Heparin, located in mast cells and basophilic granulocytes, is widely used as an anticoagulant. It belongs to a class of linear polysaccharides called glycosaminoglycans (GAGs). Using phage display technology, we have selected 19 unique human antiheparin antibodies. Some antibodies react almost exclusively with heparin, others also react with the structurally related heparan sulfate, and some with chondroitin sulfate. In all cases, sulfate groups are essential for binding. For activity of some antibodies, O-sulfation is more important than N-sulfation. Antibodies are reactive with heparin in mast cells. Each antibody showed a defined staining pattern on cryosections of rat kidney, pancreas, and testis. Enzymatic digestion with glycosidases on tissue sections further indicated that the antibodies are specific for GAGs. All antibodies recognize a unique epitope. The effect of the antibodies on heparin as an anticoagulant was also studied. There were 3 antibodies that were very effective inhibitors of heparin action in the activated partial thromboplastin time (APTT) clotting assay, and their effect was related to the amount of heparin bound. Some antibodies reacted strongly with the pentasaccharide, which interacts with antithrombin III. The human antibodies selected represent unique tools to study the structure, location, and function of heparin and related GAGs, and some may be used as blocking agents.
Introduction
Heparin, located in mast cells and basophilic granulocytes, and heparan sulfate (HS), located on cell surfaces and in the extracellular matrix, are comprised of structurally similar, linear polysaccharides. They interact with a wide variety of proteins including growth factors, chemokines, enzymes, enzyme inhibitors, and viral proteins (reviewed in Salmivirta et al1 and Kjellen and Lindahl2). Heparin is best known for its ability to activate antithrombin III, resulting in prevention of blood clotting. Heparin/HS and heparin-binding proteins are involved in many basic processes such as growth, migration, and differentiation. For instance, in angiogenesis, but also in tumor growth,3,4 heparin/HS and a number of heparin-binding proteins like fibroblast growth factors,3 vascular endothelial growth factors,5 angiogenin,6 and midkine7 are involved. Attachment and infection by bacteria, protozoa, and viruses are also mediated by heparin-binding proteins.8,9 Consequently, heparin/HS, their analogues, and their inhibitors may have potential as therapeutic agents. Inhibition of dengue virus infectivity by both a heparin decasaccharide and a polysulphonated heparin analogue, suramin, has been reported.8 Suramin and pentosan polysulfate can inhibit some processes involved in metastatis.10 11
The ability of heparin/HS to inhibit blood coagulation has been known for a long time. Its major clinical applications include the prevention of postoperative thrombosis and the treatment of acute venous thrombosis. Briefly, heparin/HS catalyzes the inactivation of serine proteases, particularly thrombin and factor Xa, by activating the serine protease inhibitor antithrombin III.12 It is believed that the biologic activity of heparin/HS largely depends on the amount and distribution of the sulfate groups which provide specific binding sites for proteins (reviewed in Salmivirta et al1 and Kjellen and Lindahl2). Identification of oligosaccharide sequences that provide specific binding sites is crucial for the understanding of GAG-protein interactions. One strategy to study structural aspects of heparin/HS is the use of antibodies specific for domains within heparin/HS. Since heparin/HS is generally not antigenic, the number of specific antibodies is currently limited.13,14 The antibody phage display system has been adopted to generate anti-HS antibodies.15 16 In this study we have created a sublibrary of phages expressing antiheparin antibodies, and we have characterized them. We furthermore investigated the use of these antibodies in inhibiting the anticoagulant effect of heparin.
Materials and methods
Materials
The synthetic single-chain variable fragment (scFv) library no. 1,17 recombinant heparinase III (from Flavobacterium heparinum) and synthetic heparin pentasaccharide (SR90107A/ORG31540) were generous gifts from Dr G. Winter (Cambridge University, Cambridge, United Kingdom), from IBEX Technologies (Montreal, QC, Canada), and from Sanofi-Synthelabo (Choay, France), respectively.
For phage display, 2 Escherichia coli strains were used: the suppressor strain TG1 (K12, supE, hsdΔ5,thi, Δ[lac-proAB], F'[traD36,proAB+, lacIq,lac-ZΔM15]) and the nonsuppressor strain HB2151 (K12,ara, thi, Δ[lac-pro], F'[proAB+, lacIq, ZΔM15]). Helper phage VCS-M13 was from Stratagene (La Jolla, CA).
All chemicals used were purchased from Merck (Darmstadt, Germany) unless stated otherwise. Bacterial medium (2xTY) was from Gibco BRL (Paisley, Scotland); chemically modified heparin kit, anti–chondroitin sulfate (CS)/dermatan sulfate (DS) “stub” antibody (2B6), and anti-HS “stub” antibody (3G10) were from Seikagaku Kogyo (Tokyo, Japan); heparin from porcine intestinal mucosa, HS from bovine kidney and from porcine intestinal mucosa, chondroitin 4-sulfate from whale cartilage, chondroitin 6-sulfate from shark cartilage, hyaluronate from human umbilical cord, DNA from calf thymus, dextran sulfate, phenylmethylsulfonyl fluoride (PMSF), N-ethylmaleimide, aprotinin, sodium azide, pepstatin A, activated partial thromboplastin time (APTT) reagent, normal reference plasma, bovine serum albumin (fraction V), and chondroitinase ABC (from Flavobacterium heparinum) were from Sigma (St Louis, MO); Microlon 96-well microtiter plates were from Greiner (Frickenhausen, Germany); polystyrene Maxisorp Immunotubes were from Nunc (Roskilde, Denmark); anti–c-Myc tag mouse monoclonal IgG (clone 9E10), anti-VSV tag mouse monoclonal IgG (clone P5D4), and (5)6-carboxyfluorescein-N-hydroxysuccinimide ester were from Boehringer Mannheim (Mannheim, Germany); anti–c-Myc tag rabbit polyclonal IgG (A-14) were from Santa Cruz Biotechnology (Santa Cruz, CA); alkaline phosphatase–conjugated rabbit antimouse IgG and mouse antihuman mast cell tryptase were from Dakopatts (Glostrup, Denmark); Alexa 488-conjugated goat antimouse IgG and Alexa 594–conjugated goat antimouse IgG were from Molecular Probes (Eugene, OR); Mowiol (4-88) was from Calbiochem (La Jolla, CA); Protein A Sepharose was from Kem-En-Tec A/S (Copenhagen, Denmark); Ni-NTA agarose was from Qiagen (Hilden, Germany); and ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit was from PE Applied Biosystems (Norwalk, CT).
