomed 96-well plate in RPMI 1640 medium (Gibco BRL, Gaithersburg, MD) containing 10% fetal calf serum (FCS; Hyclone, Logan, UT), and 2 mmol/L l-glutamine (Biowhittaker, Walkersville, MD), as well as 1 μg/mL cyclosporin A (Sandoz Pharmaceuticals, Washington, DC). Cells were fed weekly until lymphoblastoid cell lines (LCLs) were established.
Generation and transduction of EBV-specific CTL cultures
Peripheral blood-derived mononuclear cells (2 × 106) were cocultured with 5 × 104γ-irradiated (40 Gy) autologous LCLs per well in a 24-well plate. Starting on day 10, the responder cells were restimulated weekly with irradiated LCLs at a responder-to-stimulator ratio of 4:1. Two weekly doses of recombinant human interleukin-2 (IL- 2) (rhIL-2; 40 IU/mL) were added from day 14. Twenty-four hours after the third stimulation, the cells were transferred to a 24-well plate precoated with OKT-3 (1 μg/mL; Ortho Pharmaceuticals, Raritan, NJ) and anti-CD28 antibody (1 μg/mL; Pharmingen, San Diego, CA) at 1 × 106 cells/well and incubated for 48 hours. Cells were transduced in 24-well plates (Becton Dickinson, Franklin Lakes, NJ), coated with recombinant FN CH-296 (Retronectin, Takara Shuzo, Otsu, Japan) at a concentration of 4 μg/cm2. The prestimulated CTLs were resuspended at 1 × 106 cells/mL in culture medium containing rhIL-2 (100 IU/mL), and incubated with equal volumes of freshly generated viral supernatant for 36 hours at 37°C and 5% CO2.
Flow cytometry
Cells were stained with fluorescein-conjugated monoclonal antibodies (Becton Dickinson, San Jose, CA) directed against CD3, CD4, CD8, CD16, CD56, and CD25 surface proteins. For each sample, 10 000 cells were analyzed by FACSCalibur with the Cell Quest Software (Becton Dickinson, San Jose, CA). Surface expression of 14.G2a-ζ was analyzed after incubation of CTLs (1 × 106) with 14.G2a anti-idiotypic antibody 1A7 (200 ng/5 × 105 cells) in the presence of normal goat serum for 20 minutes on ice, followed by incubation with fluorescein isothiocyanate (FITC)-labeled goat antimouse antibody (Becton Dickinson, San Jose, CA). For tetramer staining, CTLs from HLA-A2+ donors were incubated with phycoerythrin (PE)-labeled LMP-2/HLA-A2 tetramer at a final concentration of 50 mg/mL in phosphate-buffered saline (PBS) plus 2% FCS for 30 minutes on ice, then washed and stained with peridinin chlorophyll protein (PerCP)-labeled anti-CD8 antibody for 20 minutes. One million events were acquired and analyzed.
Measurement of cytokine production
Duplicate samples of transduced effector cells (5 × 104/well) were cocultured with various tumor cells or EBV-transformed LCLs at target-to-effector ratios of 3:1 and 1:3 in 96-well round bottomed plates. After 24 hours, the supernatants were harvested and analyzed for human interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-4, IL-10, and IL-12 (Pharmingen) or granulocyte-macrophage colony-stimulating factor (GM-CSF; R & D Systems, Minneapolis, MN) by ELISA according to the manufacturer's instructions.
Cytotoxicity assays
Cytotoxic specificity was determined in standard51Cr release assays. Various numbers of T-effector cells were coincubated in triplicate with 5000 target cells labeled with 100 μCi (3.7 MBq) 51Cr in a total volume of 200 μL in a V-bottomed 96-well plate. At the end of a 4-hour period at 37°C and 5% CO2, supernatants were harvested, and radioactivity was counted in a gamma counter. Maximum release was determined by lysis of target cells with Triton X. To determine HLA class I or II restriction of cytolysis, target cells were preincubated for 30 minutes with 16.5 ng/mL W6/32 or CR3/43 antibodies (Dako, Carpinteria, CA). For cold target inhibition assays, unlabeled inhibitor cells (cold targets) were seeded in plates at various cold-to-hot target ratios. Effector cells were then added and incubated for 30 minutes at 37°C before labeled target cells (hot targets) were added.
Proliferation assays
Transduced T lymphocytes were coincubated in triplicate at 5 × 104 cells/well with various tumor cell targets or autologous or allogeneic EBV-LCLs at a 4:1 stimulator-to-responder ratio. Following a 72-hour coincubation period, wells were pulsed with 2.5 μCi (0.0925 MBq) [3H]-thymidine for 18 hours, and the samples were harvested onto glass fiber filter paper for β scintillation counting.
Results
Transduced EBV-specific CTLs express the chimeric receptor while maintaining their immunophenotype
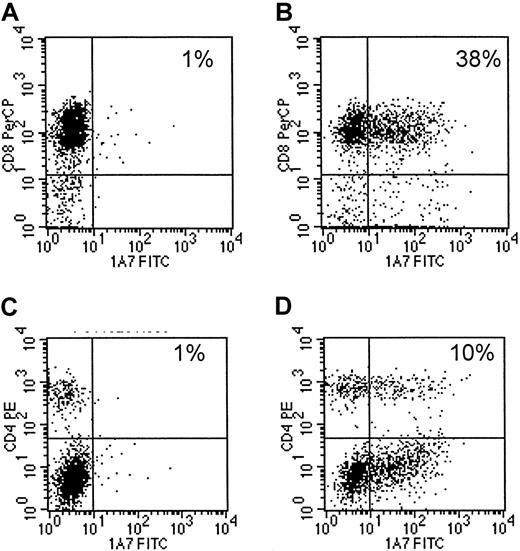
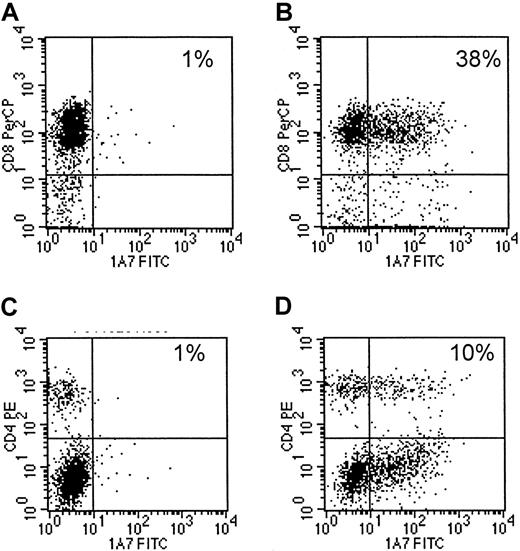
Eight EBV-specific CTL lines, generated from 4 different seropositive healthy donors13,14 were transduced with 14.G2a-ζ chimeric receptor genes. This receptor is derived from the 14.G2a monoclonal antibody that recognizes GD2, a ganglioside antigen present on tumors of neural crest origin,17 22 including neuroblastoma and small cell lung cancer, as well as glioblastoma and melanoma. Flow cytometric analysis of CTLs stained with anti-14.G2a idiotype-specific antibody identified chimeric receptors on 10.2% to 43.1% of the CTLs (mean, 16.5%). Chimeric receptor expression was maintained over the entire period of culture (up to 45 days) without any apparent down-regulation. CD4+ and CD8+ T lymphocytes within the cultured cell population were transduced equally well (Figure1).
