Gemtuzumab ozogamicin (Mylotarg) targets leukemia cells expressing the CD33 receptor by means of a monoclonal antibody conjugated to a cytotoxic agent, calicheamicin. Treatment of acute myeloid leukemia (AML) with gemtuzumab ozogamicin may result in liver injury. We reviewed the course of 23 patients who were given gemtuzumab ozogamicin for AML that had relapsed after hematopoietic cell transplantation. Liver toxicity was assessed through physical examination, serum tests, histologic examination, and hepatic venous pressure measurements. Liver injury developed in 11 patients after gemtuzumab ozogamicin administration; it was manifested as weight gain, ascites, and jaundice in 7 patients. Seven patients died with persistent liver dysfunction and either multiorgan failure or sepsis at a median of 40 days after gemtuzumab ozogamicin infusion. Portal pressure measurements were elevated in 2 patients. Results of liver histologic examination in 5 patients showed sinusoidal injury with extensive sinusoidal fibrosis, centrilobular congestion, and hepatocyte necrosis. Six patients experienced AML remission that was sustained for at least 60 days after gemtuzumab ozogamicin infusion. In summary, hepatic sinusoidal liver injury developed after gemtuzumab ozogamicin infusion. Histology showed striking deposition of sinusoidal collagen, suggesting that gemtuzumab ozogamicin targets CD33+ cells residing in hepatic sinusoids as the mechanism for its hepatic toxicity.
Introduction
In May 2000, the United States Food and Drug Administration approved gemtuzumab ozogamicin (Mylotarg; Wyeth-Ayerst Pharmaceuticals, Radnor, PA) for the treatment of relapsed CD33+ acute myeloid leukemia in patients older than 60. Gemtuzumab ozogamicin uses monoclonal antibody technology to target leukemia cells expressing the CD33 receptor by means of a humanized antibody conjugated to a modified cytotoxic agent, calicheamicin.1,2 On binding to the CD33 antigen, the calicheamicin–antibody complex is internalized into target cells, and calicheamicin is released intracellularly.3 In phase 2 clinical trials involving 188 patients, the frequencies of gemtuzumab ozogamicin-related elevated bilirubin levels greater than 3 mg/dL and of serum aminotransferase enzyme levels greater than 5 times normal were 8% and 16%, respectively (Sievers et al,2 Sievers et al,4 and unpublished observations (March 2001), Wyeth-Ayerst Pharmaceuticals). One patient died with persistent ascites and hepatosplenomegaly.4 Several additional case reports of gemtuzumab ozogamicin-related liver toxicity have been reported, some in association with other chemotherapy agents.5,6 In patients administered gemtuzumab ozogamicin after myeloablative therapy and hematopoietic cell transplantation, the reported frequency of posttransplantation clinical illness resembling veno-occlusive disease of the liver was 6 of 38 (16%) and included 3 fatalities (Sievers et al7 and unpublished observations (March 2001), Wyeth-Ayerst Pharmaceuticals).
This report describes the frequency and course of liver injury in 23 consecutive patients given gemtuzumab ozogamicin for the treatment of relapsed leukemia after hematopoietic stem cell transplantation. Liver injury in these patients affected hepatic sinusoids and was accompanied by signs of portal hypertension as the dominant clinical feature. We suggest that this form of liver toxicity results from the delivery of calicheamicin to CD33+ cells that reside in the sinusoids of the liver (ie, Kupffer cells8,9 and leukemia cells10 and possibly sinusoidal endothelial cells11 and stellate cells).
Patients, materials, and methods
Patient selection
We reviewed all patients at the Fred Hutchinson Cancer Research Center and University of Washington Medical Center who were given Mylotarg for the treatment of acute myeloid leukemia (AML) that had relapsed after myeloablative therapy and hematopoietic cell transplantation. The retrospective review of patient records for this report was performed under a protocol approved by our Institutional Review Board.