All experiments were performed at ambient temperature (22°C), unless stated otherwise.
Selection of antiheparin antibodies
The human synthetic single-chain variable fragment library no. 117 was used in this study. This library contains 108 different clones with 50 different human VHgenes with synthetic random complementarity-determining region 3 (CDR3) segments and one VL gene. Selection of phages displaying scFv antibodies was as described.16 To obtain phages expressing antiheparin antibodies, immunotubes were coated with heparin at a concentration of 10 μg/mL overnight. Phages (5 × 1012 in phosphate-buffered saline [PBS] containing 2% Marvel [dried skimmed milk]) were added to the tubes, and allowed to bind to heparin under continuous rotation for 30 minutes, followed by standing for an additional 90 minutes. Tubes were washed 5 times with PBS containing 0.05% (wt/vol) Tween-20 and 5 times with PBS, and bound phages were eluted by addition of 1 mL 100 mM triethylamine. After neutralization with 0.5 mL of 1 M Tris/HCl, pH 7.4, phages were allowed to infect E coli TG1. Bacteria were grown and the phages produced used for a new round of panning. A total of 4 rounds of selection was performed. After the last round of selection, phages were used to infect E coli HB2151, which allows for production of soluble antibodies rather than phage-bound antibodies.
Infected bacteria were plated and single colonies were picked and induced with isopropyl-β-D-thiogalactopyranoside (IPTG) to produce antibodies. After overnight induction at 30°C, the bacterial supernatants containing the antibodies were evaluated for reactivity with heparin by enzyme-linked immunosorbent assay (ELISA) (see below). Phagemid DNA of positive clones was isolated and digested with restriction enzymes NotI and NcoI to obtain the DNA encoding the scFv. This fragment was subcloned into vector pUC119 His VSV (J. M. H. Raats, unpublished data, September, 1996), which is similar to the original pHEN1 vector17 18 but does not contain the c-Myc tag and pIII gene. Instead, this vector contains a polyhistidine and VSV-tag for detection and purification of the antibodies. Both strands of the antibody DNA were sequenced using primers PelB-seq (5′-CCGCTGGATTGTTATTACTC-3′) (located within the PelB leader sequence), and For Link-seq RIC (5′-GCCACCTCCGCCTGAACC-3′) (located in the linker region between the VH and the VL genes).
Source of antibodies
To produce large amounts of antibodies, periplasmic fractions from infected bacteria were isolated. A quantity of 500 mL 2xTY medium containing 0.1% (wt/vol) glucose and 100 μg ampicillin/mL was inoculated with an overnight bacterial culture and grown at 37°C until an optical density (OD600) of 0.6 was reached. Induction was obtained by addition of IPTG (final concentration 1 mM). After 3 hours of incubation at 30°C, the culture was cooled on ice for 20 minutes and cells were centrifuged at 3000g for 10 minutes at 4°C. The pellet was resuspended in 5 mL ice-cold 200 mM sodium-borate buffer (pH 8.0) containing 160 mM NaCl, and an ethylenediaminetetraacetic acid (EDTA)–free protease inhibitor cocktail (Boehringer Mannheim). After centrifugation at 5000g for 30 minutes at 4°C, the supernatant (representing the periplasmic fraction) was filtered through a 0.45-μm filter and dialyzed against PBS.
Evaluation of specificity by ELISA
To evaluate the specificity of antibodies, their reactivity with the following molecules was evaluated: heparin, HS (from bovine kidney and from intestinal mucosa), chondroitin 4-sulfate, chondroitin 6-sulfate, dextran sulfate, hyaluronate, and DNA. Wells were coated with the various molecules by incubation with 100 μL of a 10 μg/mL solution in wells of a 96-well microtiter plate. The wells were rinsed with PBS and blocked with 1% bovine serum albumin (BSA) in PBS containing 0.1% (vol/vol) Tween-20 (PBST). Periplasmic fractions containing antibodies were added and allowed to bind for 90 minutes. Bound antibodies were detected by incubation with 2-fold diluted mouse anti-VSV monoclonal antibody P5D4 followed by incubation with 1:1000 diluted alkaline-phosphatase conjugated rabbit antimouse IgG. The plates were rinsed 6 times with PBST following each incubation. Enzyme activity was detected using 100 μL 1 mg p-nitrophenyl phosphate/mL 1 M diethanolamine/0.5 mM MgCl2, pH 9.8, as a substrate. Absorbance was read at 405 nm. All assays were performed at least 3 times and representative results are shown.
To evaluate which groups in heparin are important for recognition of the antibodies, an inhibition ELISA with chemically modified heparins (from porcine intestine) was performed, including heparins that were completely desulfated and N-sulfated, completely desulfated and N-acetylated, and N-desulfated and N-acetylated. Periplasmic fractions containing antibodies were preincubated overnight with various concentrations of chemically modified heparin followed by transfer to 96-well microtiter plates previously coated with heparin. The plates were washed and ELISA was performed as described above.
To evaluate if the antibodies could bind to the antithrombin III-binding pentasaccharide in heparin, a competition ELISA was performed. Periplasmic fractions containing antibodies were incubated with various concentrations of the pentasaccharide in wells coated with heparin. The plates were washed and ELISA was performed as described above.
Evaluation of specificity by immunohistochemistry
Tissue specimens from rat kidney, pancreas, and testis were snap-frozen in liquid isopentane, cooled with liquid nitrogen, and stored at −80°C. Cryosections (5 μm) were rehydrated for 10 minutes in PBS, blocked with PBS containing 1% (wt/vol) BSA for 30 minutes, and incubated with 1:1 diluted antibodies for 90 minutes. Bound antibodies were detected by incubation with 1:1 diluted mouse anti-VSV monoclonal antibody P5D4, followed by goat antimouse IgG Alexa 488, both for 60 minutes. After each incubation, cryosections were washed with PBS (3 × 5 minutes). Finally, the sections were fixed in 100% methanol, air-dried, and embedded in Mowiol (10% [wt/vol] in 0.1 M Tris-HCl, pH 8.5, 25% glycerol [vol/vol], 2.5% NaN3 [wt/vol]). As a control, cryosections were stained with an irrelevant antibody TSCO1 (CDR3 sequence LGFHS, VH3 family, germline segment DP 40).