Transduced CTLs express surface 14.G2a-ζ chimeric receptors.
Cells of a representative EBV-specific CTL line, 5 days after retroviral transduction with 14.G2a-ζ chimeric receptor genes, were stained with 14.G2a idiotype-specific monoclonal antibody 1A7, followed by incubation with FITC-labeled goat antimouse antibody, and then PerCP-labeled anti-CD or anti-CD4 antibody. Surface immunofluorescence was analyzed by flow cytometry. Panels A and C are nontransduced CTLs; panels B and D are transduced CTLs.
Transduced CTLs express surface 14.G2a-ζ chimeric receptors.
Cells of a representative EBV-specific CTL line, 5 days after retroviral transduction with 14.G2a-ζ chimeric receptor genes, were stained with 14.G2a idiotype-specific monoclonal antibody 1A7, followed by incubation with FITC-labeled goat antimouse antibody, and then PerCP-labeled anti-CD or anti-CD4 antibody. Surface immunofluorescence was analyzed by flow cytometry. Panels A and C are nontransduced CTLs; panels B and D are transduced CTLs.
After 3 stimulations with autologous LCLs, the majority of the CTL lines had a characteristic immunophenotype12-15: CD3+ CD8+ on 80% to more than 90% of the cells and CD3+ CD4+ (T-cell helper) on 4% to 21%. Fewer than 5% of the cells showed an immunophenotype characteristic of natural killer (NK) cells (CD3−CD16+ CD56+). One cell line was predominantly CD3+ CD4+. Transduction of CTLs did not result in any changes of cellular immunophenotypes by comparison with nontransduced cells (not shown). Hence, introduction and expression of the chimeric receptor does not shift the phenotype of EBV-specific CTLs from that known to be able to expand, persist, and induce tumor regression following infusion in vivo.12-15
Triggering of the native T-cell receptor but not the ζ chimera induces proliferation and expansion of modified EBV-specific CTLs
To confirm that signaling through the chimeric receptor alone provides a proliferation stimulus insufficient for in vitro expansion of the cells, we cultured 14.G2a-ζ transduced and nontransduced EBV-CTLs in the presence of LCLs, irradiated G (LAN-2), and G (A-204) tumor cell targets and rhIL-2 (40 IU/mL), or EBV-CTLs alone. Incubation with the tumor targets did not elicit a proliferative response from either the modified or unmodified cells, as demonstrated by [3H]-thymidine incorporation (Figure2A). In contrast, autologous LCLs triggered substantial [3H]-thymidine uptake by both types of CTL lines. Furthermore, whereas transduced CTLs continued to expand in response to stimulation with irradiated autologous LCLs, with kinetics similar to those of nontransduced cells, the transduced CTLs could not be maintained in culture for longer than 4 weeks when stimulated with tumor cells alone (Figure 2B). These results are compatible with in vivo data showing that chimeric receptor stimulation alone is inadequate to maintain T-cell proliferation and expansion.8 9
Proliferation and expansion of 14.G2a-ζ–transduced CTLs.
(A) 14.G2a-ζ–transduced CTLs proliferate in response to EBV but not tumor targets. 14.G2a-ζ–transduced (CTL/chRec) and nontransduced EBV-specific CTLs (CTL/NT) were stimulated with irradiated (40 Gy) autologous or mismatched allogeneic LCLs, or with G (LAN-1) or G (A-204) tumor cells at a 1:4 stimulator-to-responder ratio. Proliferative responses were assessed by measurement of [3H]-thymidine uptake. (B) 14.G2a-ζ–transduced CTLs expand in response to EBV but not tumor targets. EBV-specific CTL cultures, either nontransduced or transduced with the chimeric receptor gene 14.G2a-ζ or with a control gene encoding enhanced green fluorescent protein (EGFP), received weekly stimulations with irradiated (40 Gy) autologous LCL or G tumor cell targets at a 1:4 stimulator-to-responder ratio. Cells were fed twice weekly with medium containing rhIL-2 (40 IU/mL), and their growth was assessed. A representative experiment (of 4) is shown.
Proliferation and expansion of 14.G2a-ζ–transduced CTLs.
(A) 14.G2a-ζ–transduced CTLs proliferate in response to EBV but not tumor targets. 14.G2a-ζ–transduced (CTL/chRec) and nontransduced EBV-specific CTLs (CTL/NT) were stimulated with irradiated (40 Gy) autologous or mismatched allogeneic LCLs, or with G (LAN-1) or G (A-204) tumor cells at a 1:4 stimulator-to-responder ratio. Proliferative responses were assessed by measurement of [3H]-thymidine uptake. (B) 14.G2a-ζ–transduced CTLs expand in response to EBV but not tumor targets. EBV-specific CTL cultures, either nontransduced or transduced with the chimeric receptor gene 14.G2a-ζ or with a control gene encoding enhanced green fluorescent protein (EGFP), received weekly stimulations with irradiated (40 Gy) autologous LCL or G tumor cell targets at a 1:4 stimulator-to-responder ratio. Cells were fed twice weekly with medium containing rhIL-2 (40 IU/mL), and their growth was assessed. A representative experiment (of 4) is shown.
Coculture with tumor cells does not result in CTL lysis or growth inhibition
To exclude the possibility that coculture with G tumor cells is toxic to T cells independent of chimeric receptor triggering, we performed control experiments by testing the cytotoxicity of G tumor cells toward chimeric receptor-transduced CTLs in standard 51Cr release assays, and by comparing the expansion of CTLs in the presence of EBV and tumor targets.
The EBV-specific CTLs were not susceptible to cytolysis by JF or LAN-1 tumor cells after coincubation periods of up to 18 hours (not shown). Furthermore, CTL expansion was not decreased in the presence of tumor cells, when compared to allogeneic LCLs or absence of stimulator cells, indicating that the tumor cells did not actively inhibit CTL expansion (not shown).
