Abstract
B7 molecules provide important costimulatory signals to T cells, and B7 genes have been introduced into B7-negative tumor cells to enhance their immunogenicity. However, the role of B7 molecules in inducing tumor immunity is controversial because of conflicting results and reports of differential signaling through the B7 molecules and their ligands CD28 and CTLA-4. In this study, we compared the effect of B7-1 (CD80) and B7-2 (CD86) on the induction of T-cell immunity to C1498, a murine myelogenous leukemia. When cultured with exogenous cytokines in vitro, C1498/B7-1 and C1498/B7-2 induced syngeneic CD8+ T cells to kill parental C1498. In vivo, C1498/B7-1 grew progressively after subcutaneous injection, whereas C1498/B7-2 completely regressed after transient growth in naive mice. Spontaneous rejection of C1498/B7-2 resulted in immunity to challenge doses of C1498 and C1498/B7-1. Antibody-depletion studies in vivo showed that CD8+ T cells rejected C1498/B7-2, whereas only natural killer cells affected the growth of C1498/B7-1. Two approaches were used to determine whether preferential interaction of B7-1 with CTLA-4 contributed to the failure of C1498/B7-1 to activate CD8+ T cells in vivo. First, CTLA-4 specific monoclonal antibody was used to block B7-1–CTLA-4 interaction. Second, CTLA-4 deletional mutant (−/−) bone marrow chimeras were used as tumor hosts. In both systems, there was a significant increase in the rate of rejection of C1498/B7-1 tumors. Resistance to C1498/B7-1 in CTLA-4−/− hosts was mediated by CD8+ T cells. Blocking or deletion of CTLA-4 did not affect the growth of parental C1498, indicating that B7-1 was important for the induction of CD8+ T-cell immunity in the absence of CTLA-4.
Introduction
Two independent signals are necessary for effective T-cell activation.1-3 Signal 1 is an antigen-specific interaction between the peptide–major histocompatibility complex (MHC) on antigen-presenting cells (APCs) and the T-cell receptor (TCR) on T lymphocytes. Signal 2 (costimulation) is antigen-nonspecific and is delivered by accessory receptors on T cells after their engagement with counter-receptor ligands expressed on APCs, such as activated B cells, macrophages, and dendritic cells.4 Among the most well-studied costimulatory interactions are those between the B7 costimulatory molecules and their counter-receptors, CD28 and CTLA-4 (CD152).
The introduction of genes encoding the B7 molecules into tumor cells and the use of blocking antibodies to CTLA-4 has been therapeutically effective in the enhancement of antitumor immunity,5-9presumably by making the tumor cells more efficient at antigen presentation. However, this approach has given mixed results with respect to whether B7-1 or B7-2 is dominant in facilitating the generation of antitumor reactivity.10-16 In some systems in which B7 expression is inadequate to generate tumor immunity, anti–CTLA-4 monoclonal antibodies (mAbs) have been used to enhance the antitumor response in vivo.9 17 However, the mechanism behind the enhanced tumor immunity in the presence of CTLA-4 mAbs is not completely clear. In the current study, we compared the effects of B7-1 and B7-2 expression on the generation of an antitumor immune response against C1498, a murine myelogenous leukemia. Our results show that B7-1 and B7-2 costimulatory molecules are not equivalent in their ability to induce tumor-specific responses in vivo. C1498 cells expressing B7-2 induced effective CD8+ T-cell–mediated, tumor-specific immunity to wild-type C1498 myeloid leukemia, whereas C1498 cells expressing B7-1 did not. At a low tumor burden, regression of C1498/B7-1 was mediated solely by natural killer (NK)1.1+ cells. C1498/B7-1 failed to activate CD8+ T cells in vivo. Using a CTLA-4–specific blocking mAb and host mice deficient in CTLA-4, we determined that the failure of C1498/B7-1 to induce tumor-specific CD8+ T cells was a result of the specific interaction between B7-1 on the tumor vaccine and CTLA-4 on responding T cells. These data provide new information as to the mechanisms by which the expression of B7-1 or B7-2 may have diverse effects on the induction of antitumor responses in vivo.
Materials and methods
Mice
C57BL/6 (H-2b; Thy 1.2+) (B6), B6.PL-Thy1a (H-2b; Thy 1.1+), and C57BL/6 nu/nu mice (6-10 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). CTLA-4−/− and littermate control CTLA-4+/+,+/− mice (2.5-4 weeks old) were obtained from Elizabeth A. Tivol (Blood Center of Southeastern Wisconsin, Milwaukee).18 All mice were housed in the Medical College of Wisconsin's Animal Resource Center, which is accredited by the American Association for the Accreditation of Laboratory Animal Care.
Characterization of C1498 tumor cell lines
C1498 arose spontaneously in a 10-month-old female C57BL/6 mouse in 1941 and was characterized as a myelomonocytic leukemia.19,20 C1498 lacks cell surface expression of MHC class II, NK1.1, common T-cell, and B-cell markers, but it is positive for MHC class I (Figure 1A), CD1, ICAM-1, ICAM-2, and LFA-1 (data not shown and 21). C1498 is negative for CD80 (B7-1) and CD86 (B7-2) (Figure 1A and data not shown). The C1498 used in this study was obtained from the American Type Culture Collection (ATCC, Rockville, MD). C1498 expressing B7-1 (C1498/B7-1) and B7-2 (C1498/B7-2) were generated as previously reported.16 Briefly, C1498/B7-1 was generated by retroviral transduction, and C1498/B7-2 was generated by plasmid transfection with vectors encoding for B7-1 and B7-2, respectively. Multiple high B7 expressing C1498/B7-1 and C1498/B7-2 lines were isolated and established using limiting-dilution techniques. Multiple clones for each tumor line were expanded in vitro, and frozen stocks were prepared for use in subsequent experiments. Flow cytometric analysis of the 3 tumor lines indicated equivalent cell surface expression of MHC class I molecules and equivalent levels of the B7 ligand on C1498/B7-1 and C1498/B7-2 (Figure 1).
Surface expression of MHC and B7 molecules on tumor lines used in this study.
Expression levels of the MHC class I molecules H-2Kb and H-2Db are shown by shaded histograms; open histograms show background staining with isotype control mAb. Expression of B7-1 and B7-2 (shaded histograms) was detected using CTLA4-Ig + goat anti–human IgG biotin + SA-PE. Open histograms represent control staining with CTLA4-Ig + SA-PE. In addition to using CTLA4 immunoglobulin, tumors used in each experiment were stained directly with anti-CD80 and anti-CD86 mAbs to confirm B7-1 and B7-2 surface expression, respectively (data not shown).