Assessment of liver injury
Patients were examined daily during their hospitalization and weekly while they were observed as outpatients. Serum bilirubin, unconjugated serum bilirubin, serum aspartate aminotransferase (AST), and alkaline phosphatase levels were measured 3 times weekly. Patients who showed evidence of liver injury were further evaluated with viral serology, hepatitis B virus DNA, hepatitis C virus RNA, liver imaging tests, and serial examinations. Liver tissue for analysis was available from 6 patients (3 after death, 3 before death). Liver biopsy specimens were cultured for herpesviruses and adenovirus.12-14 Liver tissue was fixed in B5 and in buffered formalin, and sections were stained with hematoxylin and eosin, Mallory trichrome, and reticulin stains. Two patients underwent measurement of the wedged hepatic venous pressure gradient during transvenous liver biopsy.15
Evaluation of risk factors for liver injury
Details of hematopoietic cell transplantation preceding gemtuzumab ozogamicin therapy were recorded, specifically the conditioning regimen, donor type, drugs used for prophylaxis against graft-versus-host disease (GVHD), and development of any hepatobiliary problem after transplantation.16 Before the first dose of gemtuzumab ozogamicin, liver-related laboratory test results, concurrent medications, and comorbid medical problems were noted.
Assessment of acute myeloid leukemia response
Bone marrow aspirates were performed, when possible, 7 days after the administration of gemtuzumab ozogamicin. Clearance of blasts was defined as less than 5% blasts identified by morphologic evaluation.
Statistical considerations
In analyzing possible risk factors for the development of gemtuzumab ozogamicin liver toxicity, the Fisher exact test was used to compare categoric variables and the Wilcoxon 2-sample test was used to compare continuous variables, recognizing that the small sample sizes would preclude definitive analysis.
Results
Patient characteristics
Between April 1995 and June 2000, 23 patients were given gemtuzumab ozogamicin for the treatment of recurrent AML after transplantation. The median age was 43 years (range, 26-60 years). There were 9 men and 14 women. Thirteen patients received grafts from a matched sibling, 8 received them from other allogeneic donors, and 2 received autologous grafts. Pretransplantation myeloablative conditioning therapy consisted of busulfan plus cyclophosphamide (10 patients), cyclophosphamide plus total body irradiation (10 patients), busulfan plus total body irradiation (2 patients), and busulfan plus etoposide (1 patient). Eight (35%) patients had evidence of veno-occlusive disease of the liver after transplantation; jaundice and weight gain caused by veno-occlusive disease resolved in all patients before they were administered gemtuzumab ozogamicin. Nineteen patients had evidence of acute GVHD at some time point after transplantation; 9 of them had GVHD at the time of gemtuzumab ozogamicin infusion. The median interval between transplantation and infusion of gemtuzumab ozogamicin was 131 days (range, 17-967 days). The median dose of gemtuzumab ozogamicin given to these patients was 4 mg/m2(range, 0.25-9 mg/m2); 14, 7, and 2 patients received 1, 2, and 3 doses, respectively.
Incidence and clinical presentation of liver injury after Mylotarg therapy
After Mylotarg infusion, 11 patients had no overt liver abnormalities, but one patient had clinical and histologic evidence of GVHD involving the liver. Eleven (48%) patients had evidence of liver toxicity after gemtuzumab ozogamicin administration. The frequency of liver abnormalities, the peak values, and the timing of these abnormalities in these patients are given in Table1. Hepatomegaly and either weight gain greater than 5% or ascites developed in 8 patients within 7 to 10 days of gemtuzumab ozogamicin infusion, followed by elevation of AST, bilirubin, and alkaline phosphatase levels. Peak values for these tests occurred at medians of 8 to 22 days after gemtuzumab ozogamicin infusion. Ascites was clinically obvious in 5 of 7 patients. Wedged hepatic venous pressure gradients were measured at the time of transvenous liver biopsy in 2 patients, and both measurements were elevated (18 and 29 mm Hg, respectively; normal level, 6 mm Hg or less15). Encephalopathy developed in one patient 13 days after gemtuzumab ozogamicin, but it responded to oral lactulose therapy. Liver tissue was available from 3 of these 8 patients, each showing evidence of sinusoidal injury (see below). Three patients had abnormal liver test results after gemtuzumab ozogamicin infusion, but they had no evidence of weight gain, ascites, or hepatomegaly. Liver histologic examination in 2 of these patients showed sinusoidal injury (see below).
Course of putative Mylotarg-related liver injury
Of 11 patients in whom liver injury temporarily followed gemtuzumab ozogamicin infusion, 9 have died and 2 are alive. Two patients recovered from liver dysfunction and their ascites resolved, but they died of leukemic relapse 55 and 167 days, respectively, after gemtuzumab ozogamicin infusion. Seven patients died with persistent liver dysfunction and either multiorgan failure or sepsis at a median of 40 days after gemtuzumab ozogamicin infusion (range, 8-47 days).
Liver histology
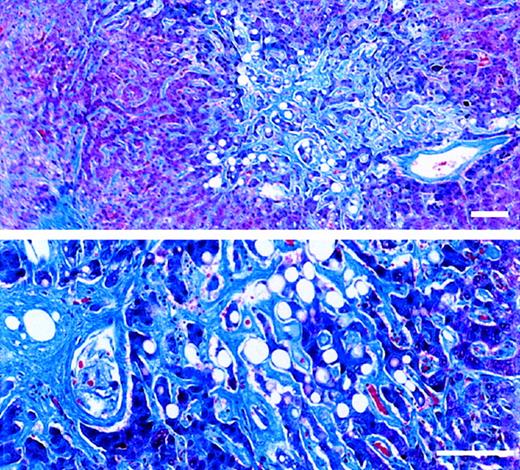
Liver tissue was available from 6 of 23 patients, one showing only bile duct abnormalities typical of GVHD. Five patients had evidence of injury to zone 3 of the liver acinus, with varying degrees of congestion and hepatocyte necrosis, sinusoidal fibrosis, and venular abnormalities (subendothelial edema, phlebosclerosis, fibrotic venular occlusion). There appeared to be a relation between certain histologic findings and the time between gemtuzumab ozogamicin exposure and tissue sampling. Zone 3 sinusoidal fibrosis was extensive in 3 patients whose time intervals from gemtuzumab ozogamicin exposure to tissue sampling were 37, 28, and 24 days, respectively, but it was absent in 2 patients whose time intervals were 6 and 12 days, respectively. The sinusoidal fibrosis illustrated in Figure 1 was that of a patient with a 37-day interval from first gemtuzumab ozogamicin exposure. Subendothelial edema in venules and zone 3 congestion, hemorrhage, and hepatocyte necrosis were more prominent when the time interval was shorter. Only the patient with the 37-day interval had phlebosclerosis or venular fibrotic occlusion; by quantitative count, fibrotic occlusion affected only 29% of her venules at autopsy, whereas most lobules had evidence of sinusoidal fibrosis (Figure 1). One patient had evidence of sinusoidal injury and bile duct damage consistent with GVHD.
Trichrome-stained sections of necropsied liver.
The patient died of multiorgan failure 37 days after receiving the first of 2 doses of Mylotarg (6 mg/m2). Standard bar equals 100 μm. (upper panel) Extensive sinusoidal fibrosis adjacent to a nonoccluded sublobular venule. (lower panel) Zone 3 region containing atrophic hepatocyte cords surrounded by markedly widened sinusoids filled with dense collagen. An adjacent central venule shows eccentric perivenular fibrosis.
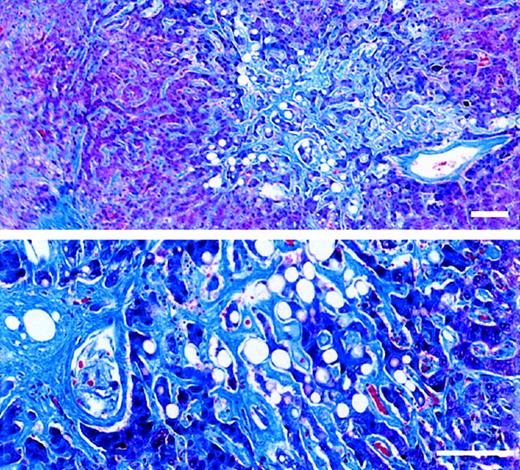
Trichrome-stained sections of necropsied liver.
The patient died of multiorgan failure 37 days after receiving the first of 2 doses of Mylotarg (6 mg/m2). Standard bar equals 100 μm. (upper panel) Extensive sinusoidal fibrosis adjacent to a nonoccluded sublobular venule. (lower panel) Zone 3 region containing atrophic hepatocyte cords surrounded by markedly widened sinusoids filled with dense collagen. An adjacent central venule shows eccentric perivenular fibrosis.
