We have discovered a novel canine hereditary bleeding disorder with the characteristic features of Scott syndrome, a rare defect of platelet procoagulant activity. Affected dogs were from a single, inbred colony and experienced clinical signs of epistaxis, hyphema, intramuscular hematoma, and prolonged bleeding with cutaneous bruising after surgery. The hemostatic abnormalities identified were restricted to tests of platelet procoagulant activity, whereas platelet count, platelet morphology under light microscopy, bleeding time, clot retraction, and platelet aggregation and secretion in response to thrombin, collagen, and adenosine diphosphate stimulation were all within normal limits. Washed platelets from the affected dogs demonstrated approximately twice normal clotting times in a platelet factor 3 availability assay and, in a prothrombinase assay, generated only background levels of thrombin in response to calcium ionophore, thrombin, or combined thrombin plus collagen stimulation. While platelet phospholipid content was normal, flow cytometric analyses revealed diminished phosphatidylserine exposure and a failure of microvesiculation in response to calcium ionophore, thrombin, and collagen stimulation. Pedigree studies indicate a likely homozygous recessive inheritance pattern of the defect. These findings confirm the importance of platelet procoagulant activity for in vivo hemostasis and provide a large animal model for studying agonist-induced signal transduction, calcium mobilization, and effector pathways involved in the late platelet response of transmembrane phospholipid movement and membrane vesiculation.
Introduction
Platelets play a critical role in the initiation and regulation of hemostasis. Vascular injury triggers a series of platelet reactions culminating in platelet aggregation and formation of a localized primary hemostatic plug. In the course of activation, the platelet outer membrane is transformed to a catalytic surface for assembly of active coagulation complexes. The tenase complex, consisting of factors IXa and VIIIa, proteolytically activates factor X. In a similar fashion, the prothrombinase complex, consisting of factor Xa and its cofactor Va, catalyzes conversion of prothrombin to thrombin. Activated platelets support tenase and prothrombinase assembly primarily through membrane surface exposure of a negatively charged phospholipid, phosphatidylserine (PS). In a temporally related process, activated platelets shed small membrane particles, microvesicles, which also present surface PS. Thus, PS expression by activated platelets and microvesicles is the source of platelet procoagulant activity.1-4
The specific receptors and signaling pathways required for platelet procoagulant activity, and their relationship to the other receptor-mediated platelet responses of aggregation and secretion, are now being investigated. In nonactivated platelets, the aminophospholipids PS and phosphatidylethanolamine (PE) are essentially restricted to the inner membrane leaflet while the outer leaflet consists predominately of phosphatidylcholine (PC) and sphingomyelin (SM).5,6 This membrane lipid asymmetry requires the action of a Mg2+ and adenosine triphosphate (ATP)–dependent aminophospholipid translocase, which transports PS and PE to the inner membrane.7,8 The rapid loss of membrane asymmetry, with movement of PS to the outer membrane leaflet, is mediated by a Ca++-dependent phospholipid scramblase.9,10Platelet membrane PS exposure and microvesicle release can be induced in vitro through a variety of stimuli that cause sustained elevation in platelet cytosolic ionized calcium concentration ([Ca++]i).11-13 It is believed that high [Ca++]i activates scramblase with concomitant inhibition of translocase, resulting in rapid bidirectional movement of phospholipids and the appearance of surface PS. Microvesiculation apparently depends on membrane PS exposure but also requires protein tyrosine phosphatase and calpain activation.14 The physiologic platelet agonists, collagen and thrombin, are capable of inducing PS exposure and microvesiculation; however, the rate and extent of response vary greatly depending on reaction conditions.15-18
Studies of Scott syndrome, a rare bleeding disorder associated with deficiency of platelet procoagulant activity, have been used to explore the basis of membrane PS movement.19-22 Characteristics of this syndrome include a moderate to severe bleeding tendency with normal platelet aggregation and secretion response but defective membrane transbilayer movement of PS and abnormal microvesiculation.4,23,24 Molecular analyses of the candidate gene scramblase in a single family, and in lymphocytes derived from the original patient, have not revealed the molecular basis of Scott syndrome.25 26 We report here the phenotypic characterization of a naturally occurring bleeding disorder of dogs caused by a lack of platelet procoagulant activity. Clinically affected dogs have normal platelet aggregation and secretion. Platelet prothrombinase activity, however, is markedly reduced, and platelets demonstrate impaired PS exposure and microvesiculation in response to both physiologic agonists and calcium ionophore stimulation.
Materials and methods
Experimental animals
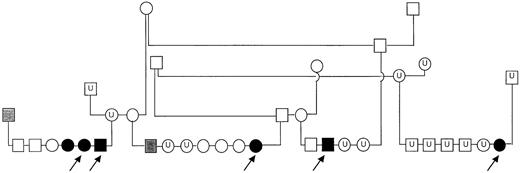
We studied 5 related adult German shepherd dogs (GSDs) (Figure1). The dogs (3 female, 2 male) ranged from 1.5 to 3 years of age and were from a closed colony with an average inbreeding coefficient of 22.5%. Clinical expression of their bleeding diathesis included epistaxis, hyphema, and perioperative bleeding with prominent bruising along suture lines. Two of the 3 females studied had hemoabdomen after ovariohysterectomy, and a male developed a large scrotal hematoma after castration. An intramuscular (quadriceps) hematoma developed in 1 affected dog. Two dogs had epistaxis of more than 3 days' duration that responded to fresh whole blood transfusion. Physical examination and medical histories of the 5 dogs (and other affected dogs from the colony) revealed no abnormalities other than the bleeding tendency. The dogs referred to our laboratory were fed commercial dry dog food ad libitum and were housed in facilities approved by the American Association for Accreditation of Laboratory Animal Care. All studies followed protocols approved by an institutional animal care and use committee and were performed more than 2 months after transfusion or administration of any medication or vaccination.
Pedigree of inbred GSDs with platelet procoagulant deficiency.
Solid symbols represent dogs with clinical signs of bleeding and high residual serum prothrombin; shaded symbols represent dogs with high residual serum prothrombin (but no history of abnormal bleeding); open symbols represent clinically normal dogs with normal residual serum prothrombin; U indicates dogs unavailable for testing. Arrows indicate the 5 dogs examined in this report.
Pedigree of inbred GSDs with platelet procoagulant deficiency.
Solid symbols represent dogs with clinical signs of bleeding and high residual serum prothrombin; shaded symbols represent dogs with high residual serum prothrombin (but no history of abnormal bleeding); open symbols represent clinically normal dogs with normal residual serum prothrombin; U indicates dogs unavailable for testing. Arrows indicate the 5 dogs examined in this report.