Surface expression of MHC and B7 molecules on tumor lines used in this study.
Expression levels of the MHC class I molecules H-2Kb and H-2Db are shown by shaded histograms; open histograms show background staining with isotype control mAb. Expression of B7-1 and B7-2 (shaded histograms) was detected using CTLA4-Ig + goat anti–human IgG biotin + SA-PE. Open histograms represent control staining with CTLA4-Ig + SA-PE. In addition to using CTLA4 immunoglobulin, tumors used in each experiment were stained directly with anti-CD80 and anti-CD86 mAbs to confirm B7-1 and B7-2 surface expression, respectively (data not shown).
The C1498 cell lines were maintained at 37°C in 5% CO2and were grown in RPMI 1640 with 10% heat-inactivated fetal bovine serum supplemented with 5 × 10−5 M 2-mercaptoethanol (Sigma, St Louis, MO), HEPES buffer (10 mM), sodium pyruvate (1 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), L-glutamine (2 mM), and MEM amino acids (referred to as RPMI-10). All components were obtained from Gibco BRL (Grand Island, NY) unless otherwise specified. C1498/B7-1 was cultured in RPMI-10 and 0.4 mg/mL G418 (Gibco BRL), and C1498/B7-2 was cultured in RPMI-10 and 0.8 mg/mL G418. Cells were passaged every 3 to 4 days and were cultured in vitro for no longer than 30 days before they were re-established from frozen stocks. Experiments were performed using frozen stocks from multiple clones.
Tumor model
Tissue culture–derived tumor cells were routinely checked for the expression of B7 molecules before injection. Host mice were transiently anesthetized with halothane (Halocarbon Laboratories, River Edge, NJ) long enough to arrest movement. Their backs were shaved with clippers, and tumor cells in serum-free medium were injected subcutaneously at the doses indicated into the suprascapular region in a volume of 0.2 mL. Tumor growth was monitored over time on anesthetized mice. Data for tumor size represent the product of 2 perpendicular measurements made with vernier calipers (Bel-Art Products, Pequannock, NJ). Mice were killed when their tumors grew to 200 to 250 mm2 or became ulcerated.
Monoclonal antibodies and flow cytometric analysis
Antibodies specific for the following molecules were used for flow cytometric analysis: H-2Kb (AF6-88.5; biotin), H-2Db (34-2-12; biotin), CD80 (16-10A1; biotin), CD86 (GL1; biotin), CD3ε (145-2C11; phycoerythrin [PE]), CD4 (GK1.5; PE), CD8 (53-6.7; fluorescein isothiocyanate [FITC]), CD11b (M1/70; FITC), CD11c (HL3; FITC), NK1.1 (PK136; PE), CD1 (1B1; biotin), ICAM-1 (3E2; PE), Thy1.2 (30-H21; FITC), Thy1.1 (OX-7; PE), and B220 (RA3-6B2; FITC, Caltag Laboratories, San Francisco, CA). Staining with biotinylated mAbs was followed by PE-conjugated streptavidin (SA-PE) as a second-step reagent. All antibodies were from PharMingen (San Diego, CA) unless otherwise stated. For assessing B7 expression, 1 μg purified CTLA-4 immunoglobulin fusion protein (kindly provided by Arthur Hurwitz, State University of New York, Upstate Medical University, Syracuse) was used with biotinylated goat anti–human immunoglobulin (Sigma) plus SA-PE. Cells were washed 3 times with phosphate-buffered saline (PBS) plus 0.1% sodium azide. Flow cytometry was performed on a Becton Dickinson (San Jose, CA) FACScan flow cytometer.
Mixed-lymphocyte tumor reactions and cell-mediated lysis assays
Single-cell suspensions of splenocytes from naive mice were prepared as previously described.22 Responder splenocytes (2 × 105) were cocultured in round-bottomed 96-microwell plates with 2.0 × 104 irradiated (10 000 cGy) tumor cells. Syngeneic erythroleukemia, FBL-3, was used as control tumor cell targets (kindly provided by Martin Cheever, University of Washington, Seattle, WA). Cultures were incubated in 10% lymphocyte-conditioned medium and 5 IU/mL human recombinant interleukin-2 (rh-IL-2; Chiron, Emeryville, CA) for the first 7 days and in RPMI-10 and 20 IU/mL rh-IL-2 thereafter. Lymphocyte-conditioned medium was RPMI-10 plus 10% Rat T-Stim (Collaborative Biomedical Products, Bedford, MA). Every 5 days, flow cytometry and cell-mediated lysis assays were performed with half of each culture while the remaining responders were restimulated with irradiated tumor cells. Half the media were replaced in each culture every 3 to 4 days.
Standard chromium-release assays were used to assess the tumor-specific cytotoxicity of the responder populations from mixed-lymphocyte tumor reaction (MLTR) cultures as previously described.22Briefly, target cells were labeled with 400 μCi (15 MBq) chromium Cr 51 as sodium chromate for 70 minutes at 37°C. Six wells were set up with targets alone and with targets plus detergent to determine spontaneous release (SR) and maximum release (MR) values, respectively. The percentage of specific lysis was calculated by the formula: % Specific Lysis = 100 (cpm experimental − cpm SR)/(cpm MR − cpm SR)
The number of cells that lysed 30% of the targets per million cells (LU30) was then calculated using the following formula: LU30 = 1 × 106/(no. targets × χ), where χ is the E/T ratio yielding 30% lysis.
In vivo lymphocyte subset depletions
The following mAbs were used in vivo in the form of culture supernatants partially purified from miniPERM bioreactor (Sartorius AG, Göttingen, Germany) by ammonium sulfate precipitation23: anti-CD4 (GK1.5, ATCC), anti-CD8 (2.43, ATCC), anti-NK1.1 (PK136, ATCC), anti-Thy 1.2 (30-H12, ATCC), and anti–CTLA-4 (UC10-4F10-11, ATCC). Protein concentration of each mAb stock was determined using the BCA Protein Assay Kit (Pierce, Rockford, IL).