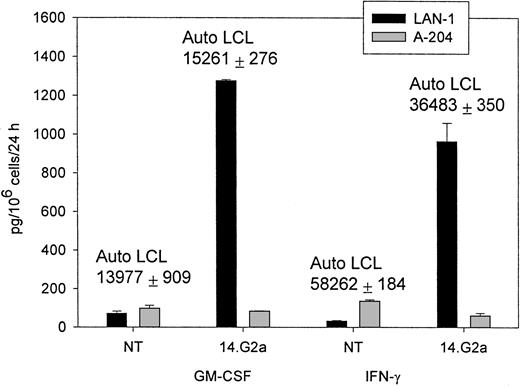
Chimeric receptor-modified CTLs specifically release cytokines in response to autologous EBV and G tumor targets
Having demonstrated that chimeric EBV-specific T cells could be readily expanded ex vivo, we next compared functional activation of transduced EBV-specific CTLs by their native T-cell receptor and chimeric receptor-defined cellular targets. We determined the pattern of cytokine release by modified T lymphocytes after incubation with G and G tumor target cells, as well as with autologous EBV-infected LCLs.
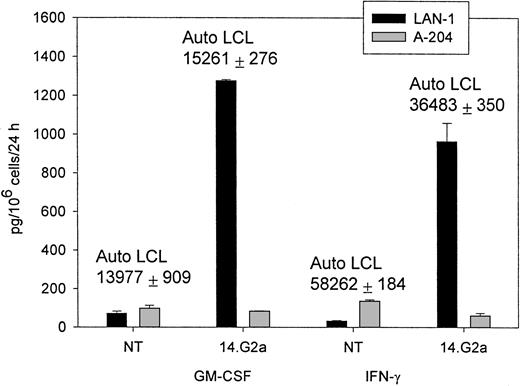
The CTL cultures containing 5% to 15% 14.G2a-ζ–transduced T lymphocytes released IFN-γ (up to 2982 pg/mL × 106cells/24 hours) and GM-CSF (up to 1278 pg/mL × 106cells/24 hours), as well as trace amounts of TNF-α on stimulation with G target cells in the absence of specific cytokine release during incubation with G tumor cell targets (Figure 3). Nontransduced CTLs and cell lines transduced with the truncated chimeric receptor variant 14.G2a-Δ did not release cytokines in response to incubation with G tumor targets. IL-4, IL-10, and IL-12 were not detected in the supernatants of the stimulated cells. A similar pattern of cytokine release was observed when cells were cultured with autologous EBV-LCLs. No differences in the cytokine response to EBV targets were found between nontransduced and transduced CTL. Nonetheless, the quantity of specific IFN-γ and GM-CSF release by CTLs in response to autologous LCL targets exceeded the cytokine release triggered by stimulation of transduced CTL populations with G tumor targets by 8- to 49-fold (Figure 3). Hence, whereas chimeric receptor-positive EBV-infected CTLs retain the capacity to respond to stimulation of the native receptor, chimeric receptor engagement results in comparatively low levels of specific IFN-γ and GM-CSF secretion.
IFN-γ and GM-CSF release by CTLs in response to coincubation with tumor target cells.
Nontransduced (NT) CTLs or CTLs transduced with SFG/14.G2a-ζ were cocultured with tumor cells at a 3:1 target-to-effector ratio for 24 hours. LAN-1 is a G neuroblastoma cell line and A-204 is a G rhabdomyosarcoma cell line. Numbers above columns indicate the amount of cytokine secretion in response to autologous LCL targets for each population. Data shown are representative of independent experiments performed with CTL lines from 3 donors.
IFN-γ and GM-CSF release by CTLs in response to coincubation with tumor target cells.
Nontransduced (NT) CTLs or CTLs transduced with SFG/14.G2a-ζ were cocultured with tumor cells at a 3:1 target-to-effector ratio for 24 hours. LAN-1 is a G neuroblastoma cell line and A-204 is a G rhabdomyosarcoma cell line. Numbers above columns indicate the amount of cytokine secretion in response to autologous LCL targets for each population. Data shown are representative of independent experiments performed with CTL lines from 3 donors.
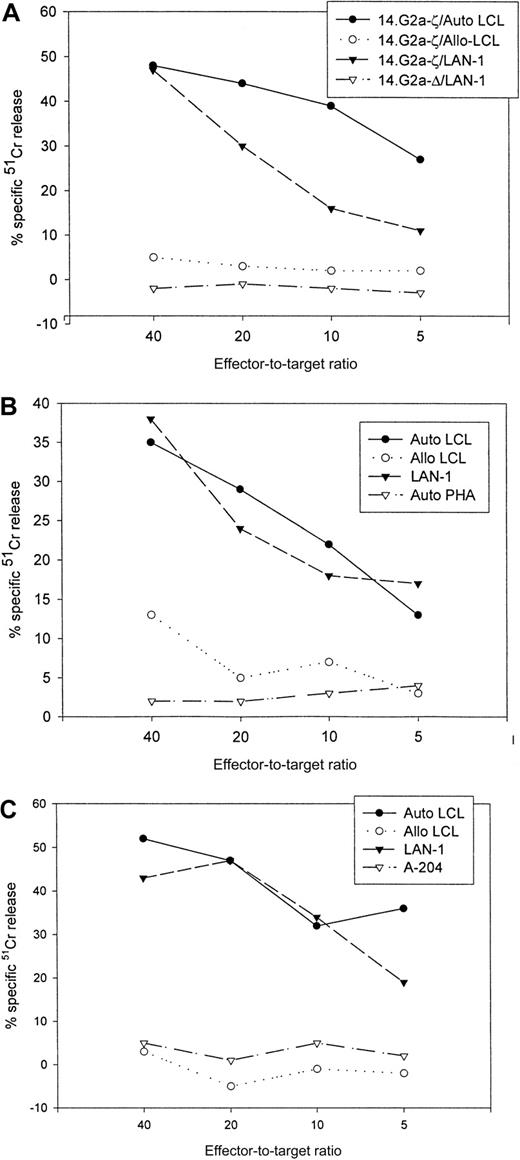
Chimeric receptor-modified CTLs specifically lyse autologous EBV and G tumor targets
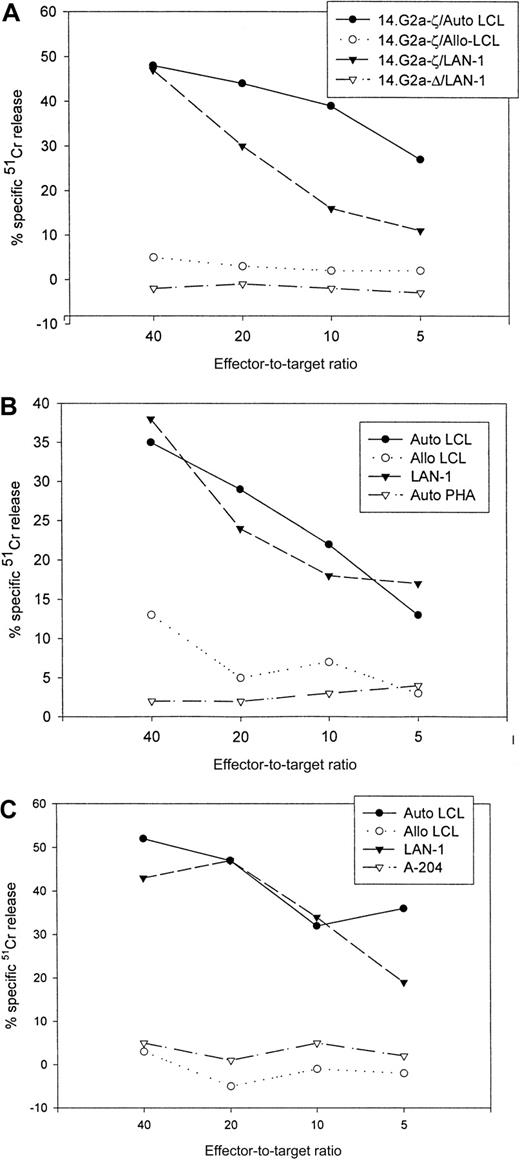
The cytolytic specificities of nontransduced and transduced cells were compared in standard 4-hour 51Cr release assays (Table1). Following transduction, the CTLs had received at least 1 and up to 4 further weekly stimulations with autologous EBV targets before cytotoxic activity was assessed. The corresponding nontransduced CTLs had received an equal number of restimulations. At an effector-to-target ratio of 40:1, the percentage of autologous LCL targets lysed by nontransduced CTL lines ranged from 27% to 81% (mean, 49.6%), including one CTL line with a high percentage of CD4+ T cells. After transduction with chimeric receptor genes, 30% to 66% (mean, 47.6%) of autologous LCL targets were lysed by these lines. Under the same conditions, only 5% to 23% (mean, 10.1%) of HLA-mismatched EBV-transformed targets were lysed by nontransduced CTLs, compared with 3% to 19% (mean, 6.7%) by 14.G2a-ζ–transduced cells. None of the lines had discernible activity against autologous phytohemagglutinin-stimulated lymphoblasts. Whereas nontransduced CTLs were incapable of lysing tumor targets (0%-5%), 21% to 50% (mean, 36.6%) of G LAN-1 tumor cells were lysed during coincubation with 14.G2a-ζ–transduced CTLs. No cytolytic activity of transduced cells against the G tumor target A-204 was seen. The specificity of the interaction with the GD2-expressing tumor cell targets was further confirmed by preincubation of G target cells with 14.G2a monoclonal antibody, resulting in up to 81% inhibition of lysis by 14.G2a-ζ–transduced cells (not shown). The cytolytic activity of cell lines tested for up to 29 days after transduction (4 stimulations) was comparable to that of lines tested over shorter intervals. Retesting of a CTL line after an additional round of restimulation with autologous LCLs resulted in comparable cytolytic activity against both EBV and tumor targets (Table 1, CTL lines 5 and 10).
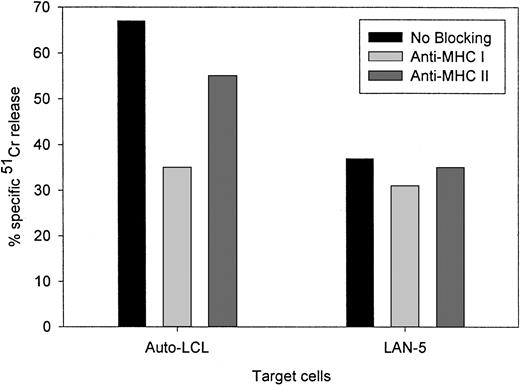
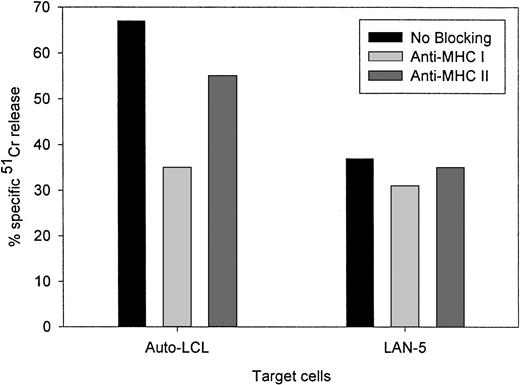
Figure 4 compares the cytolytic activities of 3 different 14.2a-ζ–transduced CTL lines against EBV and tumor cell targets. The chimeric receptor-bearing T cells recognized and lysed G tumor cells (LAN-1) and autologous LCLs with similar efficiency over the entire range of effector-to-target ratios, but responded poorly to G tumor cells (A-204). Inhibition studies with anti-MHC antibodies identified MHC class I as the major restriction element for LCL lysis by nontransduced and 14.G2a-ζ–transduced CD8+ CTL lines. However, preincubation with HLA class I and II blocking antibodies did not affect the lysis of LAN-1 target cells, indicating a lack of MHC restriction (Figure5). These results establish that the EBV-specific chimeric T cells kill their EBV targets through their (MHC-restricted) conventional receptor, and their tumor targets through the (MHC-unrestricted) chimeric receptor.
Cytolytic activity of 3 EBV-specific CTL lines against EBV and tumor targets.
Transduced CTLs were tested against 51Cr-labeled autologous LCLs, class I mismatched allogeneic LCLs, Gneuroblastoma tumor cells (LAN-1), Grhabdomyosarcoma cells (A-204), or autologous phytohemagglutinin-stimulated lymphoblasts. (A) 14.G2a-ζ– and 14.G2a-Δ–transduced CTL line no. 2, (B) 14.G2a-ζ–transduced CTL line no. 4, (C) 14.G2a-ζ–transduced CTL line no. 8.
Cytolytic activity of 3 EBV-specific CTL lines against EBV and tumor targets.
Transduced CTLs were tested against 51Cr-labeled autologous LCLs, class I mismatched allogeneic LCLs, Gneuroblastoma tumor cells (LAN-1), Grhabdomyosarcoma cells (A-204), or autologous phytohemagglutinin-stimulated lymphoblasts. (A) 14.G2a-ζ– and 14.G2a-Δ–transduced CTL line no. 2, (B) 14.G2a-ζ–transduced CTL line no. 4, (C) 14.G2a-ζ–transduced CTL line no. 8.
Lack of MHC restriction in chRec-mediated tumor cell killing by transduced CTLs.
51Cr-labeled G LAN-5 neuroblastoma cells and autologous EBV-LCLs were preincubated for 30 minutes with monoclonal antibodies recognizing monomorphic determinants of HLA class I or HLA class II, then coincubated for 4 hours with 14.G2a-ζ–transduced CTL at a 20:1 effector-to-target ratio.
Lack of MHC restriction in chRec-mediated tumor cell killing by transduced CTLs.
51Cr-labeled G LAN-5 neuroblastoma cells and autologous EBV-LCLs were preincubated for 30 minutes with monoclonal antibodies recognizing monomorphic determinants of HLA class I or HLA class II, then coincubated for 4 hours with 14.G2a-ζ–transduced CTL at a 20:1 effector-to-target ratio.
14.G2a-ζ–transduced EBV-specific CTLs have functional specificity for both EBV and tumor antigens
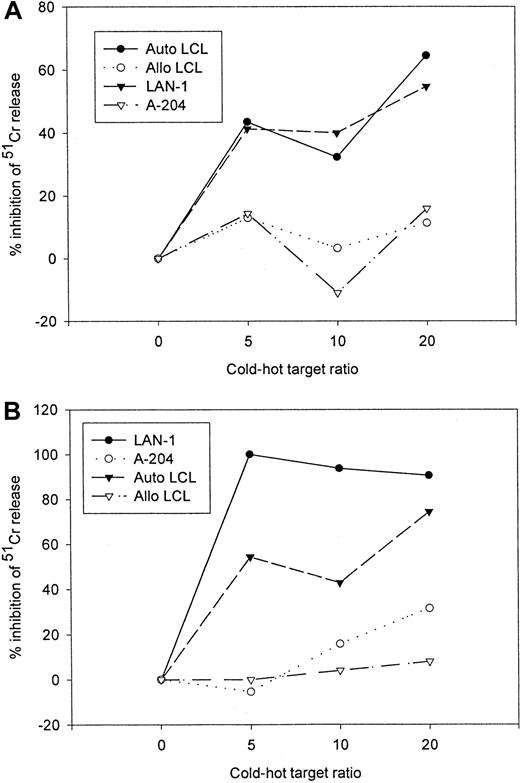
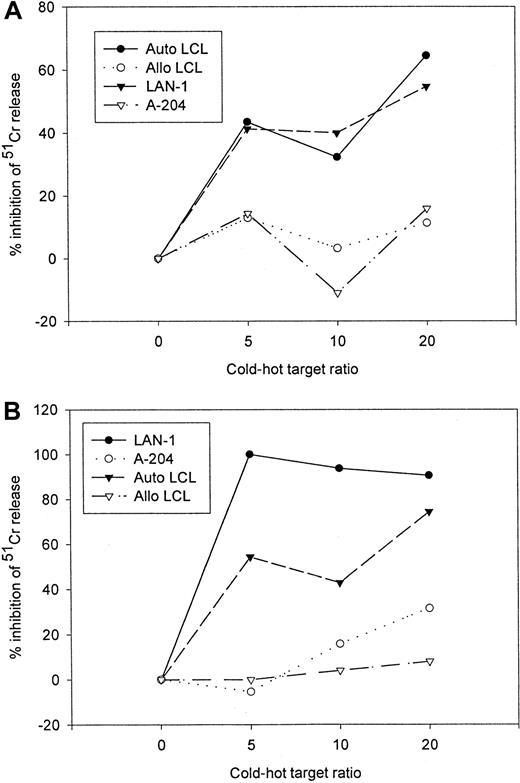
Because of the superior in vivo functional capabilities of EBV-specific cell lines over single epitope specific clones,23 24 the preceding studies were performed on mixed populations of CTLs. Therefore to demonstrate that transduced populations of EBV-specific CTLs are functionally bispecific for the native T-cell receptor antigen and the chimeric receptor target, rather than recognizing such targets independently of one another, we performed cold target inhibition assays. The antigen specificity of both tumor cell and LCL lysis by gene-modified EBV-specific CTL lines was determined by analyzing the capacity of unlabeled autologous LCLs and G tumor cells to block lysis of tumor cells and LCLs, respectively. Although lysis of the G tumor target LAN-1 by 14.G2a-ζ–transduced EBV-specific CTLs was HLA independent, it could be inhibited by addition of nonlabeled EBV target cells, whereas lysis of EBV+ targets could be diminished by competition from nonlabeled tumor cells (Figure6). Neither allogeneic EBV-LCL nor G cold targets had an effect on the lysis of autologous EBV-LCL and G hot targets.
Cold target inhibition assay.
14.G2a-ζ–transduced EBV-specific CTLs were preincubated with unlabeled autologous LCL (Auto-LCL), HLA-mismatched allogeneic LCL (Allo LCL), G (LAN-1), or G(A-204) tumor cells at various cold-to-hot target ratios. Cytotoxic activity was then determined against 51Cr-labeled autologous LCL (A) and G LAN-1 tumor cells (B) at an effector-to-target ratio of 40:1.
Cold target inhibition assay.
14.G2a-ζ–transduced EBV-specific CTLs were preincubated with unlabeled autologous LCL (Auto-LCL), HLA-mismatched allogeneic LCL (Allo LCL), G (LAN-1), or G(A-204) tumor cells at various cold-to-hot target ratios. Cytotoxic activity was then determined against 51Cr-labeled autologous LCL (A) and G LAN-1 tumor cells (B) at an effector-to-target ratio of 40:1.
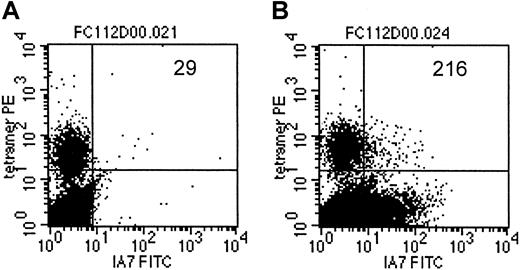
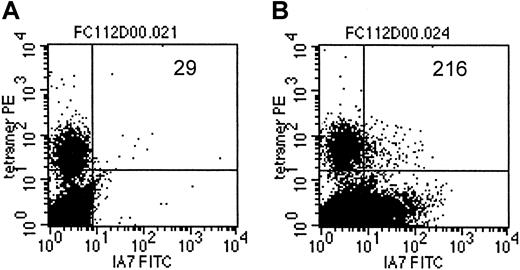
As an additional demonstration of coexpression of the T-cell receptor, specific for an EBV-encoded antigen, as well as the 14.G2a-ζ chimeric receptor on individual cells within the CTL culture population, we costained a transduced CTL line from an HLA-A2+ donor with LMP-2 specific tetramers and with anti-14.G2a-idiotype antibody 1A7 (Figure 7). Slightly more than 7% of the cells specific for LMP-2 had detectable levels of 14.G2a-ζ surface expression, comparable to the bulk CTL population, which contained 5.8% 1A7+ CTLs. Taken together, our functional observations from cold target inhibition experiments as well as the receptor coexpression studies provide evidence that the same population of cells is responsible for killing both EBV and tumor targets, and that this effect is associated with coexpression of EBV-specific and tumor-specific receptors on the same cells.
LMP-2/HLA-A2 tetramer+ CTLs coexpress the 14.G2a-ζ chimeric receptor.
On day 14 after transduction, CTLs were stained with monoclonal antibody 1A7 and FITC-labeled goat antimouse antibody, then incubated with PE-labeled LMP-2/HLA-A2 tetramer, followed by staining with PerCP-labeled anti-CD8 antibody. One million events were acquired and analyzed. Indicated are the absolute numbers of events for tetramer-positive cells that express detectable levels of 14.G2a-ζ in a population of nontransduced CTLs (A) or 14.G2a-ζ–transduced CTLs (B).
LMP-2/HLA-A2 tetramer+ CTLs coexpress the 14.G2a-ζ chimeric receptor.
On day 14 after transduction, CTLs were stained with monoclonal antibody 1A7 and FITC-labeled goat antimouse antibody, then incubated with PE-labeled LMP-2/HLA-A2 tetramer, followed by staining with PerCP-labeled anti-CD8 antibody. One million events were acquired and analyzed. Indicated are the absolute numbers of events for tetramer-positive cells that express detectable levels of 14.G2a-ζ in a population of nontransduced CTLs (A) or 14.G2a-ζ–transduced CTLs (B).
Chimeric receptor-modified CTLs are rescued to proliferate by stimulation with autologous EBV-LCLs
The clinical success of transduced EBV-CTLs will likely depend in part on these cells being able to proliferate when restimulated by autologous EBV targets following exposure to tumor cells. We therefore compared the proliferative responses of the CTLs to tumor targets and to EBV targets after repeated stimulation with either autologous LCLs, allogeneic LCLs, G tumor cells, or G tumor cells, and in the absence of stimulation. Confirming our previous data, autologous LCLs alone were capable of inducing the effector cells to proliferate above their low background level. However, CTL exposed to tumor cells for 1 to 2 weeks still showed a strong proliferative response when restimulated with autologous LCLs, comparable to the response obtained in cells receiving weekly stimulations with EBV targets (Figure8).
Transduced cells are rescued to proliferate by stimulation with autologous EBV-LCLs.
14.G2a-ζ–transduced (A) and nontransduced (B) EBV-specific CTLs were stimulated with irradiated autologous (Auto) or mismatched allogeneic (Allo) LCLs, or with G (LAN-1) or G (A-204) tumor cells at a 1:4 stimulator-to-responder ratio. After 7 to 14 days, CTLs were stimulated with autologous LCLs (Auto/Auto, LAN-1/Auto, JF/Auto, A-204/Auto, Allo/Auto) or stimulated as before (LAN-1/LAN-1, JF/JF, A-204/A-204, Allo/Allo). Proliferative responses were then assessed by measurement of [3H]-thymidine uptake. Shown is a representative experiment of 2.
Transduced cells are rescued to proliferate by stimulation with autologous EBV-LCLs.
14.G2a-ζ–transduced (A) and nontransduced (B) EBV-specific CTLs were stimulated with irradiated autologous (Auto) or mismatched allogeneic (Allo) LCLs, or with G (LAN-1) or G (A-204) tumor cells at a 1:4 stimulator-to-responder ratio. After 7 to 14 days, CTLs were stimulated with autologous LCLs (Auto/Auto, LAN-1/Auto, JF/Auto, A-204/Auto, Allo/Auto) or stimulated as before (LAN-1/LAN-1, JF/JF, A-204/A-204, Allo/Allo). Proliferative responses were then assessed by measurement of [3H]-thymidine uptake. Shown is a representative experiment of 2.
Discussion
Adoptive immunotherapy with chimeric receptor-modified T lymphocytes has shown promise in preclinical studies as a means to combat infectious and malignant diseases.4 However, the first clinical evaluation of chimeric receptor-modified cells revealed a disappointing lack of correlation between in vivo and in vitro cytotoxicity.6,7 One of the major factors limiting successful therapeutic use of modified T cells is their failure to expand and their short life span in vivo, even in the absence of any immune response directed against the chimeric T cells. CD4+helper function plays a crucial role in establishing or maintaining CD8+ CTL-mediated antiviral or antitumoral immunity,27-29 and long-term maintenance of engineered T cells is clearly improved if both CD8+ and CD4+transduced T cells are infused, rather than CD8 cells alone.6,7 Previous clinical trials in human immunodeficiency virus (HIV) infection have demonstrated prolonged, high-level persistence of chimeric receptor-modified CD4+and CD8+ T cells for at least 1 year. However, no significant mean change in plasma HIV RNA or blood proviral DNA was observed in patients with persisting modified T lymphocytes. No analysis of in vivo function of modified T cells over time has been conducted in this study. An explanation for this observation is that even in the continued presence of detectable chimeric receptor-modified cells in vivo, the surviving T lymphocytes may lose their ability to produce cytokines and to lyse their targets, reflecting functional inactivation of the modified cells. Supporting this concept are studies in a transgenic mouse model that showed that chimeric receptor-mediated signaling was not sufficient to trigger activation of resting primary T cells.8,9 Although the lack of coreceptor signaling by most tumor targets probably contributes to this effect,10it is also likely that chimeric receptors provide only limited access to downstream signaling pathways.8,9 The pattern of T-cell activation triggered by chimeric receptor engagement that we observed in our study, including efficient target cell lysis, reduced levels of specific cytokine release, and lack of cellular proliferation, is reminiscent of the T-cell response to altered peptide ligands as a consequence of incomplete phosphorylation of TCR-associated proximal activation motifs.30 31
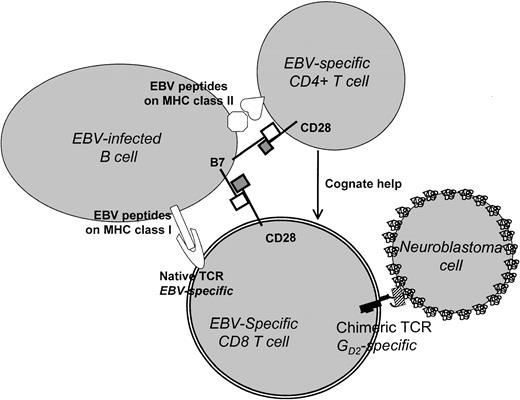
Taken together, the above results indicate that efficient and sustained expansion and activation of transfused chimeric effector T lymphocytes in vivo will require (1) T-cell helper activity provided in a cognate fashion, (2) signaling through the native TCR/CD3 complex, and (3) the presence of costimulatory signals and cytokines. Our results suggest that the introduction of chimeric receptors into ex vivo–generated EBV-specific T-cell lines will meet all of these requirements (Figure9). These cell lines contain antigen-specific CD4+ helper T cells that contribute to immune control of EBV latency by providing growth factors capable of maintaining both CD4+ and CD8+ cells, as well as CD8+ cytotoxic T cells.14 The target cells are EBV+ B lymphocytes, which present antigens extremely well. They express both class I and class II MHC-restricted antigenic epitopes, facilitating cognate interactions between CD44and CD8+ T cells, and are rich in costimulator molecule expression.12-15
Use of EBV-infected B lymphocytes and chimeric TCRs to target cancer cells.
In this model, CD8 T cells bearing a GD2-specific chimeric TCR are activated by EBV antigen binding to a native TCR and are costimulated through the interaction of B7/CD28. They may receive additional cognate help from EBV-specific CD4+ T cells. The stimulated chimeric receptor-positive cells are able to recognize and lyse GD2+ tumor cells (such as neuroblastoma) via the corresponding epitope of the chimeric TCR.
Use of EBV-infected B lymphocytes and chimeric TCRs to target cancer cells.
In this model, CD8 T cells bearing a GD2-specific chimeric TCR are activated by EBV antigen binding to a native TCR and are costimulated through the interaction of B7/CD28. They may receive additional cognate help from EBV-specific CD4+ T cells. The stimulated chimeric receptor-positive cells are able to recognize and lyse GD2+ tumor cells (such as neuroblastoma) via the corresponding epitope of the chimeric TCR.
What is the evidence that the properties of such T-cell lines will be reiterated in vivo? In patients given gene-marked EBV-specific CTLs, we can detect a high degree of in vivo expansion, resulting in long-term persistence and antiviral activity for more than 6 years.12-15 Expression of chimeric receptor genes in EBV-specific CTLs does not interfere with the ability of the cells to proliferate or to respond to autologous EBV-infected targets (Figure2A,B). Their ability to kill tumor cell targets through the chimeric receptor is retained even after expansion driven through the EBV-antigen specific native receptors (Table 1, Figure 4). Following exposure to tumor cells in culture, transduced CTLs can be rescued to proliferate and expand by stimulation through their EBV-specific receptor (Figure 8). In our system, none of the tumor cell targets proved toxic to the CTLs or inhibitory to their expansion. We therefore postulate that as a result of the continued presence of viral antigens in an EBV-infected host, engineered antitumor T lymphocytes with native specificity for EBV antigens will survive for extended periods. Such an effect can result only if the same T cell is both EBV and tumor specific, a requirement that was met in the present study, both phenotypically (as demonstrated by fluorescent analysis with an anti-idiotype monoclonal antibody and an EBV tetramer) and functionally (as shown by cross-inhibition of each target cell with either EBV-infected B lymphocytes or tumor cells). Although cross-inhibition can be demonstrated in short-term assays in vitro, it should not prove a significant limitation in vivo because a single T cell can kill multiple cellular targets sequentially, disengaging from each once killing has been achieved. This effect is illustrated by the ability of chimeric EBV-CTLs that have been repeatedly stimulated by EBV-LCLs, to subsequently kill tumor targets (Figure 3). Hence, after infusion of chimeric EBV-specific T cells into EBV+ individuals, there should be lifelong in vivo restimulation via the native T-cell receptor in the presence of adequate costimulation as illustrated in Figure 6. This will prevent functional inactivation of the cells and should enable them to continuously lyse any chimeric receptor target cells they encounter.
Without performing a clinical study, we cannot be certain that there will be sufficient stimulation by EBV antigens in vivo to produce expansion and maintenance of chimeric T cells at a sufficient level to produce antitumor effects. Nonetheless, recent evidence from our current adoptive transfer studies of EBV CTLs suggests that expansion and functional maintenance of these cells will occur even in patients with “normal” levels of EBV DNA and without evident EBV+ malignancy (C.R. and Barbara Savoldo, unpublished observations, August 2001). This effect is probably a consequence of periodic reactivation of EBV (and other herpesviruses). Serologic evidence suggests that reactivation after primary infection is a frequent event,32 and recent measures of serum EBV-DNA levels have produced similar results.33 34Should the number of functionally activated EBV-specific CTLs and their level of activation in the absence of massive EBV reactivation prove to be too low to provide a stimulus of sufficient strength for chimeric-mediated tumor cell lysis, it may prove possible to boost T-cell responses by immunization with autologous irradiated LCLs.
In principle, the above strategy may be used for any tumor target to which a chimeric receptor can be made. However, one of the major advantages of chimeric T cell–based therapies is that they obviate the need to select and expand the scanty tumor-specific T cells present in the circulation. The EBV-specific CTL chimeras we describe here would seem to remove that advantage, because an antigen-specific selection and expansion process will be required after all. But, in practical terms, the expansion of EBV CTLs and the expansion of tumor-specific CTLs are 2 quite separate propositions. The high frequency of EBV-specific precursor cells in peripheral blood and the excellent antigen-presenting capacity of EBV-infected B cells makes this a robust system. Over the past 6 years, we have successfully generated EBV-specific CTL lines from 138 of 140 donors, including cancer patients pretreated with chemotherapy.35 More than 100 patients with EBV-associated infections or malignancy have received EBV-CTL infusions with no serious adverse effects. These results have been confirmed by others.36 Moreover, because the infused cells are expected to expand markedly in vivo and persist in the circulation for extended periods, only limited numbers of cells may need to be grown and infused.12-15 The use of EBV-specific cell lines is to be preferred to the use of clones because (1) they are simpler to prepare, (2) the combination of CD4 and CD8 cells present produces a more sustained immune response than CD8+ clones alone,12,14,24 and (3) EBV antigen escape mutants are less likely to arise.23 Because high-efficiency cytolysis was achieved with cultures containing up to 90% nontransduced CTLs, there would be no need to coexpress marker or selection genes—a major source of immunogenicity after chimeric T-lymphocyte infusion. The individual components of this proposed system have already been safely used in humans; the chimeric receptor in the form of monoclonal antibodies administered to patients with malignancy,37,38 and the EBV-CTLs given to patients at risk of EBV-lymphoma or with Hodgkin disease.12 14 Our findings suggest that combining these components as antitumor chimeric receptors expressed by EBV-specific T cells may overcome many of the current limitations of chimeric T-cell immunotherapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Malcolm K. Brenner, Center for Cell and Gene Therapy, 6621 Fannin St, MC3-3320, Houston, TX 77030; e-mail:mbrenner@bcm.tmc.edu.

![Fig. 2. Proliferation and expansion of 14.G2a-ζ–transduced CTLs. / (A) 14.G2a-ζ–transduced CTLs proliferate in response to EBV but not tumor targets. 14.G2a-ζ–transduced (CTL/chRec) and nontransduced EBV-specific CTLs (CTL/NT) were stimulated with irradiated (40 Gy) autologous or mismatched allogeneic LCLs, or with GD2+ (LAN-1) or GD2− (A-204) tumor cells at a 1:4 stimulator-to-responder ratio. Proliferative responses were assessed by measurement of [3H]-thymidine uptake. (B) 14.G2a-ζ–transduced CTLs expand in response to EBV but not tumor targets. EBV-specific CTL cultures, either nontransduced or transduced with the chimeric receptor gene 14.G2a-ζ or with a control gene encoding enhanced green fluorescent protein (EGFP), received weekly stimulations with irradiated (40 Gy) autologous LCL or GD2+ tumor cell targets at a 1:4 stimulator-to-responder ratio. Cells were fed twice weekly with medium containing rhIL-2 (40 IU/mL), and their growth was assessed. A representative experiment (of 4) is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.2009/6/m_h80622263002.jpeg?Expires=1769145644&Signature=CbpoBv6dWWqHBwusURHVciU1XI1lHNNdSTF37zZyfW94kTLdd5uK0otH4f5UqtO5OesppN9kTQ55fOLCPtVCxRWreoPj4gjUObMTWTg~SbyUP-VTWQi5~-U4BReV-ugRVPkss4kDKGNGUbnfrRmBcdwDRoWD5FjhAS8IKh-ZICDrlNJB5pZdtrREsPWtJAcU3Az8LGm4bkGWwrQHIGqnGUK5zROdkNWmo9pGOJ4dqfkBcdHvbOtKyhslgrg0rY1~0jPpqv0wQzWj5ZwNiEuy-rq8LQ9-O2lnCjUUqHhW2VKnkbkjbcLhzUax9tcXiGQ3VZRsvEe1mZbQ-1i8~uvqlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Transduced cells are rescued to proliferate by stimulation with autologous EBV-LCLs. / 14.G2a-ζ–transduced (A) and nontransduced (B) EBV-specific CTLs were stimulated with irradiated autologous (Auto) or mismatched allogeneic (Allo) LCLs, or with GD2+ (LAN-1) or GD2− (A-204) tumor cells at a 1:4 stimulator-to-responder ratio. After 7 to 14 days, CTLs were stimulated with autologous LCLs (Auto/Auto, LAN-1/Auto, JF/Auto, A-204/Auto, Allo/Auto) or stimulated as before (LAN-1/LAN-1, JF/JF, A-204/A-204, Allo/Allo). Proliferative responses were then assessed by measurement of [3H]-thymidine uptake. Shown is a representative experiment of 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.2009/6/m_h80622263008.jpeg?Expires=1769145644&Signature=TNVkcykelwYX4r-UVMtUe1aW7pvQ-C2xjv~niZOpcpNepzHtgxYbYtJSMo-7AYlRLK5caf7Li49Ved73rLq8GGbusHA5RfD1-u5xsyNOWnXe40bXbiMKiGjiKVsRjdEG1ShtFBBxWmBMZY4rxVsYjnLs2I5k0U1~1D4bXl3vibSe1GxZ9f6EhOVhMKB6-lxNkbKhyRHOCut30wGXvFOVr66Fd33gcrr1Be-N8MrC-h6YoK-xFB-OWWrfpYyxvHSrFnodR~k83CBU8MragZ~hM3av9ufkDkmu5DL02X52-aeHP0W-0L8tOD0cbeXXp4GjF-OrlRs4fIb~SYjZpOU7Ug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Proliferation and expansion of 14.G2a-ζ–transduced CTLs. / (A) 14.G2a-ζ–transduced CTLs proliferate in response to EBV but not tumor targets. 14.G2a-ζ–transduced (CTL/chRec) and nontransduced EBV-specific CTLs (CTL/NT) were stimulated with irradiated (40 Gy) autologous or mismatched allogeneic LCLs, or with GD2+ (LAN-1) or GD2− (A-204) tumor cells at a 1:4 stimulator-to-responder ratio. Proliferative responses were assessed by measurement of [3H]-thymidine uptake. (B) 14.G2a-ζ–transduced CTLs expand in response to EBV but not tumor targets. EBV-specific CTL cultures, either nontransduced or transduced with the chimeric receptor gene 14.G2a-ζ or with a control gene encoding enhanced green fluorescent protein (EGFP), received weekly stimulations with irradiated (40 Gy) autologous LCL or GD2+ tumor cell targets at a 1:4 stimulator-to-responder ratio. Cells were fed twice weekly with medium containing rhIL-2 (40 IU/mL), and their growth was assessed. A representative experiment (of 4) is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.2009/6/m_h80622263002.jpeg?Expires=1769544313&Signature=CPUE~1UjE3IaR36qTayEptFmxNADvq6LeSq2W-uvS6GF0Iw5D7CnRyyDKJf50xK5oaGpMQ0rMticSWGHBAoCvf4UwUA6FOMUVm9ZjI35MwcpEamDR6Ozij9MMn8qi814WwCGxRhepcnUdGEBM6YMv2LDyEfd1A3bQpiBWGClpdEG2Jfy5X8EF~moECYTtkURyqG4M2ukIdzfsLR1HTJe8j90-E14hLImTpC9RYJDI86lvFlacD0eAFLAWL2-htuA2z86uCiQg31nq0JmLbKswLko7bNBMgJyDpU0p6-127uN8NIf9b3TaJsHoJ~ukr63SKrDRf17yYjMumCc8DUQMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Transduced cells are rescued to proliferate by stimulation with autologous EBV-LCLs. / 14.G2a-ζ–transduced (A) and nontransduced (B) EBV-specific CTLs were stimulated with irradiated autologous (Auto) or mismatched allogeneic (Allo) LCLs, or with GD2+ (LAN-1) or GD2− (A-204) tumor cells at a 1:4 stimulator-to-responder ratio. After 7 to 14 days, CTLs were stimulated with autologous LCLs (Auto/Auto, LAN-1/Auto, JF/Auto, A-204/Auto, Allo/Auto) or stimulated as before (LAN-1/LAN-1, JF/JF, A-204/A-204, Allo/Allo). Proliferative responses were then assessed by measurement of [3H]-thymidine uptake. Shown is a representative experiment of 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/6/10.1182_blood.v99.6.2009/6/m_h80622263008.jpeg?Expires=1769544313&Signature=UabPatUwugbnGNlWuCUZ-UaG6l8Ro9jblfo82nhsJ6SiCRRr9YOczbc-y29Bl~KN-2rxcz9LDk-Wuinur3nd8rneEpTOO81im1nI5nSTWYWEyC8xgThphX1FlrQu7O2292G~fl0AnrCwq3rX~TQYYTzWbGGa7LWRi7PB2njm5mjW~jI1cbbk-3NjUzo0bEpATZbZ690C0hIwYIGigszgWQ32pT7xuQUsslR9U1CFGVX7Y4AR3GzWlnMUPJ567B6Lrae9vcYCWgs1BvBddCs0nxy3YmqrXA8nr87KXpo0OG1ShNDibk0bfMsLwpKW5k7Q~AKxsOVFtUm5771-6k6ysw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