To evaluate the specificity of the antibodies toward tissue-associated GAGs, cryosections were digested with the glycosidases heparinase III (digests HS; 0.02 IU/mL in 25 mM Tris-HCl, pH 8.0, 22 hours at 37°C) and chondroitinase ABC (digests CS and DS; 1 unit/mL in 25 mM Tris-HCL, pH 8.0, 22 hours at 37°C). Combined digestions of enzymes were performed using the same concentrations in 25mM Tris HCl, pH 8.0. As a control, cryosections were incubated in reaction buffer without enzyme. After washing 3 times with PBS and blocking with PBS containing 1% (wt/vol) BSA, sections were incubated with antibodies and processed for immunofluorescence as described above. The efficiency of heparinase III and chondroitinase ABC treatment was evaluated by incubation of cryosections with antibodies against GAG-“stubs,” generated by the glycosidases. For HS stubs the antibody 3G10 was used, for chondroitin sulfate stubs the antibody 2B6 was used. All tests were performed at least 3 times.
To evaluate whether the antibodies recognize heparin in situ, cryosections of human lung, which contain mast cells, were used. Cryosections (5 μm) were rehydrated for 10 minutes in PBS, blocked with PBS containing 1% (wt/vol) BSA for 30 minutes, and incubated with 1:1 diluted antibodies for 90 minutes. Bound antibodies were detected using 1:500 diluted anti–c-Myc rabbit polyclonal antibody A-14 and goat antirabbit IgG Alexa 488, each for 60 minutes. For detection of mast cell tryptase, 1:500 diluted mouse antihuman mast cell tryptase and goat antimouse IgG Alexa 594 were included in the incubations. After each incubation, cryosections were washed with PBS (3 × 5 minutes). Finally, the sections were fixed in 100% methanol, air-dried, and embedded in Mowiol. As a control, cryosections were stained with an irrelevant antibody TSCO1.
Activated partial thromboplastin time clotting assay
Heparin, an anticoagulant, prolongs clotting time in proportion to its concentration.19 To evaluate if antibodies could inhibit the effect of heparin on clotting time, an APTT clotting assay was performed. Antibodies were purified from periplasmic fractions by binding to Protein A Sepharose or Ni-NTA agarose. Since only antibodies of the VH3 family can bind to protein A, 15 antibodies could be purified using Protein A Sepharose. Some antibodies of the VH3 family (EW3D10, EW3E4, EW4B5, EW4G2) and of other VH families (EW4A4, EW4B7, EW4B10, EW4E10) were purified using Ni-NTA agarose. To allow binding, 300 μL of a periplasmic fraction was added to 300 μL of Protein A Sepharose beads in a total volume of 900 μL PBS, and rotated for 3 hours at 4°C. Beads were washed 3 times with PBS. For binding to Ni-NTA agarose, an identical procedure was used, only 50 mM NaH2PO4, 300 mM NaCl, pH 8.0, was used as binding buffer. To check for purity, bead-bound antibodies were resuspended in 2x sample buffer (2% sodium dodecyl sulfate [SDS], 62,5 mM Tris pH 6.8, 5% 2-mercaptoethanol, 0.001% bromofenolblue) and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). A single band of 30 kd, representing the antibody, was observed after silver staining of the gel (data not shown). For the APTT clotting assay, 50 μL Protein A Sepharose or Ni-NTA agarose-bound antibodies was mixed with 50 μL of 10 μg/mL heparin and allowed to interact for 60 minutes. Next, 100 μL human reference plasma was added, incubated for 1 minute at 37°C, followed by incubation with 100 μL APTT reagent for 3 minutes at 37°C. Finally, 100 μL of 20 mM CaCl2 was added, and the clotting time recorded according to the manufacturer's instructions.
Labeling of heparin with a fluorescent marker
Heparin (10 mg), dissolved in 250 μL 0.2 M Na2CO3/NaHCO3 (pH 8.5), and 250 μg (5)6-carboxyfluorescein-N-hydroxysuccinimide ester (FLUOS), dissolved in 200 μL dimethylsulphoxide, were mixed and incubated for 1 hour while shaking. The reaction was stopped by applying the reaction mixture onto a milli-Q water-equilibrated Sephadex G-25 column. Heparin was eluted with milli-Q. Fractions containing fluorescence were pooled. Heparin-FLUOS was precipitated by addition of 0.5 mL 5 M NaCl, 50 μL 1 M Tris pH 7.0, and 25 mL ice-cold ethanol, followed by incubation at −20°C for 16 hours, and centrifugation at 6000g for 30 minutes. The pellet was washed with ice-cold 70% ethanol and resuspended in 1 mL milli-Q. The heparin concentration was determined by dimethylmethylene blue staining in glycine buffer as described.20
Binding of fluorescent heparin to antibodies bound to beads
To evaluate the amount of heparin bound to antibodies immobilized on Protein-A or Ni-NTA beads, a binding assay with fluorescent heparin was performed identical to the one used for the APTT clotting assay, except that heparin was replaced by heparin-FLUOS. After binding, beads were washed, dissolved in 1 mL PBS, and the fluorescence measured using an RF-5301PC spectrofluorophotometer (Shimadzu, Tokyo, Japan) at an excitation wavelength of 488 nm and an emission wavelength of 520 nm. A 10-nm slot was used. The amount of heparin-FLUOS bound by the antibodies attached to beads was calculated using a calibration curve of known concentrations of heparin-FLUOS to which beads had been added.
Results
Selection of antiheparin antibodies
We performed 4 rounds of biopanning of the synthetic scFv library no. 1 against immobilized heparin, which resulted in a 6 × 104-fold increase of phage titer. The second round of selection resulted in a substantial increase of 103-fold. The phage titers after selection rounds 1, 2, 3, and 4 were 5.0 × 103, 5.8 × 106, 9.0 × 107, and 3.1 × 108 colony-forming units, respectively. Antibody supernatants derived from induced individual clones of the third and fourth selection were tested for reactivity with heparin in an ELISA. Of the 188 scFv antibody preparations screened, 36 reacted with heparin. DNA sequence analysis of the VH gene of these 36 clones revealed a set of 19 unique antiheparin antibodies (Table 1).
