Members of the Src family of kinases are abundant in platelets. Although their localization is known, their role(s) in platelet function are not well understood. Lyn is a Src-family kinase that participates in signal transduction pathways elicited by collagen-related peptide; it has also been implicated through biochemical studies in the regulation of von Willebrand factor signaling. Here, we provide evidence that Lyn plays a role in γ-thrombin activation of platelets. Unlike the wild-type platelets, platelets from Lyn-deficient mice do not undergo irreversible aggregation, produce thromboxane A2, or secrete adenosine diphosphate in response to submaximal γ-thrombin concentrations that cause secretion-dependent irreversible aggregation. Phosphorylation of Akt, a downstream effector of phosphatidylinositol 3-kinase, also requires a higher concentration of γ-thrombin in Lyn-deficient platelets than in wild-type platelets. These findings demonstrate that Lyn signaling is required for thrombin induction of secretion-dependent platelet aggregation. Specifically, Lyn is required under these conditions to enable thrombin-induced TxA2 production and adenosine diphosphate secretion, necessary steps in secretion-dependent platelet aggregation.
Introduction
Src family kinases are a group of closely related nonreceptor protein tyrosine kinases that participate in the signal transduction pathways of various hemopoietic cells.1-3Recent findings in vitro and in vivo have shown that the Src family kinases Src and Hck are direct effectors of G proteins.4Six of the 8 Src family kinases—Fgr, Fyn, Lck, Lyn, Src, and Yes—are present in platelets.5 Results of recent studies have shown that Lyn and Fyn play a role in glycoprotein (GP) VI–mediated platelet activation.6-8 Lyn has also been implicated in GP Ib-IX-V–mediated signaling by biochemical analysis.9However, the roles of these kinases in platelet activation initiated by protease-activated receptors coupled to G proteins have not been elucidated.
Thrombin stimulates platelet activation by 2 types of receptors—protease-activated receptors (PARs) coupled to G proteins10 and the glycoprotein complex Ib-IX-V.11,12 Thrombin stimulation of platelets causes the activation of phosphoinositol-3 kinase (PI3K), the release of adenosine diphosphate (ADP), the production of thromboxane (Tx) A2, the activation of fibrinogen receptors, and platelet aggregation, though the order of these events is unknown. Various data suggest that PI3K activation is required for the sustained activation of the platelet-specific integrin αIIbβ3 (GP IIb/IIIa) and the accumulation of phosphatidylinositol 3,4-bisphosphate, both of which appear to be required for irreversible aggregation of platelets treated with thrombin.13-17 Activation of G-protein–coupled receptors (GPCRs) by thrombin can cause the activation of PI3Kβ through G protein βγ dimers.18,19 Active PI3Kβ causes the activation of protein kinase B (Akt).20Moreover, under certain conditions, generation of the PI3K products required for irreversible aggregation is dependent on synergistic signals from secreted ADP and TxA2.21-23
We studied platelets from Lyn-deficient mice to elucidate the role of Lyn in γ-thrombin–induced platelet activation. A proteolytic derivative of α-thrombin, γ-thrombin was used because it activates platelets but does not convert fibrinogen to fibrin, thereby precluding clot formation. Our findings demonstrate that Lyn is required for irreversible aggregation induced by γ-thrombin at concentrations that cause secretion-dependent aggregation and that ADP secretion and TxA2 production are Lyn-dependent in this thrombin-activated GPCR-mediated pathway. Further, our results suggest that ADP and TxA2 produce the signal amplification required for secretion-dependent irreversible aggregation in response to γ-thrombin. This signal amplification may, among other things, facilitate irreversible aggregation by activating PI3K.
Materials and methods
Arachidonic acid, U46619, α-thrombin, and the Gly-Pro-Arg-Pro–amide were purchased from Sigma Chemical (St Louis, MO). PAR4 peptide (AYPGKF) was a gift from COR Therapeutics (San Francisco, CA).
Lyn knockout mice
C57/BL6 Lyn knockout mice were generated and characterized as previously described.24 Wild-type (Lyn+/+) C57/BL6 mice were studied in parallel.