Risk factors for putative Mylotarg-related liver injury
We compared patients who had liver injury after gemtuzumab ozogamicin (n = 11) with those who did not (n = 12) according to the variables listed in Table 2. The only factor possibly related to development of liver injury was the total dose of gemtuzumab ozogamicin, which was marginally higher among patients with liver injury (median, 9 mg/m2; range, 5-18 mg/m2) than among those without it (median, 6 mg/m2; range, 0.25-18 mg/m2).
Response rate of acute myeloid leukemia after Mylotarg
Twenty-two patients were evaluable for antileukemic response to gemtuzumab ozogamicin monotherapy (one patient underwent conventional chemotherapy 3 days after receiving 0.25 mg/m2 gemtuzumab ozogamicin). Ten patients had no more than 5% blasts in the bone marrow after at least one dose of gemtuzumab ozogamicin. Sustained remissions of at least 60 days were observed in 6 of these 10 patients, 2 of whom then received donor lymphocyte infusions and remained disease free for 8 and 48 months, respectively, before experiencing extramedullary relapses of AML. The actuarial survival of patients in this series was 23% at 1 year. Product limit estimates show that survival of these 23 patients who had AML relapses after hematopoietic cell transplantation and who were treated with gemtuzumab ozogamicin did not differ significantly from our historical experience of 333 similar patients who were not treated with gemtuzumab ozogamicin (data not shown).
Discussion
Liver injury after gemtuzumab ozogamicin infusion in our patients was usually manifested by signs of portal hypertension—hepatomegaly, weight gain, ascites, and elevated portal pressure in the 2 patients in whom pressure was measured. We cannot be certain that some patients in this series did not have concomitant GVHD of the liver, but the development of portal hypertension is unusual in patients with hepatic GVHD.14,17 We have termed this liver injury sinusoidal obstruction syndrome because clinical and histologic findings point to hepatic sinusoids as the anatomic target of this unusual toxicity. There was evidence of hepatocyte necrosis, but elevated levels of serum AST developed days after exposure and often followed the development of hepatomegaly and fluid retention, suggesting that hepatocyte necrosis was caused by ischemia related to sinusoidal injury rather than to a direct toxic effect of calicheamicin on hepatocytes.18Other antitumor drugs may also cause acute portal hypertension, hepatomegaly, and ascites, a syndrome usually called veno-occlusive disease or hepatoportal sclerosis.19,20 The term veno-occlusive disease is a misnomer because the primary site of injury from toxins that are metabolized by hepatocytes in zone 3 of the liver acinus is the sinusoidal endothelium. Hepatic venules are not always involved, and prognosis is related more to the extent of hepatocyte necrosis than to venular abnormalities.21-23
There are 3 potential mechanisms by which gemtuzumab ozogamicin might cause injury to cells in hepatic sinusoids: (1) exposure to unconjugated calicheamicin in the circulation; (2) nonspecific uptake of the antibody–calicheamicin complex by Kupffer cells, similar to the mechanism of liver injury from another antibody–toxin conjugate, LMB-224,25; and (3) receptor-mediated uptake of the antibody–calicheamicin complex through CD33 expression on one or more of the cell populations of the liver. These are not mutually exclusive hypotheses. We speculate that the most likely explanation is receptor-mediated uptake of gemtuzumab ozogamicin, for the following reasons. The calicheamicin in gemtuzumab ozogamicin is not the natural, highly toxic product but is an acetylated derivative that is less toxic. Although there is a free N-acetyl-γ-calicheamicin derivative in gemtuzumab ozogamicin infusates, the quantities are in the range of 0.4 to 0.6 μm/mg protein, or approximately 5 μm per vial of gemtuzumab ozogamicin (unpublished observations (March 2001), Wyeth-Ayerst Pharmaceuticals). Infusions of gemtuzumab ozogamicin in animals that do not express CD33 recognized by the humanized antibody26 did not produce hepatic disease that resembled that seen in our patients. For example, rats given weekly doses of gemtuzumab ozogamicin showed only minor elevations of serum aminotransferase enzymes compared to controls and no evidence of hepatomegaly, ascites, or portal hypertension at necropsy, and studies in cynomolgus monkeys yielded similar findings and no evidence of death after repeated high-dose exposure (unpublished observations, March 2001, Wyeth-Ayerst Pharmaceuticals). These studies suggest that neither free calicheamicin derivative nor nonspecific uptake of the antibody–calicheamicin conjugate causes the sort of sinusoidal injury seen in some humans treated with gemtuzumab ozogamicin. Animal studies, however, are not exactly analogous to the posttransplantation situation, in which the liver may have been injured before exposure to gemtuzumab ozogamicin. The cells in the normal human liver that express CD33 are Kupffer cells, possibly hepatocytes near portal areas, and possibly sinusoidal endothelial cells—each of these populations can be derived from bone marrow progenitors.8,9,11,27,28 In patients with AML, there are likely to be CD33+ leukemia cells in hepatic sinusoids10 and CD33+hematopoietic cell precursor cells in the liver as part of extramedullary hematopoiesis.29 We could not demonstrate a relation between leukocyte or blast counts in the circulation and development of sinusoidal obstruction syndrome in our patients, but it is possible that leukemia cells were sequestered in hepatic sinusoids out of proportion to peripheral blood blast counts.10There is no literature on the expression of CD33 on isolated human stellate cells, and immunohistochemistry studies of human liver tissue has not shown quiescent stellate cells to be CD33+. However, because of the central role of stellate cells in the generation of extracellular matrix30 and the striking deposition of matrix in the sinusoids in the livers of 5 patients in this series, the possibility of CD33 expression on activated human stellate cells should be examined. We speculate, without any direct evidence, that the anti-CD33–calicheamicin conjugate targets Kupffer cells in the sinusoids of the liver, stimulating them to release cytokines in a paracrine manner to activate stellate cells that then lay down matrix in sinusoids.30 This may be a self-perpetuating process, in that ischemia is a fibrogenic stimulus.30 We speculate that gemtuzumab ozogamicin triggers a series of cellular events that lead to sinusoidal obstruction rather than directly causing hepatocyte necrosis.
Although there have been reports of liver injury resembling veno-occlusive disease of the liver after monotherapy with gemtuzumab ozogamicin infusion,5,6 the prevalence of this sort of injury is less than 5% in patients with AML who have not undergone transplantation.2,4 After multidrug chemotherapy that includes gemtuzumab ozogamicin or treatment schedules of gemtuzumab ozogamicin monotherapy with more time-intensive dosing intervals, the frequency of liver injury may be higher.5 In the patients reported here with available liver histology findings, sinusoidal fibrosis was the most common lesion. Fibrous venular occlusion was seen in only 1 of 5 patients with clinical evidence of portal hypertension, and in that patient most of the venules were patent. No matter what the name, drug toxicity to cells in hepatic sinusoids and the resultant sinusoidal obstruction syndrome may not be readily identified by screening tools used in phase 1 and 2 drug studies because signs of portal hypertension (hepatomegaly, renal sodium retention, rapid weight gain, ascites) may develop in the absence of jaundice and the elevation of serum aminotransferase enzyme levels.31
The high prevalence of liver injury after gemtuzumab ozogamicin in our series suggests that there is something about the hepatic milieu in patients who have survived hematopoietic stem cell transplantation that makes the liver more susceptible to gemtuzumab ozogamicin-related sinusoidal injury. There are 4 potential explanations: (1) high-dose myeloablative conditioning therapy damages sinusoidal endothelial cells and activates stellate cells, making both these nonhepatocyte cell types more susceptible to subsequent injury21,32; (2) after allografting, Kupffer cells (derived from donor hematopoietic cells33) may be more susceptible to injury and more prone to occlude sinusoidal blood flow34; (3) sinusoidal endothelial cells might express CD33 (an untested hypothesis) because of their derivation from donor hematopoietic stem cells11; and (4) inflammatory cells related to acute GVHD, retained in the liver, may have activated Kupffer cells and stellate cells before gemtuzumab ozogamicin infusion.35 36
In summary, some patients given gemtuzumab ozogamicin for AML relapse after hematopoietic stem cell transplantation developed a sinusoidal obstruction syndrome characterized by portal hypertension, jaundice, and elevated serum AST level. This form of liver toxicity may be caused by targeted delivery of calicheamicin to CD33+cells in the hepatic sinusoids—ie, Kupffer cells and leukemia cells and possibly sinusoidal endothelial cells and stellate cells.
Supported by grants CA15704 and CA18029 from the National Institutes of Health, National Cancer Institute.
Correspondence:George B. McDonald, Gastroenterology/Hepatology Section (D2-190), Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, PO Box 19024, Seattle, WA 98109-1024.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.