Coagulation, von Willebrand factor, and fibrinolysis assays
We measured whole blood clotting time (CT) and activated CT according to previously published methods.27,28 Plasma samples for all other tests were prepared from whole blood collected via cephalic venipuncture directly into 3.8% sodium citrate (1 vol citrate: 9 vol blood). CTs in coagulation screening tests were determined using commercial reagents as follows: Actin FS, Dade Behring, Marburg, Germany, Thromboplastin-L, Pacific Hemostasis, Huntersville, NC, and Russell's Viper Venom, American Diagnostica, Greenwich, CT. We also measured clottable (Clauss) fibrinogen (STA Fibrinogen [100 U human thrombin per mL], Diagnostica Stago, Parsippany, NJ), thrombin time (Fibriquick [25 U/mL], Organon Teknika, Durham, NC), and factor II coagulant activity using a modified prothrombin time (PT) and human factor II-deficient substrate plasma (Sigma, St Louis, MO). We used a previously described enzyme-linked immunosorbent assay29 to measure plasma von Willebrand factor (VWF) concentration and determined VWF multimer distribution via immunoelectrophoresis and Western blotting.30,31 We assayed ristocetin cofactor activity (RCof) by measuring agglutination of formalin-fixed human platelets (Biodata, Horsham, PA) in dilutions of test plasma. We measured plasma antithrombin, protein C, plasminogen, and antiplasmin activities using chromogenic substrate kits (STAChrom, Diagnostica Stago) and the manufacturer's automated analyzer (STA Compact). Tests were performed according to manufacturer's recommendations except for a long venom activation time in the protein C assay and the substitution of urokinase for streptokinase activation32 in the plasminogen assay. Results of VWF antigen and all factor activity assays were reported as the percentage of a pooled canine plasma standard having an assigned value of 100%. Plasma fibrin and fibrinogen degradation product (FDP) and D-dimer concentration were measured using latex agglutination kits (FDP Plasma, Diagnostica Stago; Accuclot D-dimer, Sigma).
Plasma lipid analyses
Plasma cholesterol and triglyceride concentrations were measured using an automated chemistry analyzer and the manufacturer's reagents and reaction conditions (Roche/Hitachi 917, Roche Diagnostics, Indianapolis, IN).
Platelet function testing
Platelet count and mean platelet volume were measured in ethylenediaminetetraacetic acid whole blood samples using an automated hematology analyzer (Advia 120, Bayer, Tarrytown, NY), and Wright-stained blood films were reviewed by a veterinary clinical pathologist to assess platelet morphology. We measured bleeding time after incision of the buccal mucosa with a template device (Surgicutt, Organon Teknika).33 Clot retraction was measured in saline-dilute blood combined with 1 U thrombin. Platelet-rich plasma (PRP) for aggregation and secretion studies was prepared by centrifuging whole blood collected into 3.8% citrate at 650g at room temperature for 3 separate 3-minute intervals. The resultant PRP was pooled and adjusted to a platelet count of 300 × 109/L (300 000/μL) with autologous platelet-poor plasma (PPP) obtained by centrifuging blood samples at 2200g for 15 minutes. The PRP was allowed to rest at room temperature for 30 minutes prior to use. Platelet aggregation studies were performed as previously described34 using a lumiaggregometer (Model 500 Ca, Chrono-log, Havertown, PA). Agonist-induced dense granule secretion was detected as luminescence (Chrono-lume reagent, Chrono-log) by monitoring ATP release under conditions that minimize potentiation of canine platelet aggregation.35 Aggregation and secretion studies were completed within 2 hours of PRP preparation.
Platelet factor 3 availability
We used a reported method for measuring kaolin-induced platelet factor 3 availability (PF3a).20 Briefly, recalcification time was determined for a mixture of kaolin-activated PPP and PRP. The PRP was prepared from whole blood collected in 3.2% sodium citrate (1 vol citrate: 9 vol blood) and adjusted to a platelet count of 300 × 109/L (300 000/μL) with autologous PPP. Substitution studies were performed by combining suspensions of washed platelets from affected GSDs in PPP from a control dog and washed platelets from a control dog in PPP from affected GSDs.
Prothrombin consumption test
We measured serum CT as an index of prothrombin consumption.36 Serum aliquots were collected after 1, 2, and 7 hours of incubation (at 37°C) and immediately combined with 3.2% sodium citrate (1 vol citrate: 9 vol serum). For the PT assay, the citrate serum was added to a bovine fibrinogen solution (Sigma) containing a final concentration of fibrinogen of 1.5 mg/mL. The time for clot formation was then measured after the addition of a thromboplastin and calcium reagent (Thromboplastin-L, Pacific Hemostasis). We compared each serum PT to the PT of a plasma sample drawn at the time of whole blood collection. The residual serum prothrombin was expressed as a serum PT ratio (serum PT % = plasma PT/serum PT × 100). In a series of mixing studies, we determined the residual serum PT for affected GSD blood combined with increasing proportions of normal dog blood (mixtures containing from 10% to 90% normal dog blood). We also measured the serum PT percentage of all available parents, grandparents, and littermates of the 5 affected dogs and all dogs in 3 litters sired by 1 of the affected dogs. Samples for these analyses were shipped frozen on dry ice to our laboratory and assayed within 3 days of collection.