Specificity of antiheparin antibodies
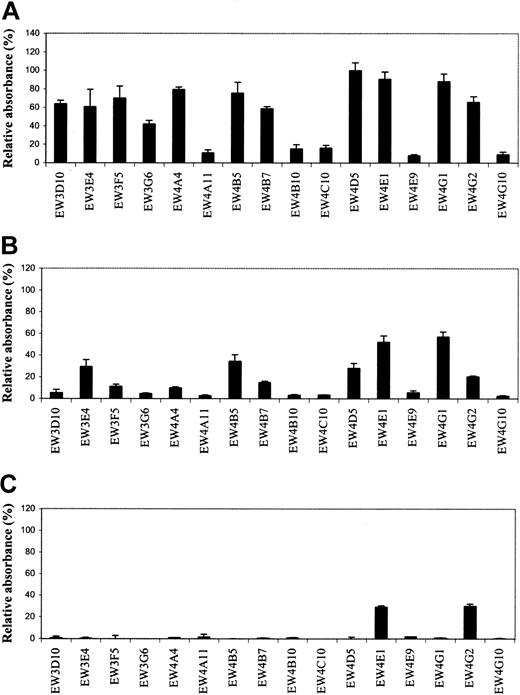
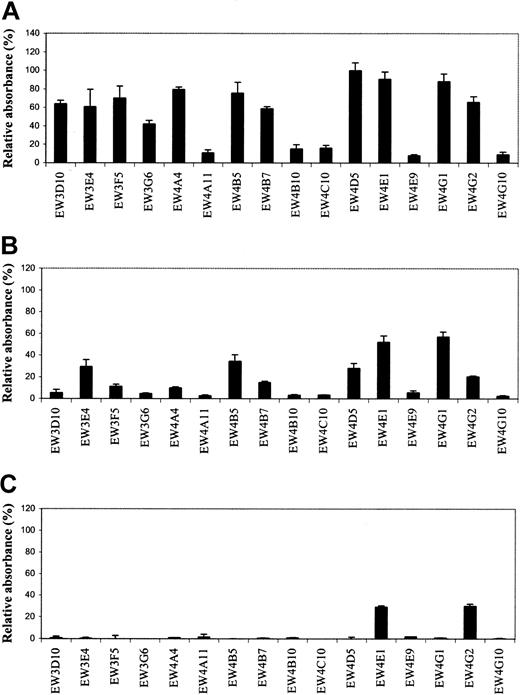
Antibodies were tested for their reactivity with different GAGs and related molecules. All 19 antibodies reacted with heparin in ELISA. Since 3 antibodies (EW3H12, EW4D2, and EW4E10) were also selected and characterized in previous studies,15,21 22 we only evaluated the remaining 16 antibodies for their specificity and affinity. No reactivity was observed with dextran sulfate, hyaluronate, and DNA, which are all strongly negatively charged molecules. Most antibodies showed a different reactivity with HS from bovine kidney, HS from porcine intestinal mucosa, and chondroitin 6-sulfate (Figure1A-C). Antibodies EW4A11, EW4B10, EW4C10, EW4E9, and EW4G10 preferentially reacted with heparin; EW3D10, EW3F5, EW3G6, EW4A4, and EW4B7 also reacted with HS from bovine kidney, but hardly with HS from porcine intestinal mucosa. EW4E1 and EW4G2 reacted, next to heparin and HS, with chondroitin 6-sulfate. EW4G2 also reacted with chondroitin 4-sulfate (data not shown).
Evaluation of antiheparin antibody specificity by ELISA.
Periplasmic fractions containing antiheparin antibodies were applied to various GAG species coated on microtiter plates. Bound antibodies were detected using anti-VSV mouse monoclonal antibody P5D4, followed by alkaline phosphatase–conjugated rabbit antimouse IgG. Enzymatic activity was measured using p-nitrophenyl phosphate as a substrate. Bars represent the mean reactivity ± SD (n = 3) of the antibodies in percent relative to their reactivity with heparin. (A) Reactivity toward HS from bovine kidney. (B) Reactivity toward HS from porcine intestinal mucosa. (C) Reactivity toward chondroitin-6 sulfate from shark cartilage.
Evaluation of antiheparin antibody specificity by ELISA.
Periplasmic fractions containing antiheparin antibodies were applied to various GAG species coated on microtiter plates. Bound antibodies were detected using anti-VSV mouse monoclonal antibody P5D4, followed by alkaline phosphatase–conjugated rabbit antimouse IgG. Enzymatic activity was measured using p-nitrophenyl phosphate as a substrate. Bars represent the mean reactivity ± SD (n = 3) of the antibodies in percent relative to their reactivity with heparin. (A) Reactivity toward HS from bovine kidney. (B) Reactivity toward HS from porcine intestinal mucosa. (C) Reactivity toward chondroitin-6 sulfate from shark cartilage.