Platelet aggregation studies
Blood was drawn from the abdominal aortas of methoxyflurane-anesthetized mice into syringes that contained 100 μL/mL 3.8% sodium citrate, pH 7.4. Platelet-rich plasma was prepared by differential centrifugation. Aggregation tests were performed by using 300 μL platelet-rich plasma adjusted to contain approximately 106 platelets/μL in the Lumi-aggregometer. Gly-Pro-Arg-Pro peptide was used to block fibrin polymerization and thus to prevent fibrin clot formation in the presence of α-thrombin.25
γ-Thrombin
A low concentration of γ-thrombin was less than 225 nM; a medium concentration was 225 to 250 nM; a high concentration of γ-thrombin was more than 250 nM. The γ-thrombin solution also contained 0.6% α-thrombin. It was a generous gift of Dr John Fenton (New York State Department of Health, Albany).
Measurement of adenosine triphosphate secretion and TxA2 production
Adenosine triphosphate (ATP) secretion was measured by using the CHORONO-LUME reagent (Chrono-Log, Havertown, PA) according to the manufacturer's protocol, with minor variations. After 4 to 6 minutes of platelet activation by stirring (1200 rpm), 10 μL luciferase–luciferin was added directly to the cuvettes. Luminescence intensity was measured at a luminescence setting of 0.001×. ATP secretion was expressed as percentage secretion in the Lumi-aggregometer. TxA2 was assayed by removing the platelets at the end of the 4- to 6-minute aggregation period. The TxB2 enzyme immunoassay kit (Assay Designs, Ann Arbor, MI) was used according to the manufacturer's protocol to indirectly measure free TxA2. Samples were diluted 1:50 with the supplied assay buffer.
Studies of Ca++ flux
We incubated platelet-rich plasma (106platelets/μL) with 10 μM INDO-1 (am ester; Molecular Probes, Eugene, OR) for 45 minutes at 37°C. After incubation, platelets were diluted to 2 × 106/mL with CAT buffer (0.002 M CaCl2, 0.01 M Tris, 0.15 M NaCl, pH 7.4) and were subjected to laser excitation at 488 nm and 357 nm. The ratio of the fluorescence level at these wavelengths was calculated as a function of time over a period of 512 seconds.
Immunoblotting
Aggregated platelet samples were washed and suspended in EHS buffer (1 mM Na2 EDTA, 10 mM HEPES, 0.15 M NaCl), pH 7.6, solubilized in one-third volume of Laemmli sample buffer with fresh dithiothreitol (24.6 mg/mL), boiled for 5 minutes, and loaded onto a 7.5%–15% sodium dodecyl sulfate–polyacrylamide, linear gradient gel. Proteins were transferred to a nitrocellulose membrane, treated with anti–phospho-Akt (New England Biolabs, Beverly, MA) and subsequently were treated with a secondary peroxidase-conjugated antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). The phospho-Akt (Ser 473) antibody detects Akt only when phosphorylated at Ser 473. The membrane was developed with enhanced chemiluminescence (Amersham, Piscataway, NJ) or Supersignal (Pierce, Rockford, IL) to allow Akt detection. After Akt detection, membranes were stripped and incubated with anti-actin antibody (BMB, Indianapolis, IN) to confirm uniform protein loading.