Platelet phospholipid composition
We collected 12 mL of blood directly into acid citrate dextrose (ACD) (National Institutes of Health formula A: 1 vol ACD: 5 vol blood) from 2 affected GSDs and 2 control dogs and prepared PRP (as described above) except for the addition of 1 μM prostaglandin E1 (PGE1). The PGE1-treated PRP was centrifuged (21°C) at 16 000g for 20 seconds, and the resultant platelet pellets were washed twice in platelet wash buffer (113 mM NaCl, 4.3 mM K2HPO4, 4.2 mM Na2HPO4, 24.4 mM NaH2PO4, 5.5 mM glucose, 1 μM PGE1, pH 6.3).37 After the second wash, platelets were resuspended to a final concentration of 1 × 109 platelets per 1.0 mL wash buffer and recentrifuged. The supernatants were discarded, and the platelet pellets were frozen at −70°C until analysis. Platelet pellets were prepared and analyzed from each dog on 2 different dates. Lipids from each platelet pellet were chloroform-extracted38 and then separated using a Shimadzu high-performance liquid chromatography system (consisting of 2 Model LC-10A pumps, a Model SIL-10A autosampler, system controller Model SCL-10A, and an SPD-10AV UV detector, Columbia, MD). Bovine phospholipid standards,l-α-PI, l-α-PS, l-α-PE,l-α-PC, and SM, were used at 10 mg/mL stock solutions (Avanti Polar Lipids, Alabaster, AL). Phospholipid separation was achieved on a stainless steel μ-Porasil 3.9 × 300 mm I.D. analytic column (10 μ particle size, 125 Å pore size [Waters, Milford, MA]). The mobile phase was acetonitrile:methanol:85% phosphoric acid (100:5:1.5). Analyses were performed in isocratic mode at a flow rate of 1.0 mL/min and pressure of 40 kilogram-force/cm2. Detector wavelength was 203 nM, and the system was washed between runs with 60 mL methanol and 25 mL 2-propanol. Major phospholipid classes were resolved within 23 minutes in a single run. Retention times for PI, PS, PE, PC, and SM were 3.6, 5.9, 7.7, 11.7, and 20.1, respectively. The phospholipid concentrations and peak-area values exhibited a linear relationship in the range of 0 to 8.0 μg/10μL of the standard mixtures. Phospholipid content (mole percentage) was determined by peak-area integration (CLASS—VP 4.2 software).
Platelet suspensions for determination of prothrombinase activity and flow cytometric analyses
For these studies, PRP (3.2% citrate) was prepared as described above. After the addition of PGE1 (1 μM final), platelets were washed twice in platelet wash buffer and then resuspended to a final concentration of 25 × 109/L (2.5 × 107/mL) in resuspension buffer (137 mM NaCl, 4 mM KCl, 0.5 mM MgCl, 0.5 mM Na2HPO4, 0.1% glucose, 0.1% bovine serum albumin, and 10 mM HEPES, pH 7.4). Extracellular ionized calcium was adjusted to 2 mM by the addition of 1.5 M CaCl2, and platelets were equilibrated at room temperature for 30 minutes. Vehicle or agonist was then added to 1.0 mL aliquots of the platelet suspension in plastic tubes and gently mixed (Nutator, Becton Dickinson, Sparks, MD) at room temperature for 25 minutes. We tested pharmacologic and physiologic agonists previously shown to be inducers of membrane PS exposure and vesiculation:calcium ionophore (A23187 3 μM), thrombin (0.5 U/mL) collagen (24 μg/mL), and tetracaine (2 mM), a membrane-active agent that strongly induces PS exposure with only limited vesiculation.39
Prothrombinase activity assay
We measured platelet prothrombinase activity with minor modification of a previously reported method.40 Briefly, 0.25 U bovine factor Xa (Haematologic Technologies, Essex Junction, VT) was added to 1.0 mL unstimulated or agonist-treated platelets (2.5 × 107 cells in resuspension buffer containing 2 mM Ca++) followed by the immediate addition of 10 μg bovine prothrombin (Haematologic Technologies). At various times, 50 μL aliquots were removed and added to 550 μL stop buffer (0.15 M NaCl, 20 mM ethylenediaminetetraacetic acid, 5 mg/mL ovalbumin [Sigma], and 20 mM Tris-HCl, pH 7.9) to halt prothrombin conversion. Thrombin activity was measured by the addition of 50 μL thrombin substrate S-2238 (0.1 mM final, DiaPharma, West Chester, OH) to microtiter plate wells containing 200 μL sample or standard. Changes in absorbance values at 405 nM were recorded at 10-second intervals in the kinetic mode of an enzyme-linked immunosorbent assay plate reader (EL 312, BioTek Instruments, Winooski, VT) using the supplied Kinetic-Calc software. Thrombin concentration in each sample was determined from a calibration curve derived from purified bovine α-thrombin (Haematologic Technologies).
Red blood cell prothrombinase activity assay
We measured erythrocyte prothrombinase activity of 2 affected GSDs and 2 control dogs. For these studies, red cells were washed twice in platelet wash buffer, resuspended to a final concentration of 10 × 109/L in resuspension buffer containing 2 mM Ca++, and then reacted at 37°C with either 3 μM A23187 or a dimethyl sulfoxide vehicle control. The prothrombinase assay37 was configured with bovine prothrombin factors Va and Xa (Haematologic Technologies), and the nanomoles of thrombin generated per 107 red cells was measured as described above, after reaction times of 0, 30, 60, and 90 minutes.
Flow cytometry
We analyzed control and agonist-treated platelet suspensions from affected GSDs and healthy control dogs in a FACSCalibur cytometer (Becton Dickinson, San Jose, CA) using logarithmic gain settings for light scatter and fluorescence. Parameter settings were adjusted so that the cell population of PRP of each dog was centered on the second decade of the 4-decade log scales of the forward versus side light scatter dot plot. For each sample, 10 000 total events were acquired without gating. Forward and side scatter and fluorochrome binding were used to assess platelets and derived microparticles.39 41PS exposure was detected by annexin V–fluorescein isothiocyanate (FITC) binding (TACS Annexin V-FITC, R&D Systems, Minneapolis, MN) and membrane surface glycoprotein (GP) IIIa by CD61-FITC binding (anti-CD61, clone Y2/51, Dako, Carpentieria, CA). In 100 μL reaction volumes, we labeled 10 μL aliquots of the platelet suspensions (containing 10 × 109/L [1 × 107/mL] platelets) using 0.1 μg CD61-FITC or isotype control antibody (mouse IgG1-FITC, clone DAK-GO1, Dako) or 0.0125 μg annexin V–FITC. After incubation in the dark for 30 minutes at 4°C (CD61-FITC and isotype control) or 15 minutes at room temperature (annexin V–FITC), 400 μL of suspension buffer was added to each reaction tube and the samples were analyzed within 30 minutes.
Clot signature analysis
We examined the platelet function and coagulant activity of flowing, nonanticoagulated whole blood using a point-of-care device (Clot Signature Analyzer, Xylum, Scarsdale, NY). Under conditions that simulate a small arterial bleeding site, the instrument displays tracings that represent shear-induced platelet activation with subsequent platelet plug formation and development of an occlusive fibrin clot.42 The clot signature analysis (CSA) tracings and numeric values of platelet-mediated hemostasis time (PHT) and CT of the 5 affected GSDs were compared with those of 6 healthy mixed breed dogs.
Results
The results of coagulation, fibrinolysis, and VWF assays, platelet count, and mean platelet volume determination were all within normal limits (Table 1), as were mean plasma cholesterol (6.00 mM/L; reference range 3.20 to 8.65) and mean plasma triglyceride (0.54 mM/L; reference range 0.29 to 1.22). Under light microscopy, affected GSD platelets appeared normal, and the GSDs and control dogs had similar VWF multimer distribution and hybridization intensities (data not shown). The 5 affected GSDs had normal bleeding times, clot retraction, and platelet aggregation. Platelet secretion response of the 3 affected GSDs studied was within normal limits (Table 1).
Results of coagulation, fibrinolysis, and platelet function studies of GSDs with platelet procoagulant deficiency
| Tests . | Affected GSD (mean of 5 dogs) . | Canine reference range . |
|---|---|---|
| Platelet count, 109/L | 220 | 170-510 |
| Mean platelet volume, fL | 11.6 | 8.4 -13.2 |
| Bleeding time, min | 3.5 | 2 -4 |
| Activated CT, s | 121 | 60 -180 |
| Whole blood CT, min | 7.8 | 6 -8 |
| Activated partial thromboplastin time, sec | 11.8 | 10 -17 |
| PT, sec | 14.0 | 14 -18 |
| Thrombin CT, sec | 6.8 | 5 -9 |
| Russell viper venom time, sec | 21.0 | 19 -23 |
| Fibrinogen, g/L | 3.6 | 1.5 -4.8 |
| Factor II, % | 107 | 50 -150 |
| Antithrombin, % | 97 | 70 -125 |
| Protein C, % | 111 | 60 -160 |
| Plasminogen, % | 94 | 60 -170 |
| Antiplasmin | 106 | 65 -115 |
| FDP, mg/L | < 5 | < 5 |
| D-dimer, μg/L | < 250 | < 250 |
| VWF: antigen, % | 87 | 70 -180 |
| VWF: Rcof, % | 93 | 50 -170 |
| Platelet aggregation: | ||
| Adenosine diphosphate (5 μM), % | 77 | > 5 |
| Adenosine diphosphate (10 μM), % | 90 | > 60 |
| Collagen (12 ug/mL), % | 63 | > 60 |
| Collagen (24 ug/mL), % | 95 | > 60 |
| Platelet secretion, μM ATP release per 1011 platelets | (mean of 3 dogs) | |
| Adenosine diphosphate (10 μM), μM | 0.07 | 0.06 -0.10 |
| Collagen (12 ug/mL), μM | 0.9 | 0.41 -1.05 |
| Collagen (24 ug/mL), μM | 1.01 | 0.68 -1.43 |
| Bovine α-thrombin (1 U/mL), μM | 1.35 | 1.21 -1.63 |
| Tests . | Affected GSD (mean of 5 dogs) . | Canine reference range . |
|---|---|---|
| Platelet count, 109/L | 220 | 170-510 |
| Mean platelet volume, fL | 11.6 | 8.4 -13.2 |
| Bleeding time, min | 3.5 | 2 -4 |
| Activated CT, s | 121 | 60 -180 |
| Whole blood CT, min | 7.8 | 6 -8 |
| Activated partial thromboplastin time, sec | 11.8 | 10 -17 |
| PT, sec | 14.0 | 14 -18 |
| Thrombin CT, sec | 6.8 | 5 -9 |
| Russell viper venom time, sec | 21.0 | 19 -23 |
| Fibrinogen, g/L | 3.6 | 1.5 -4.8 |
| Factor II, % | 107 | 50 -150 |
| Antithrombin, % | 97 | 70 -125 |
| Protein C, % | 111 | 60 -160 |
| Plasminogen, % | 94 | 60 -170 |
| Antiplasmin | 106 | 65 -115 |
| FDP, mg/L | < 5 | < 5 |
| D-dimer, μg/L | < 250 | < 250 |
| VWF: antigen, % | 87 | 70 -180 |
| VWF: Rcof, % | 93 | 50 -170 |
| Platelet aggregation: | ||
| Adenosine diphosphate (5 μM), % | 77 | > 5 |
| Adenosine diphosphate (10 μM), % | 90 | > 60 |
| Collagen (12 ug/mL), % | 63 | > 60 |
| Collagen (24 ug/mL), % | 95 | > 60 |
| Platelet secretion, μM ATP release per 1011 platelets | (mean of 3 dogs) | |
| Adenosine diphosphate (10 μM), μM | 0.07 | 0.06 -0.10 |
| Collagen (12 ug/mL), μM | 0.9 | 0.41 -1.05 |
| Collagen (24 ug/mL), μM | 1.01 | 0.68 -1.43 |
| Bovine α-thrombin (1 U/mL), μM | 1.35 | 1.21 -1.63 |
Tests of platelet procoagulant activity
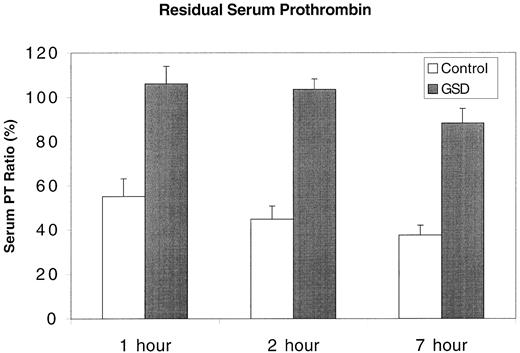
All GSDs had long CTs in the PF3a assay. In mixing studies, CT was prolonged when the assay was configured with GSD platelets suspended in control dog plasma; however, CT was within normal limits when control dog platelets were suspended in GSD plasma (Table2). Prothrombin times of GSD serum samples were nearly identical to their plasma PT. Consequently, serum PT ratio for these dogs was initially close to 100% and fell to only approximately 90% after 7 hours. In contrast, the mean serum PT ratio of 15 control dogs (including 5 healthy GSDs unrelated to the affected dogs) was less than 60% by 1 hour (Figure2) and progressively fell at the 2-hour and 7-hour time points. The 1-hour serum PT ratio of mixtures combining whole blood from affected GSDs and control dogs fell to less than 60% only when the proportion of control dog blood was 75% or greater.
PF3a values
| Subject . | PF3a, sec . |
|---|---|
| Affected GSDs (n = 5) | |
| Mean | 38.9 |
| SD | 3.8 |
| Range | 35.0-44.0 |
| Control dogs (n = 15) | |
| Mean | 25.5 |
| SD | 2.9 |
| Range | 19.5-28.0 |
| GSD platelets combined with control dog plasma | 41.0 |
| GSD plasma combined with control dog platelets | 27.0 |
| Subject . | PF3a, sec . |
|---|---|
| Affected GSDs (n = 5) | |
| Mean | 38.9 |
| SD | 3.8 |
| Range | 35.0-44.0 |
| Control dogs (n = 15) | |
| Mean | 25.5 |
| SD | 2.9 |
| Range | 19.5-28.0 |
| GSD platelets combined with control dog plasma | 41.0 |
| GSD plasma combined with control dog platelets | 27.0 |
Residual serum prothrombin of control dogs and affected GSDs.