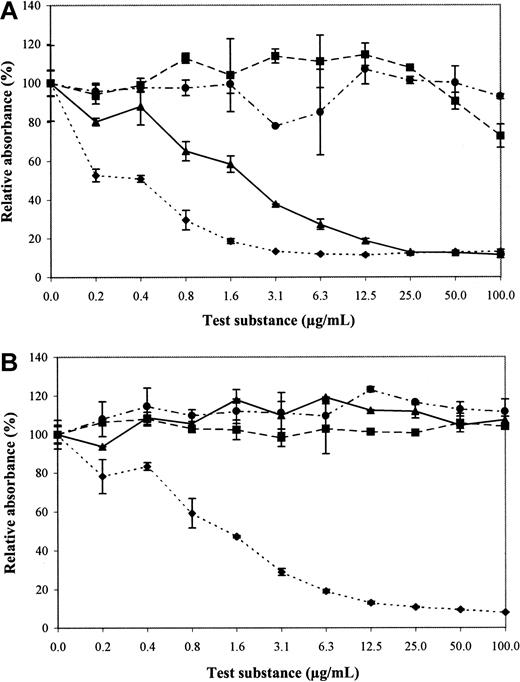
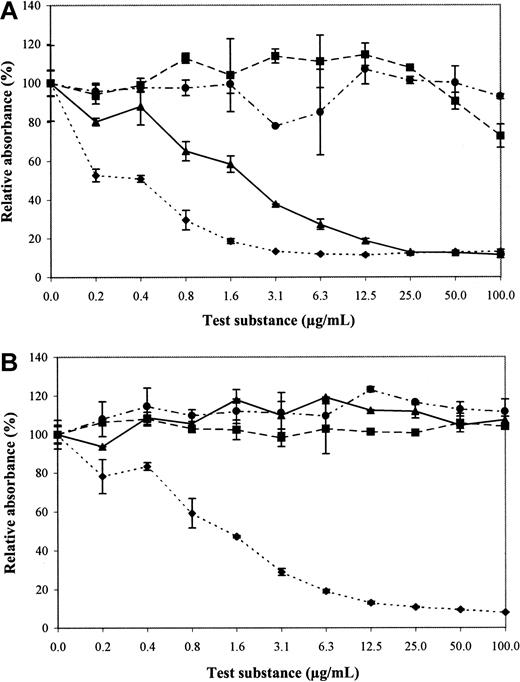
To determine which epitopes on heparin are important for recognition, we tested all 16 antibodies for reactivity with chemically modified heparin preparations. Completely desulfated and N-sulfated heparin, completely desulfated and N-acetylated heparin, and N-desulfated and N-acetylated heparin were not able to inhibit the binding of the antibodies to heparin (Figure 2A). Binding of EW3F5 and EW3G6 to heparin, however, was partially inhibited by N-desulfated, N-acetylated heparin. The concentration at which an inhibition of 50% binding was observed (IC50) was about 2 μg/mL for EW3F5, and 6 μg/mL for EW3G6 (Figure 2B), indicating affinity for this modified heparin. For heparin itself, the IC50 was about 0.4 μg/mL, and 0.6 μg/mL respectively, indicating that the antibodies bind N-desulfated, N-acetylated heparin, but the affinity is higher for unmodified heparin.
Reactivity of antibodies with modified heparin preparations (inhibition ELISA).
Periplasmic fractions containing antibodies were allowed to bind to chemically modified heparin preparations for 16 hours, and then transferred to heparin-coated wells. Antibodies bound to heparin were detected using anti-VSV mouse monoclonal antibody P5D4, followed by alkaline phosphatase–conjugated rabbit antimouse IgG. Enzymatic activity was measured using p-nitrophenyl phosphate as a substrate. Values represent the reactivity in percent relative to heparin. Chemically modified heparin preparations used were: completely desulfated, N-sulfated heparin (■); N-desulfated and N-acetylated heparin (▴); completely desulfated and N-acetylated heparin (●); unmodified heparin (♦). (A) Antibody EW3F5. (B) Antibody EW4A4. Note reactivity of EW3F5, but not EW4A4, with N-desulfated and N-acetylated heparin.
Reactivity of antibodies with modified heparin preparations (inhibition ELISA).
Periplasmic fractions containing antibodies were allowed to bind to chemically modified heparin preparations for 16 hours, and then transferred to heparin-coated wells. Antibodies bound to heparin were detected using anti-VSV mouse monoclonal antibody P5D4, followed by alkaline phosphatase–conjugated rabbit antimouse IgG. Enzymatic activity was measured using p-nitrophenyl phosphate as a substrate. Values represent the reactivity in percent relative to heparin. Chemically modified heparin preparations used were: completely desulfated, N-sulfated heparin (■); N-desulfated and N-acetylated heparin (▴); completely desulfated and N-acetylated heparin (●); unmodified heparin (♦). (A) Antibody EW3F5. (B) Antibody EW4A4. Note reactivity of EW3F5, but not EW4A4, with N-desulfated and N-acetylated heparin.
Reactivity of antiheparin antibodies with rat renal cortex, pancreas, and testis
To further evaluate the reactivity of antiheparin antibodies, we performed immunohistochemistry on cryosections of rat renal cortex, pancreas, and testis. Each antibody shows a defined pattern of reactivity (Figures 3,4, and5). Antibody EW3E4 does not react with any of the investigated organs, whereas the reaction with heparin and HS in ELISA is clearly positive. Antibodies EW3F5, EW3H12, EW4B7, EW4D2, EW4E1, EW4E10, and EW4G1 react with all the morphologic structures analyzed, indicating that these antibodies detect general epitopes. Antibodies EW3G6, EW4A11, and EW4G10 detect only a few structures and show some specificity, indicating that these antibodies detect relatively rare epitopes. Antibody EW3G6 detects epitopes in renal cortex, but not in pancreas and testis. Antibody EW4A11 detects epitopes in renal cortex and testis, but not in pancreas. Of the renal tubules, only the collecting ducts are stained. Antibody EW4G10 stains only pancreas and testis, but not renal cortex. Subtle differences were observed in the intensity of staining by the investigated antibodies (Figure 3).
Immunofluorescence staining of various tissues with antiheparin antibodies.
Cryosections of rat renal cortex, pancreas, and testis were stained with periplasmic fractions containing the antibodies. Bound antibodies were visualized by incubation with anti-VSV mouse monoclonal antibody P5D4, followed by Alexa 488–conjugated goat antimouse IgG. Staining intensity: ++, strong; +, moderate; +−, weak; −, absent (n=3). (a) Collecting ducts only.
Immunofluorescence staining of various tissues with antiheparin antibodies.
Cryosections of rat renal cortex, pancreas, and testis were stained with periplasmic fractions containing the antibodies. Bound antibodies were visualized by incubation with anti-VSV mouse monoclonal antibody P5D4, followed by Alexa 488–conjugated goat antimouse IgG. Staining intensity: ++, strong; +, moderate; +−, weak; −, absent (n=3). (a) Collecting ducts only.
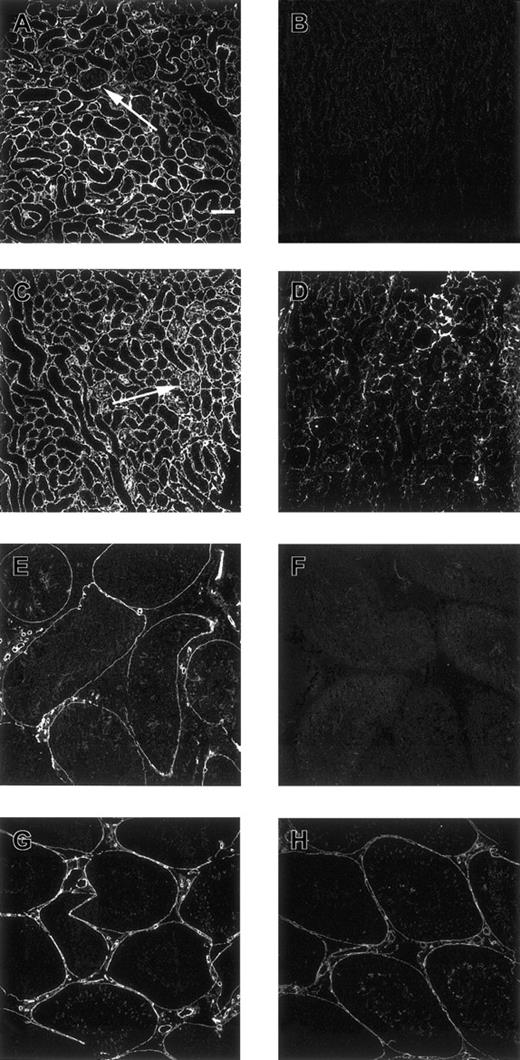
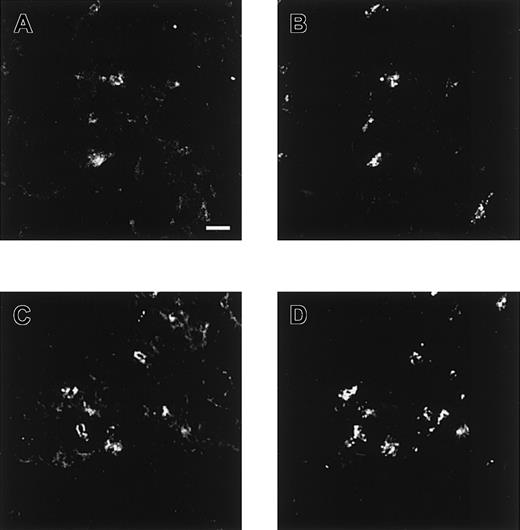
Confocal microscopical analysis of renal cortex and testis stained with antiheparin antibodies and its sensitivity to heparinase III digestion.
Nontreated (A, C) and heparinase III–treated (B, D) cryosections of rat renal cortex, and nontreated (E, G) and heparinase III–treated (F, H) cryosections of rat testis were incubated with periplasmic fractions containing antiheparin antibody EW4B5 (A, B), antiheparin antibody EW4E1 (C-F) or antiheparin antibody EW4G2 (G, H). Bound antibodies were visualized by incubation with anti-VSV mouse monoclonal antibody P5D4, followed by Alexa 488–conjugated goat antimouse IgG. Note the difference in staining of the glomerulus in A and C and the difference in staining intensity after heparinase III digestion in B, D, F, and H. Scale bar, 100 μm.
Confocal microscopical analysis of renal cortex and testis stained with antiheparin antibodies and its sensitivity to heparinase III digestion.
Nontreated (A, C) and heparinase III–treated (B, D) cryosections of rat renal cortex, and nontreated (E, G) and heparinase III–treated (F, H) cryosections of rat testis were incubated with periplasmic fractions containing antiheparin antibody EW4B5 (A, B), antiheparin antibody EW4E1 (C-F) or antiheparin antibody EW4G2 (G, H). Bound antibodies were visualized by incubation with anti-VSV mouse monoclonal antibody P5D4, followed by Alexa 488–conjugated goat antimouse IgG. Note the difference in staining of the glomerulus in A and C and the difference in staining intensity after heparinase III digestion in B, D, F, and H. Scale bar, 100 μm.
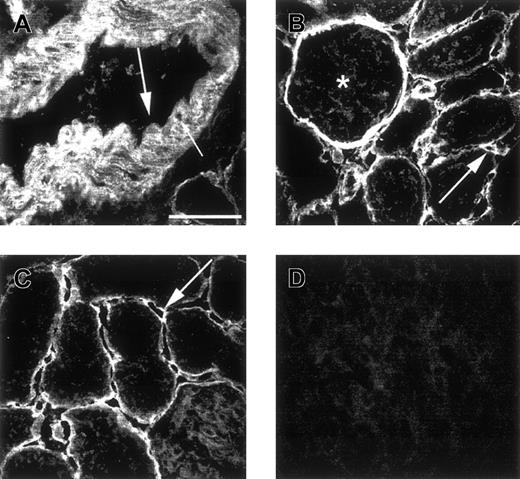
Microscopical analysis of renal cortex and pancreas stained with antiheparin antibodies.
Cryosections of rat renal cortex (A-C) and pancreas (D) were incubated with periplasmic fractions containing antiheparin antibody EW4D5 (A, B) or antiheparin antibody EW4E9 (C, D). Bound antibodies were visualized by incubation with anti-VSV mouse monoclonal antibody P5D4, followed by Alexa 488–conjugated goat antimouse IgG. Small arrow in A indicates smooth muscle cells; large arrow in A, endothelium; arrow in B, peritubular capillary; asterisk in B, glomerulus; arrow in C, peritubular capillary. Note the absence of staining of endothelium in A, but staining of endothelium in B. Scale bar, 50 μm.
Microscopical analysis of renal cortex and pancreas stained with antiheparin antibodies.
Cryosections of rat renal cortex (A-C) and pancreas (D) were incubated with periplasmic fractions containing antiheparin antibody EW4D5 (A, B) or antiheparin antibody EW4E9 (C, D). Bound antibodies were visualized by incubation with anti-VSV mouse monoclonal antibody P5D4, followed by Alexa 488–conjugated goat antimouse IgG. Small arrow in A indicates smooth muscle cells; large arrow in A, endothelium; arrow in B, peritubular capillary; asterisk in B, glomerulus; arrow in C, peritubular capillary. Note the absence of staining of endothelium in A, but staining of endothelium in B. Scale bar, 50 μm.
To further investigate the specificity of the antibodies, cryosections of kidney and testis were treated with heparinase III, chondroitinase ABC, or a combination of heparinase and chondroitinase prior to incubation with antibody. Staining is absent or strongly decreased after treatment with heparinase III. For antibodies EW4E1 (kidney but not testis) and EW4G2 (kidney and testis) some staining still remains after treatment with heparinase III (Figure 4D,H). This staining was lost after combined treatment with heparinase III and chondroitinase ABC (data not shown).
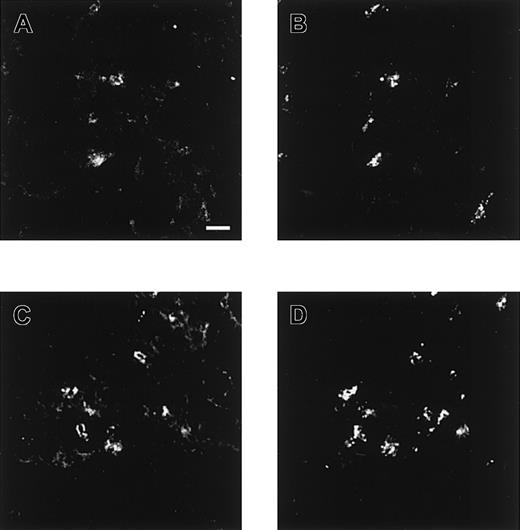
To investigate the binding of the antiheparin antibodies to heparin in mast cells, cryosections of human lung were incubated with 2 antibodies (EW4E1 or EW4G1). Mast cells were identified using anti–mast cell tryptase. Both antiheparin antibodies reacted strongly with mast cells (Figure 6).
Confocal microscopical analysis of lung stained with antiheparin antibodies.
Cryosections of human lung were incubated with periplasmic fractions of antiheparin antibodies EW4E1 (A) and EW4G1 (C). Bound antibodies were visualized by incubation with rabbit polyclonal anti–c-Myc IgG followed by Alexa 488–conjugated goat antirabbit IgG. Tryptase, present in mast cells, was visualized using mouse antihuman mast cell tryptase followed by Alexa 594–conjugated goat antimouse IgG (B, D). Both antiheparin antibodies react strongly with mast cells. Scale bar, 100 μm.
Confocal microscopical analysis of lung stained with antiheparin antibodies.
Cryosections of human lung were incubated with periplasmic fractions of antiheparin antibodies EW4E1 (A) and EW4G1 (C). Bound antibodies were visualized by incubation with rabbit polyclonal anti–c-Myc IgG followed by Alexa 488–conjugated goat antirabbit IgG. Tryptase, present in mast cells, was visualized using mouse antihuman mast cell tryptase followed by Alexa 594–conjugated goat antimouse IgG (B, D). Both antiheparin antibodies react strongly with mast cells. Scale bar, 100 μm.
Inhibition of heparin effect on activated partial thromboplastin time by antiheparin antibodies
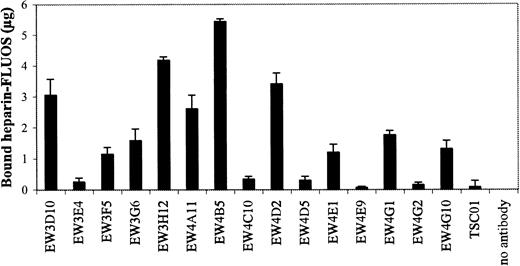
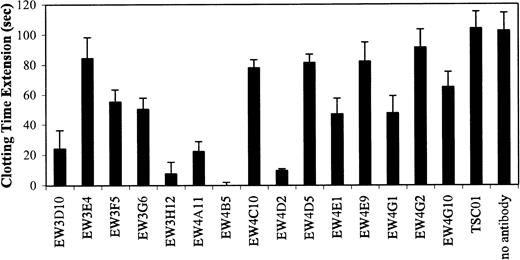
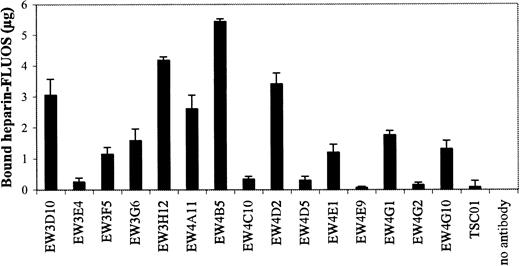
Since the antibodies were selected against heparin, we investigated whether they were able to inhibit the biologic activity of heparin. One characteristic of heparin is its inhibition of blood coagulation by binding to, and activation of, antithrombin III.23 All antibodies were evaluated for their capacity to block the effect of heparin on coagulation using a standard APTT assay. In this assay, heparin prolongs the time of coagulation. We used beads to purify our antibody preparations. Antibodies of the VH3 family could be purified using Protein A Sepharose, whereas all antibodies could be purified using Ni-NTA agarose. Of the 15 antibodies bound to Protein A, EW3D10, EW3H12, EW4A11, EW4B5, and EW4D2 extensively inhibited the anticoagulation effect of heparin (Figure7). A complete inhibition was accomplished by antibody EW4B5. By the use of Ni-NTA agarose, similar results were obtained (data not shown). The inhibitory effect of the antibodies was related to the amount of heparin bound by the bead-bound antibodies (Figure 8). For example, antibody EW4B5 bound the largest amount of heparin and showed the strongest effect. The amount of bound heparin is a result of the amount of antibody used and the binding affinity of the antibody. The amount of antibody used in the assay, however, is difficult to determine.
Blocking of anticoagulant effect of heparin by antiheparin antibodies.
Periplasmic fractions containing antiheparin antibodies were allowed to bind to Protein A Sepharose. The beads were washed and the antibody-bead complexes were used in an APTT assay. Note the complete inhibition of the anticoagulant effect of heparin by EW4B5. Values are mean ± SD (n = 3).
Blocking of anticoagulant effect of heparin by antiheparin antibodies.
Periplasmic fractions containing antiheparin antibodies were allowed to bind to Protein A Sepharose. The beads were washed and the antibody-bead complexes were used in an APTT assay. Note the complete inhibition of the anticoagulant effect of heparin by EW4B5. Values are mean ± SD (n = 3).
Determination of the amount of heparin bound by Protein A Sepharose–bound antibodies.
Periplasmic fractions of antiheparin antibodies were allowed to bind to Protein A Sepharose beads. The beads were washed and the antibody-bead complexes were incubated with FLUOS-labeled heparin. After washing, the fluorescence of the bound heparin was determined.
Determination of the amount of heparin bound by Protein A Sepharose–bound antibodies.
Periplasmic fractions of antiheparin antibodies were allowed to bind to Protein A Sepharose beads. The beads were washed and the antibody-bead complexes were incubated with FLUOS-labeled heparin. After washing, the fluorescence of the bound heparin was determined.
To investigate if the anticoagulation capacity of the antibodies was due to interference of the binding of heparin to antithrombin, 4 antibodies were tested for their reactivity with the antithrombin III-binding pentasaccharide of heparin. EW4D2 and EW4E1, both having anticoagulation capacity, bound to the pentasaccharide (IC50 of about 1.2 μg/mL and 0.16 μg/mL, respectively; the IC50 for heparin was about 0.4 μg/mL and 0.17 μg/mL respectively, indicating that especially the affinity of EW4E1 for the pentasaccharide is high). EW4B5, also displaying anticoagulation capacity, however, did not bind to the pentasaccharide. EW4G2, having no anticoagulation effect, was also not reactive.
Discussion
In this study we have used the phage display technique to select antibodies against heparin from the semisynthetic scFv library no. 1. The use of a semisynthetic library has advantages because GAGs, including heparin, are largely nonimmunogenic, and therefore the use of conventional antibody generation techniques is generally unsuccessful. Using this library, we have selected 19 antibodies all with unique amino acid sequences (Table 1). Antibody EW3H12 with CDR3 sequence GMRPRL was also selected in 3 previous studies.15,21,22 In 2 studies performed in our laboratory, this antibody, named RB4CD12 and HS3A8, was selected against isolated GAGs from mouse muscle and HS from bovine kidney, respectively, and reacted with heparin and HS from various sources.15,22 In another study, the same antibody was selected for its reactivity with high-molecular-weight melanoma-associated antigen, a chondroitin sulfate proteoglycan.21 Perhaps the epitope, recognized by this antibody, is also located in this proteoglycan. Alternatively, the high-molecular-weight melanoma-associated antigen could also contain HS chains. Remarkable is the predominance of DP segment 38 of the VH3 family in the antibodies selected. The antibodies from the third round of selection all contain the DP 38. At present, we have no explanation for this.
The specificity of the antibodies was determined by ELISA and immunocytochemistry. Data indicate that all antibodies recognize a unique epitope. Some antibodies react with different classes of GAGs (eg, heparin and CS), indicating common epitopes within these classes. In this respect, an analogy may be drawn with the hepatocyte growth factor which binds to HS as well as DS.24
To test the importance of sulfate groups in the binding of antibodies, we used modified heparins. None of the antibodies is able to bind to completely desulfated and N-acetylated heparin or completely desulfated and N-sulfated heparin. Apparently, sulfate groups are essential for binding of all antibodies tested. Only EW3F5 and EW3G6 reacted with N-desulfated, N-acetylated heparin, indicating that the presence of N-sulfate groups in heparin is not essential for binding to these antibodies.
Heparin is widely used as an anticoagulant (eg, in the prophylactic treatment of patients at risk for embolism) in postoperative prevention of thromboembolism, and in prevention of clotting and thrombus formation resulting from interventions in the circulatory system.25
Heparin is also used in many other procedures including extracorporeal blood circulation, such as hemodialysis, use of artificial organs, and organ transplantation. The effect of heparin must be controllable (ie, neutralized when necessary). Currently, protamine sulfate is the most frequently used compound to reverse heparin-induced anticoagulation. The use of protamine, however, occasionally results in adverse hemodynamic and hematologic side effects such as hypotension, bradycardia, pulmonary artery hypertension, depressed oxygen consumption, thrombocytopenia with pulmonary platelet sequestration, and leukopenia.25 Furthermore, many patients suffer from adverse immunologic reactions to protamine. There is clearly a need for a safer, less-toxic agent for inhibition of heparin activity. We therefore tested if the antiheparin antibodies could block the effect of heparin on coagulation. Some, but not all, antibodies were able to inhibit the effect of heparin on coagulation in vitro. Antibody EW4B5 was able to completely neutralize the effect of heparin. Antibodies EW4D2 and EW3H12 were also very potent inhibitors. This may be accomplished by competition of the antibody for binding sites of (anti)coagulation factors, or by modifying the secondary structure of the heparin molecule, and thereby modulating its function. To address the first possibility, we tested if the antibodies that inhibit the anticoagulant effect of heparin were reactive with the antithrombin III-binding pentasaccharide of heparin. Antibodies EW4D2 and EW4E1 bound to the pentasaccharide, but antibody EW4B5 did not. The latter antibody may exert its anticoagulant effect by binding to a heparin epitope very close to the pentasaccharide (sterical hindrance), or by preventing the binding of thrombin to heparin, thereby preventing its interaction with antithrombin-III.
The antibodies, which reduce or inhibit the anticoagulant activity of heparin, and which are of human origin, may provide a good alternative to the use of protamine as a heparin-blocking agent.
Next to its effect on coagulation, heparin has a number of other biologic activities.26-28 These include antiatherosclerotic activities (binding of lipoprotein lipase, inhibition of proliferation of smooth muscle cells), angiogenic activities (binding growth factors), and the ability to inhibit complement activation. It is becoming increasingly clear that all these activities are mediated by different domains on the heparin molecule. The antibodies described here may be useful tools to further pinpoint which domain (ie, which monosaccharide sequence) is important for a certain biologic effect.
We express our gratitude to Dr G. Winter (Cambridge University, Cambridge, United Kingdom) for providing the phage display library. We thank Dr J. M. H. Raats (Department of Biochemistry, Faculty of Sciences, Nijmegen, The Netherlands) for providing the pUC119 His VSV vector; IBEX Technologies (Montreal, QC, Canada) for providing recombinant heparinase III derived from Flavobacterium heparinum, and Sanofi-Synthelabo (Choay, France) for providing the pentasaccharide.
Supported by the Dutch Cancer Society (Project KUN 98-1801) and the Dutch Kidney Foundation (C 96.1587).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toin H. van Kuppevelt, Department of Biochemistry 194, University Medical Center Nijmegen, NCMLS, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: a.vankuppevelt@NCMLS.kun.nl.