Mice depleted of CD4+, CD8+, CD4+/CD8+, and NK1.1+ populations received intraperitoneal injections of 250 μg specific mAb on days −6, −4, and −1 before tumor inoculation and every 6 to 7 days, thereafter, for the remainder of the experiment. Anti-CD4 and anti-CD8 depletions routinely gave more than 95% depletion of CD4+and CD8+ T cells from splenocyte populations by flow cytometric analysis. Anti-NK1.1 depletion routinely gave more than 95% abrogation of NK-mediated cell lysis of NK-sensitive YAC-1 murine lymphoma cells.22 Before challenge, randomly selected mice were analyzed by flow cytometry to ensure expected levels of immunodepletion. Mice in control groups were injected with PBS.
In vivo growth kinetics of the tumor lines were measured by injecting tumor cells into immunocompromised animals. Syngeneic B6 mice were depleted of T cells by sublethal total body irradiation (325 cGy γ-irradiation) followed by treatment with cyclophosphamide (150 μg/kg on day −1) and anti-Thy1.2 mAb (500 mg on day +14).
For in vivo blockade studies, mice were injected intraperitoneally with 250 μg anti–CTLA-4 mAb on days −6, −4, −2, and +1. Anti–CTLA-4 mAb (250 μg) was also given subcutaneously with the injection of tumor. Mice in control groups were injected with PBS.
Creation of CTLA-4–deficient and control bone marrow chimeras
Recipient B6 mice were conditioned 24 hours before bone marrow (BM) transplantation with 1100 cGy total body irradiation split into 2 equal doses given 4 hours apart using a Shepherd Mark I cesium irradiator (J.L. Shepherd and Associates, San Fernando, CA). BM was obtained from B6 donor CTLA-4+/+, CTLA-4+/−, and CTLA-4−/− mice by flushing the femurs and tibias with Dulbecco minimum essential medium (Gibco) containing antibiotics, HEPES buffer, and sodium pyruvate. Irradiated mice received a single intravenous injection (0.3 mL) containing 5.0 to 8.5 × 106 viable BM cells. BM chimeras were not injected with tumor cells until 30 days after transplantation to allow time for reconstitution of the peripheral T-cell compartment with marrow-derived cells. Reconstitution with CTLA-4–deficient T cells was confirmed in recipients of CTLA-4−/− BM by flow cytometry of polyclonally activated cells using immobilized anti-CD3 mAb (20 μg/mL; clone 145-2C11, ATCC) in flat-bottomed microwell plates and staining for membrane expression of CTLA-4.
Statistical analysis
Kaplan-Meier survival curves were compared using log-rank statistical analysis. The Fischer exact test was used to analyze the percentage of mice that progressed with tumor in individual experiments. Kruskal-Wallis analysis of variance was used to compare tumor size in the anti–CTLA-4 mAb experiments.
Results
C1498/B7-1 and C1498/B7-2 induce tumor-specific CD8+ cytotoxic T lymphocytes in vitro
Splenocytes from B6 mice were stimulated in MLTR, in the presence of exogenous T-cell growth factors, to compare the ability of C1498/B7-1 and C1498/B7-2 to generate tumor-specific cytotoxic T lymphocytes. Two endpoints were measured: (1) proliferation of CD4+ and CD8+ T-cell subsets and (2) lysis of parental C1498 tumor cells. As shown in Table1, a dramatic increase in the percentage of CD8+ T cells was detected in MLTR cultures stimulated for 5 or 15 days with either C1498/B7-1 or C1498/B7-2. Selective stimulation of CD8+ T cells is in accordance with the fact that C1498 is MHC class II-negative (data not shown). The percentage of CD8+ T cells stimulated with parental C1498 increased over time, but not to the level of cultures stimulated with B7-expressing tumor cells (day 15, 54% vs 85%-88%). C1498/B7-1 consistently induced greater lytic activity in vitro than C1498/B7-2 (Table 1 and additional data not shown). Although the precise nature of the tumor antigens on C1498 is unknown, the lytic activity increased in favor of the specific target (C1498) compared with that of a syngeneic control erythroleukemia (FBL-3). MLTR established with parental C1498 tumor cells as stimulators failed to reach the same lytic potential found in the B7-costimulated cultures. These data indicate that C1498/B7-1 and C1498/B7-2 were superior to parental C1498 in their ability to activate tumor-specific CD8+ CTL in vitro.
C1498/B7-1 and C1498/B7-2 induce tumor-specific CD8+ CTL in vitro
| Days in culture* . | Day 0 . | Day 5 . | Day 15 . | ||||
|---|---|---|---|---|---|---|---|
| Stimulator† . | — . | C1498 . | C1498/B7-1 . | C1498/B7-2 . | C1498 . | C1498/B7-1 . | C1498/B7-2 . |
| %CD4+ | 16.6 | 32.2 | 8.2 | 9.4 | 26.1 | 1.8 | 2.3 |
| %CD8+ | 11.4 | 20.7 | 31.5 | 40.2 | 53.5 | 88.0 | 85.3 |
| LU30 vs C1498‡ | 0.0 | 0.0 | 248.8 | 60.9 | 52.3 | 642.0 | 501.3 |
| LU30 vs FBL-3 | 0.0 | 0.0 | 92.9 | 17.4 | 0.02 | 18.1 | 11.6 |
| Days in culture* . | Day 0 . | Day 5 . | Day 15 . | ||||
|---|---|---|---|---|---|---|---|
| Stimulator† . | — . | C1498 . | C1498/B7-1 . | C1498/B7-2 . | C1498 . | C1498/B7-1 . | C1498/B7-2 . |
| %CD4+ | 16.6 | 32.2 | 8.2 | 9.4 | 26.1 | 1.8 | 2.3 |
| %CD8+ | 11.4 | 20.7 | 31.5 | 40.2 | 53.5 | 88.0 | 85.3 |
| LU30 vs C1498‡ | 0.0 | 0.0 | 248.8 | 60.9 | 52.3 | 642.0 | 501.3 |
| LU30 vs FBL-3 | 0.0 | 0.0 | 92.9 | 17.4 | 0.02 | 18.1 | 11.6 |
Naive splenocytes were cocultured with irradiated C1498, C1498/B7-1, or C1498/B7-2 stimulator cells in MLTR cultures. Every 5 days, half the contents of the culture wells was harvested for phenotypic analysis and lytic assays.
Cells remaining in the culture wells were restimulated with 2 × 104 irradiated C1498, C1498/B7-1, or C1498/B7-2 stimulator cells.
LU30 = number of lytic units per 1 × 106 cells. Data are representative of 3 separate experiments.
C1498/B7-2, but not C1498 or C1498/B7-1, induces effective antitumor immunity in vivo
To assess the effect of B7-1 and B7-2 costimulatory molecules on the induction of an antitumor response in naive mice, immunocompetent B6 mice were given live tumor cells by subcutaneous injection. As shown in Figure 2, the mean survival time (MST) for mice injected with C1498/B7-1 was significantly longer (P < .005) than for those given the parent C1498 line (32 ± 8 and 23 ± 3 days, respectively). However, all mice injected with C1498/B7-1 eventually died or were killed because of progressive tumor growth. In contrast, none of the mice injected subcutaneously with C1498/B7-2 had progressive tumors. Differences in tumor progression with C1498/B7-1 and C1498/B7-2 were not the result of loss of expression of B7-1 in vivo because flow cytometric analysis of single-cell suspensions prepared from excised tumors in this and subsequent experiments showed no decrease in B7-1 expression in vivo (data not shown).
C1498/B7-2, but not C1498 or C1498/B7-1, induced effective tumor rejection in vivo.
Individual C57BL/6 mice were injected subcutaneously with 1 × 106 live C1498 (n = 16), C1498/B7-1 (n = 13), or C1498/B7-2 (n = 13) tumor cells into the suprascapular region. Survival curves represent the combined results of 4 separate experiments in which all groups were included. Mice that did not die with tumor were killed when the tumor mass was larger than 200 mm2. The lone death on day 50 in the C1498/B7-2 group was not caused by tumor growth. P values represent statistical significance with respect to the C1498-injected group (log-rank test).
C1498/B7-2, but not C1498 or C1498/B7-1, induced effective tumor rejection in vivo.
Individual C57BL/6 mice were injected subcutaneously with 1 × 106 live C1498 (n = 16), C1498/B7-1 (n = 13), or C1498/B7-2 (n = 13) tumor cells into the suprascapular region. Survival curves represent the combined results of 4 separate experiments in which all groups were included. Mice that did not die with tumor were killed when the tumor mass was larger than 200 mm2. The lone death on day 50 in the C1498/B7-2 group was not caused by tumor growth. P values represent statistical significance with respect to the C1498-injected group (log-rank test).
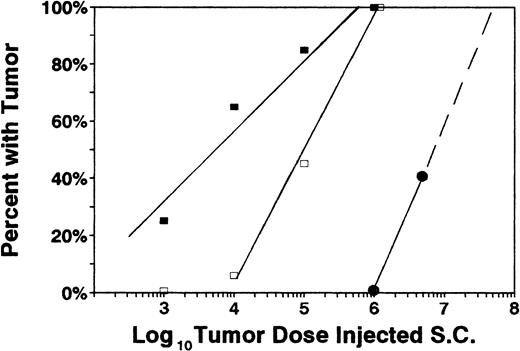
To quantify the effect of B7-1 and B7-2 molecules on tumor resistance, dose titration experiments were conducted (Figure3). Minimal lethal dose (MLD) for the parental C1498 tumor was calculated to be approximately 6.0 × 105 cells by subcutaneous injection. C1498/B7-1 cells were rejected more efficiently than the parental line (MLD, approximately 1.0 × 106) but were rejected almost 2 orders of magnitude less efficiently than C1498/B7-2 (MLD > 6.0 × 107).
Dose titration of individual tumor lines.
Various doses of tumor cells were subcutaneously injected into naive recipients: 5 × 106 (5 mice/group), 1 × 106 (8 mice/group), 1 × 105 (10 mice/group), 1 × 104 (10 mice/group), and 1 × 103 (10 mice/group). Percentages of mice that developed and progressed with tumor are indicated for each challenge dose. ■ = C1498, □ = C1498/B7-1, and ● = C1498/B7-2. All mice that progressed with tumor died or were electively killed because of tumor size larger than 250 mm2. Combined results of 3 independent experiments are shown.
Dose titration of individual tumor lines.
Various doses of tumor cells were subcutaneously injected into naive recipients: 5 × 106 (5 mice/group), 1 × 106 (8 mice/group), 1 × 105 (10 mice/group), 1 × 104 (10 mice/group), and 1 × 103 (10 mice/group). Percentages of mice that developed and progressed with tumor are indicated for each challenge dose. ■ = C1498, □ = C1498/B7-1, and ● = C1498/B7-2. All mice that progressed with tumor died or were electively killed because of tumor size larger than 250 mm2. Combined results of 3 independent experiments are shown.
To rule out the possibility that regression of C1498/B7-2 and progression of C1498/B7-1 was simply caused by altered growth kinetics in vivo, groups of immunocompromised B6 mice (see “Materials and methods”) were injected with each tumor construct (1 × 106 tumor cells, 3 per group), and tumor-free survival was measured. All tumor lines grew at a rate that killed all immunosuppressed mice within 15 to 23 days after injection: MST (±SD) for C1498 = 21.0 (± 1.7) days; for C1498/B7-1 = 20.3 (± 4.6) days; and for C1498/B7-2 = 21.0 (± 0.0) days (P > .05). Similar results were found in a parallel experiment using B6 nu/nu mice that lacked functional T cells as hosts (5 per group): C1498 = 18.8 (± 2.4) days; C1498/B7-1 = 19.0 (± 3.0) days; and C1498/B7-2 = 23.8 (± 2.6) days (P > .05). As a note, we observed equivalent growth kinetics and rejection rates in vivo for vector-alone tumor lines compared with wild-type C1498, indicating that the tumor cells were not significantly altered by the method of gene transfer (data not shown). Notably, the in vitro (Table 1) and in vivo (Figure 2) data are paradoxical. In vitro, C1498/B7-1 is more efficient in eliciting CTL responses than C1498/B7-2, whereas opposite results are obtained with respect to the capacity of these transfectants to induce CTL immunity in vivo. In view of these observations, we examined the effector cells involved in the response to B7-1+ and B7-2+C1498 in vivo.