The PT of citrate plasma was compared with that of serum allowed to clot for 1, 2, or 7 hours. The results are expressed as a serum PT ratio (%) representing plasma PT/serum PT × 100. Control = mean + SD of 15 healthy dogs; GSD = mean + SD of 5 clinically affected GSDs.
Residual serum prothrombin of control dogs and affected GSDs.
The PT of citrate plasma was compared with that of serum allowed to clot for 1, 2, or 7 hours. The results are expressed as a serum PT ratio (%) representing plasma PT/serum PT × 100. Control = mean + SD of 15 healthy dogs; GSD = mean + SD of 5 clinically affected GSDs.
Platelet phospholipid composition
The proportional phospholipid content of platelets from the affected GSDs and control dogs were similar (Table3). In particular, the PS content of GSD platelets (11.1%) was nearly identical to that of control dogs (10.7%) and to a previously reported value of canine platelet PS content (11.8%).43
Platelet phospholipid composition
| Phospholipid . | Mean content, mol % . | |
|---|---|---|
| GSDs . | Control dogs . | |
| PE | 32.2 | 33.3 |
| SM | 25.8 | 24.7 |
| PC | 23.6 | 23.2 |
| PS | 11.1 | 10.7 |
| PI | 7.5 | 8.1 |
| Phospholipid . | Mean content, mol % . | |
|---|---|---|
| GSDs . | Control dogs . | |
| PE | 32.2 | 33.3 |
| SM | 25.8 | 24.7 |
| PC | 23.6 | 23.2 |
| PS | 11.1 | 10.7 |
| PI | 7.5 | 8.1 |
Mean content values were derived from assays of platelet samples from 2 affected GSDs and 2 control dogs drawn and analyzed on 2 separate dates.
Platelet prothrombinase activity
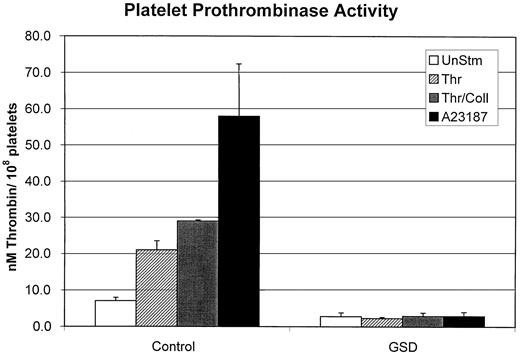
In our assay system, stirred unstimulated control dog platelets generated approximately 8 nM thrombin at 10 minutes' reaction time (Figure 3). A stimulated response was apparent in control platelets treated with calcium ionophore and the physiologic agonists thrombin and collagen. As previously reported for human platelets, ionophore induced maximal response, followed by combined thrombin plus collagen and then thrombin alone.39In contrast, GSD platelets demonstrated minimal prothrombinase activity throughout a 10-minute reaction time, with indistinguishable values for unstimulated versus agonist-treated platelets (Figure 3). In preliminary experiments, the addition of 0.85 μg bovine factor Va to the reaction mixture failed to correct the lack of prothrombinase activity of the GSD platelets (data not shown).
Platelet prothrombinase activity in response to agonist treatment.
Platelet suspensions from control dogs or affected GSDs were either treated with dimethyl sulfoxide (UnStm) or stimulated with 0.5 U/mL thrombin (Thr), 0.5 U/mL thrombin combined with 24 μg/mL collagen (Thr/Coll), or 3 μM calcium ionophore (A23187). Platelets were stirred with the various compounds for 25 minutes and then tested in the prothrombinase assay as described in “Materials and methods.” The nanomoles of thrombin generated by 108 platelets was determined for a 10-minute prothrombinase assay reaction time. Data represent mean values (with SD) obtained from testing 5 control dogs and 5 affected GSDs.
Platelet prothrombinase activity in response to agonist treatment.
Platelet suspensions from control dogs or affected GSDs were either treated with dimethyl sulfoxide (UnStm) or stimulated with 0.5 U/mL thrombin (Thr), 0.5 U/mL thrombin combined with 24 μg/mL collagen (Thr/Coll), or 3 μM calcium ionophore (A23187). Platelets were stirred with the various compounds for 25 minutes and then tested in the prothrombinase assay as described in “Materials and methods.” The nanomoles of thrombin generated by 108 platelets was determined for a 10-minute prothrombinase assay reaction time. Data represent mean values (with SD) obtained from testing 5 control dogs and 5 affected GSDs.
Red cell prothrombinase activity
The affected GSDs and control dogs had essentially identical red cell prothrombinase response. The prothrombinase activity of ionphore-treated control and GSD red cells increased over time, with a range of 44 to 74 nM thrombin per 107 cells at 30 minutes and 158 to 210 nM thrombin per 107 cells at 90 minutes. All ionophore-treated red cell suspensions (controls and GSDs) became hemolyzed over the 90-minute reaction time. Pending additional studies, we therefore cannot differentiate prothrombinase activity induced by ionophore-stimulated transbilayer PS movement from the nonspecific effects of red cell membrane rupture.
Flow cytometry analyses
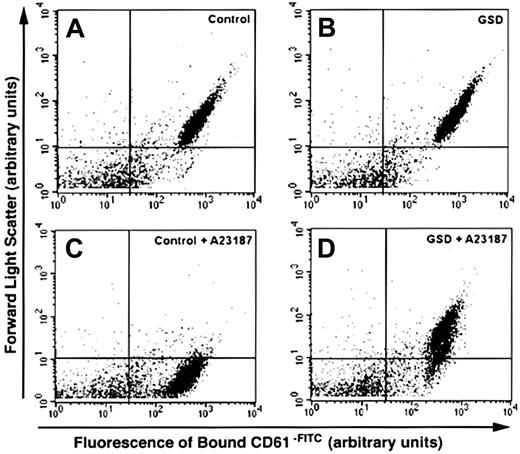
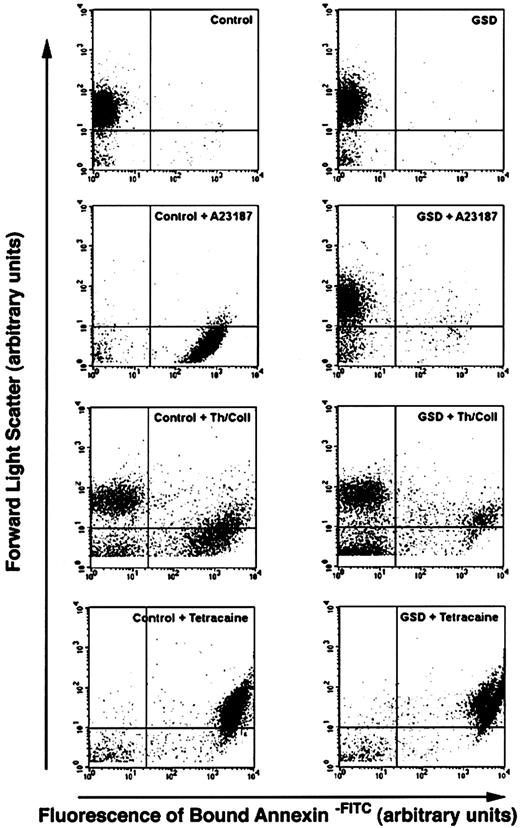
Dot plots of unstimulated control dog and GSD platelet suspensions had similar size (assessed by forward scatter) and fluorescence intensity patterns. The presence of GPIIIa was evident by high-intensity labeling with CD61-FITC (Figure4). In response to calcium ionophore stimulation (Figure 4C,D), the control dog platelet suspension showed a dramatic decrease in forward scatter. The CD61-FITC+population now extended in an apparent continuum from the lower boundary of unstimulated platelets to the threshold of forward scatter detection. In contrast, the GSD platelet suspensions demonstrated a relatively small decrease in forward scatter for only a minor proportion of the CD61-FITC+ population. Annexin V did not bind to unstimulated control dog or GSD platelets (Figure5A,B). Annexin V–FITC labeling of the ionophore-stimulated control dog platelet suspension (Figure 5C) showed dramatic decrease in forward scatter, with high-intensity fluorescence of this new population indicating the appearance of surface PS. The GSD suspensions showed almost no change in either forward scatter or fluorescence intensity in response to ionophore stimulation. When platelet suspensions were treated with thrombin plus collagen (Figure5E,F), both control dogs and GSDs developed high-intensity–labeled annexin V–FITC subpopulations. However, fewer GSD platelets bound annexin V–FITC, and these platelets did not demonstrate the logfold decrease in forward scatter as did control platelets. The dot plots in response to tetracaine treatment (Figure 5G,H) were similar for control and GSD platelet suspensions, with essentially the entire population of platelets now demonstrating high-intensity labeling, denoting extensive surface PS exposure. Forward scatter of the major population of control and GSD platelets, however, did not decrease in response to tetracaine.
Flow cytometry analyses of unstimulated and calcium ionophore–treated platelets from control and affected dogs labeled to detect platelet membrane GPIIIa.
Dot plots depict platelet suspensions from a healthy dog (control) and an affected GSD labeled with CD61-FITC, a fluorescein-conjugated monoclonal antibody directed against the β3 subunit of GPIIb/IIIa. Unstimulated platelet suspensions (A,B) and calcium ionophore–treated suspensions (C,D) were stirred in a buffer containing 2 mM CaCl2 in the absence or presence of 3 μM A23187. Control and GSD platelets demonstrate high-intensity labeling; however, only control platelets developed a marked decrease in forward scatter in response to ionophore treatment. Arrows indicate increasing forward light scatter intensity and increasing green fluourescence intensity. Data were collected without gating. The horizontal quadrant marker is set at the lower boundary of forward scatter for unstimulated platelets, and the vertical marker is set at the upper boundary of fluorescence for 95% of the isotype control–labeled particles (indicating nonspecific fluorescence). Particles in the lower left quadrant of each plot represent debris and machine noise. These plots correspond to a single analysis, representative of results obtained from ionophore treatment of all 5 affected GSDs.
Flow cytometry analyses of unstimulated and calcium ionophore–treated platelets from control and affected dogs labeled to detect platelet membrane GPIIIa.
Dot plots depict platelet suspensions from a healthy dog (control) and an affected GSD labeled with CD61-FITC, a fluorescein-conjugated monoclonal antibody directed against the β3 subunit of GPIIb/IIIa. Unstimulated platelet suspensions (A,B) and calcium ionophore–treated suspensions (C,D) were stirred in a buffer containing 2 mM CaCl2 in the absence or presence of 3 μM A23187. Control and GSD platelets demonstrate high-intensity labeling; however, only control platelets developed a marked decrease in forward scatter in response to ionophore treatment. Arrows indicate increasing forward light scatter intensity and increasing green fluourescence intensity. Data were collected without gating. The horizontal quadrant marker is set at the lower boundary of forward scatter for unstimulated platelets, and the vertical marker is set at the upper boundary of fluorescence for 95% of the isotype control–labeled particles (indicating nonspecific fluorescence). Particles in the lower left quadrant of each plot represent debris and machine noise. These plots correspond to a single analysis, representative of results obtained from ionophore treatment of all 5 affected GSDs.
Flow cytometry analyses of unstimulated and agonist-treated platelets from control and affected dogs labeled to detect surface exposure of PS.
Dot plots depict platelet suspensions from a healthy dog (control) and an affected GSD labeled with annexin V-FITC, a fluorescein-conjugated protein with high affinity for membrane surface aminophospholipid. The platelet suspensions were stirred in a buffer containing 2 mM CaCl2, in the absence of agonist compounds (A,B) or in the presence of one of the following treatments: A23187 (3 μM A23187, 25 minutes at room temperature); Th/Coll (0.5 U/mL thrombin plus 24 μg/mL collagen, 25 minutes at room temperature); tetracaine (2 mM tetracaine, 60 minutes at 37°C). Unstimulated control and GSD platelets are not labeled, indicating a lack of surface PS. All 3 treatments induced surface PS exposure in control dogs, denoted by the appearance of high-intensity fluorescence. The ionophore A23187 and thrombin plus collagen also induced a marked decrease in forward scatter for all or a portion of the labeled control platelets. The GSD platelets demonstrated virtually no increase in surface PS in response to A23187 and a relatively minor increase in PS exposure in response to thrombin plus collagen. Exposure of PS in response to tetracaine was equivalent to that of control dogs. Arrows indicate increasing forward light scatter intensity and increasing green fluourescence intensity. Data were collected without gating. The horizontal quadrant marker is at the lower boundary of forward scatter for unstimulated platelets, and the vertical marker is set at the upper boundary of fluorescence for more than 95% of the unstimulated platelets (indicating nonspecific fluorescence). Particles in the lower left quadrant of each plot represent debris and machine noise. These plots correspond to a single analysis, representative of results obtained from agonist treatment of all 5 affected GSDs.
Flow cytometry analyses of unstimulated and agonist-treated platelets from control and affected dogs labeled to detect surface exposure of PS.
Dot plots depict platelet suspensions from a healthy dog (control) and an affected GSD labeled with annexin V-FITC, a fluorescein-conjugated protein with high affinity for membrane surface aminophospholipid. The platelet suspensions were stirred in a buffer containing 2 mM CaCl2, in the absence of agonist compounds (A,B) or in the presence of one of the following treatments: A23187 (3 μM A23187, 25 minutes at room temperature); Th/Coll (0.5 U/mL thrombin plus 24 μg/mL collagen, 25 minutes at room temperature); tetracaine (2 mM tetracaine, 60 minutes at 37°C). Unstimulated control and GSD platelets are not labeled, indicating a lack of surface PS. All 3 treatments induced surface PS exposure in control dogs, denoted by the appearance of high-intensity fluorescence. The ionophore A23187 and thrombin plus collagen also induced a marked decrease in forward scatter for all or a portion of the labeled control platelets. The GSD platelets demonstrated virtually no increase in surface PS in response to A23187 and a relatively minor increase in PS exposure in response to thrombin plus collagen. Exposure of PS in response to tetracaine was equivalent to that of control dogs. Arrows indicate increasing forward light scatter intensity and increasing green fluourescence intensity. Data were collected without gating. The horizontal quadrant marker is at the lower boundary of forward scatter for unstimulated platelets, and the vertical marker is set at the upper boundary of fluorescence for more than 95% of the unstimulated platelets (indicating nonspecific fluorescence). Particles in the lower left quadrant of each plot represent debris and machine noise. These plots correspond to a single analysis, representative of results obtained from agonist treatment of all 5 affected GSDs.
Clot signature analyses
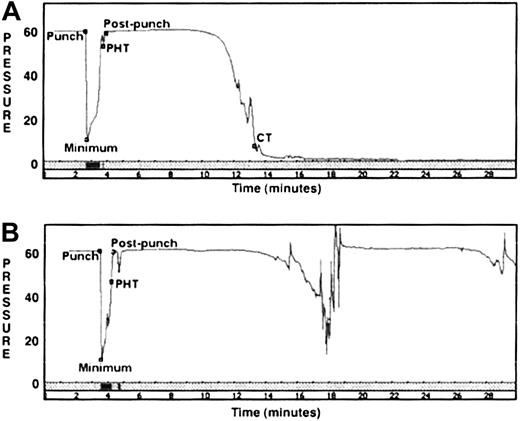
The control dog CSA tracings (Figure6A) depicted a rapid return of pressure to baseline values as a platelet plug sealed the needle punch holes in the instrument tubing. A gradual drop in pressure followed as fibrin clot formation subsequently occluded the tubing lumen. Because no additions are made to the nonanticoagulated whole blood in this assay system, activated platelets are the primary source of phospholipid membrane for assembly of the coagulation factor complexes responsible for fibrin clot formation. The quantitative parameters PHT and CT were derived from 9 CSA evaluations of 6 healthy dogs. The mean PHT was 1.2 minutes (median 1.1 minute), and mean CT was 12 minutes (median 12.9 minutes). The CSA tracings of 15 determinations from the 5 affected GSDs demonstrated qualitatively normal platelet plug formation, with mean PHT of 1.8 minutes (median 1.2 minutes). The portion of the tracing representing fibrin clot formation was consistently abnormal for all GSDs, with CT of more than 30 minutes (the upper limit of instrument observation time). During this period, the GSD tracings demonstrated long intervals of sustained high pressure interrupted by one or more transient drops in pressure (Figure 6B).
CSAs.
Pressure time tracings are depicted for a healthy control dog (A) and an affected GSD (B). The CSA measures pressure (mm Hg) in a cassette chamber containing nonanticoagulated blood flowing in plastic tubing. The labeled events include the following: punch = lumen pierced by fine needle; minimum = low pressure caused by blood exiting punch hole; PHT (pressure is restored by thrombi sealing punch hole); postpunch = return to baseline blood flow; CT (luminal flow is occluded by clot). Control dog: PHT = 1.1 minutes, CT = 13.4 minutes; GSD: PHT = 0.7 minutes; CT = more than 30 minutes. The 5 affected GSDs demonstrated a variable number of transient pressure drops in the postpunch period; however, all consistently failed to form an occlusive clot.
CSAs.
Pressure time tracings are depicted for a healthy control dog (A) and an affected GSD (B). The CSA measures pressure (mm Hg) in a cassette chamber containing nonanticoagulated blood flowing in plastic tubing. The labeled events include the following: punch = lumen pierced by fine needle; minimum = low pressure caused by blood exiting punch hole; PHT (pressure is restored by thrombi sealing punch hole); postpunch = return to baseline blood flow; CT (luminal flow is occluded by clot). Control dog: PHT = 1.1 minutes, CT = 13.4 minutes; GSD: PHT = 0.7 minutes; CT = more than 30 minutes. The 5 affected GSDs demonstrated a variable number of transient pressure drops in the postpunch period; however, all consistently failed to form an occlusive clot.
Pedigree studies
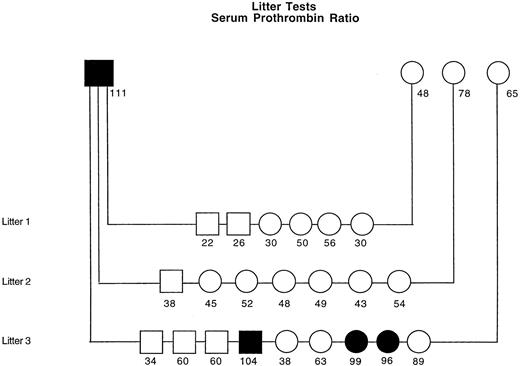
The serum PT ratio was used to evaluate platelet procoagulant activity of 3 GSD litters sired by 1 affected male (Figure7). This male had been bred to 3 clinically normal females and produced a total of 22 offspring. All offspring in 2 of these litters had no signs of abnormal bleeding by 1.5 years of age and had serum PT ratios within a reference range of 21% to 62% (derived from 2-hour serum CTs of 25 healthy dogs). In the third litter, 3 of 9 pups had prolonged bleeding after surgery or minor trauma, and all 3 had marked elevation of their serum PT ratios (range 96%-104%). The dam of this litter when bred to a different, clinically normal male produced a litter consisting of 4 pups with no clinical signs and 2 pups that experienced abnormal bleeding.
Pedigree studies of residual serum prothrombin in 3 litters sired by a single affected male GSD.
Solid symbols represent dogs with clinical signs of abnormal bleeding. The 2-hour serum prothrombin ratio (%), performed as described in “Materials and methods,” is listed below each symbol. Reference range for healthy control dogs (n = 25) is 21% to 62%.
Pedigree studies of residual serum prothrombin in 3 litters sired by a single affected male GSD.
Solid symbols represent dogs with clinical signs of abnormal bleeding. The 2-hour serum prothrombin ratio (%), performed as described in “Materials and methods,” is listed below each symbol. Reference range for healthy control dogs (n = 25) is 21% to 62%.
Discussion
Many hereditary hemostatic disorders have been identified in dogs. While canine hemophilia and von Willebrand disease are common, a variety of clotting factor deficiencies and platelet function defects caused by surface glycoprotein abnormalities, storage pool defects, and failure of signal transduction occur in this species.44-46Comprehensive testing of the GSDs described in this report revealed a new canine hemostatic defect with features comparable to Scott syndrome of human beings. The affected dogs experienced a mild to moderate bleeding tendency but had normal in vitro coagulation and normal response to tests of platelet adhesion, aggregation, and secretion. In contrast, abnormal values were consistently found in all tests of platelet procoagulant activity. The association of a bleeding tendency with deficiency of platelet procoagulant activity, in both dogs and human beings, underscores the importance of this platelet response for supporting in vivo hemostasis.
The presence of a bleeding disorder in this colony was evident through at least 5 generations. Although attempts were made to exclude affected dogs from the breeding program, many dogs subsequently confirmed as carriers and a few affected dogs were bred, thereby disseminating the defect throughout the closed colony. Based upon pedigree inspection and assessment of residual serum prothrombin (PT ratio), the hemostatic defect in this colony appears to be an autosomal recessive trait. Affected males and females have been identified in roughly equal numbers, with no obvious decrease in litter size or excess neonatal mortality in litters with affected pups. Obligate carrier males and females demonstrate normal residual serum prothrombin and no evidence of a bleeding tendency, even after surgery. Progeny tests of 3 litters sired by an affected male (bred to 3 different clinically normal females) produced 3 of 22 pups with high serum PT ratios and expression of a bleeding tendency. This proportion of affected pups is lower than expected for an autosomal dominant trait, assuming either homozygosity or heterozygosity of the affected male. Confirmation of autosomal recessive inheritance and expression will require evaluation of outcross and backcross litters and, ultimately, identification of the underlying molecular defect.
The affected dogs' specific lack of platelet procoagulant activity appears to be caused by an intrinsic platelet defect. Washed platelets from affected GSDs do not support kaolin-activated clot formation when combined with normal dog plasma in the PF3a assay. In contrast, assays configured with control dog platelets and affected GSD plasma as source of clotting factors have normal PF3a. In a prothrombinase assay system, washed stimulated GSD platelets combined with an excess of activated bovine factors are unable to generate thrombin beyond the background levels of unstimulated platelets. This lack of prothrombinase activity cannot be attributed to membrane PS deficiency because GSD and control platelets have the same PS content. Furthermore, GSD platelets treated with tetracaine express surface PS in a similar manner as control platelets, as evidenced by their high-intensity labeling with an annexin V fluorescent probe. This normal response to tetracaine, a local anesthetic whose actions include direct exchange with membrane PS,47 contrasts with GSD platelets' abnormal membrane response to calcium ionophore and the physiologic platelet agonists thrombin and collagen.
Flow cytometry dot plots of control dog platelets stimulated with ionophore A23187 exhibited the same qualitative changes described for human platelets, ie, annexin V binding and decreased forward light scatter.39 The decrease in forward scatter was pronounced, and the scatter plots consisted primarily of events with high prevalence surface GPIIIa and PS, denoting extensive platelet membrane microvesiculation.41 Ionophore treatment of GSD platelets induced virtually no PS exposure and only a minor shift in forward scatter of the GPIIIa+ events. This change in forward scatter is compatible with platelet shape change and granule release rather than microvesiculation.48,49 As described for human platelets,14 39 thrombin plus collagen stimulation induced PS exposure and microvesiculation in only a subpopulation of the control dog platelets. Some surface exposure of PS was also apparent in a subpopulation of the thrombin plus collagen–treated GSD platelets, in contrast to their lack of PS exposure in response to ionophore. The GSD suspensions, however, had relatively fewer platelets exposing surface PS and little evidence of microvesiculation, compatible with their failure to generate thrombin in the prothrombinase assay system.
Collagen is a critical physiologic stimulus of platelet membrane PS exposure and microvesiculation.39,50,51 The collagen-evoked responses are mediated by sustained high [Ca++]i and are associated with activation of both protein tyrosine kinases and tyrosine phosphatases.52,53 Although the biochemical and molecular basis of Scott syndrome is unknown, transformed Scott lymphoblasts display defective capacitative Ca++ entry54and Scott lymphoblasts, red cells, and platelets have abnormal patterns of protein tyrosine phosphorylation.22,54,55 The GSD platelets demonstrate normal aggregation and secretion in response to collagen, indicating that the collagen receptor, signal transduction, and effector pathways leading to release of intracellular Ca++ stores and platelet shape change are intact. Some of the thrombin plus collagen–stimulated GSD platelets appear to be capable of PS exposure, albeit with minimal evidence of vesiculation. In vitro, normal human platelets can be induced to expose membrane PS without vesiculation under reaction conditions that limit Ca++ influx, block activation of protein tyrosine phosphatases, or inhibit μ-calpain activity.14 52Studies of these processes in GSD platelets are likely to help elucidate pathways that are unique to or predominate in the platelet membrane changes resulting in platelet procoagulant activity. In addition, the discovery of a canine model will allow application of candidate gene and mapping strategies to define a molecular defect capable of producing the characteristic Scott syndrome phenotype.
The authors thank Drs Harvey Weiss and Bruce Lages for their critical review and comments.
Supported in part by the National Institutes of Health (GM58516) and Sidney Kimmel Foundation for Cancer Research (H.A.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marjory Brooks, Comparative Coagulation Section, Diagnostic Laboratory, Cornell University, Upper Tower Rd, Ithaca, NY 14853; e-mail: mbb9@cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal