Abstract
All-trans retinoic acid (ATRA) is a potential therapeutic agent for the treatment of hematopoietic malignancies, because of its function as an inducer of terminal differentiation of leukemic blasts. Although the efficacy of ATRA as an anticancer drug has been demonstrated by the successful treatment of acute promyelocytic leukemia (APL), the molecular mechanisms of ATRA-induced cell cycle arrest of myeloid cells have not been fully investigated. In this study, we show that the onset of ATRA-induced G0/G1 arrest of human monoblastic U-937 cells is linked to a sharp down-regulation of c-Myc and cyclin E levels and an increase in p21WAF1/CIP1 expression. This is followed by an increase in p27Kip1 protein expression due to enhanced protein stability. The importance of an early decrease in Myc expression for these events was demonstrated by the failure of a U-937 subline with constitutive exogenous expression of v-Myc to cell cycle arrest and regulate cyclin E and p27Kip1 in response to ATRA. Preceding the initiation of G1 arrest, a transient rise in retinoblastoma protein (pRb), p107, and cyclin A levels was detected. Later, a rapid fall in the levels of cyclins A and B and a coordinate dephosphorylation of pRb at Ser780, Ser795, and Ser807/811 coincided with the accumulation of cells in G1. These results thus identify a decrease in c-Myc and cyclin E levels and a posttranscriptional up-regulation of p27Kip1 as important early changes, and position them in the complex chain of events regulating ATRA-induced cell cycle arrest of myeloid cells.
Introduction
Hematopoietic tumors often arise as a consequence of uncontrolled proliferation of immature blasts, failing to terminally differentiate into mature blood cells.1 A hallmark of terminal differentiation is an irreversible arrest in the G0/G1 phase of the cell cycle. This arrest involves the coordinate regulation of signals that negatively control the cell cycle machinery and inhibit the G1/S transition. At the heart of the cell cycle lies the regulation of cyclin-dependent kinases (CDKs), which control the phosphorylation of several substrates, including retinoblastoma protein (pRb) family members, and thus the transcriptional activation or repression of E2F target genes important for cell cycle progression.2-4 The G1/S transition is believed to be sequentially controlled by D-type cyclins, which activate CDK4 and CDK6, and subsequently by cyclin E, which associates with CDK2.5 CDK activity is dependent on association with cyclins and subsequent phosphorylation-dephosphorylation events, and may be inhibited by 2 different groups of CDK inhibitors (CKIs), namely the Ink4 family and the Cip/Kip family.6 Thus, growth arrest associated with differentiation could be achieved by several mechanisms including the down-regulation of cyclins or up-regulation of CKIs or both. The critical events leading to a cell cycle arrest are, however, likely to be specific for each mode of induction of differentiation and may differ for each particular cell type.
Proliferation and differentiation of hematopoietic cells can be regulated by a number of physiologic agents, including all-trans retinoic acid (ATRA). ATRA treatment triggers terminal differentiation and growth arrest of several established human myeloid cell lines in vitro and has also proven to be effective in the clinical treatment of acute promyelocytic leukemia (APL) by inducing differentiation and apoptosis of the immature blasts.7,8Although the biologic effects of ATRA are well characterized, the molecular mechanisms regulating these processes are largely unknown. The cell cycle arrest associated with ATRA-induced differentiation may involve regulation of the expression of both cyclins and CKIs, affect phosphorylation status of CDKs, and ultimately trigger dephosphorylation of pRb pocket proteins. However, although previous studies on ATRA-induced growth arrest of myeloid cells have implicated individual proteins, for example, p21WAF1/CIP1,9-11 a more complete investigation of the regulation of the cell cycle machinery is required to understand the relative importance of different events during the differentiation process.
To gain further insight into the ATRA-induced events leading to G0/G1 arrest during terminal differentiation of human myeloid cells, we have performed a comprehensive analysis of the regulation of CKIs, cyclins, CDKs, and pRb pocket proteins during terminal differentiation. The well-characterized human U-937 cell line12 was chosen as a model system. This established model of monocytic differentiation can be induced to undergo terminal differentiation by 12-O-tetradecanoyl phorbol-13-acetate (TPA), vitamin D3, and ATRA, resulting in G0/G1-arrested cells with distinct phenotypes.13-15 By kinetically correlating messenger RNA (mRNA) and protein expression levels of important cell cycle regulatory genes with the cell cycle distribution in U-937 cells after ATRA induction, we determined the relative order of these events. This enabled us to identify a temporal pattern of changes in gene expression: (1) a rapid up-regulation of p21 and down-regulation of c-Myc; (2) a down-regulation of cyclin E and up-regulation of p27, at the onset of the cell cycle arrest; (3) an early, transient increase in cyclins, pRb, and p107 expression; and finally (4) down-regulation of cyclins A and B and dephosphorylation of pRb, coincidental to the accumulation of cells in the G1 phase of the cell cycle. Taken together our results demonstrate that a series of complex regulatory events lead to an irreversible cell cycle arrest associated with ATRA-induced myeloid differentiation, in which a rapid reduction of c-Myc and cyclin E levels in conjunction with a posttranscriptional increase in p27 expression appears to be pivotal.
Materials and methods
Cell culture and ATRA induction
U-937-1,12,16 HL-60,17NB-4,18 U-937-myc-2,19 containing the OK10 v-myc oncogene as part of the retroviral construct MMCV-neo, and the parental control U-937-GTB cells19 were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Sigma, St Louis, MO), glutamine, and antibiotics in a humidified 5% CO2 in air atmosphere at 37°C. ATRA (Sigma) was dissolved in ethanol-dimethyl sulfoxide (1:5) at 50 mM concentration and stored in aliquots at −70°C. Inductions were done on exponentially growing cells using 1 μM ATRA for the indicated time periods.
Cell cycle and immunofluorescence analysis
Analysis of cell cycle phase distribution and preparation of nuclei were performed according to Vindelov et al.20Briefly, cells were induced with 1 μM ATRA for the indicated time, washed once in phosphate-buffered saline (PBS), and treated with 0.03 mg/mL trypsin (Sigma) for 10 minutes at room temperature. Then RNase A (Sigma, 0.08 mg/mL) and trypsin inhibitor (Sigma, 0.5 mg/mL) were added, and cells were incubated for an additional 10 minutes at room temperature. Finally, prepared nuclei were stained with propidium iodide (PI; Sigma, 0.2 mg/mL). Stained nuclei were analyzed using a FACScan (Becton Dickinson, Mountain View, CA) and the MacCycle software (Phoenix Flow Systems, San Diego, CA).
The surface antigen expression was analyzed by immunofluorescence using flow cytometry (FACScan; Becton Dickinson). Briefly, the cells were washed twice with PBS, incubated with phycoerythrin (PE)–conjugated antibodies on ice for 30 minutes and finally washed twice with PBS plus 0.5% fetal calf serum (FCS). The PE-conjugated antibodies used were Leu M5 (CD11c) (Becton Dickinson) and IgG2b (Dako, Glostrup, Denmark).
Statistical analysis
RNA isolation and RNAse protection assay
Total RNA was isolated using the RNAgents Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's instructions. RNA concentration was determined by measuring the absorbance at 260 nm using a spectrophotometer.
The RNAse protection assay (RPA) was done with 7 μg of total RNA using the RiboQuant Multi-Probe RNase Protection Assay system (Pharmingen, Becton Dickinson, Stockholm, Sweden) according to the manual and 32P-UTP (Sigma) labeled multitemplate probes (Pharmingen). Multitemplate probes hCC-2 (p130, pRb, p107, p53, p57, p27, p21, p19, p18, p16, p14/15, L32, GAPDH) and hCYC-1 (cyclin A, cyclin B, cyclin C, cyclin D1, cyclin D2, cyclin D3, cyclin A1, L32, GAPDH) were used to analyze expression of CKI and cyclin mRNA.
Cell lysates and Western blot analysis
Cells were collected by centrifugation at 1500 rpm, washed once with PBS, lysed in 1% NP-40, 0.1 M Tris-HCl, pH 8.0, 0.15 M NaCl, and 5 mM EDTA with complete protease inhibitor (Boehringer Mannheim, Mannheim, Germany), 1 mM Dithiotreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1 mM NaVO3, 10 mM NaF, 50 μM NaMoO4, and 1 mM ZnCl2, and incubated on ice for 15 minutes. Cell lysates were spun 15 minutes at 14 000 rpm at 4°C in a chilled microcentrifuge, supernatants were collected, and the protein content was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's recommendations. Equal amounts (10-15 μg) of extracts were fractionated on Novex NuPAGE (4%-12%, 10%) precast gels using the Novex electrophoresis and blotting system (Novex, San Diego, CA). The membrane (Hybond-C extra, Amersham, Uppsala, Sweden) was blocked in 5% dry milk in .1% Tween-Tris buffered Saline (TTBS) for 1 hour at room temperature. Incubation with primary antibody was done at 4°C overnight, and then the membranes were washed 5 times in TTBS, and incubated with secondary horseradish peroxidase (HRP)–linked antibodies (Dako) for 1 hour at room temperature. After washing the membranes extensively in TTBS, antibody binding was detected using enhanced chemoluminescence plus (Amersham Pharmacia Biotech, Uppsala, Sweden). Primary antibodies used were α-cyclin A (C-19), α-cyclin B (H-20), α-p27 (C-19), α-cyclin D2 (C-17), α-cyclin D3 (C-16), α-cyclin E (HE12), α-p107 (C-18), α-p130 (211.6), and α-actin (I-19) (Santa Cruz Biotechnology, Santa Cruz, CA) and the Phosphoplus Rb (Ser780, Ser795, Ser807/811) antibody kit (Cell Signaling Technology, Beverly, MA). Western blots were performed with extracts from at least 3 independent ATRA-induced kinetic experiments and routinely stained by Ponceau S to control for protein loading differences.
Assay for p27Kip1 protein stability
Exponentially growing U-937 cells and cells induced to differentiate in medium containing 1 μM ATRA for 72 hours were analyzed. To inhibit new protein synthesis the cells were treated with 100 μg/mL cycloheximide (Sigma) at time zero and p27 degradation followed for 8 hours. Whole cell lysates were prepared at indicated times and subjected to Western blot analysis as described above using primary antibodies directed against p27 or actin. The bands were quantified and the p27 protein half-life was estimated from the slope of the curve.
Immunoprecipitation
Cell lysates were prepared as described above and equal amounts of protein were incubated with rabbit pan-Myc antibodies IG921 and protein A-agarose beads at 4°C overnight. The immunocomplexes were washed 3 times in lysis buffer, and boiled at 95°C for 5 minutes prior to electrophoresis on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. The fractionated protein was transferred from the gel to a nitrocellulose membrane and subjected to immunoblotting as described, using biotin-coupled C-33 monoclonal pan-Myc antibodies (Santa Cruz Biotechnology) followed by streptavidin-HRP conjugates. The C-33 antibodies were conjugated with 10 μg biotin-X-NHS (Calbiochem-Novabiochem, La Jolla, CA) per milligram antibody in 0.1 M NaHCO3, pH 7.4, 0.1 M NaCl for 1 hour at room temperature followed by dialysis in PBS.
In vitro kinase assay
Whole cell lysates were prepared as described above, with the exception of the use of 150 mM NaCl, 0.5% NP-40, and 50 mM Tris pH 6.8 lysis buffer. Equal amounts of protein were subjected to immunoprecipitation with 2 μg CDK2, CDK4, or CDK6 antibodies for 2 to 3 hours at 4°C, and then protein G-Sepharose beads were added before a second incubation 1 to 2 hours at 4°C. The immunoprecipitates were washed 3 times in lysis buffer and 3 times in kinase buffer (20 mM Tris pH 7.5, 4 mM MgCl2). The beads were suspended in 10 μL reaction buffer containing 4 mM Tris, pH 7.5, 0.8 mM MgCl2, 1 μg recombinant pRb (769, Santa Cruz) and32P-γ-adenosine triphosphate, and incubated for 30 minutes at 37°C. Prior to loading on 4% to 12% NuPAGE gels (Novex), the samples were suspended in sample buffer with reducing agent (Novex), boiled at 95°C for 5 minutes, and briefly centrifuged. The amount of labeled pRb was quantified using a Fuiji Bas 2000 Imaging System (Fuiji, Tokyo, Japan).
Results
ATRA-induced G0/G1 arrest in U-937 cells is associated with reduced levels of cyclins A, B, D3, and E
The kinetics of ATRA-induced cell cycle arrest were determined by flow cytometric analysis of PI-labeled nuclei. U-937 cells were induced by ATRA and harvested at the indicated times; cell cycle distribution was analyzed as described in “Materials and methods” (Figure1A). Cells started to accumulate in the G0/G1 phase of the cell cycle between 24 and 48 hours of ATRA treatment, and the percentage of cells in the G0/G1 phase continued to increase to over 90% after 96 hours of induction. To further analyze the kinetics of ATRA-induced differentiation, the expression of the surface antigen p150.95/CD11c, indicative of monocyte/macrophage differentiation,22 was determined by FACS analysis after 24, 48, 72, and 96 hours of ATRA treatment (Figure 1B). The expression of CD11c inversely correlated with the percentage of cycling cells (S+G2/M phase cells), coupling differentiation to accumulation of cells in the G0/G1 phase of the cell cycle.
U-937 cells arrest in the G0/G1phase of the cell cycle and up-regulate the differentiation marker CD11c in response to ATRA treatment.
(A) The graph indicates the percentage of cells in the S, G2, and M phases during ATRA differentiation. U-937 cells were grown in the presence of ATRA and collected at the indicated times, and the distribution of cells in different cell cycle phases was determined by flow cytometry of PI-stained nuclei. Mean ± SD (n = 4). (B) Induction of CD11c expression during ATRA-induced differentiation. Cells were induced by ATRA, collected at the indicated times, and analyzed by flow cytometry. Data are presented as CD11c mean fluorescence intensity (MFI) corrected for background binding by subtracting the MFI value for isotype-specific IgG2b control antibody. Mean ± SD (n = 3).
U-937 cells arrest in the G0/G1phase of the cell cycle and up-regulate the differentiation marker CD11c in response to ATRA treatment.
(A) The graph indicates the percentage of cells in the S, G2, and M phases during ATRA differentiation. U-937 cells were grown in the presence of ATRA and collected at the indicated times, and the distribution of cells in different cell cycle phases was determined by flow cytometry of PI-stained nuclei. Mean ± SD (n = 4). (B) Induction of CD11c expression during ATRA-induced differentiation. Cells were induced by ATRA, collected at the indicated times, and analyzed by flow cytometry. Data are presented as CD11c mean fluorescence intensity (MFI) corrected for background binding by subtracting the MFI value for isotype-specific IgG2b control antibody. Mean ± SD (n = 3).
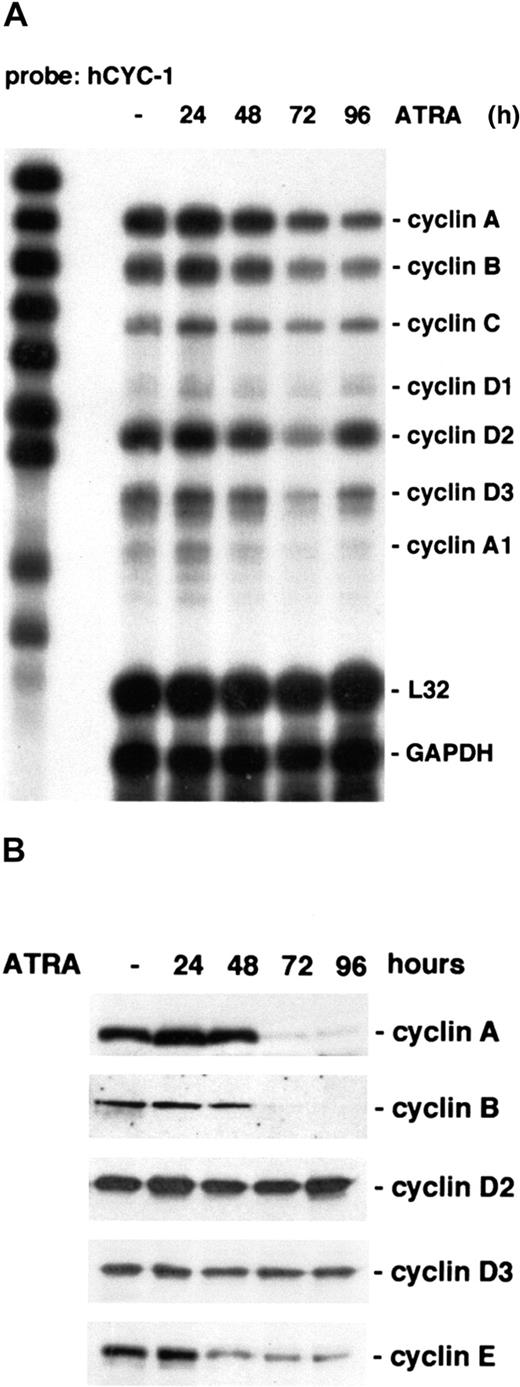
Cell cycle progression is controlled by cyclins and CKIs, which determine the activity of cyclin-dependent kinases. To determine the expression of cyclins during U-937 G0/G1 arrest after ATRA induction, RPA was performed using RNA from untreated or treated cells and a multitemplate cyclin probe (h-CYC-1). A marked reduction in cyclin A and cyclin B mRNA was observed after 72 hours of ATRA treatment (Figure 2A). Both cyclin A and cyclin B levels are associated with the G2 phase of the cell cycle and are down-regulated in G1.2 The levels of cyclins C, A1, D1, and D2 mRNA were not appreciably affected by ATRA treatment when comparing several experiments, whereas cyclin D3 mRNA levels were slightly down-regulated during differentiation.
Cyclins A, B, D3, and E are down-regulated during ATRA-induced differentiation of U-937 cells.
(A) Cells were induced by ATRA and harvested at the indicated times, and total mRNA was prepared. The regulation of cyclin mRNA during ATRA treatment was analyzed by RPA using the hCYC-1 multiprobe template. (B) The protein levels of cyclins A, B, D2, D3, and E during ATRA-induced differentiation were determined by Western blot analysis using whole cell lysates.
Cyclins A, B, D3, and E are down-regulated during ATRA-induced differentiation of U-937 cells.
(A) Cells were induced by ATRA and harvested at the indicated times, and total mRNA was prepared. The regulation of cyclin mRNA during ATRA treatment was analyzed by RPA using the hCYC-1 multiprobe template. (B) The protein levels of cyclins A, B, D2, D3, and E during ATRA-induced differentiation were determined by Western blot analysis using whole cell lysates.
Western blot analysis confirmed a sharp decrease in both cyclin A and B protein levels occurring after 72 hours of ATRA induction (Figure2B). The levels of cyclins are controlled both by transcriptional and posttranscriptional mechanisms; therefore, the protein levels of G0/G1 cyclins D1, D2, and D3 were also analyzed. Whereas cyclin D1 was not detectable in these cells, the level of cyclin D2 was unchanged, and the cyclin D3 protein level was decreased slightly during differentiation. Cyclin E is known to play a role in the G0/G1 transition, but there was no probe for this cyclin in the RPA template set used. Instead, the cyclin E protein level was determined by Western blot analysis. Interestingly, coinciding with the onset of cell cycle arrest, cyclin E was found to be down-regulated after 24 to 48 hours of ATRA stimulation (Figure 2B).
CKIs p21WAF1/CIP1 and p27Kip1 are up-regulated by ATRA treatment of U-937 cells, correlating to the onset of G0/G1 arrest
The levels of CKIs were monitored using a multitemplate CKI probe (hCC-2; Figure 3A). An increase in p21 mRNA was detected after 24 hours of ATRA induction, as previously reported by Liu et al.9 In addition, we could detect a slight down-regulation of p16, a CDK inhibitor associated with D-type cyclins. The levels of p130, pRb, p53, p57, p27, p19, p18, and p14/15 mRNA remained unchanged.
ATRA-induced cell cycle arrest is associated with up-regulation of CKIs p21 and p27.
(A) RPA analysis of different CKIs during ATRA-induced differentiation was done using the hCC-2 multitemplate probe and total mRNA from ATRA-treated cells. (B) ATRA-induced regulation of p27 protein levels was determined by Western blot analysis using whole cell lysates of ATRA-treated cells harvested at the indicated times. (C) To determine the turnover of p27 protein, cells were grown with ATRA or left untreated, cycloheximide (CHX) was added after 72 hours of induction, and cells were harvested at 0, 1, 2, 4, 6, and 8 hours after CHX treatment. Whole cell lysates were prepared and analyzed by Western blot using antibodies directed against p27 or actin.
ATRA-induced cell cycle arrest is associated with up-regulation of CKIs p21 and p27.
(A) RPA analysis of different CKIs during ATRA-induced differentiation was done using the hCC-2 multitemplate probe and total mRNA from ATRA-treated cells. (B) ATRA-induced regulation of p27 protein levels was determined by Western blot analysis using whole cell lysates of ATRA-treated cells harvested at the indicated times. (C) To determine the turnover of p27 protein, cells were grown with ATRA or left untreated, cycloheximide (CHX) was added after 72 hours of induction, and cells were harvested at 0, 1, 2, 4, 6, and 8 hours after CHX treatment. Whole cell lysates were prepared and analyzed by Western blot using antibodies directed against p27 or actin.
The RPA analysis showed that p27 mRNA levels remained unchanged after ATRA induction. However, p27 up-regulation can occur by posttranscriptional mechanisms, and has previously been reported to be induced by vitamin D3 treatment of U-937 cells.23 Therefore, the p27 protein level was monitored during ATRA-induced differentiation of U-937 cells. Western blot analysis showed an increase in p27 protein starting after 48 hours of induction (Figure 3B), coinciding with cell cycle arrest. To determine if ATRA treatment affected p27 protein stability, the half-life of p27 was analyzed at 72 hours of induction by following protein levels after 1, 2, 4, 6, and 8 hours after blocking protein synthesis by cycloheximide treatment. The estimated half-life of p27 was approximately 3 to 4 hours in exponentially growing U-937 cells, whereas there was a clear stabilization of p27 protein in cells differentiated by ATRA for 72 hours, consistent with reported observations in ATRA-differentiated neuroblastoma cells.24
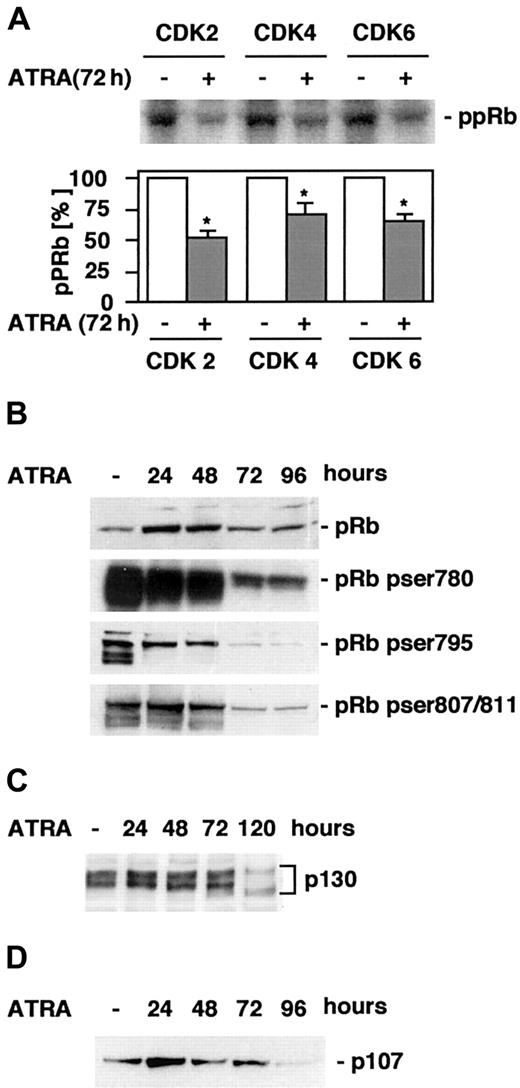
Changes in the levels of cyclins and CKIs are reflected by down-regulation of CDK 2–, 4–, and 6–associated activity and dephosphorylation of pRb
Cyclins and CKIs regulate the activity of CDKs, which in turn regulate phosphorylation of several substrates, including pRb. Phosphorylation of specific pRb serine or threonine residues has been shown to regulate binding to different targets, including E2F and the c-Abl tyrosine kinase.25,26 Because we observed down-regulation of cyclins A, B, D3, and E and up-regulation of p21 and p27, which may potentially alter the activities of CDKs 2, 4, and 6, the activities of these CDKs were determined through an in vitro kinase assay using pRb as a substrate. A significant (P < .05) reduction in both CDK4, CDK6, and, most markedly, CDK2 activity was detected after 72 hours of ATRA stimulation (Figure4A). Next, to directly determine the kinetics of changes in pRb phosphorylation associated with ATRA-induced differentiation, cells were induced by ATRA, whole cell lysates were prepared at the indicated times, and Western blot analysis was performed using antibodies directed against pRb phosphorylated on Ser780 or Ser795, important for binding to E2F,25 or Ser-807/811, regulating binding to c-Abl,26 or total levels of pRb (Figure 4B). As expected, differentiation as the result of ATRA treatment was linked to the disappearance of hyperphosphorylated pRb, specifically involving dephosphorylation of Ser780, Ser795 and Ser807/811 with very similar kinetics.
ATRA treatment of U-937 cells results in decreased CDK activity and a reduction in hyperphosphorylated pRb.
(A) CDK2, 4, and 6 kinase activity was measured in an in vitro kinase assay using pRb as a substrate. Cells were grown in the presence or absence of ATRA for 72 hours, whole cell lysates were prepared, and the activities of CDK2, CDK4, and CDK6 were determined as described in “Materials and methods.” The graph indicates quantification of the bands correlated to control. Mean ± SD (n = 4). The asterisk indicates significant differences from the corresponding controls (P < .05). (B-D) The levels of total pRb or Ser780, Ser795, or Ser807/811 phosphorylated pRb (B), p130 (C), or p107 (D) during ATRA-induced differentiation were determined by Western blot of whole cell lysates prepared at the indicated times using specific antibodies.
ATRA treatment of U-937 cells results in decreased CDK activity and a reduction in hyperphosphorylated pRb.
(A) CDK2, 4, and 6 kinase activity was measured in an in vitro kinase assay using pRb as a substrate. Cells were grown in the presence or absence of ATRA for 72 hours, whole cell lysates were prepared, and the activities of CDK2, CDK4, and CDK6 were determined as described in “Materials and methods.” The graph indicates quantification of the bands correlated to control. Mean ± SD (n = 4). The asterisk indicates significant differences from the corresponding controls (P < .05). (B-D) The levels of total pRb or Ser780, Ser795, or Ser807/811 phosphorylated pRb (B), p130 (C), or p107 (D) during ATRA-induced differentiation were determined by Western blot of whole cell lysates prepared at the indicated times using specific antibodies.
The other members of the retinoblastoma family of pocket proteins, p107 and p130, have also been shown to be important in cell cycle regulation and differentiation.27 28 Similar to the situation for pRb, the function of p130 in cell cycle regulation is determined by both the actual protein level and the phosphorylation status. To determine if there was a change in the level of p130 or its phosphorylation status during differentiation, whole cell lysates of ATRA-induced cells were subjected to electrophoresis on a low-density gel followed by Western blot analysis using p130 antibodies (Figure4C). There was a slight increase in p130 levels transiently after 24 hours of ATRA treatment, and a drop in both phosphorylated and total p130 after 120 hours of induction. Interestingly, p107 protein levels were regulated in a similar fashion, increasing transiently after 24 hours of ATRA induction, a point before any apparent accumulation in the G1 phase of the cell cycle can be observed, and the p107 expression thereafter continues to decline throughout the 96-hour period of ATRA induction studied (Figure 4D). However, only p107 appeared to be regulated on the mRNA level (Figure 2B).
ATRA induces down-regulation of cyclin E and up-regulation of p27KIP1 in the ATRA-responsive human myeloid leukemic cell lines HL-60 and NB-4
Next, we wanted to determine whether the regulation of p27 and cyclin E, similar to the more well-established changes in Myc29,30 and p21,9-11 is a common event linked to cell cycle arrest during myeloid differentiation induced by ATRA. Therefore, we studied the regulation of these proteins in the ATRA-sensitive human myeloid cell lines HL-60 and NB-4. HL-60 cells arrest in the G0/G1 phase of the cell cycle in response to ATRA treatment, whereas NB-4 cells arrest in a non–G1-restricted manner, at all points in the cell cycle31 32 (and data not shown). After 96 hours of ATRA stimulation, the level of p27 protein was increased in both NB-4 and HL-60 cells, whereas the cyclin E level was decreased (Figure5).
HL-60 and NB-4 cells respond to ATRA treatment by down-regulation of cyclin E and up-regulation of p27.
Cells were induced by ATRA for 96 hours, whole cell lysates were prepared, and the protein levels of cyclin E and p27 were analyzed by Western blot.
HL-60 and NB-4 cells respond to ATRA treatment by down-regulation of cyclin E and up-regulation of p27.
Cells were induced by ATRA for 96 hours, whole cell lysates were prepared, and the protein levels of cyclin E and p27 were analyzed by Western blot.
Down-regulation of c-Myc precedes the G0/G1arrest and is necessary for regulation of cyclin E and p27Kip1
Previous reports have identified c-Myc as a regulator of cyclin E-CDK2 activity through induction of cyclin E expression and inhibition of the association of p27 with cyclin E-CDK2 complexes.33,34 Also, cyclin E-CDK2 complexes have been shown to regulate p27 protein levels through phosphorylation on Thr187.35 This phosphorylation is a prerequisite for the proteosome-dependent degradation of p27.36 Therefore, the observed ATRA-induced decrease in cyclin E levels and subsequent increase in p27 protein may be due to a down-regulation of c-Myc. To determine the protein levels of c-Myc during differentiation, lysates from ATRA-treated cells were immunoprecipitated using pan-Myc antibodies, and analyzed by Western blot. A decrease in the c-Myc protein levels was initiated after 12 hours of ATRA treatment and continued throughout differentiation (Figure6A), preceding U-937 cell cycle arrest and changes in the levels of cyclin E and p27. This raised the question of whether there is a causal relationship between the reduction of Myc and the down-regulation of cyclin E, and the following up-regulation of p27. To address this possibility we used a U-937 subline constitutively expressing v-Myc, U-937-myc-2.19 37 The U-937-myc-2 and the parental control cells, U-937-GTB, were induced by ATRA and the cell cycle distribution was analyzed during 96 hours. Only a small reduction in the percentage of cycling cells was observed for the U-937-myc-2 cells, whereas U-937-GTB cells became G1arrested (Figure 6B). Figure 6C shows that U-937-myc-2 cells fail to down-regulate the expression of cyclin E and up-regulate p27 at 72 hours after induction, suggesting that the down-regulation of c-Myc is a prerequisite for these changes.
Exogenous expression of Myc inhibits ATRA-induced cell cycle arrest and the associated down-regulation of cyclin E and up-regulation of p27.
(A) The expression of c-Myc is down-regulated in response to ATRA treatment in U-937 cells. Cells were treated with ATRA for 0, 12, 24, 48, and 72 hours as indicated; whole cell lysates were prepared and subjected to immunoprecipitation using pan-Myc antibodies. The immunocomplexes were collected, separated by SDS-PAGE, and analyzed by Western blot using biotin-coupled pan-Myc antibodies and streptavidin-HRP. (B) U-937-myc-2 cells and the parental subline U-937-GTB were grown in the presence of ATRA, collected at the indicated times, and the distribution of cells in different cell cycle phases was determined by flow cytometry of PI-stained nuclei. Mean ± SD (n = 3). The asterisk indicates significant differences from the corresponding U-937-GTB controls. (C) The protein levels of cyclin E and p27 after 72 hours of ATRA treatment in U-937-GTB and U-937-myc-2 cells were analyzed by Western blot.
Exogenous expression of Myc inhibits ATRA-induced cell cycle arrest and the associated down-regulation of cyclin E and up-regulation of p27.
(A) The expression of c-Myc is down-regulated in response to ATRA treatment in U-937 cells. Cells were treated with ATRA for 0, 12, 24, 48, and 72 hours as indicated; whole cell lysates were prepared and subjected to immunoprecipitation using pan-Myc antibodies. The immunocomplexes were collected, separated by SDS-PAGE, and analyzed by Western blot using biotin-coupled pan-Myc antibodies and streptavidin-HRP. (B) U-937-myc-2 cells and the parental subline U-937-GTB were grown in the presence of ATRA, collected at the indicated times, and the distribution of cells in different cell cycle phases was determined by flow cytometry of PI-stained nuclei. Mean ± SD (n = 3). The asterisk indicates significant differences from the corresponding U-937-GTB controls. (C) The protein levels of cyclin E and p27 after 72 hours of ATRA treatment in U-937-GTB and U-937-myc-2 cells were analyzed by Western blot.
Discussion
The successful treatment of APL patients with ATRA demonstrates that external signals can overcome the block of differentiation in myeloid leukemic cells in vivo.7 Although leukemias and lymphomas represent a diverse group of tumors that can arise as a consequence of several different genetic changes, extensive work done in established leukemic cell lines suggests that ATRA or synthetic retinoid derivatives may be effective as therapeutic agents in the treatment also of other types of myeloid malignancies than APL. Identifying the critical subset of cell cycle control genes involved in ATRA-induced growth arrest of myeloid cells is important for the understanding of the molecular events underlying the therapeutic potential of ATRA treatment.
Much research interest has been focused on the CKIs with respect to the molecular events initiating a G1 cell cycle arrest. During normal myeloid differentiation from CD34+ hematopoietic progenitors a progressive increase in p21 mRNA and protein is observed.38 Similarly, an early ATRA-induced up-regulation of p21 has been described in leukemic cell lines, for example, U-937, HL-60, and NB-4 cells.9-11,39 The promoter of p21 has been shown to contain a retinoic acid response element (RARE), directly linking activation of the RAR/RXR nuclear receptor complex to induction of p21 expression.9 However, the ATRA-induced level of p21 protein in U-937 cells is relatively low as compared to TPA-induced U-937 cells, and only detectable after immunoprecipitation in our system (data not shown). Therefore, we expected that other CKIs would also be up-regulated by ATRA and that they would contribute to the G0/G1 arrest. The level of p27 mRNA was found to be constant during differentiation. However, the half-life for the p27 protein increased in ATRA-differentiated U-937 cells. This indicates that the mechanism for p27 up-regulation is, at least partially, due to a stabilization of the p27 protein, consistent with findings in ATRA-induced neuroblastoma.24 During hematopoiesis a low expression of p27 protein is detected in early progenitors and continues throughout myeloid differentiation with increased levels or subcellular redistribution (or both) at later stages.40,41 Moderate levels of p27 protein are found in undifferentiated U-937 cells, and it is likely that the observed reduction in cyclin E levels contributes to the following increase in p27 stability after ATRA treatment, because cyclin E-CDK2 complexes can target p27 for degradation through phosphorylation on Thr187.35 36 The general relevance of cyclin E and p27 regulation for differentiation-associated growth arrest of myeloid cells was confirmed in ATRA-induced NB-4 and HL-60 cells.
Down-regulation of c-Myc is an important early event that has been connected to terminal differentiation and growth arrest of several cell types,29,30 including U-937.19,37 We observe a decrease in c-Myc levels within 12 hours of ATRA stimulation and a continued down-regulation throughout differentiation. Myc plays a dual role in regulating the activity of cyclin E–CDK2 complexes, primarily via the inhibition of p27, either by induction of cyclin D1 and cyclin D2 resulting in p27 sequestration33,34,42,43 or by direct repression of the p27 promoter,44 but also through direct or indirect transcriptional activation of the cyclin E gene.34 Consequently, changes resulting from the early down-regulation of c-Myc may directly affect the cell cycle machinery and be important for the initiation of G0/G1arrest. Two observations place c-Myc upstream of the other changes in cell cycle control proteins. First, the kinetics of c-Myc down-regulation is rapid and precedes subsequent changes in cyclin E and p27. Second, we provide evidence that constitutive expression of av-myc gene prevents reduction of cyclin E and increase in p27 and the consecutive G1 arrest.
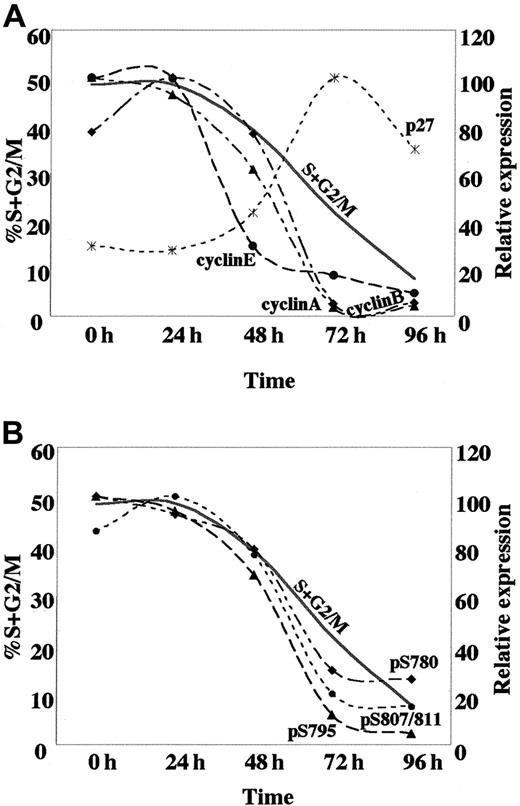
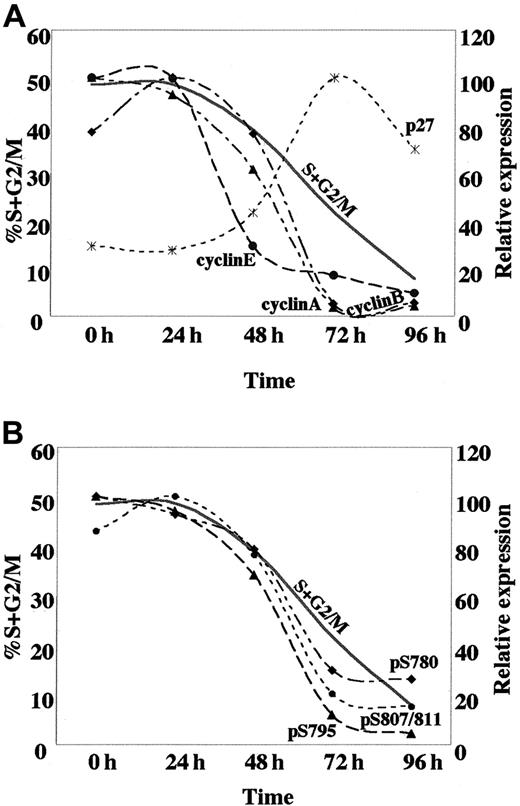
An early reduction in CDK activity, initiating the G0/G1 arrest in response to ATRA treatment of U-937 cells, appears to be largely dependent on the marked down-regulation of cyclin E in combination with up-regulation of p21 and p27. This is later followed by a fall in cyclin A and B expression, coincident with the accumulation of cells in G1 (Figure7A). The observed pattern of cyclin E, cyclin A, and cyclin B expression in ATRA-induced U-937 cells is reminiscent of that in cells undergoing myeloid differentiation from normal hematopoietic progenitors, with a high expression during the proliferative phase followed by down-regulation at the terminal stage.45 In contrast, the sustained expression of cyclin D2 and the marginal reduction of cyclin D3 levels during ATRA-induced cell cycle arrest is clearly different as compared to normal myeloid differentiation or, for example, to the G1 arrest that is induced in response to the withdrawal of mitogenic signals (mainly affecting D-type cyclins).5 This difference may either be the consequence of the leukemic nature of the U-937 cells or a particular feature of ATRA-induced differentiation.
Kinetics of the expression of cell cycle regulatory proteins and dephosphorylation of pRB, associated with ATRA-induced differentiation.
(A) The temporal changes in protein expression of the indicated cell cycle regulators based on the quantification of Western blot data from Figures 2B and 3B; cyclin A (▴), cyclin B (♦), cyclin E (●), p27 (*). (B) Kinetics of pRb dephosphorylation at specific sites based on the quantification of Western blot data from Figure 4B; Ser780 (♦), Ser795 (▴), Ser807/811 (●). Quantified data are displayed with the peak value for each series arbitrarily set to 100. For cycling cells the actual percentage of cells in the S+G2/M phases of the cell cycle is shown.
Kinetics of the expression of cell cycle regulatory proteins and dephosphorylation of pRB, associated with ATRA-induced differentiation.
(A) The temporal changes in protein expression of the indicated cell cycle regulators based on the quantification of Western blot data from Figures 2B and 3B; cyclin A (▴), cyclin B (♦), cyclin E (●), p27 (*). (B) Kinetics of pRb dephosphorylation at specific sites based on the quantification of Western blot data from Figure 4B; Ser780 (♦), Ser795 (▴), Ser807/811 (●). Quantified data are displayed with the peak value for each series arbitrarily set to 100. For cycling cells the actual percentage of cells in the S+G2/M phases of the cell cycle is shown.
Changes in the expression of cyclins and CKIs in response to ATRA treatment were followed by down-regulation of CDK2, CDK4, and CDK6 activity and dephosphorylation of pRb at specific serine residues. The most apparent dephosphorylation of pRb occurs after 72 hours of induction, although an elevation in hypophosphorylated pRb is evident already after 24 hours of ATRA induction, when taking the increase in total pRb into account (Figure 4 and Figure 7B). During the cell cycle progression, pRb is sequentially phosphorylated by different cyclin-CDK complexes.46 Down-regulation of cyclin E precedes dephosphorylation of pRb at Ser780, Ser795, and Ser807/811, and could therefore potentially affect other phosphoacceptor sites. Phosphorylation of pRb by cyclin E–CDK2 has been suggested to prevent E2F binding; therefore, a reduction in cyclin E–CDK2 activity would be predicted to inhibit E2F function and expression of S-phase genes.3 47 Thus, the presence of hypophosphorylated pRb could potentially be important to preclude re-entry into S phase and thereby establish an irreversible cell cycle exit in the terminally differentiated cell.
The intimate link between terminal differentiation and cell cycle arrest makes it difficult to assign a particular function of an individual protein to either of the 2 processes. Also, several of the cell cycle regulatory proteins may have dual functions. For instance, the function of pRb family proteins has also been linked to differentiation in various cell systems.27 Interestingly, expression of an antisense pRb construct in U-937 cells, which decreased pRb levels, resulted in defects in ATRA-induced differentiation, but not cell cycle arrest.48Up-regulation of p21 and p27 may also impinge on the differentiation process; for example, up-regulation of p21 in APL cells has been reported to be important for commitment to differentiation, but not required for cell cycle arrest.39 Forced expression of p21 or p27 has been suggested to induce markers of differentiation in U-937 cells,49 supporting this notion. Typically, it appears that during induced differentiation myeloid cells traverse one or several cell cycles, before the associated G1 arrest is established.39,49,50 Moreover, the proliferative burst after vitamin D3 treatment of U-937 cells is accompanied by a transient up-regulation of cyclins, p27, and p21.49 In agreement with this observation, we observed a transient up-regulation of cyclins A and D2, as well as pRb and p107 within the first 24 hours of ATRA treatment. A transient up-regulation of p107 has recently been suggested to be important for adipocyte differentiation.51It remains to be shown if similar links between the transient up-regulation of cell cycle control proteins and terminal differentiation exist in ATRA-induced U-937 cells.
The main new finding in this paper is that the initiation of ATRA-induced cell cycle arrest in U-937 cells is preceded by down-regulation of c-Myc, necessary for a coordinate fall in cyclin E levels and up-regulation of p27. Although a schematic blueprint of differentiation-associated regulation of cell cycle control proteins in myeloid cells has been drawn by this study, the molecular mechanism of ATRA-induced regulation of cyclins, CDKs, and CKIs warrants further investigation
We thank Lina Dimberg for critical reading of the manuscript.
Supported by the Swedish Cancer Society, the Magnus Bergwall Foundation, the HKH Lovisa/Tielman Foundation, the Hans von Kantzow Foundation, the King Gustaf V's 80 Year Foundation, and the Åke Wiberg Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Fredrik Öberg, Department of Genetics and Pathology, Rudbeck Laboratory, Uppsala University, S-751, 85 Uppsala, Sweden; e-mail: fredrik.oberg@genpat.uu.se.