Results
Defective aggregation of platelets from Lyn-deficient mice
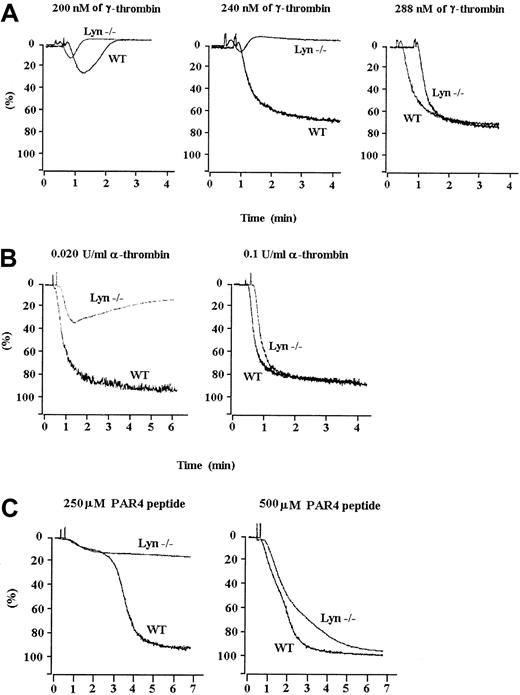
We investigated the role of Lyn in thrombin receptor-mediated signaling by stimulating wild-type and Lyn-deficient platelets with increasing concentrations of γ-thrombin. Unlike control platelets which aggregated irreversibly, Lyn-deficient platelets aggregated reversibly in response to a medium concentration of γ-thrombin (Figure 1A); however, a high level of γ-thrombin caused irreversible aggregation of both types of platelets. The defective aggregation was not unique to γ-thrombin; it was also observed at a low concentration of α-thrombin or protease-activated receptor 4 (PAR4)-specific peptide. In contrast to wild-type platelets, which aggregated irreversibly when stimulated with a low concentration of α-thrombin or the PAR4 peptide, Lyn-deficient platelets aggregated reversibly (Figure 1B,C). A high concentration of these agonists, however, induced irreversible aggregation of Lyn-deficient and the wild-type platelets.
Lyn-deficient platelets aggregate abnormally in response to thrombin receptor-activating agonists.
Lyn-deficient and wild-type murine platelets were treated with increasing concentrations of γ-thrombin (A), α-thrombin (B), or PAR4 peptide (C). Lyn-deficient platelets aggregated irreversibly only when a high concentration of agonist was used. Results are representative of 5 separate experiments.
Lyn-deficient platelets aggregate abnormally in response to thrombin receptor-activating agonists.
Lyn-deficient and wild-type murine platelets were treated with increasing concentrations of γ-thrombin (A), α-thrombin (B), or PAR4 peptide (C). Lyn-deficient platelets aggregated irreversibly only when a high concentration of agonist was used. Results are representative of 5 separate experiments.
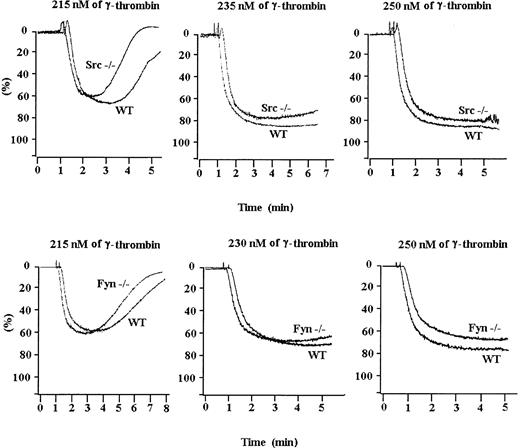
Unlike platelets from Lyn knockout mice, platelets from Src or Fyn knockout mice responded indistinguishably from wild-type platelets to a broad range of γ-thrombin (Figure 2). Therefore, not all Src family kinases present in platelets were required for thrombin-induced platelet aggregation.
Platelets from Src- and Fyn-deficient mice aggregate normally in response to γ-thrombin.
Src-deficient or Fyn-deficient platelets were exposed to increasing concentrations of γ-thrombin. Both types of platelets aggregated normally in response to low, medium, or high concentrations of γ-thrombin. Results are representative of 3 separate experiments.
Platelets from Src- and Fyn-deficient mice aggregate normally in response to γ-thrombin.
Src-deficient or Fyn-deficient platelets were exposed to increasing concentrations of γ-thrombin. Both types of platelets aggregated normally in response to low, medium, or high concentrations of γ-thrombin. Results are representative of 3 separate experiments.
Role of Lyn is limited to GPCR activated by γ-thrombin
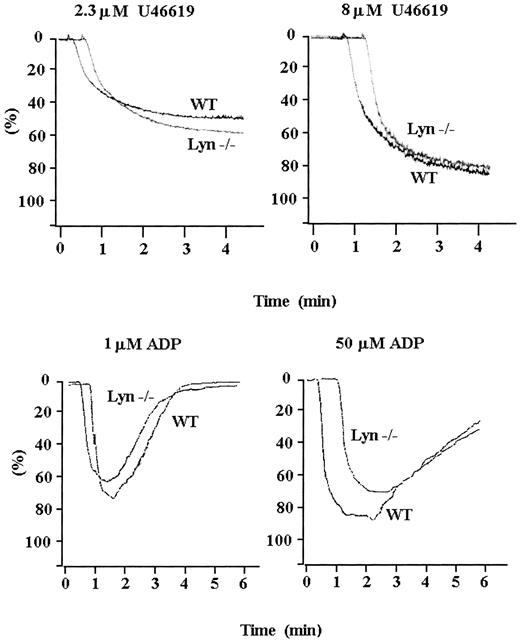
Lyn-deficient platelets were tested with 2 other GPCR-activating agonists—U46619, a stable TxA2 analog, and ADP—to distinguish whether the involvement of Lyn kinase is general to all GPCR signaling or is specific to thrombin PARs coupled to G proteins. Increasing concentrations of U46619 and ADP produced only normal aggregation of Lyn-deficient platelets (Figure 3). Thus, Lyn-deficient platelets do not aggregate abnormally in response to all GPCR signaling.
Responses of Lyn-deficient and wild-type platelets to agonists of other G-protein–coupled receptors.
Lyn-deficient and wild-type murine platelets were activated with U46619or ADP at low and high concentrations. Lyn-deficient platelets aggregated normally in response to low and high concentrations ofU46619 and ADP; therefore, Lyn kinase function is not required for all GPCR signaling. Results are representative of 5 separate experiments.
Responses of Lyn-deficient and wild-type platelets to agonists of other G-protein–coupled receptors.
Lyn-deficient and wild-type murine platelets were activated with U46619or ADP at low and high concentrations. Lyn-deficient platelets aggregated normally in response to low and high concentrations ofU46619 and ADP; therefore, Lyn kinase function is not required for all GPCR signaling. Results are representative of 5 separate experiments.
TxA2 and adenosine diphosphate are required for irreversible aggregation in response to medium γ-thrombin concentrations
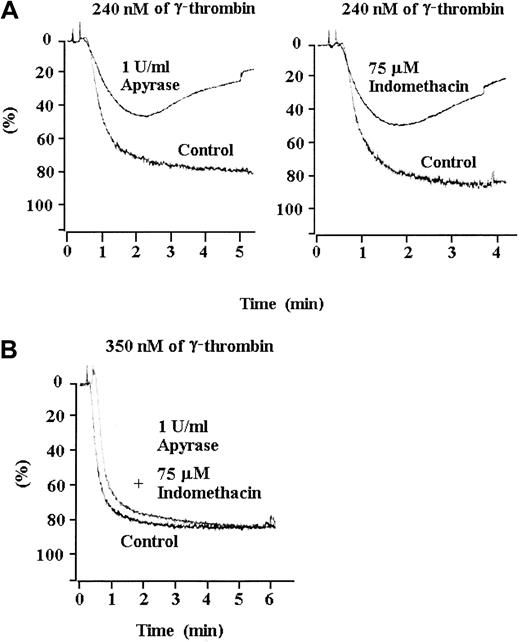
There is evidence that ADP and TxA2 play a synergistic role in thrombin activation.21 22 We pretreated the wild-type platelets with indomethacin or apyrase to establish whether TxA2 and ADP are required for irreversible aggregation in response to a medium concentration of γ-thrombin. Pretreatment of platelets with indomethacin completely inhibited TxA2 production and converted irreversible aggregation to reversible aggregation (Figure4A). Similarly, apyrase inhibited irreversible aggregation of the wild-type platelets in response to a medium concentration of γ-thrombin (Figure 4A). Aggregation in response to a medium concentration of γ-thrombin remained reversible in the presence of apyrase and indomethacin (data not shown). However, aggregation was irreversible when the platelets were pretreated with apyrase or indomethacin in response to a high concentration of γ-thrombin (Figure 4B). The requirement for ADP and TxA2 in response to medium but not high concentrations of γ-thrombin suggests that ADP and TxA2 play a signal amplification role in γ-thrombin–induced aggregation and provides the basis for a plausible explanation of the effect of Lyn deficiency on thrombin-induced platelet aggregation.
Effect of apyrase and indomethacin on the aggregation of wild-type platelets.
After pre-incubation with apyrase (in phosphate-buffered saline) or indomethacin (in dimethyl sulfoxide), wild-type platelets were activated with medium or high concentrations of γ-thrombin. Apyrase and indomethacin individually (A) and together (not shown) converted irreversible aggregation induced by a medium concentration of γ-thrombin to reversible aggregation. However, pre-incubation with apyrase, indomethacin, or both (B) did not prevent irreversible aggregation in response to a high concentration of γ-thrombin. Platelets were pre-incubated with the buffer in which apyrase and indomethacin were dissolved as a control.
Effect of apyrase and indomethacin on the aggregation of wild-type platelets.
After pre-incubation with apyrase (in phosphate-buffered saline) or indomethacin (in dimethyl sulfoxide), wild-type platelets were activated with medium or high concentrations of γ-thrombin. Apyrase and indomethacin individually (A) and together (not shown) converted irreversible aggregation induced by a medium concentration of γ-thrombin to reversible aggregation. However, pre-incubation with apyrase, indomethacin, or both (B) did not prevent irreversible aggregation in response to a high concentration of γ-thrombin. Platelets were pre-incubated with the buffer in which apyrase and indomethacin were dissolved as a control.
Dense granule secretion and TxA2 production are abnormal in Lyn-deficient platelets in response to γ-thrombin
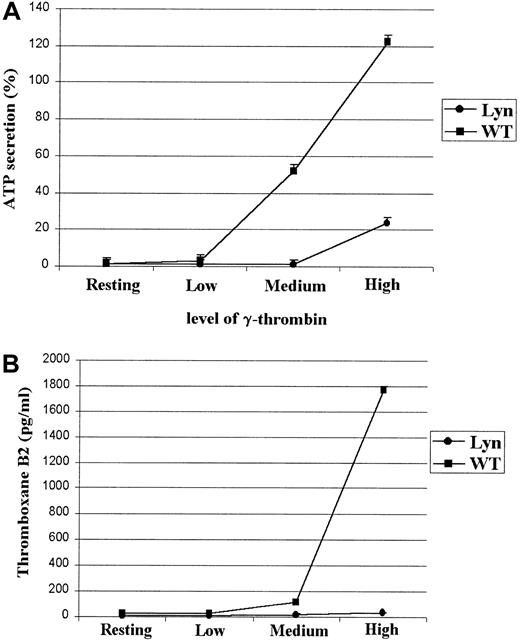
Because Lyn deficiency and treatment with indomethacin and apyrase converted irreversible aggregation to reversible aggregation in response to a medium concentration of thrombin, we hypothesized that Lyn deficiency alters aggregation by adversely affecting thrombin-induced TxA2 production and dense granule secretion. This hypothesis was tested by measuring ATP release and TxA2 production by Lyn-deficient platelets in response to increasing concentration of γ-thrombin. No ATP secretion was observed from Lyn-deficient platelets in response to a medium dose of γ-thrombin (Figure5A), which causes a secretion-dependent irreversible aggregation of wild-type platelets. ATP secretion was consistently lower from Lyn-deficient platelets than from wild-type platelets in response to higher concentrations of γ-thrombin (Figure5A). In addition, TxA2 production by the Lyn-deficient platelets was barely detectable, even in the presence of high concentrations of γ-thrombin, which causes irreversible aggregation (Figure5B).
Secretion of ATP and TxA2 in response to γ-thrombin is greatly diminished in Lyn-deficient platelets.
ATP secretion was measured by luminescence detection at a setting of 0.001X with the addition of 10 μL CHRONO-LUME reagent after the completion of aggregation and expressed as percent secretion. Because TxA2 has a half-life of 37 seconds and is rapidly converted to the stable product TxB2, TxA2 was measured as TxB2. Lyn-deficient platelets did not secrete ATP (A) or generate TxA2 (B) in response to a medium concentration of γ-thrombin. ATP secretion and TxA2 production were greatly diminished in response to high concentrations of γ-thrombin, though Lyn-deficient and wild-type platelets aggregated similarly.
Secretion of ATP and TxA2 in response to γ-thrombin is greatly diminished in Lyn-deficient platelets.
ATP secretion was measured by luminescence detection at a setting of 0.001X with the addition of 10 μL CHRONO-LUME reagent after the completion of aggregation and expressed as percent secretion. Because TxA2 has a half-life of 37 seconds and is rapidly converted to the stable product TxB2, TxA2 was measured as TxB2. Lyn-deficient platelets did not secrete ATP (A) or generate TxA2 (B) in response to a medium concentration of γ-thrombin. ATP secretion and TxA2 production were greatly diminished in response to high concentrations of γ-thrombin, though Lyn-deficient and wild-type platelets aggregated similarly.
To better understand the defect of TxA2 generation and ADP secretion in Lyn-deficient platelets, we treated Lyn-deficient platelets with free arachidonic acid to see whether they still had the capacity to synthesize TxA2. Arachidonic acid caused normal aggregation, ADP secretion, and TxA2 production in Lyn-deficient and wild-type platelets. Collagen stimulation of Lyn-deficient platelets also induced ADP secretion and TxA2 production (data not shown). These results demonstrated that Lyn-deficient platelets can synthesize TxA2 and release ADP. Therefore, Lyn is required for the regulation of TxA2 synthesis and for maximal dense granule release in γ-thrombin–stimulated platelets, but not for irreversible aggregation of platelets in response to high concentrations of γ-thrombin. Furthermore, the fact that indomethacin and apyrase recapitulated the effect of Lyn deficiency on platelet aggregation demonstrates the physiological significance of Lyn in platelet activation (Figures 1, 4).
Diminished Akt phosphorylation in Lyn-deficient platelets is associated with reversible aggregation
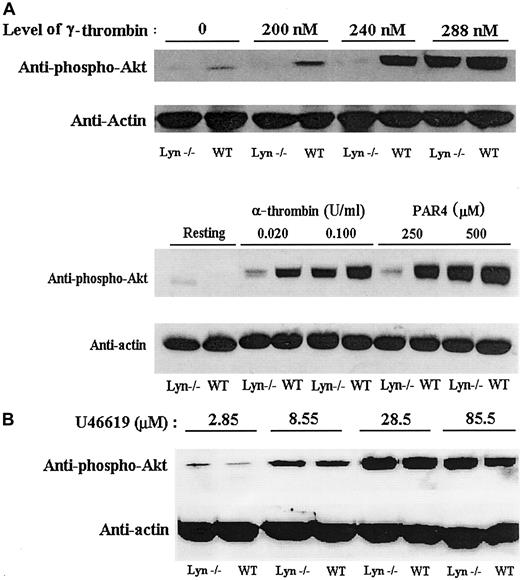
Because PI3K activation may be necessary for irreversible aggregation in response to the thrombin receptor activation peptide, we examined Akt phosphorylation, which occurs in response to PI3K activation.20,26 We demonstrated by Western blot analysis that Lyn deficiency reduced the phosphorylation of Akt (Figure6A). Akt was phosphorylated less extensively in Lyn-deficient platelets than in wild-type platelets in response to γ-thrombin, α-thrombin, or PAR4 peptide levels that caused secretion-dependent aggregation. The defect caused by Lyn deficiency was corrected at concentrations of the agonists that caused secretion-independent irreversible aggregation. These results are consistent with previous reports that sustained PI3K activation is required for the induction of irreversible aggregation by thrombin receptor activation peptide.15 As a control, Lyn-deficient platelets were treated with increasing concentrations of U46619 to determine whether Lyn deficiency also directly affects TxA2 receptor–induced Akt phosphorylation. Akt phosphorylation levels were similar in Lyn-deficient and wild-type platelets treated with U46619(Figure 6B). Therefore, Lyn deficiency did not directly affect Akt phosphorylation, and it is clear that Lyn deficiency does not directly affect signaling through the TxA2 receptor.
Akt phosphorylation is decreased in Lyn-deficient platelets relative to wild-type platelets in response to thrombin and the PAR4 peptide.
Lyn-deficient platelets diminished Akt phosphorylation in response to γ-thrombin, α-thrombin, or PAR4 peptide concentrations that produce secretion-dependent aggregation (A). However, high concentrations of γ-thrombin, α-thrombin, or PAR4 peptide induced Akt phosphorylation in Lyn-deficient platelets. Immunodetection of actin was performed to show that a similar amount of protein was present in each lane. As a control, increasing concentrations of U46619 were used to activate Lyn-deficient and wild-type platelets (B). Akt phosphorylation by Lyn-deficient platelets was normal in response to U46619, so the diminished Akt phosphorylation was specific to thrombin receptor signaling.
Akt phosphorylation is decreased in Lyn-deficient platelets relative to wild-type platelets in response to thrombin and the PAR4 peptide.
Lyn-deficient platelets diminished Akt phosphorylation in response to γ-thrombin, α-thrombin, or PAR4 peptide concentrations that produce secretion-dependent aggregation (A). However, high concentrations of γ-thrombin, α-thrombin, or PAR4 peptide induced Akt phosphorylation in Lyn-deficient platelets. Immunodetection of actin was performed to show that a similar amount of protein was present in each lane. As a control, increasing concentrations of U46619 were used to activate Lyn-deficient and wild-type platelets (B). Akt phosphorylation by Lyn-deficient platelets was normal in response to U46619, so the diminished Akt phosphorylation was specific to thrombin receptor signaling.
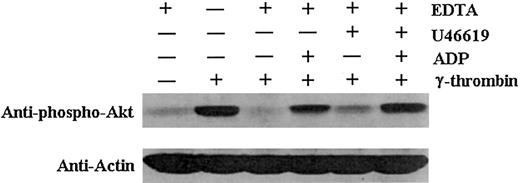
The indirect effect of Lyn deficiency on Akt phosphorylation might result from the lack of aggregation, the absence of TxA2 production and ADP secretion, or a combination of both. Alternative explanations were evaluated by treating wild-type platelets with γ-thrombin with or without EDTA to prevent aggregation with or without U46619 and ADP. Results clearly showed that aggregation is not required; the presence of the agonists resulted in Akt phosphorylation even in the absence of aggregation (Figure 7). Therefore, it is clear that PI3K activation is adversely affected by Lyn deficiency because of the absence of TxA2 and ADP, rather than being directly caused by the absence of aggregation or of Lyn kinase.
ADP and TxA2 enhance Akt phosphorylation.
EDTA treatment of wild-type platelets inhibited aggregation induced by a medium concentration of γ-thrombin. In the absence of aggregation, platelets were also activated with 5 μM ADP or 0.05 μM U46619, or both, in the presence of a medium concentration of γ-thrombin. Akt phosphorylation was restored by the addition of ADP and U46619 in the γ-thrombin–stimulated platelets in the absence of aggregation. Thus, aggregation is not absolutely required for Akt phosphorylation.
ADP and TxA2 enhance Akt phosphorylation.
EDTA treatment of wild-type platelets inhibited aggregation induced by a medium concentration of γ-thrombin. In the absence of aggregation, platelets were also activated with 5 μM ADP or 0.05 μM U46619, or both, in the presence of a medium concentration of γ-thrombin. Akt phosphorylation was restored by the addition of ADP and U46619 in the γ-thrombin–stimulated platelets in the absence of aggregation. Thus, aggregation is not absolutely required for Akt phosphorylation.
Discussion
Although it has long been known that Src family kinases are expressed in platelets, the functional roles of these nonreceptor tyrosine kinases are only now being elucidated. Our investigation of the Src family kinases in thrombin-induced GPCR signaling showed that Lyn, but not Src or Fyn, plays an important role in ADP secretion and TxA2 production in response to concentrations of γ-thrombin that induce secretion-dependent, irreversible aggregation. We also established that the defective signaling in the Lyn- deficient platelets is part of a GPCR signaling pathway because Lyn deficiency affected platelets similarly, whether thrombin or the PAR4 peptide was used as the agonist.10,27 However, our data do not exclude a role of Lyn in thrombin-induced GP Ibα–mediated signaling.11 12 Additional work is required to resolve that issue.
Huang et al4 reported that Src tyrosine kinase is a direct effector of G proteins in NG 108 cells. We do not know whether Lyn kinase is a direct effector of G proteins coupled to thrombin receptors in platelets. However, because Lyn is not essential for irreversible aggregation in response to high concentrations of γ-thrombin, Lyn clearly cannot be an indispensable effector of G proteins in platelets. Although Lyn-deficient platelets aggregate normally in response to concentrations of thrombin that cause secretion-independent aggregation, ADP secretion is diminished, and little TxA2 is produced under those conditions. However, when wild-type and Lyn-deficient platelets were exposed to increasing concentrations of γ-thrombin to measure the cytosolic Ca++ increase, there was no evidence that Lyn was involved in γ-thrombin–induced calcium mobilization, even at a level of γ-thrombin that induced a small Ca++increase in control platelets (data not shown). These results show that not all responses to thrombin are impaired by Lyn deficiency, and they imply that Lyn-dependent and Lyn-independent pathways are involved in thrombin receptor signaling.
To understand the difference between the Lyn-dependent and Lyn-independent pathways, we used apyrase to inhibit ADP signaling and indomethacin to inhibit TxA2 signaling. We observed that higher concentrations of thrombin are required to cause irreversible aggregation when ADP and TxA2 are absent than when they are present. This observation is consistent with previous reports that suggest that ADP and TxA2 provide synergistic autocrine stimulation for the formation of PI3K products in response to thrombin and that PI3K activation and the products of its activation play an important role in thrombin-induced irreversible aggregation.15 17
We measured Akt phosphorylation to evaluate PI3K activation in the Lyn-dependent and Lyn-independent pathways. Akt, also known as protein kinase B, plays a central role in apoptosis, and its phosphorylation is stimulated by the activation of PI3K as the result of signaling mediated by GPCRs. We found that the extent of Akt phosphorylation is dependent on thrombin concentration. The apparent absence or reduction of Akt phosphorylation in Lyn-deficient platelets in response to concentrations of thrombin or PAR4 peptide that cause secretion-dependent aggregation was associated with diminished ADP secretion, TxA2 production, and reversible aggregation. This effect of Lyn deficiency was eliminated by exogenous ADP and U46619 (Figure 7), demonstrating that these secreted agonists provide an important amplification signal that enhances subsequent PI3K activation. Therefore, as suggested elsewhere,21-23 ADP, TxA2, or both may be required for prolonged PI3K activation in response to a level of thrombin concentration that causes secretion-dependent, irreversible aggregation, and PI3K activation or its products of activation may support irreversible aggregation by helping to stabilize αIIbβ3 in an active conformation. However, further work is required to establish this point.
In conclusion, ADP and TxA2 are not required for γ-thrombin–induced irreversible aggregation in the presence of high concentration of γ-thrombin, though they appear to enable platelets to aggregate irreversibly in response to medium concentrations of γ-thrombin by amplifying the G-protein signaling induced by thrombin. This explanation is supported by our observation that a medium level of γ-thrombin can induce ADP secretion, TxA2 synthesis, and irreversible aggregation by wild-type but not Lyn-deficient platelets. Accordingly, ADP and TxA2 would facilitate irreversible aggregation of wild-type platelets by amplifying γ-thrombin–initiated signaling. In contrast, wild-type and Lyn-deficient platelets aggregate irreversibly in response to high concentrations of γ-thrombin despite the barely detectable production of TxA2, because TxA2 is not required for signal amplification under those conditions. A high concentration of γ-thrombin may directly dissociate enough G protein βγ subunits to activate PI3K sufficiently to support irreversible aggregation without requiring amplification of the signal by TxA2-stimulated G protein activation.
We thank Richard Ashmun (St Jude Children's Research Hospital) for his technical support on flow cytometry.
Supported in part by grants from the National Heart, Lung, and Blood Institute (HL56369, HL54476, and HL63216); the National Cancer Institute, Public Health Services (P01CA20180 and Cancer Center Support P30CA21765); the National Institutes of Health (CA21765); the American Lebanese Syrian Associated Charities; and the W. Harry Feinstone Center for Genomic Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
T. Kent Gartner, Department of Microbiology and Molecular Cell Sciences, University of Memphis, Memphis, TN 38152; e-mail: tgartner@memphis.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal