Abstract
We have characterized a splice variant (isoform) of the human CD28 T cell costimulatory receptor. The nucleotide sequence of this CD28 isoform was identical to that of CD28 in the signal peptide, the transmembrane domain, and the cytoplasmic tail, but it was missing a large segment of the extracellular ligand-binding domain, which is encoded by the second exon. This isoform (CD28i), whose message level exceeded 25% of CD28, was a transmembrane homodimer. CD28i was found noncovalently associated with CD28 and was tyrosine-phosphorylated/PI3-kinase–complexed following the crosslinking of CD28, and the CD28 costimulatory signal was enhanced in T cells expressing CD28i. These data demonstrate that CD28i, via noncovalent association with CD28, plays a role as a costimulatory signal amplifier in human T cells.
Introduction
CD4+ T cells play a key role in the immune surveillance of pathogens, and are involved in the recognition of peptide antigens presented on class II major histocompatibility complex (MHC) of antigen-presenting cells (APCs) through the T-cell receptor (TCR). Mounting evidence indicates that T-cell activation is incomplete when initiated by the TCR signal alone, and that the costimulatory CD28 receptor must generate an additional signal for antigen-specific activation.1
CD28 is a heavily glycosylated 90-kd homodimeric glycoprotein present on the surface of almost all CD4+CD8+thymocytes, mature CD4+ T cells, and CD11b−CD8+ T cells.2,3 The physiologic ligands for CD28 are B7-1 (CD80) and B7-2 (CD86), which are expressed on APCs such as macrophages, dendritic cells, and B cells.4,5 Crosslinking CD28 with specific antibody (Ab) or CD80 and CD86 in the absence of TCR stimulation does not trigger T-cell activation. By comparison, crosslinking of CD28 with TCR stimulation results in a rapid induction of cell proliferation and cytokine production, and promotes the survival of T cells.2 6
CD28 is a type I transmembrane receptor belonging to the immunoglobulin superfamily. It has 5 cystines (Cys) in the extracellular domain. Of these cystines, 2 located near the N-terminus form a Cys-Cys bond-mediated loop.2 Other cystines are involved in homodimer formation.2 CD28 is composed of 220 amino acids with an 18-residue signal peptide at its N-terminus, a 28-residue membrane-spanning domain beginning at Pro134, and a 41–amino acid cytoplasmic tail.3 The cytoplasmic tail has a signaling motif, pY173MNM, identical to the binding site in growth factor receptors for the p85α subunit of phosphatidylinositol-3-phosphate (PI3) kinase.7,8 The adapter protein Grb-2, which complexes with Sos, is also known to bind to the same motif.8 Other signaling molecules known to be regulated in T cells following CD28 stimulation are protein tyrosine kinases (PTKs),8 sphingomyelinase,9,10 S6 kinase,8 cyclic adenosine monophosphate (AMP)–responsive element-binding protein,11 cyclic AMP-specific phosphodiesterase-7,12 serine/threonine phosphatase PP2A,13 PKC-θ,14,15 as well as p21-activated kinase and MEK kinase-1.16 We have also presented data indicating that CD28 stimulation promotes molecular changes which simulate focal adhesion by T cells.17 The F-actin formation and the accumulation of Cdc42 and Rac1 small Rho-family G proteins were prominent at the sites where T cells made contact with CD86 on transfected Chinese hamster ovary (CHO) cells. Together with the recent finding that CD28 stimulation accumulates the membrane rafts to the T cell–APC contact sites,18 there is a possibility that the TCR signal and the CD28 signal merge primarily at the level of the membrane raft and cytoskeletal signaling pathways.
The expression of truncated CD28 messages, which could encode the soluble ligand-binding proteins in human T cells, was reported recently.19 The data seem to illuminate intriguing mechanisms that the secretory type isoforms of CD28 bound to B7 ligands possibly interfere with costimulation via CD28 in human T cells. A previous report also showed a truncated human CD28 message that could be expressed as the transmembrane protein.20 Collectively, the signaling by CD28 thus may result from a delicate tune with these various CD28 isoforms.
In this communication, we report the unique function of a transmembrane CD28 isoform (CD28i). CD28i lacks an extracellular ligand-binding region; however, it is transactivated by CD28, and enhances the costimulatory signaling in human T cells. Our data highlighted a novel signaling mechanism of CD28 in human T cells, in which a transmembrane isoform serves as the signal amplifier for CD28. Also documented is data that, for the first time, demonstrates a heteromolecular receptor complex consisting of splice variants which functionally corporate to amplify the signal initiated by the wild-type molecule.
Materials and methods
Preparation of peripheral blood T cells
Human peripheral blood was treated with RosetteSep for T-cell purification (StemCell Technology, Vancouver, BC, Canada) and then T cells were separated with Ficoll-PaquePLUS (Amersham Pharmacia Biotechnology, Uppsala, Sweden) according to the manufacturer's instructions. CD3-specific cell-surface staining and flow cytometry showed that the purity of peripheral blood T (PBL-T) cells was generally more than 97%.
Detection of CD28 isoform mRNAs and the construction of expression vectors
The mRNAs of CD28 and CD28i were reverse trancriptase–polymerase chain reaction (RT-PCR)–amplified originally from cytoplasmic RNA of human PBL-T cells using CD28-specific primers: sense 5′-ttggcaggtgcgtctttcagttcc-3′ and antisense 5′-agtgatattgagcagatgggggct-3′. To generate C-terminal fusions of each isoform with green fluorescence protein (GFP), RT-PCR was performed using the following primers: sense 5′-GGCGCTAGCAGatgctcaggctgctcttggctc-3′ and antisense 5′-CCCCTCGAGggagcgataggctgcgaagtcgcg-3′, (capital letter sequences indicate restriction sites) and CD28 or CD28i cDNA were inserted into the multiple cloning site of pEGFP (Clontech B-D, Palo Alto, CA) using the NheI and XhoI sites. To make the inserts of CD28 or CD28i with the hemophilus influenza hemaglutinin (HA) tag (YPYDVPDYA: tatccatatgatgttccagattatgct), 5′-sequence was PCR-prepared from the CD28 cDNA template with sense 5′-taatacgactcactataggg-3′ and antisense 5′-taatctggaacatcatatggatagtttcctgttacttgaat-3′. The 3′-sequence was similarly prepared using sense 5′-catatgatgttccagattatgctaagattttggtgaagcag-3′ and antisense 5′-tagaaggcacagtcgagg-3′ primers. The resulting PCR product was used to further PCR-amplify the CD28 or CD28i insert with the HA epitope at the N-terminus next to the first amino acid (Asp) using CD28 or CD28i cDNA templates and primers (sense 5′-tcaagtaacaggaaactatccatatgatgttccagattatg-3′ and antisense 5′-tagaaggcacagtcgagg-3′).
To make the final inserts of CD28 or CD28i with the HA tag, PCR reactions were performed using primers, (sense 5′-GGCGCTAGCAGatgctcaggctgctcttggctc-3′ and antisense 5′-CCCCTCGAGtcaggagcgataggctgcgaagtc-3′) and were ligated intopcDNA3.1 (Invitrogen Canada, Burlington, ON, Canada) using the NheI and XhoI restriction sites.
Construction of N-linked glycosylation-deficient CD28i-HA expression vector
Site-directed mutagenesis of the human cDNA was performed using the PCR overlap extension technique to eliminate all out of 2 putative N-linked glycosylation sites (N19Q and N27Q) of CD28i. The oligonucleotide primers, which contain the desired mutations between 25 bp and 40 bp were used to generate a mutant CD28i fragment that was then used to replace the wild-type sequence of CD28i. The DNA sequence of the mutant CD28i was verified by DNA sequencing. To make the final insert of the mutant with the HA tag, PCR reactions were performed using primers (sense 5′-AGGCTCGAGatgctcaggctgctcttggct-ctc-3′ and antisense 5′-CGTCTAGAtcaggagcgataggctgcgaagtc-3′) and were ligated into mammalian expression vector pcDNA3 using theXhoI and XbaI restriction sites, and subsequently sequenced.
Semiquantitative PCR analysis for CD28i message
To study CD28i mRNA levels semiquantitatively, we prepared specific 5′-primers (5′-aagtattcctacaatctcttctca for CD28, 5′-atgcggtcaaccttagctacaatg for CD28i). PCR reactions were performed using each specific primer (sense) and the common antisense, 5′-agtgatattgagcagatgggggct-3′. One reaction of each experimental group was terminated at every 2 cycles of reaction from 21 to 31 cycles. Each sample was electrophoresed on agarose gel and stained with ethidium bromide (EtBr) to detect a 600-bp band (CD28) and a 368-bp band (CD28i) of amplified fragment. The intensity of the EtBr luminescence was measured by CCD image sensor (Bio-rad, Richmond, CA). All samples reached the plateau level in 10 cycles after the band intensity reached the lower limit of detection. To estimate the efficiency of amplification in every cycle, regression equations of the form y = I × En, where y is the intensity, n is the number of cycles, constant I is each amount of the original template, and constant E is each efficiency of amplification every cycle, were fitted to the data in the linear portion the semilogarithmic graphs.21 Accordingly, the amplification efficiency of CD28 was 1.80, and that of CD28i was 1.82. We calculated the ratios of each CD28 isoform original template from these results on the basis of the efficiency of amplification: I (CD28i) / I (CD28) = y (CD28i) / y (CD28) × (1.80 / 1.82) n, where I (CD28) indicates the original template amount of CD28; I (CD28i), original template amount of CD28i; y (CD28), intensity of CD28; I (CD28i), and intensity of CD28i.
Detection of isoform proteins and the other cell-surface molecules, and the detection of tyrosine phosphorylation
The Abs specific for CD28 C-terminal (sc-1623; Santa Cruz Biotechnology, Santa Cruz, CA), human CD28 (L293; B-D Pharmingen, Mississauga, ON, Canada), HA tag (3F10; Roche, Mannheim, Germany), CD43 (DF-T1; Santa Cruz), and CD4 (Q4120; Sigma, St Louis, MO) were used to stain or immunoprecipitate cell-surface receptors or CD28i-HA. Cell extracts were prepared using lysis buffer16 containing 1% Nonidet P40 or 1% digitonin. To immunoprecipitate CD28i-HA with anti-HA Ab (rat IgG1), protein L agarose beads (sc-2336; Santa Cruz) were used. The whole cell lysates or immunoprecipitates were fractionated by 10% NuPAGE (Novex, Helixx Technology, Scarborough, ON, Canada) using 2-(N-morpholino) ethanesulfonic acid (MES) or 3-(N-morpholino) propanesulfonic acid (MOPS) buffer under reduced or nonreduced conditions. Fractionated proteins were blotted onto a polyvinylidenefluoride (PVDF) membrane (Bio Trace; PALL, Ann Arbor, MI) and probed with specific Abs and secondary detection Abs as described previously.16 In some studies, in order to analyze immunoprecipitated CD28i, immunoprecipitation Ab only was fractionated in pilot lanes next to the sample lanes. Protein blots were transferred to a PVDF membrane and the area of pilot lanes was excised and the position of Ig light chains was determined by Ig-specific Western blotting. Based upon these data, the membrane region that was expected to contain Ig light chain blots was excised from the sample lanes before CD28 C-terminal–specific Western blotting.
To examine the tyrosine phosphorylation of CD28i in Jurkat T-cell transfectant, cells were serum starved overnight and then incubated with anti-CD28 Ab (5 μg/mL) (L293) for one hour on ice. Cells were then washed and incubated at 37°C with 50 μg/mL anti–mouse IgG Ab for the periods indicated. Cell extracts in 1% Nonidet P40 buffer were fractionated by 10% NuPAGE using MES buffer under the reduced condition. Fractionated proteins were blotted onto a PVDF membrane and protein tyrosine phosphorylation was detected using a recombinant anti–P-Tyr reagent, RC20 (B-D Transduction Laboratory). The approximate molecular size for each protein was estimated by the prestained marker proteins (SeeBlue, Novex, Helixx).
Detection of PI3-kinase p85α association with CD28i
To investigate the PI3-kinase p85α association with CD28i in T cells stimulated via CD28, CD28i-HA–transfected Jurkat cells were serum starved overnight and then incubated at 37°C with twice the number of CHO cells or CHO-B7-1 cells for 5 minutes. Cell extracts in 1% Nonidet P40 buffer were immunoprecipitated with anti-HA Ab, and fractionated by 10% NuPAGE using MOPS buffer under the reduced condition. Fractionated proteins were blotted onto a PVDF membrane and PI3-kinase p85α was detected using anti–PI3-kinase p85 (UB93-3; Upstate Biotechnology, Lake Placid, NY). The approximate molecular size for each protein was estimated by the prestained marker proteins (SeeBlue, Novex, Helixx).
Laser confocal microscopic study and flow cytometric analysis
To examine the distribution of CD28 and CD28i in T cells, GFP-fusion proteins encoding CD28 or CD28i were transfected into Jurkat T cells by electroporation (800 μF, 300 V). Cells were subsequently incubated overnight at 37°C and monitored by laser confocal microscope (LSM 510; Carl Zeiss, Thornwood, NY). Cells were stained with RPE–antihuman CD28 Ab (MCA 709PE; Serotec, Kidlington, Oxford, United Kingdom), RPE–anti-CD4 Ab (Q4120; Sigma), or anti-CD43 Ab plus Alexa–anti–mouse IgG (Molecular Probes, Eugene, OR) prior to confocal microscopic analysis to monitor the distribution of CD28i in relation to CD28, CD4, or CD43.
The stable transfectant of Jurkat T cells expressing HA-tagged CD28i was established by transfecting pcDNA3.1 harboring CD28i-HA, selecting transfectants in 2 mg/mL G418. The cell-surface expression of CD28i of the transfectants was determined by flow cytometry using anti-HA Ab (Y-11; Santa Cruz) and fluorescein isothiocyanate (FITC)–anti–rabbit IgG.
Interleukin-2 promoter reporter assay
To investigate the function of CD28i in CD28-dependent costimulatory signaling, Jurkat T cells or DC28 cells were transiently transfected using DEAE-dextran22 with interleukin-2 (IL-2) promoter reporter plasmid (IL-2-luc)23 in conjunction withpcDNA3.1-based expression vector harboring CD28i-HA. After an overnight culture at 37°C, cells were stimulated with phorbol myristate acetate (PMA) (10 ng/mL) plus phytohemaglutinin (PHA) (10 μg/mL), or antimouse CD3 Ab (5 μg/mL) (2C11; B-D Pharmingen) in the presence of anti-CD28 Ab (33740D; B-D Pharmingen), anti-HA Ab, anti-CD7 Ab, or anti-CD2 Ab. CHO-B7-1 cells were used to crosslink human CD28 when DC28 cells were studied. Cells were incubated with various stimulating reagents for 6 hours and then the cell lysates were subjected to the luciferase assay (Promega, Madison, WI). Data represent fold induction of the PMA-plus-PHA or anti-CD3 Ab response, which was set at 100% for each cell group.
Results
Identification of CD28 isoforms
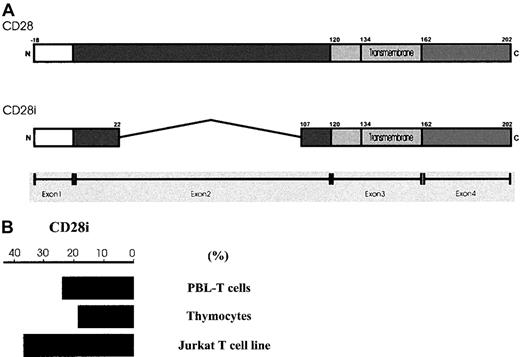
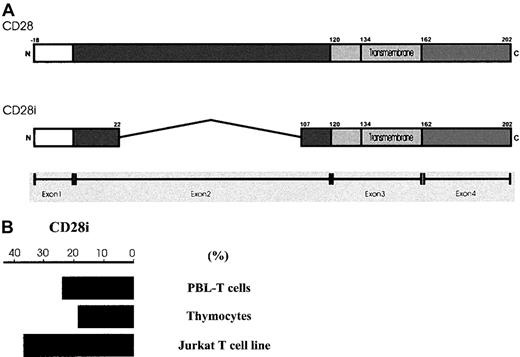
In humans, CD28 is encoded by a single gene that consists of 4 exons.20 The first exon encodes for the signal peptide whereas the second, and largest, exon encodes almost the entire extracellular domain, including amino acid motifs involved in ligand binding. The third and fourth exons encode the transmembrane domain and cytoplasmic tail, respectively. While characterizing the message level of CD28 by RT-PCR in human PBL-T cells, we observed multiple amplified DNA fragments smaller than that of CD28. We hypothesized that these messages were CD28 variants, possibly resulting from alternative splicing like those previously reported.19,20 Indeed, the sequencing results of the PCR products suggested that they were CD28 splice isoforms and a few of them were novel isoforms (GenBank accession numbers AF222341, AF222342, and AF222343) (manuscript in preparation). The most prominent PCR product has the identical sequence to what was previously reported20 and was designated as CD28i in our study. As shown in Figure1A, CD28i lacks large portions of the extracellular domain of CD28 arising from deletions within exon 2. The predicted amino acid sequence suggested that CD28i could be a transmembrane protein.
Scheme of CD28i and semiquantitative measurement of CD28i mRNA levels.
(A) Schematic representation of human CD28 and CD28i. Exon 1, exon 2, exon 3, and exon 4 are shaded in different tones. The transmembrane domains in exon 3 are indicated. (B) Semiquantitative measurement of CD28i. The cytoplasmic RNAs from PBL-T cells, thymocytes, and Jurkat T cells were examined by RT-PCR for CD28i messages using a specific primer. EtBr-stained PCR products were estimated semiquantitatively after 21, 23, 25, 27, 29, and 31 cycles to ensure near-linear amplification. The percent values indicate the proportional amplification compared with the similarly amplified CD28 message that scores 100%. The data represent the 3 experiments with similar results.
Scheme of CD28i and semiquantitative measurement of CD28i mRNA levels.
(A) Schematic representation of human CD28 and CD28i. Exon 1, exon 2, exon 3, and exon 4 are shaded in different tones. The transmembrane domains in exon 3 are indicated. (B) Semiquantitative measurement of CD28i. The cytoplasmic RNAs from PBL-T cells, thymocytes, and Jurkat T cells were examined by RT-PCR for CD28i messages using a specific primer. EtBr-stained PCR products were estimated semiquantitatively after 21, 23, 25, 27, 29, and 31 cycles to ensure near-linear amplification. The percent values indicate the proportional amplification compared with the similarly amplified CD28 message that scores 100%. The data represent the 3 experiments with similar results.
To measure the amounts of CD28i message specifically, we generated specific 5′-primer spanning the junctional DNA sequence, which resulted from the alternative splicing. This primer was used to investigate the mRNA expression levels of CD28i in cytoplasmic RNA samples from human PBL-T cells, thymocytes, and human Jurkat T cells using a semiquantitative PCR-based assay. Expression levels relative to the message level of CD28 (100%), which was amplified from the same cytoplasmic RNA using a specific 5′-primer, are shown in Figure 1B. In T cells, the CD28i mRNA level is approximately 30% of that seen with CD28. These data suggest that CD28i was expressed at different levels among normal PBL-T cells, thymocytes, and an in vitro–maintained T-cell line such as Jurkat cells.
CD28i is a homodimeric glycoprotein targeted to the cell surface
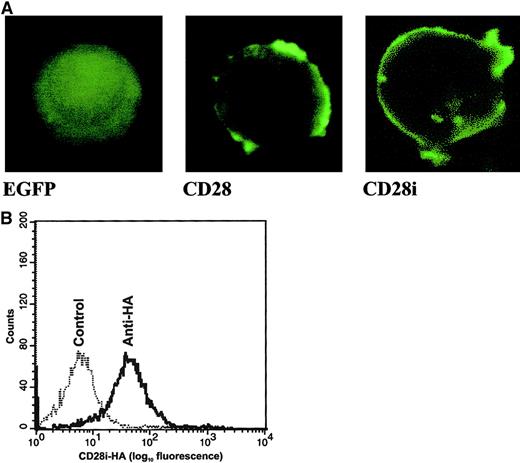
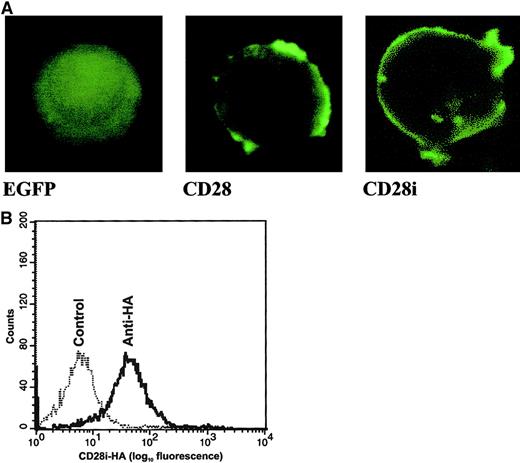
Having isolated CD28i cDNA, we next investigated how CD28i was localized in T cells by preparing expression vector–encoding C-terminal GFP conjugate. As shown in Figure2A, CD28i is targeted to the cell membrane. To further investigate if CD28i is indeed expressed as a transmembrane protein on the surface of T cells, we modified the CD28i cDNA such that an HA epitope tag was inserted at its N-terminus. A Jurkat T cell transfected with this HA-tagged CD28i (CD28i-HA) was stained with anti-HA Ab for analysis by flow cytometry (Figure 2B). The data clearly showed that CD28i is a cell-surface molecule in T cells. It is noteworthy that the monoclonal Abs currently available for cell-surface staining of CD28 are unlikely to detect CD28i because of the deletion in exon 2. In fact, none of the commercial anti-CD28 Abs tested detected cell-surface CD28i on the transfected cells (data not shown).
Characterization of CD28 isoform proteins in transfected Jurkat T cells.
(A) Distribution of CD28 isoforms in transfected Jurkat T cells. The cDNAs encoding CD28 and CD28i were subcloned into pEGFP to generate C-terminal fusions of CD28 and CD28i with EGFP. Jurkat T cells were transfected with pEGFP or those pEGFP-based plasmids, and cells were monitored 12 hours later by laser confocal microscope. Original magnification × 400. (B) Detection of HA-tagged CD28i on the Jurkat T cell transfectant by cell-surface staining. Jurkat T cells were transfected with the expression vector harboring HA-tagged CD28i. The stable transfectant was stained with rabbit anti-HA Ab and FITC–goat anti–rabbit IgG Ab, then subjected to analysis with flow cytometry. The control was stained with FITC–goat anti–rabbit IgG Ab alone.
Characterization of CD28 isoform proteins in transfected Jurkat T cells.
(A) Distribution of CD28 isoforms in transfected Jurkat T cells. The cDNAs encoding CD28 and CD28i were subcloned into pEGFP to generate C-terminal fusions of CD28 and CD28i with EGFP. Jurkat T cells were transfected with pEGFP or those pEGFP-based plasmids, and cells were monitored 12 hours later by laser confocal microscope. Original magnification × 400. (B) Detection of HA-tagged CD28i on the Jurkat T cell transfectant by cell-surface staining. Jurkat T cells were transfected with the expression vector harboring HA-tagged CD28i. The stable transfectant was stained with rabbit anti-HA Ab and FITC–goat anti–rabbit IgG Ab, then subjected to analysis with flow cytometry. The control was stained with FITC–goat anti–rabbit IgG Ab alone.
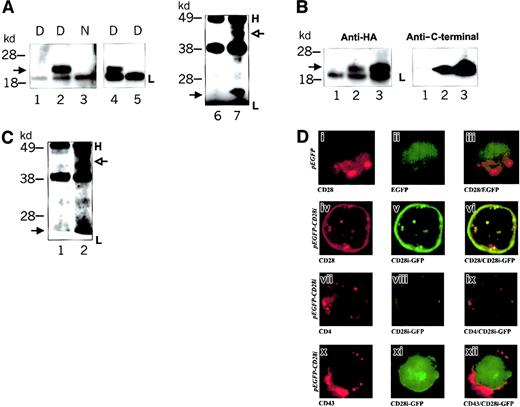
The predicted amino acid sequence of CD28 comprises 202 amino acid residues. Although the amino acid sequence suggests a protein of approximately 23 kd, owing to the heavy N-glycosylation, the molecular mass of CD28 is approximately 42 kd to 46 kd on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The nucleotide sequence of CD28i predicts 118 amino acid residues possessing 2 N-glycosylation sites in the extracellular region, rather than 5 in CD28. Although the deduced amino acid sequence predicts a molecular mass of approximately 13 kd, subsequent glycosylation may modify the size significantly. The biochemical nature of CD28i was therefore evaluated in the transfected cells expressing HA-tagged CD28i. Initially, we tried to determine the apparent molecular weight of CD28i, and also to test if CD28i is a heavily glycosylated protein. To reduce the glycosylation modulation, we mutated CD28i at 2 N-glycosylation sites (N19 and N27), and stably gene-transfected it into Jurkat T cells. Under reduced conditions, an approximately 23-kd protein was detected in CD28i-HA transfected cells whereas N-linked glycosylation-deficient CD28i-HA–transfected cells expressed an approximately 14-kd protein (Figure3A). The data suggested that nearly 40% of CD28i molecular mass is carbohydrate nature. We furthered the characterization of CD28i if it homodimerizes by analyzing the whole-cell lysates of CD28i-HA–transfected Jurkat T cells in nonreduced conditions on immunoblots probed with anti-HA Ab (Figure3A). Under the nonreduced condition, a protein of approximately 46 kd was detected, a result consistent with the formation of CD28i homodimers (Figure 3A).
Protein analysis of CD28i in PBL-T cells and Jurkat T cells.
(A) SDS-PAGE analysis of HA-tagged CD28i protein in Jurkat T-cell transfectants. Stable transfectants of Jurkat T cells harboring CD28i-HA or N-linked glycosylation-deficient CD28i-HA were lysed in 1% Nonidet P40. Whole-cell lysates were fractionated by SDS-PAGE in reduced or nonreduced conditions and immunoblots were performed using anti-HA Ab. Arrows in lane 1 and lane 3 indicate CD28i-HA monomers. Arrows in lane 4 and lane 5 indicate N-linked glycosylation-deficient CD28i-HA monomers and CD28i-HA dimers, respectively. Lane 2 and lane 6 show samples of native Jurkat T cells. (B) The detection of CD28i protein in PBL-T cells and Jurkat T cells with CD28 C-terminal–specific Western blotting. The 1% Nonidet P40 cell lysates of PBL-T cells and Jurkat T cells were immunoprecipitated with a CD28 C-terminal–specific Ab (lanes 2 and 4) or the control Ab (lanes 1 and 3). Immunoprecipitates were fractionated by SDS-PAGE in the reduced condition and CD28 C-terminal–specific Western blotting was performed. Shown in lanes 5 and 6 are the data indicating the HA-tagged CD28i and HA-tagged CD28, which were visualized by HA-specific Western blotting. Lanes 1 and 2 are the data of human PBL-T cells, and lanes 3 and 4 are the data of Jurkat T cells. CD28 is indicated by an open arrow, and CD28i is indicated by a filled arrow in lanes 2 and 4. H indicates the position of the Ig heavy chain, and L indicates the position of the Ig light chain. The data represent 3 experiments with similar results.
Protein analysis of CD28i in PBL-T cells and Jurkat T cells.
(A) SDS-PAGE analysis of HA-tagged CD28i protein in Jurkat T-cell transfectants. Stable transfectants of Jurkat T cells harboring CD28i-HA or N-linked glycosylation-deficient CD28i-HA were lysed in 1% Nonidet P40. Whole-cell lysates were fractionated by SDS-PAGE in reduced or nonreduced conditions and immunoblots were performed using anti-HA Ab. Arrows in lane 1 and lane 3 indicate CD28i-HA monomers. Arrows in lane 4 and lane 5 indicate N-linked glycosylation-deficient CD28i-HA monomers and CD28i-HA dimers, respectively. Lane 2 and lane 6 show samples of native Jurkat T cells. (B) The detection of CD28i protein in PBL-T cells and Jurkat T cells with CD28 C-terminal–specific Western blotting. The 1% Nonidet P40 cell lysates of PBL-T cells and Jurkat T cells were immunoprecipitated with a CD28 C-terminal–specific Ab (lanes 2 and 4) or the control Ab (lanes 1 and 3). Immunoprecipitates were fractionated by SDS-PAGE in the reduced condition and CD28 C-terminal–specific Western blotting was performed. Shown in lanes 5 and 6 are the data indicating the HA-tagged CD28i and HA-tagged CD28, which were visualized by HA-specific Western blotting. Lanes 1 and 2 are the data of human PBL-T cells, and lanes 3 and 4 are the data of Jurkat T cells. CD28 is indicated by an open arrow, and CD28i is indicated by a filled arrow in lanes 2 and 4. H indicates the position of the Ig heavy chain, and L indicates the position of the Ig light chain. The data represent 3 experiments with similar results.
We next investigated whether the CD28i isoform is expressed in PBL-T cells or in native Jurkat T cells. Immunoprecipitates were prepared from 1% Nonidet P40 cell lysates using a CD28 C-terminal–specific Ab, fractionated by SDS-PAGE in reduced conditions, then Western-blotted using a C-terminal–specific Ab. Immunoprecipitated proteins of approximately 23 kd were detected immediately above the Ig light chain of the immunoprecipitation Ab in both samples from PBL-T cells (Figure3B, lanes 1 and 2) and the Jurkat T cell (Figure 3B, lanes 3 and 4). Since the control Ab did not precipitate this protein, it is likely that these 23-kd proteins are endogenous CD28i isoforms.
CD28i associates with CD28
The data in the previous section revealed that CD28i is a homodimeric transmembrane protein in T cells. The study however has left the possibility of heterodimer formation between CD28i and CD28 unresolved. Notably, Cys147 is located within the extracellular domain near the membrane-spanning region which corresponds to the CD28 homodimerization domain, and is retained in CD28i. Therefore, it is possible that CD28i complexes with CD28 via the Cys-Cys bond. Initial attempts to coimmunoprecipitate transfected CD28i-HA with a CD28-specific Ab using cell lysates prepared with 1% Nonidet P40 were unsuccessful. However, CD28i-HA and CD28 coimmunoprecipitation was demonstrated using 1% digitonin cell lysates (Figure4A). The isotype control and anti-CD43 Ab did not coimmunoprecipitate the CD28i in the similar assay, although the anti-CD43 Ab was capable of immunoprecipitating CD43 from the same cell lysate (data not shown). Furthermore, neither anti-CD2 Ab, anti-CD4 Ab, nor anti-CD7 Ab coimmunoprecipitated CD28i-HA in the similar assay (data not shown). The immunoprecipitation with anti-HA Ab also showed coimmunoprecipitated CD28 (Figure 4A). The approximately 23-kd proteins coimmunoprecipitated with CD28 were detected by reprobing with CD28 C-terminal–specific Western blotting (Figure 4B). The data further confirmed the evidence that CD28i associates with CD28. Finally, the CD28 C-terminal–specific Western blotting showed CD28i in anti-CD28 immunoprecipitate derived from 1% digitonin cell lysates of PBL-T cells (Figure 4C). The data clearly indicated that CD28i associates with CD28, although it is unlikely that CD28i complexes with CD28 via the Cys-Cys covalent bond like the immunoglobulin heavy chain and light chain complex. To understand the association between CD28i and CD28 in a comprehensive fashion, we investigated the cell-surface distribution of CD28i in relation to CD28 by confocal microscope. The confocal microscope photographs in Figure4D showed the red fluorescence by Ab staining specific to CD28, CD4, or CD43 (left photographs), and green fluorescence of CD28i-GFP or GFP alone (middle photographs). On the right are the merged images of green fluorescence and red fluorescence. When cells were expressing GFP alone, and stained with anti-CD28, green fluorescence and red fluorescence segregated independently. However, in cells that express CD28i-GFP, the staining by anti-CD28 Ab revealed a distinct colocalization between CD28 and CD28i. The colocalization was observed in a CD28-specific fashion as the CD4-specific Ab or CD43-specific Ab did not result in yellow fluorescence and the CD4 or CD43 (red) and CD28i (green) are clearly separated. Collectively, both the immunoprecipitation experiments and confocal microscopy indicate that there is a cell-surface association of CD28 and CD28i in T cells.
Association of CD28i with CD28.
(A) Coimmunoprecipitation of CD28i with CD28 in 1% digitonin cell extract. Extracts from CD28i-HA–transfected Jurkat T cells were prepared using 1% Nonidet P40 or 1% digitonin buffer, immunoprecipitated with anti-CD28 Ab (L293) that only reacts with CD28, or an Ab specific for CD43. Immunoprecipitates were fractionated by SDS-PAGE. Western blot membranes were probed with anti-HA Ab. The anti-CD28 only precipitated HA-tagged CD28i from 1% digitonin extract but not from 1% Nonidet P40 extract. In parallel, extracts of CD28i-HA–transfected Jurkat T cells were prepared with 1% digitonin and immunoprecipitated with anti-HA Ab or control Ab. Subsequently, the CD28 C-terminal–specific Western blotting was performed and is shown in the 2 lanes at the right end. Lanes 1 and 6: immunoprecipitated by the control Ab. Lanes 2, 3, and 4: immunoprecipitated by anti-CD28 Ab. Lane 5: immunoprecipitated by anti-CD43 Ab. Lane 7: immunoprecipitated by anti-HA Ab. D indicates digitonin buffer cell extract and N indicates Nonidet P40 buffer cell extract. Filled arrows indicate CD28i-HA in lanes 2, 4, and 7. In lane 7, the open arrow indicates CD28. H indicates the position of the Ig heavy chain, and L indicates the position of the Ig light chain. (B) Detection of HA-tagged CD28i with anti-CD28 C-terminal Ab. One percent digitonin cell extracts of CD28i-HA–transfected Jurkat T cells were immunoprecipitated with anti-CD28 Ab (L293) or anti-HA Ab. Western blot membrane was probed first with anti-HA Ab (left panel). To avoid the background by Ig light chain at approximately 20 kd, the Ig light chain blots were excised before the membrane was reprobed with anti-CD28 C-terminal Ab (right panel). Lane 1: immunoprecipitated with the control Ab. Lane 2: immunoprecipitated with anti-CD28 Ab. Lane 3: immunoprecipitated with anti-HA Ab. In the left panel, the filled arrow indicates CD28i-HA; in the right panel, the filled arrow indicates anti-CD28 C-terminal Ab reactive proteins. L indicates the position of the Ig light chain. (C) Coimmunoprecipitation of CD28i with CD28 in PBL-T 1% digitonin cell extract. One percent digitonin cell extract of PBL-T cells was immunoprecipitated with anti-CD28 Ab (L293). Western blot membrane was probed with anti-CD28 C-terminal Ab. To avoid the background by Ig light chain, the Ig light chain blots were excised prior to film exposure. Lane 1: immunoprecipitated with the control Ab. Lane 2: immunoprecipitated with anti-CD28 Ab. In lane 2, the open arrow indicates CD28. H indicates the position of the Ig heavy chain, and L indicates the position of the Ig light chain. (D) Confocal microscopic study of GFP-fusion proteins of CD28i in Jurkat T cells stained with anti-CD28 Ab. Jurkat T cells were transfected with pEGFP(i-iii) or pEGFP-CD28i (CD28i-GFP) (iv-xii). Sixteen hours later, cells were stained with RPE-conjugated anti-CD28 Ab, which detects CD28 but not CD28i, RPE–anti-CD4, or anti-CD43 Ab-plus Alexa-anti–mouse IgG, then analyzed by confocal microscope. CD28 (i and iv), CD4 (vii) and CD43 (x) were shown in red fluorescence. Only green fluorescence (GFP alone or CD28i-GFP) was detected in the middle panels (ii, v, viii, xi). Red fluorescence and green fluorescence were merged and are shown in the right panels (iii, vi, ix, xii). Only (vi) shows yellow patches resulting from a close association of CD28i-GFP and RPE–anti-CD28 Ab. Transfected plasmids are indicated on the left.
Association of CD28i with CD28.
(A) Coimmunoprecipitation of CD28i with CD28 in 1% digitonin cell extract. Extracts from CD28i-HA–transfected Jurkat T cells were prepared using 1% Nonidet P40 or 1% digitonin buffer, immunoprecipitated with anti-CD28 Ab (L293) that only reacts with CD28, or an Ab specific for CD43. Immunoprecipitates were fractionated by SDS-PAGE. Western blot membranes were probed with anti-HA Ab. The anti-CD28 only precipitated HA-tagged CD28i from 1% digitonin extract but not from 1% Nonidet P40 extract. In parallel, extracts of CD28i-HA–transfected Jurkat T cells were prepared with 1% digitonin and immunoprecipitated with anti-HA Ab or control Ab. Subsequently, the CD28 C-terminal–specific Western blotting was performed and is shown in the 2 lanes at the right end. Lanes 1 and 6: immunoprecipitated by the control Ab. Lanes 2, 3, and 4: immunoprecipitated by anti-CD28 Ab. Lane 5: immunoprecipitated by anti-CD43 Ab. Lane 7: immunoprecipitated by anti-HA Ab. D indicates digitonin buffer cell extract and N indicates Nonidet P40 buffer cell extract. Filled arrows indicate CD28i-HA in lanes 2, 4, and 7. In lane 7, the open arrow indicates CD28. H indicates the position of the Ig heavy chain, and L indicates the position of the Ig light chain. (B) Detection of HA-tagged CD28i with anti-CD28 C-terminal Ab. One percent digitonin cell extracts of CD28i-HA–transfected Jurkat T cells were immunoprecipitated with anti-CD28 Ab (L293) or anti-HA Ab. Western blot membrane was probed first with anti-HA Ab (left panel). To avoid the background by Ig light chain at approximately 20 kd, the Ig light chain blots were excised before the membrane was reprobed with anti-CD28 C-terminal Ab (right panel). Lane 1: immunoprecipitated with the control Ab. Lane 2: immunoprecipitated with anti-CD28 Ab. Lane 3: immunoprecipitated with anti-HA Ab. In the left panel, the filled arrow indicates CD28i-HA; in the right panel, the filled arrow indicates anti-CD28 C-terminal Ab reactive proteins. L indicates the position of the Ig light chain. (C) Coimmunoprecipitation of CD28i with CD28 in PBL-T 1% digitonin cell extract. One percent digitonin cell extract of PBL-T cells was immunoprecipitated with anti-CD28 Ab (L293). Western blot membrane was probed with anti-CD28 C-terminal Ab. To avoid the background by Ig light chain, the Ig light chain blots were excised prior to film exposure. Lane 1: immunoprecipitated with the control Ab. Lane 2: immunoprecipitated with anti-CD28 Ab. In lane 2, the open arrow indicates CD28. H indicates the position of the Ig heavy chain, and L indicates the position of the Ig light chain. (D) Confocal microscopic study of GFP-fusion proteins of CD28i in Jurkat T cells stained with anti-CD28 Ab. Jurkat T cells were transfected with pEGFP(i-iii) or pEGFP-CD28i (CD28i-GFP) (iv-xii). Sixteen hours later, cells were stained with RPE-conjugated anti-CD28 Ab, which detects CD28 but not CD28i, RPE–anti-CD4, or anti-CD43 Ab-plus Alexa-anti–mouse IgG, then analyzed by confocal microscope. CD28 (i and iv), CD4 (vii) and CD43 (x) were shown in red fluorescence. Only green fluorescence (GFP alone or CD28i-GFP) was detected in the middle panels (ii, v, viii, xi). Red fluorescence and green fluorescence were merged and are shown in the right panels (iii, vi, ix, xii). Only (vi) shows yellow patches resulting from a close association of CD28i-GFP and RPE–anti-CD28 Ab. Transfected plasmids are indicated on the left.
CD28 crosslinking induces tyrosine phosphorylation and the PI3-kinase association of CD28i, and the expression of CD28i enhances costimulation via CD28
The association of CD28i with CD28 predicts that the ligand-binding capacity of CD28 will be unaffected and that the clustering of cytoplasmic tail of CD28 will be enhanced. To determine if the CD28-CD28i interaction alters the signaling of CD28, we investigated CD28 signaling in CD28i-transfected T cells.
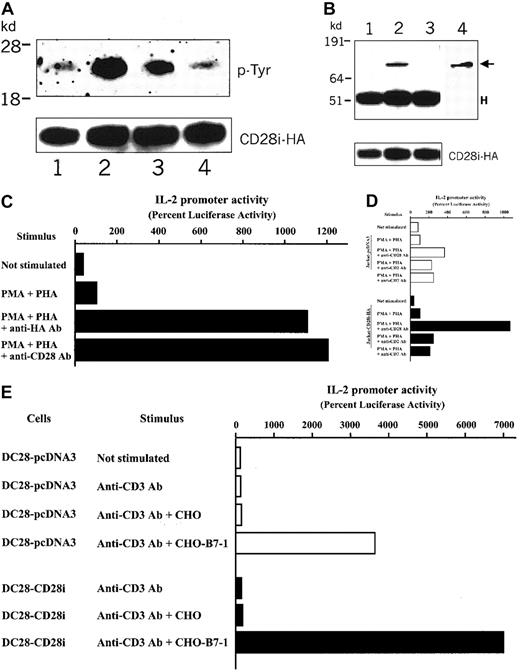
CD28 signaling involves the tyrosine phosphorylation events within the cytoplasmic tail,24,25 which promotes the PI3-kinase association. If CD28i is an integral factor of CD28 signaling, it may also be tyrosine phosphorylated and may associate with PI3-kinase upon CD28 stimulation. To investigate this possibility, Jurkat T cells expressing CD28i-HA were stimulated with anti-CD28 Ab, and HA-specific immunoprecipitates were probed on immunoblots with antiphosphotyrosine Ab blottings. To study if the association of PI3-kinase follows CD28 crosslinking, the transfectants were incubated with B7-1+CHO cells (CHO-B7-1).26 Strikingly, we observed the induction of tyrosine phosphorylation of CD28i, and its association with PI3-kinase (Figure 5A-B) followed the CD28 crosslinking, which strongly suggested that CD28i is involved in the PI3-kinase–mediated CD28 signaling. Furthermore, the transfected HA-tagged CD28i was functional, as assayed by increases in IL-2 promoter activity in response to the anti-HA Ab crosslinking (Figure 5C). Moreover, HA-tagged CD28i was able to greatly amplify the normal level of CD28 signaling (Figure 5D). The augmentation by CD28i appeared to be specific for the CD28 signal because the costimulations by CD2 and CD7 were not enhanced by the transfected CD28i expression. CD28i thus augmented the CD28-triggered costimulatory signal in T cells. The cooperation between CD28 and CD28i was further investigated in a human CD28-expressing mouse T-cell hybridoma cell, DC28,27 in which endogenous murine CD28 is at a negligible level such that it does not associate with vector-encoded CD28i (data not shown). The DC28 cell line was transfected with CD28i, and when stimulated with a mouse CD3-specific Ab in conjunction with CHO-B7-1 cells, we observed that the IL-2 promoter activity was significantly enhanced in comparison with DC28 transfected with control plasmid pcDNA3 (Figure 5E). The augmentation of CD28 signaling was not due to the increase of CD28 expression because neither transient nor stable transfection of CD28i into T cells increased the surface expression of CD28 (data not shown). Collectively, the data strongly suggested that CD28i enhances the PTK/PI3-kinase–involved costimulatory signaling pathway triggered by CD28.
CD28i functions as an integral factor to increase the CD28 costimulation signal.
(A) Tyrosine phosphorylation of CD28i induced by crosslinking of CD28. CD28i-HA–transfected Jurkat T cells were serum starved overnight. Cells were incubated with anti-CD28 Ab or anti-CD43 Ab for 30 minutes on ice. Anti–mouse IgG Ab was added to start activation at 37°C for 2 minutes and 5 minutes and then cell lysates were prepared with 1% Nonidet P40. Immunoprecipitation was performed with anti-HA Ab, and immunoprecipitates analyzed by immunoblotting. The top panel shows the data blotted with anti–P-Tyr (indicated as p-Tyr). The same membrane was blotted with anti-HA Ab and is shown in the bottom (indicated as CD28iHA). Lane 1: not stimulated. Lane 2: CD28 stimulated for 2 minutes. Lane 3: CD28 stimulated for 5 minutes. Lane 4: CD43 stimulated for 2 minutes. (B) Association of CD28i with PI3-kinase p85α induced by crosslinking of CD28. CD28i-HA–transfected Jurkat T cells were serum starved overnight. Cells were incubated with CHO cells or mouse B7-1–transfected CHO cells at 37°C for 5 minutes and then cell extracts were prepared with 1% Nonidet P40. Immunoprecipitation was performed with anti-HA Ab and immunoprecipitates analyzed by immunoblotting. The top panel shows the data blotted with anti–PI3-kinase p85α. The same membrane was blotted with anti-HA Ab and is shown in the bottom panel (indicated as CD28i-HA). Lane 1: not stimulated. Lane 2: CHO-B7-1–stimulated. Lane 3: CHO-stimulated. Lane 4: whole-cell lysate of Jurkat T cells. The arrow indicates the position of PI3-kinase p85α. H indicates the position of the Ig heavy chain. (C) HA-tagged CD28i is functional in inducing costimulation. HA-tagged CD28i and an IL-2 reporter plasmid were transfected into Jurkat T cells. Sixteen hours later, cells were stimulated for 6 hours with PHA and PMA in the presence of anti-HA Ab or anti-CD28 Ab as indicated on the left. Cell extracts were examined for IL-2 promoter activity by luciferase assays. The data represent 3 different experiments with similar results. (D) CD28i enhances CD28 signals in Jurkat T cells. The CD28i-HA or the control plasmid (pcDNA3), and the IL-2 reporter plasmid, were transfected into Jurkat T cells. Sixteen hours later, cells were stimulated for 6 hours with PHA and PMA in the presence of anti-CD28 Ab, anti-CD2 Ab, or anti-CD7 Ab as indicated on the left. Cell extracts were studied for IL-2 promoter activity by luciferase assays. The data represent 5 different experiments with similar results. (E) CD28i enhances the human CD28 signaling in a mouse T-cell hybridoma, DC28. The human CD28 transfectant of mouse T-cell hybridoma DC28 was transfected with CD28i-HA or pcDNA3 and the IL-2 reporter plasmid. Sixteen hours later, cells were stimulated for 6 hours with CHO or CHO-B7-1 in the presence of antimouse CD3 Ab as indicated on the left. The data represent 3 different experiments with similar results.
CD28i functions as an integral factor to increase the CD28 costimulation signal.
(A) Tyrosine phosphorylation of CD28i induced by crosslinking of CD28. CD28i-HA–transfected Jurkat T cells were serum starved overnight. Cells were incubated with anti-CD28 Ab or anti-CD43 Ab for 30 minutes on ice. Anti–mouse IgG Ab was added to start activation at 37°C for 2 minutes and 5 minutes and then cell lysates were prepared with 1% Nonidet P40. Immunoprecipitation was performed with anti-HA Ab, and immunoprecipitates analyzed by immunoblotting. The top panel shows the data blotted with anti–P-Tyr (indicated as p-Tyr). The same membrane was blotted with anti-HA Ab and is shown in the bottom (indicated as CD28iHA). Lane 1: not stimulated. Lane 2: CD28 stimulated for 2 minutes. Lane 3: CD28 stimulated for 5 minutes. Lane 4: CD43 stimulated for 2 minutes. (B) Association of CD28i with PI3-kinase p85α induced by crosslinking of CD28. CD28i-HA–transfected Jurkat T cells were serum starved overnight. Cells were incubated with CHO cells or mouse B7-1–transfected CHO cells at 37°C for 5 minutes and then cell extracts were prepared with 1% Nonidet P40. Immunoprecipitation was performed with anti-HA Ab and immunoprecipitates analyzed by immunoblotting. The top panel shows the data blotted with anti–PI3-kinase p85α. The same membrane was blotted with anti-HA Ab and is shown in the bottom panel (indicated as CD28i-HA). Lane 1: not stimulated. Lane 2: CHO-B7-1–stimulated. Lane 3: CHO-stimulated. Lane 4: whole-cell lysate of Jurkat T cells. The arrow indicates the position of PI3-kinase p85α. H indicates the position of the Ig heavy chain. (C) HA-tagged CD28i is functional in inducing costimulation. HA-tagged CD28i and an IL-2 reporter plasmid were transfected into Jurkat T cells. Sixteen hours later, cells were stimulated for 6 hours with PHA and PMA in the presence of anti-HA Ab or anti-CD28 Ab as indicated on the left. Cell extracts were examined for IL-2 promoter activity by luciferase assays. The data represent 3 different experiments with similar results. (D) CD28i enhances CD28 signals in Jurkat T cells. The CD28i-HA or the control plasmid (pcDNA3), and the IL-2 reporter plasmid, were transfected into Jurkat T cells. Sixteen hours later, cells were stimulated for 6 hours with PHA and PMA in the presence of anti-CD28 Ab, anti-CD2 Ab, or anti-CD7 Ab as indicated on the left. Cell extracts were studied for IL-2 promoter activity by luciferase assays. The data represent 5 different experiments with similar results. (E) CD28i enhances the human CD28 signaling in a mouse T-cell hybridoma, DC28. The human CD28 transfectant of mouse T-cell hybridoma DC28 was transfected with CD28i-HA or pcDNA3 and the IL-2 reporter plasmid. Sixteen hours later, cells were stimulated for 6 hours with CHO or CHO-B7-1 in the presence of antimouse CD3 Ab as indicated on the left. The data represent 3 different experiments with similar results.
Discussion
CD28i is a unique membrane receptor isoform
Alternative splicing to generate transcripts encoding for membrane receptor variants is a common strategy to increase the repertoire of receptors available to the cell. In this regard, the CD28i is unique since it lacks an extracellular ligand-binding motif but is expressed on the cell surface and retains the intact structure capable of transducing signals via its cytoplasmic tail. Although a similar isoform of FcγRIb2 (CD64), which lacks part of its extracellular domain, was reported, this isoform was found to no longer be expressed on the cell surface.28 Generally, many isoforms whose genes were cloned (although in many cases their proteins have not been characterized) have predicted structures as (1) secretory proteins that have lost the transmembrane domain; (2) “decoy” receptors without cytoplasmic tails; or (3) functionally altered receptors resulting from modulation of the cytoplasmic tail. For example, cDNAs encoding alternatively spliced isoforms of signalling lymphocyte activation molecule (SLAM) predict both a secretory isoform and a decoy isoform.29 In the case of CD40L, the messages and the proteins for the decoy isoform of CD40L with deletions in the cytoplasmic tail were found in a complex with human CD40L.30 Thus, no similar example to the CD28i that we have characterized here has been previously reported.
The alternative splicing of single precursor mRNA often results in a set of distinct isoforms. Human CD28 is no exception, as we have isolated an additional isoform cDNA, CD28-S1 (Genbank accession no.AF222341), CD28-S2 (Genbank accession no. AF222342), and CD28-S3 (Genbank accession no. AF222343), which lacks a large portion of extracellular and transmembrane domains. Additionally, human CD28 isoform mRNAs, which have also lost the transmembrane domain, were reported by others.19 The existence of multiple isoforms that potentially modulate CD28 receptor function in various ways suggests that the CD28 ligand binding and the resulting cytoplasmic signaling exhibit great complexity.
Possible regulation of cell activity by expression of CD28i
It is well known that expression of CD28 on the cell surface is modulated by T-cell differentiation and activation.31Although current literature attributes the change of CD28 expression to transcriptional control,32 additional mechanisms to control the amounts of CD28 and the balance between CD28 isoforms by changing the level of alternative splicing are likely. Since the CD28 signal is enhanced by increasing association with CD28i, the regulation of CD28i expression could represent a pivotal mechanism to increase or decrease the level of costimulatory signals in T cells. We therefore investigated to determine if the expression of the message for CD28i in relevant to that for CD28 was affected in T cells upon mitogenic and stress stimulation. In PBL-T cells the message levels declined by one-third after 24 hours of the mitogenic response. When Jurkat T cells were activated with PHA plus PMA, the proportion of CD28i declined by one-third. Cell stress induced by ultraviolet (UV) radiation did not result in a significant change in CD28i expression in Jurkat T cells 24 hours after UV treatment. Our observation suggested that CD28i is persistently expressed during mitogenic activation, stress, and differentiation. Interestingly, there may be typical profiles of CD28i and CD28 ratios under each stimulation circumstance. Because the splicing of the precursor message to generate individual isoforms (and concomitantly reduce the CD28 message) could take place very early upon cellular stimulation, a temporal examination of the splicing activity that generates CD28i should be investigated.
We thank Dr C. Rudd (Harvard Medical School, Boston, MA), Dr S.-C. Sun (Pennsylvania State University, Hershey, PA), and Dr T. H. Watts (Toronto University, Toronto, ON, Canada) for their generous gifts of cell lines and reagents. We are also grateful to Dr D. Kelvin, Dr B. Chan, Dr S. Meakin, and Ms. J. Barlic for valuable discussions.
Supported by grants from the Canadian Institutes of Health Research/Juvenile Diabetes Foundation International, CIHR (MT-15481), and Multi-Organ Transplant Program.
H.H., Y.M., and S.A.M. equally contributed to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Atsuo Ochi, The John P. Robarts Research Institute and the Department of Microbiology and Immunology, University of Western Ontario, 1400 Western Rd, London, Ontario, Canada N6G 2V4; e-mail: ochi@rri.on.ca.