CD8+ T cells are responsible for the eradication of C1498/B7-2 but not C1498/B7-1
To characterize the immune effector cell(s) responsible for the antitumor response induced by C1498/B7-2, T-cell subsets and NK cells were depleted in vivo using mAbs before the injection of C1498/B7-2. Depletion of CD8+ (or both CD4+ and CD8+) T cells, but not CD4+ T cells or NK cells alone, resulted in progressive tumor growth (Table2), indicating that CD8+ T cells were required for the regression of C1498/B7-2. Similar depletion experiments were performed with mice given C1498/B7-1 (Table 2). Because 1 × 106 C1498/B7-1 cells were uniformly lethal to naive mice, a dose of 1 × 105 tumor cells was used (approximately LD40) to allow us to detect a change in tumor lethality. Depletion of NK1.1+ cells led to significant tumor progression after injection of a moderate dose of C1498/B7-1 in vivo. CD8+ T cells did not contribute to the rejection of C1498/B7-1 because there was no increase in the percentage of tumor progressors compared with untreated controls. Although others have shown a role for CD4+ T cells in the eradication of MHC II-negative tumors, CD4+ cells did not play a significant role in the rejection of B7-1 or B7-2 expressing C1498.
Elimination of C1498/B7-2 dominated by CD8+ T cells and resistance to C1498/B7-1-mediated by NK cells alone
| Depleted cells . | No. tumor progressors/injected mice (%)* . | |
|---|---|---|
| C1498/B7-2 (106) . | C1498/B7-1 (105) . | |
| None | 0/8 (0) | 5/11 (45) |
| CD4 | 0/8 (0) | 2/5 (40) |
| CD8 | 8/8 (100)† | 5/12 (42) |
| CD4 + CD8 | 8/8 (100)† | Not done |
| NK1.1 | 0/8 (0) | 11/11 (100)‡ |
| Depleted cells . | No. tumor progressors/injected mice (%)* . | |
|---|---|---|
| C1498/B7-2 (106) . | C1498/B7-1 (105) . | |
| None | 0/8 (0) | 5/11 (45) |
| CD4 | 0/8 (0) | 2/5 (40) |
| CD8 | 8/8 (100)† | 5/12 (42) |
| CD4 + CD8 | 8/8 (100)† | Not done |
| NK1.1 | 0/8 (0) | 11/11 (100)‡ |
Percentage of mice that died or were killed because of progressive tumor growth after subcutaneous injection with 1 × 106 C1498/B7-2 or 1 × 105 C1498/B7-1 tumor cells. Data from two independent experiments were pooled.
Significantly different from the None, CD4, and NK1.1-depleted groups (Fischer exact test; P < .001).
Significantly different from the None, CD4, and CD8-depleted groups (Fischer exact test; P < .02).
C1498/B7-2 induces long-lasting immunologic memory
In some tumor models, B7-1 expression has been shown to induce effective short-term antitumor responses but not long-term immunity.6,24 25 Therefore, we performed tumor challenge experiments to determine whether the rejection of C1498/B7-2 resulted in systemic immunity to C1498. Live C1498/B7-2 tumor cells (1 × 106) were injected subcutaneously into naive B6 mice for immunization. After 16 to 22 weeks, primed mice were challenged with a subcutaneous injection of 1 × 106wild-type C1498 tumor cells in a different location. As shown in Table3, C1498/B7-2 induced long-lasting systemic immunity to the wild-type tumor. In a second experiment (Table3), mice that had rejected viable C1498/B7-2 tumor cells and mice that had rejected C1498/B7-2 and a challenge dose of wild-type C1498 were subsequently rechallenged with 1 × 106 C1498/B7-1 tumor cells. In every case (n = 16), mice vaccinated with C1498/B7-2 rejected a lethal dose of C1498/B7-1. Thus, C1498/B7-2 induced long-term immunity against C1498 and C1498/B7-1 tumor cells. Furthermore, though 106 C1498/B7-1 was lethal to naive B6 mice (Figure 3), it was rejected in mice with memory T cells (Table 3), indicating that it is not inherently resistant to effector T cells and that vector-specific T cells were unlikely to be responsible for the eradication of either tumor in vivo.
C1498/B7-2 induced immunologic memory against C1498 and C1498/B7-1
| Priming tumor3-150 . | Challenge tumor (1 × 106cells)3-151 . | No. tumor progressors/injected mice (%)3-152 . | MST ± SD (d)3-153 . |
|---|---|---|---|
| None | C1498 | 5/5 (100) | 27 ± 7 |
| C1498/B7-2 | C1498 | 2/14 (14)3-155 | 19 ± 1 |
| None | C1498/B7-1 | 6/6 (100) | 34 ± 5 |
| C1498/B7-2 | C1498/B7-1 | 0/5 (0)3-155 | — |
| C1498/B7-2 and C1498 | C1498/B7-1 | 0/12 (0)3-155 | — |
| Priming tumor3-150 . | Challenge tumor (1 × 106cells)3-151 . | No. tumor progressors/injected mice (%)3-152 . | MST ± SD (d)3-153 . |
|---|---|---|---|
| None | C1498 | 5/5 (100) | 27 ± 7 |
| C1498/B7-2 | C1498 | 2/14 (14)3-155 | 19 ± 1 |
| None | C1498/B7-1 | 6/6 (100) | 34 ± 5 |
| C1498/B7-2 | C1498/B7-1 | 0/5 (0)3-155 | — |
| C1498/B7-2 and C1498 | C1498/B7-1 | 0/12 (0)3-155 | — |
C1498 challenge experiment: C1498/B7-2 “primed” B6 mice had rejected a subcutaneous challenge of 1 × 106live C1498/B7-2 tumor cells 16 to 22 weeks before this experiment. On day 0, a group of naive B6 mice and the C1498/B7-2-primed mice were challenged subcutaneously with 1 × 106 parental C1498 tumor cells.
C1498/B7-1 challenge experiment: C1498/B7-2-primed B6 mice had rejected a subcutaneous challenge of 1 × 106 C1498/B7-2 tumor cells 11 weeks earlier, whereas B6 mice primed to both C1498/B7-2 and C1498 had rejected challenges of live C1498/B7-2 (1 × 106) 36 to 43 weeks earlier and of live C1498 tumor cells (1 × 106) 21 weeks earlier. On day 0 of this experiment, naive B6 mice and primed mice noted above were challenged subcutaneously with C1498/B7-1.
C57BL/6 mice were injected subcutaneously with 1 × 106 live tumor cells, as indicated.
Percentages of mice that developed progressive tumors within 60 days after tumor injection.
MST of mice that developed tumor.
Significantly different from the naive control groups challenged with either C1498 or C1498/B7-1 (Fischer exact test;P ≤ .002).
CTLA-4 blockade leads to increased tumor-free survival and delays the growth of C1498/B7-1 in vivo
Because C1498/B7-1 stimulated naive CD8+ T cells in vitro to an extent comparable to that of C1498/B7-2 (Table 1)—but failed to prime T cells in vivo, in contrast to C1498/B7-2 (Table2)—we sought to determine whether a negative signal might have down-regulated the response in vivo. CTLA-4 blockade has been shown to potentiate antitumor immunity of both B7− and B7+ tumors. However, the exact mechanism by which this occurs remains unclear. To determine whether blockade of CTLA-4 restored a tumor response to C1498/B7-1, we injected CTLA-4–specific mAb into naive B6 mice that were challenged with a lethal dose of C1498/B7-1.
Treatment of naive B6 mice with anti–CTLA-4 mAb reduced the overall percentage of mice with progressive tumors and significantly slowed the growth rate of C1498/B7-1 (Figure 4B) compared with PBS-treated C1498/B7-1 controls (Figure 4A). On day 17 after injection, 40% of the anti–CTLA-4 treated mice were tumor-free compared with 0% of controls. All untreated mice died of progressive tumor by day 37 or 38 after injection, whereas 32% of the antibody-treated mice remained tumor-free. Anti–CTLA-4 mAb-treated mice with no evidence of tumor remained tumor-free for more than 60 days after injection. The rate of tumor growth in the anti–CTLA-4 mAb-treated animals also was slower than in control animals. By day 19 after injection, 35% of the antibody-treated animals had tumors larger than 75 mm2 compared with 90% of control mice. Similar experiments were performed using wild-type C1498. Anti–CTLA-4 mAb had no apparent effect on the parental tumor (Figure 4C). PBS treatment also had no effect on the growth of the parental C1498 tumor (data not shown). Thus, the anti–CTLA-4 mAb did not induce nonspecific T-cell activation, and costimulation through B7-1 was necessary for the induction of an anti–C1498 immune response when CTLA-4 was blocked.
Blockade of CTLA-4 in vivo with mAb led to enhanced regression of C1498/B7-1 myeloid leukemia.
C57BL/6 mice were injected intraperitoneally with PBS (A) or anti–CTLA-4 mAb (B, C) before subcutaneous injections of 1 × 106 tumor cells (C1498/B7-1 in A and B and C1498 in C). Tumor growth was monitored over time, and mice that did not die directly because of tumor growth were killed when the tumor mass became greater than 250 mm2. Each circle represents an individual mouse. Black bars represent median tumor size at each time point. *Mice that died and were censored from the data. As a group, the anti–CTLA-4 mAb-treated mice (B) had significantly smaller tumors than mice in the control groups (A, C) at every time point (P ≤ .05, Kruskal-Wallis analysis of variance). Tumor growth plots represent the combined results of 2 separate experiments.
Blockade of CTLA-4 in vivo with mAb led to enhanced regression of C1498/B7-1 myeloid leukemia.
C57BL/6 mice were injected intraperitoneally with PBS (A) or anti–CTLA-4 mAb (B, C) before subcutaneous injections of 1 × 106 tumor cells (C1498/B7-1 in A and B and C1498 in C). Tumor growth was monitored over time, and mice that did not die directly because of tumor growth were killed when the tumor mass became greater than 250 mm2. Each circle represents an individual mouse. Black bars represent median tumor size at each time point. *Mice that died and were censored from the data. As a group, the anti–CTLA-4 mAb-treated mice (B) had significantly smaller tumors than mice in the control groups (A, C) at every time point (P ≤ .05, Kruskal-Wallis analysis of variance). Tumor growth plots represent the combined results of 2 separate experiments.
Absence of CTLA-4 in vivo leads to CD8+T-cell–mediated regression of C1498/B7-1
Use of mAb to block cell–cell interactions in vivo may be problematic, especially in a dynamic system in which timing may be critical.26 To more efficiently negate the effects of CTLA-4, we created BM chimeras in which the responding T cells lacked CTLA-4 and used them as tumor hosts. BM from CTLA-4 knockout (CTLA-4−/−) B6 mice was transplanted into irradiated naive B6 recipients. This allowed us to use adult mice as hosts without concern for the lymphoproliferative syndrome, which is lethal to young CTLA-4−/− mice.18,27 28 Approximately 30 days after transplantation, C1498 and C1498/B7-1 tumor cells (1 × 106) were injected subcutaneously into the CTLA-4–deficient BM chimeras, and tumor growth was monitored. Results (Table 4) show that recipients of CTLA-4−/− BM given C1498/B7-1 had a significant (P < .001) increase in tumor-free survival rate compared with mice that were reconstituted with either CTLA-4+/+ or CTLA-4+/− BM. In addition, C1498/B7-2 was injected into CTLA-4+/+ BM chimeras to ensure that the tumors behaved the same way in animals that underwent transplantations as they did in intact B6 mice. In every case, C1498/B7-2 was rejected (data not shown).
Absence of CTLA-4 in vivo leads to CD8+T-cell-mediated C1498/B7-1 regression at tumor cell numbers that normally lead to tumor progression
| Tumor hosts4-150 . | Tumor injected4-151 . | CD8+depletion4-151 . | No. tumor progressors/mice injected (%)‡ . |
|---|---|---|---|
| CTLA-4− BM chimeras | C1498/B7-1 | No | 7/24 (29)4-153 |
| CTLA-4− BM chimeras | C1498/B7-1 | Yes | 7/7 (100) |
| CTLA-4+ BM chimeras | C1498/B7-1 | No | 21/23 (91) |
| CTLA-4+ BM chimeras | C1498/B7-1 | Yes | 7/7 (100) |
| Normal B6 mice | C1498/B7-1 | No | 7/7 (100) |
| CTLA-4− BM chimeras | C1498 | No | 7/7 (100) |
| CTLA-4+ BM chimeras | C1498 | No | 4/4 (100) |
| Normal B6 mice | C1498 | No | 7/7 (100) |
| Tumor hosts4-150 . | Tumor injected4-151 . | CD8+depletion4-151 . | No. tumor progressors/mice injected (%)‡ . |
|---|---|---|---|
| CTLA-4− BM chimeras | C1498/B7-1 | No | 7/24 (29)4-153 |
| CTLA-4− BM chimeras | C1498/B7-1 | Yes | 7/7 (100) |
| CTLA-4+ BM chimeras | C1498/B7-1 | No | 21/23 (91) |
| CTLA-4+ BM chimeras | C1498/B7-1 | Yes | 7/7 (100) |
| Normal B6 mice | C1498/B7-1 | No | 7/7 (100) |
| CTLA-4− BM chimeras | C1498 | No | 7/7 (100) |
| CTLA-4+ BM chimeras | C1498 | No | 4/4 (100) |
| Normal B6 mice | C1498 | No | 7/7 (100) |
Normal B6 or Thy1 congenic B6.PL-Thy1amice were conditioned with 1100 cGy of γ-radiation 1 day before intravenous infusion of 5.0 to 8.5 × 106 BM cells from CTLA-4− (−/−) or CTLA-4+ donors (either +/− or +/+).
Mice were injected subcutaneously with 1 × 106 tumor cells.
Mice were observed up to 70 to 96 days after bone marrow transplantation (approximately 40-60 days after tumor injection).
Significantly lower than in CTLA-4+ BM chimeras (P < .001) and normal B6 mice given C1498/B7-1 as well as CTLA-4− BM chimeras given C1498 (P < .005) (Fischer exact test).
Analysis of lymphocytes from the spleens of 6 mice that received transplants of CTLA-4+ BM and 6 with CTLA-4−/− BM showed equivalent absolute numbers of T and B cells: whole spleen, 1.1 (± 0.2) × 108 versus 1.2 (± 0.4) × 108; CD3+ cells, 3.1 (± 2.1) × 107 versus 3.4 (± 1.5) × 107; CD4+ cells, 2.1 (± 1.1) × 107 versus 2.6 (± 1.0) × 107; CD8+ cells, 7.9 (± 3.8) × 106 versus 7.8 (± 3.8) × 106; B220+ B cells, 6.8 (± 1.5) × 107 versus 5.2 (± 1.8) × 107. Thus, quantitative differences in lymphoid populations did not account for the increased rate of tumor rejection in CTLA-4–deficient hosts. Peripheral T-cell chimerism in a Thy 1–congenic transplant model was used to confirm engraftment of CTLA-4−/− T cells. T cells in the spleens of B6.PL-Thy 1a mice (Thy 1.1+) given marrow from Thy 1-congenic CTLA-4−/− (Thy 1.2+) donors were predominantly Thy 1.2+ (75.6% ± 7.4%)—ie, donor derived. A small population of radioresistant host T cells (24.4% ± 7.4%) persisted in these syngeneic chimeras because there was no graft-versus-host reactivity. It has been shown that CTLA-4–deficient cells fail to induce lymphoproliferative disease in the presence of normal T cells.28 The small proportion of normal T cells may “regulate” the lymphoproliferation of T cells deficient in CTLA-4.
CD8+ T cells were responsible for the eradication of C1498/B7-1 in CTLA-4−/− BM chimeras (Table 4). As was the case with C1498/B7-2 in normal B6 mice (Table 2), CD8 immunodepletion before the injection of C1498/B7-1 tumor cells resulted in a significant increase in tumor progression. Thus, C1498/B7-1 was able to elicit a CD8+ T-cell–mediated antitumor response against a large tumor cell dose, but only in the absence of CTLA-4 on responding CD8+ T cells.
Discussion
The data presented in this report show that the costimulatory molecules B7-1 and B7-2 differ in their ability to induce an immune response to C1498, a murine myelogenous leukemia, in vivo. Although both C1498/B7-1 and C1498/B7-2 induced tumor-specific CTL in vitro (Table 1), only B7-2+ C1498 tumor cells induced an effective CD8-dependent T-cell response in vivo. Although low doses of C1498/B7-1 were eliminated by NK effector cells, an effective CD8+ T-cell–specific response was not induced in vivo, and higher doses of C1498/B7-1 easily overwhelmed the NK-mediated response. Our data show that C1498/B7-1 escaped a CD8+T-cell–mediated immune response through the negative interaction of B7-1 on tumor cells with CTLA-4 on responding T cells. If this interaction was prevented, either by blocking CTLA-4 with specific mAb (Figure 4) or by genetically deleting CTLA-4 from responding T cells, C1498/B7-1 initiated a CD8+ T-cell–mediated antitumor response in vivo (Table 4).
The inability of C1498/B7-1 to induce CD8+ CTL in vivo contrasts dramatically with its ability to induce tumor-specific CTL in MLTR cultures supplemented with exogenous IL-2 (Table 1). IL-2 is known to re-establish IL-2 receptor expression and proliferation in T cells that have undergone CTLA-4 ligation.3,29 CTLA-4 ligation inhibits CD28-dependent IL-2 production, but it does not prevent IL-2 responsiveness.29 In the presence of exogenous IL-2, CTLA-4–induced down-regulation of the T-cell response may have been circumvented, allowing for the activation and expansion of tumor-specific CD8+ CTL in vitro. Because exogenous IL-2 was not provided in vivo, the failure of C1498/B7-1 to generate a CD8+ T-cell response typically driven by IL-2 help may be a plausible explanation for these findings. Therefore, the ability of some tumor cells to activate CD4+ T-helper cells in vivo may alter the outcome of B7-1–CTLA-4 interactions. This may account for some of the discrepancies with different tumors and experimental models.
The expression of B7-1 on C1498 may have induced a state of functional unresponsiveness within the CD8+ T-cell population. Whether this resulted from anergy induction, apoptosis, or some other form of functional or deletional tolerance30,31 is unknown. In previous work by one of us (B.R.B.), syngeneic BM chimeras given C1498/B7-1 and a tumor-specific CD8+ CTL line immediately after bone marrow transplantation had lower survival than mice given either C1498 or C1498/B7-2 and the same T-cell line, implying that B7-1 impaired the activity of the tumor-specific T cells in vivo.16 The outgrowth of C1498/B7-1 in the current study was not simply the result of high B7-1 surface expression, because C1498/B7-1 expressing lower levels of B7-1 (ie, mean fluorescence intensity 399.6 vs 899.2) routinely progressed in naive animals (data not shown).
Our results differ from those of some investigators who report B7-1 to be effective at generating an antitumor immune response.10,11 In contrast to our data, B7-1 was shown by Matulonis et al10 to be superior to B7-2 in its ability to protect from a challenge of wild-type tumor and the eradication of minimal residual tumor. Gajewski11 reported that B7-1, but not B7-2, induced P815 mastocytoma-specific CTL in vitro. Additionally, B7-1 on EL-4 T-cell lymphoma cells has been shown to induce CD8+ antitumor CTL, whereas B7-2 on the same tumor cells not only failed to activate antitumor CTL but actively suppressed B7-1–induced antitumor immunity.32-34
Evidence suggesting the superiority of B7-1 over B7-2 in CD8+ T-cell activation in some tumor models is based on data from in vivo T-cell subset depletion experiments.7,35However, a contributing role for NK cells in tumor eradication was not completely ruled out in these studies. In fact, Wu et al25report that B7-1 expression on tumor cells fails to induce systemic immunity because of the activation of NK cells rather than CD8+ CTL. In another study, tumor-infiltrating lymphocytes isolated from a B7-1–transfected tumor were found to be primarily nonspecific killers (NK cells), and no CD8+ CTLs were detected.36 Others6,24,25 have reported that, although mice injected with B7-1+ tumors survived tumor free, long-lasting protective immunity against a subsequent challenge of wild-type tumor was not generated. In one study, immunization with B7-1+ tumors induced cross-protection against unrelated tumors,6 suggesting to the authors that nonspecific, NK-cell–mediated antitumor reactivity rather than tumor-specific CD8+ T cells was induced by B7-1.
The heterogeneous effects of B7-1 and B7-2 found in different model systems may depend on the inherent immunogenicity of the tumor in question and the nature of the dominant host immune response to the parental line.37 Martin-Fontecha et al14 found that B7-1 and B7-2 were equally effective at establishing antitumor immunity against immunogenic lymphoma cells, but B7-2 provided superior protective immunity in nonimmunogenic adenocarcinoma and melanoma tumor models.
It is a remote possibility that C1498/B7-1 and C1498/B7-2 express antigens specific to the vector used to introduce the B7 molecules into the tumor cells, even though animals challenged with vector control tumors all die of overwhelming tumor burden (data not shown). However, mice challenged with live C1498 or C1498/B7-1 after the spontaneous rejection of C1498/B7-2 did not develop tumors (Table 3). Additionally, in vitro activation of T cells with C1498/B7-1 or C1498/B7-2 led to an equal ability to lyse parental C1498 tumor cells that have no exogenously added genes (Table 1). Collectively, these data indicate that the B7+ tumors induced immunity against C1498-specific rather than vector-specific antigens. To this date, the tumor-specific antigens of C1498 are unknown. Regardless of the nature of these antigens, in the tumor model used in this study, the in vivo interaction between B7-1 and B7-2 on the tumor cells and CD28 and CTLA-4 on responding T cells dictated whether an antitumor response was generated.
Clearly, based on our data and those of others, B7-1 and B7-2 are not redundant costimulatory molecules.13,14,32-34 In some systems, not only was B7-2 superior to B7-1 in the induction of antitumor immunity, B7-1 induced a dominant-negative effect over B7-2–mediated costimulation when both B7 molecules were expressed on the same tumor cells.13,14 B7-1–mediated down-regulation of the immune response did not occur in trans, indicating that the B7 molecules must have simultaneously ligated receptors on the same T cell for B7-1 to be dominant over B7-2.13 This is consistent with our results in mixed tumor experiments (data not shown). Chai et al38 report that the inhibitory effect on the activation of naive transgenic CD8+ T cells in vitro was caused by the ligation of CTLA-4 with B7-1. This suggested that the up-regulation of B7-1 on T cells over time inhibited costimulation, which was initially provided by B7-2+ APCs in the cultures. Lastly, using the T-lymphoma line BW-Sp3, Raes et al39found that immunotherapy with B7-1 transfectants led to the tolerization of antitumor T cells in vivo and actually increased tumor growth above control tumor. This promotion of tumor progression, as in our study, was reversed with the in vivo blockade of CTLA-4.40
In addition to inherent immunogenicity and ability to activate T-helper cells, the growth kinetics of different tumor lines may play a role in determining the effectiveness of either B7-1 or B7-2 in eradicating tumor. Slow-growing tumors may benefit from B7-1– or B7-2–mediated antitumor responses. Lysis of tumor cells by either NK or CD8+ effector cells might allow for antigen uptake and presentation of tumor antigens by host APCs expressing B7 molecules, thus amplifying the antitumor response. In fact, B7-1 and B7-2 expression on host-derived APCs has been shown to be critical in the establishment of CD8+ T-cell–dependent immunity to B7-1+ tumor cells.41 42
Recent evidence suggests that B7-1 and B7-2 deliver different signals to the responding T cell, including quantitatively and qualitatively distinct signals after ligation with CD28.43,44Additionally, B7-1 and B7-2 differentially regulate the expression of CD28 on the surface of activated T cells.45 Molecular analyses also indicate that B7-1 and B7-2 bind to distinct, and shared, determinants on CTLA-4 and CD28.46-48 B7-1 has greater affinity for CTLA-4 than does B7-2, and B7-1–CTLA-4 complexes dissociate more slowly than do B7-2–CTLA-4 complexes.49-51 Therefore, B7-1 may differ from B7-2 in the signal it generates through its ligation with CTLA-4, and the biologically relevant role of B7-1 may be to induce a negative signal to the T cell.52 In support of this interpretation, Chai et al53 showed that B7-1, and not B7-2, up-regulation on activated T cells leads to antigen unresponsiveness through its interaction with CTLA-4 on neighboring T cells.
The data presented here are consistent with the notion that an important role of B7-2 in vivo lies in the initiation of a T-cell–mediated immune response, whereas B7-1 contributes more to the down-regulation of T-cell activation through its interaction with CTLA-4. Such a model of B7-mediated costimulation is supported by the temporal and spatial expression patterns of B7-1 and B7-2 and the molecular signaling events they induce. The influence of these molecules on the efficacy of tumor vaccines may vary depending on immunogenicity and other biologic properties of tumor cells.
Supported by United States Public Health Service grants CA39854 (R.L.T.) and CA72669 (B.R.B.) from the National Cancer Institute; program project grant AI-35225 from the National Institute for Allergy and Infectious Diseases; Midwest Athletes Against Childhood Cancer Fund (Milwaukee, WI); and Cancer Center of the Medical College of Wisconsin. J.L.L. was supported by American Cancer Society institutional research grant 86-004-14.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert L. Truitt, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: rtruitt@mcw.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal