We recently identified CD4+ T cells that are autoreactive to β2-glycoprotein I (β2GPI) and that promote antiphospholipid antibody production in patients with antiphospholipid syndrome (APS). In this study, T-cell receptor (TCR) β chains of β2GPI-reactive T cells were examined in 8 β2GPI-responders, including 5 patients with APS and 3 healthy subjects, using polymerase chain reaction and single-strand conformation polymorphism (PCR-SSCP) analysis combined with in vitro stimulation of peripheral blood T cells with recombinant β2GPI. The TCR Vβ segments that expanded oligoclonally after stimulation with β2GPI varied among responders, but the Vβ7 and Vβ8 segments were commonly detected in 6 and 4 β2GPI-responders, respectively. Analysis of the complementarity-determining region 3 sequence of β2GPI-reactive T cells revealed limited diversity, and all Vβ7+ TCRs had an amino acid motif of TGxxN/Q or minor variations. The Vβ8+ TCRs had another motif, PxAxxD/E. Surprisingly, an identical Vβ7+ TCRβ chain was used by β2GPI-reactive T cells in 3 patients with APS. There was no apparent difference in the TCRβ usage between APS patients and healthy responders. Some of the Vβ7+ TCRs with the TGxxN/Q motif detected by PCR-SSCP analysis were also used by β2GPI-specific CD4+ T-cell clones responsive to an immunodominant epitope containing the major phospholipid-binding site. Depletion of Vβ7+ or Vβ8+ T cells from the peripheral blood mononuclear cell cultures significantly inhibited in vitro anti-β2GPI antibody production in response to β2GPI. Our results indicate preferential usage of TCRβ chains by β2GPI-reactive T cells. These TCRβ chains can be reasonable targets for TCR-based immunotherapy for patients with APS.
Introduction
Antiphospholipid syndrome (APS) is characterized by arterial and venous thrombosis and by recurrent fetal loss associated with the presence of antiphospholipid antibody.1 The most common target recognized by the antiphospholipid antibody is β2-glycoprotein I (β2GPI),2,3 a plasma glycoprotein that binds various kinds of negatively charged substances, including phospholipids, lipoproteins, and activated platelets.4,5Accumulated evidence in animal models strongly suggests that anti-β2GPI antibodies are directly involved in the pathogenic process of APS.6-8 Although precise mechanisms for the thrombophilia caused by anti-β2GPI antibodies remain unclear, one possibility is that these antibodies bind to endothelial cell surfaces by recognizing the adhered β2GPI and induce endothelial cell activation, resulting in the up-regulation of procoagulant and inflammatory processes.9,10 On the other hand, it has recently been shown that anti-β2GPI antibodies bind to oxidized, low-density lipoprotein through adhered β2GPI and promote its uptake by macrophages, resulting in the promotion of atherosclerosis.11
We recently identified CD4+ T cells responsive to β2GPI in patients with APS and in some healthy subjects.12 β2GPI-reactive CD4+T cells in patients with APS promoted anti-β2GPI antibody production from autologous B cells. Analysis of CD4+ T-cell clones from APS patients specifically responsive to β2GPI revealed that the antigenic peptide encompassing amino acid residues 276-290 (p276-290), which contains the major phospholipid-binding site, is preferentially recognized by β2GPI-specific T cells.13 Helper activity that stimulates B cells to produce anti-β2GPI antibodies is mediated through T-cell–derived interleukin (IL)-6 and CD40–CD40 ligand engagement. Because of this essential role of β2GPI-reactive T cells in anti-β2GPI antibody production, the elimination or inactivation of pathogenic β2GPI-reactive T cells should inhibit anti-β2GPI antibody production and prevent thrombosis and fetal loss in patients with APS. One of the immunologic interventions to induce selective suppression of an autoantigen-specific T-cell response is a strategy targeting the variable regions of T-cell receptors (TCRs) that are preferentially used by autoantigen-reactive T cells.14 This strategy has been effective in suppressing pathogenic autoimmune responses and improving symptoms in animal models for several organ-specific autoimmune diseases.15-18 To apply this selective therapeutic strategy, it is indispensable that the set of TCRs used by autoantigen-reactive T cells be highly restricted, but the TCR chain usage of pathogenic β2GPI-reactive T cells has not been examined to date. In this study, Vβ gene usage and TCRβ complementarity-determining region 3 (CDR3) sequences of β2GPI-reactive T cells were analyzed in APS patients and healthy responders using polymerase chain reaction (PCR) and single-strand conformation polymorphism (SSCP) analysis combined with in vitro stimulation of peripheral blood T cells with recombinant β2GPI.
Patients, materials, and methods
Patients and controls
Peripheral blood T cells from 5 Japanese patients with APS were analyzed in this study. They were selected from 12 patients enrolled in our previous study based on the presence of serum anti-β2GPI antibody, β2GPI-induced T-cell proliferative response, and availability of blood samples.12 All patients fulfilled the preliminary classification criteria for APS proposed by the International Workshop.19 A diagnosis of primary APS was made in 3 of the patients, and the remaining 2 had secondary APS accompanied by systemic lupus erythematosus. Clinical and serologic characteristics of these patients were described in detail in our previous report.12 At the time of blood examination, all the patients were taking low-dose corticosteroids (less than 10 mg/d) and aspirin. Three healthy persons with β2GPI-induced T-cell proliferative response were selected from 12 volunteers analyzed in the previous study12 and were used as control subjects. All samples were obtained after the patients and control subjects gave their written informed consent, approved by the Keio University Institutional Review Board.
HLA class II allele genotyping
Flow cytometric analysis
Two-color cell staining was performed using a phycoerythrin-conjugated monoclonal antibody (mAb) to CD3 (BD PharMingen, San Diego, CA) and an anti-Vβ2, anti-Vβ7, or anti-Vβ8 mAb (Immunotech, Marseilles, France) plus a fluorescein isothiocyanate–conjugated anti-mouse immunoglobulin (Ig) G antibody (Immunotech). Cells were analyzed on a FACSCalibur flow cytometer (BD PharMingen) using the CellQuest software.
Antigen preparations
GP-F, a recombinant maltose-binding protein (MalBP) fusion protein encoding the entire amino acid sequence of human β2GPI, was prepared and used as an antigen for T-cell stimulation.12 MalBP was also prepared as a control antigen.
Cell preparations
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by Lymphoprep (Nycomed Pharma AS, Oslo, Norway) density-gradient centrifugation and were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mMl-glutamine, 10 mMN-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, 50 U/mL penicillin, and 50 μg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C. In some experiments, PBMCs were depleted of Vβ2+, Vβ7+, or Vβ8+ cells by mixing with anti-Vβ2, anti-Vβ7, or anti-Vβ8 mAb, respectively, followed by incubation with goat anti-mouse IgG antibody-coupled magnetic beads (Dynal, Oslo, Norway). Bead-bound cells were subsequently removed using a magnetic apparatus. After the depletion treatment, the remaining cells that were positive for these TCR Vβ chains constituted less than 0.1% of the population.
GP-F and MalBP-stimulated T cells
PBMCs (2 × 106/well) were cultured in 24-well plates with GP-F or MalBP (10 μg/mL). On day 3, 30 U/mL IL-2 was added to the culture. Cells were restimulated with antigen, IL-2 (100 U/mL), and 106 irradiated (40 Gy) autologous PBMCs in fresh medium at day 10. Seven days after the second stimulation, the cells were harvested and stored at −80°C until use.
β2GPI-specific CD4+ T-cell clones
Six β2GPI-specific CD4+ T-cell clones were established from patients with APS and were reported in detail previously.13 Clones KS3 and KS8 were generated from patient APS3, and clones OM2 and OM7 were generated from patient APS4. The remaining 2 T-cell clones, EY3 and EY8, were established from an another patient with APS whose blood sample was unavailable for this study. KS3, OM2, OM7, EY3, and EY8 were shown to recognize p276-290 in the context of HLA-DRB4*0103 and to induce anti-β2GPI antibody production from autologous B cells. Antigenic profile and helper function were not fully analyzed for KS8.
Detection of the TCRβ chains used by β2GPI-reactive T cells
Total RNA was extracted from PBMCs, GP-F–stimulated T cells, and MalBP-stimulated T cells using an RNeasy kit (Qiagen, Valencia, CA). cDNA was synthesized from the total RNA using Molony murine leukemia virus reverse transcriptase (Takara, Kyoto, Japan) with oligo-dT priming. cDNA was then subjected to PCR amplification using a panel of Vβ (Vβ1-24) region-specific primers in combination with a Cβ region-specific primer.22 PCR products were resolved by electrophoresis on 2% agarose gels and were visualized by staining with ethidium bromide. The intensity of individual Vβ signals was semiquantified by densitometry using Molecular Imager FX (Bio-Rad Laboratories, Hercules, CA). The relative expression of each Vβ product was calculated as a ratio to the intensity of a control Cβ product. TCR Vβ chains, which were expressed in GP-F–stimulated T cells at a level that was at least twice as high as in unstimulated T cells, were selected as candidate TCR Vβ chains used by β2GPI-reactive T cells and were further analyzed by SSCP analysis.23 Specifically, the PCR products in a denaturing solution consisting of 95% formamide and 20 mM EDTA were boiled for 5 minutes and were loaded onto 5% polyacrylamide gels containing 10% glycerol. Gels were run at 30 W constant power for 2.5 hours. After electrophoresis, the gels were stained with a Silver Stain Plus kit (Bio-Rad Laboratories) according to the manufacturer's protocol. Clonally expanded single-strand DNA bands present in GP-F–stimulated T cells, but not in unstimulated or MalBP-stimulated T cells, were regarded as coding for the TCRβ chains of β2GPI-reactive T cells.
Nucleotide sequencing of CDR3 in TCRβ chains
Individual single-strand DNA bands of interest in silver-stained gels were re-amplified with Vβ and Cβ primers using the “bandstab” technique.24 PCR products were ligated into a pGEM-T vector (Promega, Madison, WI) and were transfected into competent DH5α Escherichia coli (Toyobo, Osaka, Japan). Both strands of at least 3 independent colonies were sequenced on an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA) using BigDye Terminator Cycle Sequence Ready Reaction kit (Applied Biosystems). A nucleotide sequence obtained from 2 or more colonies was regarded as that of a TCRβ chain used by β2GPI-reactive T cells. TCRβ CDR3 nucleotide sequences of β2GPI-specific T-cell clones were also determined directly from the PCR products as described previously,25except that an ABI Prism 310 genetic analyzer (Applied Biosystems) was used instead of gel electrophoresis of radiolabeled nucleotides. CDR3 length was defined as the region starting from the amino acid residue after the CASS sequence of most Vβ segments and ending before the GxG box in the Jβ region.26
In vitro production of anti-β2GPI antibodies in PBMC cultures
In vitro assays to analyze the β2GPI-induced synthesis of anti-β2GPI antibodies in PBMC cultures were carried out as described.12 Briefly, PBMCs and Vβ2+, Vβ7+, and Vβ8+cell-depleted PBMCs were cultured in complete medium with GP-F (10 μg/mL) in the presence of pokeweed mitogen (1 μg/mL) for 10 days. IgG anti-β2GPI antibody levels in undiluted culture supernatants were measured by an enzyme-linked immunosorbent assay kit (Yamasa, Choshi, Japan), in which cardiolipin-coated plates were incubated with purified human β2GPI as a cofactor. All cultures were prepared in duplicate, and the results were expressed as the OD450 and were calculated as the mean of the duplicates minus the mean of the blank reference wells, which did not contain sample. Differences in antibody levels between samples with and without the depletion treatment were assessed by Student ttest.
Results
TCR Vβ segments used by β2GPI-reactive T cells
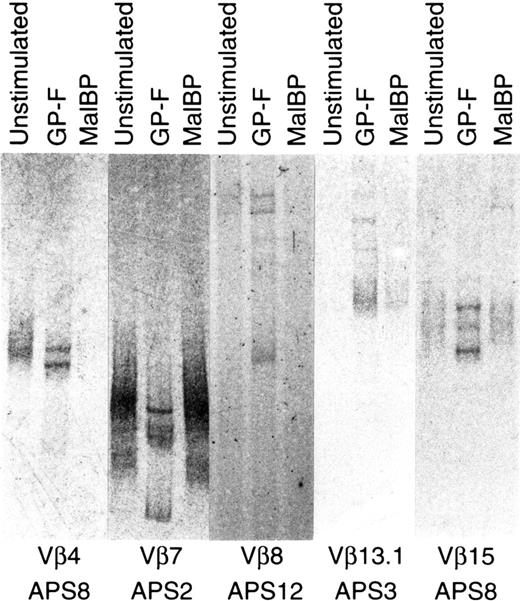
The expression of individual Vβ gene segments in unstimulated and GP-F–stimulated T cells was semiquantified by family PCR using a panel of V region–specific primers. Unstimulated T cells expressed many Vβ genes at varying levels, but a limited set of Vβ genes were expressed by GP-F–stimulated T cells. TCR Vβ chains whose expression levels were higher in GP-F–stimulated T cells than in unstimulated T cells were subjected to SSCP analysis. As shown in Figure1, oligoclonally expanded single-strand DNA bands present in the GP-F–stimulated T cells, but not in the unstimulated or MalBP-stimulated T cells, were identified as DNA encoding TCRβ of β2GPI-reactive T cells. To verify the initial selection step by densitometry, 12 randomly selected Vβ products that did not increase after GP-F stimulation were examined using SSCP analysis. Results showed that there was no oligoclonal band specifically present in GP-F–stimulated T cells. Vβ gene segments used by β2GPI-reactive T cells and HLA class II alleles in 5 APS patients and 3 healthy responders are summarized in Table1. Two or more Vβ segments were used by β2GPI-reactive T cells in all subjects except APS2. Vβ segments that were oligoclonally expanded after stimulation with β2GPI varied among subjects, but Vβ7, Vβ8, and Vβ13.1 were detected in 2 or more responders. The most frequently detected Vβ gene segment was Vβ7, which was used in 6 responders including 4 APS patients and 2 healthy donors. Vβ8 and Vβ13.1 were also detected in 4 and 2 responders, respectively. We noted that either Vβ7 or Vβ8 was used by β2GPI-reactive T cells in all β2GPI-responders. There was no apparent difference in the Vβ gene usage of β2GPI-reactive T cells between APS patients and healthy responders, though the number of subjects examined was small. No statistically significant association was found between the Vβ genes used by β2GPI-reactive T cells and HLA class II alleles, but all 4 responders with β2GPI-reactive Vβ8+ T cells were homozygous for DPB1*0501.
Oligoclonal expansion of TCRβ after stimulation with β2GPI.
PCR-amplified TCRβ products of unstimulated, GP-F–stimulated, and MalBP-stimulated T cells were fractionated on 5% polyacrylamide–glycerol gels and stained with silver. Oligoclonally expanded single-strand DNA bands present in GP-F–stimulated T cells, but not in unstimulated or MalBP-stimulated T cells, were identified as DNAs encoding TCRβ chains of β2GPI-reactive T cells.
Oligoclonal expansion of TCRβ after stimulation with β2GPI.
PCR-amplified TCRβ products of unstimulated, GP-F–stimulated, and MalBP-stimulated T cells were fractionated on 5% polyacrylamide–glycerol gels and stained with silver. Oligoclonally expanded single-strand DNA bands present in GP-F–stimulated T cells, but not in unstimulated or MalBP-stimulated T cells, were identified as DNAs encoding TCRβ chains of β2GPI-reactive T cells.
TCRβ CDR3 amino acid sequences of β2GPI-reactive T cells
SSCP bands for Vβ7, Vβ8, and Vβ13.1 were re-amplified directly from the gels, and their nucleotide sequences were determined after subcloning. The identical sequence was obtained from 2 independent SSCP-derived clones in some cases, and these corresponded to positive and negative DNA strands. We were unable to amplify some of the SSCP bands despite repeated attempts using various PCR conditions, probably because residual silver interfered with the amplification reaction. Deduced amino acid sequences of TCRβ CDR3 in β2GPI-reactive T cells are summarized in Table2. Notably, all 7 Vβ7+ TCRs obtained from 6 responders had the amino acid motif TGxxN/Q, or minor variations of it, in their CDR3 sequences. Either Vβ7.1 or Vβ7.2 was rearranged with Jβ1.2, Jβ1.5, or Jβ1.6. The CDR3 length was 12 or 13 amino acids, except in healthy donor HD4. A similar amino acid motif was also detected in 2 Vβ13.1+ TCRs, in which Vβ13.1 was rearranged with Jβ1.2 or 1.5. Surprisingly, an identical CDR3 sequence was detected in Vβ7+ TCRs obtained from 3 APS patients—APS2, APS4, and APS12. To eliminate possible PCR contamination, the same experiments were repeated in APS2 and APS4 using blood samples obtained at different time points and with entirely new reagents. As a result, Vβ7+ TCR with the identical CDR3 sequence was detected as the TCR β-chain of β2GPI-reactive T cells in these patients. Interestingly, the amino acid motif TG was primarily encoded by the germline-encoded Dβ1 segment (5′-acaggg-3′). The TGxxN/Q motif was not detected in 2 Vβ7+ TCRs that oligoclonally expanded after stimulation with MalBP in APS2. In contrast, Vβ8+ TCRs that were derived from 3 responders had the amino acid motif PxAxxD/E in their CDR3. Either Vβ8.1 or Vβ8.2 was rearranged with Jβ2.3 or Jβ2.7, and CDR3 was 9 or 11 amino acids long. The Dβ gene segment could not be definitively identified in Vβ8+ TCRs because of extensive nucleotide deletion. Nearly identical TCRβ chains were detected from APS patients and healthy responders. For example, Vβ8+ TCRs derived from APS12 and HD4 had the same Vβ8.1-Jβ2.3-Cβ2 gene rearrangement and a nearly identical CDR3 sequence, in which only one amino acid was different.
TCRβ chains in β2GPI-specific CD4+T-cell clones
TCRβ chains of 6 β2GPI-reactive T-cell clones were analyzed, and the results, in addition to the antigenic specificity and helper activity promoting anti-β2GPI antibody production, are summarized in Table 3. The Vβ–Jβ–Cβ rearrangement and the CDR3 sequence of clones KS3 and KS8 generated from APS3 were concordant with those determined by PCR-SSCP analysis in the same patient. T-cell clones OM2 and OM7 generated from APS4 had an identical Vβ7+ TCRβ chain, which was also detected in 3 APS patients, including APS4, by PCR-SSCP analysis. Furthermore, this particular Vβ7+ TCR was also detected in EY3 and EY8 generated from an APS patient who was unavailable for PCR-SSCP analysis. Identities of the TCRβ chains detected by the PCR-SSCP analysis and of the β2GPI-specific T-cell clones were further confirmed by SSCP analysis, in which TCRβ single-strand DNA had the same electrophoretic mobility on gels (results not shown). It was noted that KS3, OM2, OM7, EY3, and EY8, which recognized the immunodominant p276-290 and had helper activity, commonly used the TCRβ chains with Vβ7 and the TGxxN/Q motif.
Effects of Vβ7+ or Vβ8+ T-cell depletion on in vitro anti-β2GPI antibody production
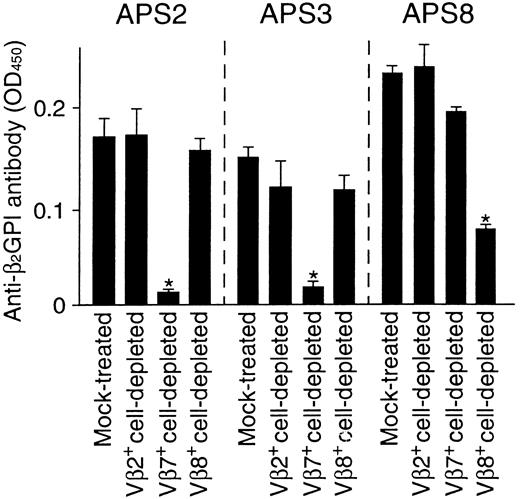
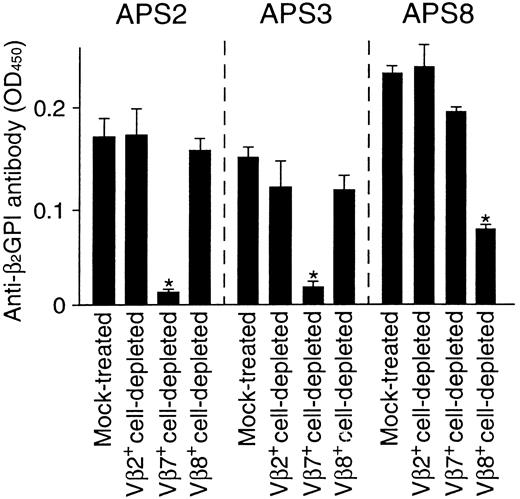
To evaluate the relative contribution of Vβ7+and Vβ8+ T cells in anti-β2GPI antibody production in APS patients, the effects of Vβ7+ and Vβ8+ T-cell depletion on in vitro anti-β2GPI antibody production in PBMC cultures were examined in samples from 3 APS patients. PBMCs depleted of irrelevant Vβ2+ T cells were also examined as a control. As shown in Figure 2, anti-β2GPI antibody production was inhibited by the depletion of Vβ7+ T cells, but not by the depletion of Vβ2+ or Vβ8+ T cells, in cultures from patients APS2 and APS3, who had β2GPI-reactive Vβ7+ T cells in circulation. On the other hand, anti-β2GPI antibody production was specifically suppressed by the depletion of Vβ8+ T cells from patient APS8, who had Vβ8+ T cells that were responsive to β2GPI. These findings strongly suggest that the β2GPI-reactive T cells with helper activity preferentially use Vβ7 or Vβ8 in patients with APS. However, the proportions of Vβ7+ and Vβ8+ T cells in the peripheral blood T cells were 1.6% to 2.1% and 3.5% to 4.7%, respectively, in 8 responders, and there was no difference in these proportions between APS patients and healthy subjects or between responders with and without β2GPI-reactive T cells with these Vβ segments.
Effects of Vβ7+ or Vβ8+T-cell depletion on in vitro anti-β2GPI antibody production.
Vβ2+, Vβ7+, and Vβ8+cell-depleted PBMCs and mock-treated PBMCs were cultured with GP-F (10 μg/mL) and pokeweed mitogen (1 μg/mL) for 10 days. Anti-β2GPI antibody levels in undiluted culture supernatants were measured by an enzyme-linked immunosorbent assay. Significant inhibition of anti-β2GPI antibody production by the depletion treatment is indicated by an asterisk. A representative result from 3 independent experiments is shown.
Effects of Vβ7+ or Vβ8+T-cell depletion on in vitro anti-β2GPI antibody production.
Vβ2+, Vβ7+, and Vβ8+cell-depleted PBMCs and mock-treated PBMCs were cultured with GP-F (10 μg/mL) and pokeweed mitogen (1 μg/mL) for 10 days. Anti-β2GPI antibody levels in undiluted culture supernatants were measured by an enzyme-linked immunosorbent assay. Significant inhibition of anti-β2GPI antibody production by the depletion treatment is indicated by an asterisk. A representative result from 3 independent experiments is shown.
Discussion
The current study demonstrates that the TCRβ chains of β2GPI-reactive T cells are highly conserved in APS patients and healthy responders. In most β2GPI-responders, β2GPI-reactive T cells used TCRβ chains with a rearranged Vβ7 segment and a TGxxN/Q motif in the CDR3 sequence. Furthermore, the shared Vβ7+ TCR chain was used by β2GPI-reactive T cells in 3 APS patients. We believe that this finding is not attributed to PCR contamination because detection of this particular Vβ7+TCR was reproducible, and Vβ7+ TCRs of MalBP-reactive T cells detected by the same method used the entirely different CDR3 sequences. On the other hand, Vβ8+ TCRs used by β2GPI-reactive T cells had a different CDR3 sequence, with a PxAxxD/E motif. It is likely that the TCRβ chains with these characteristics are predominantly used by pathogenic β2GPI-reactive T cells in most patients with APS because (1) Vβ7+ or Vβ8+ TCR was used by β2GPI-reactive T cells in all APS patients examined, (2) Vβ7+ TCRs with the TGxxN/Q motif were also detected in CD4+ T-cell clones known to be specific to β2GPI and can induce anti-β2GPI antibody production, and (3) in vitro anti-β2GPI antibody production in PBMC cultures was significantly inhibited by the depletion of Vβ7+ or Vβ8+ T cells. β2GPI-reactive T cells using Vβ segments other than Vβ7 and Vβ8 were also detected, but these appeared to be a minor repertoire of β2GPI-reactive T cells or were present only in a small subset of APS patients.
CD4+ T-cell clones responsive to p276-290 in the context of DRB4*0103 were frequently generated by patients with APS.13 Five β2GPI-specific T-cell clones with this antigenic specificity (KS3, OM2, OM7, EY3, and EY8) used the Vβ7 segment and the TGxxN/Q motif. Because the CDR3s of TCRα and TCRβ chains provide the principle peptide-binding residues in a TCR molecule, the Vβ7+ TCRs with the TGxxN/Q motif detected by the PCR-SSCP analysis may play a crucial role in recognition of the immunodominant T-cell epitope within p276-290. In addition, Vβ13.1+ TCRs detected in 2 β2GPI-responders and β2GPI-specific T-cell clone KS8 also had the TGxxN/Q motif in CDR3. Although the antigenic profile of KS8 was not fully determined, this clone was shown to recognize domain V, which includes p276-290, in an HLA-DR–restricted manner.13 Therefore, it is possible that β2GPI-reactive Vβ7+ and Vβ13.1+ T cells have the same antigenic specificity. In contrast, none of the β2GPI-specific T-cell clones analyzed used Vβ8+ TCRs with the PxAxxD/E motif. Vβ8+ TCR is presumed to recognize another epitope on β2GPI, given that β2GPI-reactive CD4+ T-cell clones were heterogeneous in terms of their antigenic specificity and that T-cell clones recognizing domain I/II in an HLA-DP–restricted manner and those recognizing domain IV in an HLA-DR–restricted manner could be also generated from patients with APS.13
APS patients and healthy responders used similar TCRβ chains for β2GPI-reactive T cells. This finding is analogous to the response of autoreactive T cells to myelin basic protein (MBP) in patients with multiple sclerosis (MS)27,28 and to topoisomerase I in patients with scleroderma.25Interestingly, the CDR3 motifs in MBP-specific T cells are shared by MS patients, healthy persons, and an animal model for MS.28Taken together with our previous finding that T cells responsive to β2GPI are present not only in APS patients but also in some healthy persons,12 the current study further supports the concept that β2GPI-reactive T cells are a component of the normal T-cell repertoire. This hypothesis could be explained by the recognition of cryptic determinants, not generated from a native molecule under normal circumstances,29 in β2GPI-reactive T cells. In fact, β2GPI-specific T-cell clones responded to reduced β2GPI and recombinant β2GPI fragments produced in bacteria, but not to native β2GPI.13 T cells responsive to cryptic self-determinants that escape negative selection in the thymus do not normally encounter the antigenic peptide in the periphery, but they would be activated if cryptic self-peptides were efficiently presented by antigen-presenting cells.30
In this study, PCR-SSCP analysis combined with in vitro stimulation of T cells with β2GPI was used to determine the TCRβ chains of β2GPI-reactive T cells. In most previous studies, the TCR usage of antigen-specific T cells was determined by analysis using antigen-specific T-cell clones.25,27 28However, the generation of antigen-specific T-cell clones requires considerable effort and time, and available T-cell clones are potentially biased because T cells with rapid proliferation kinetics tend to be selected. The current study confirmed that the TCRβ chains detected by PCR-SSCP analysis were concordant with those of β2GPI-specific T-cell clones. Thus, PCR-SSCP analysis combined with in vitro antigenic stimulation of T cells is a powerful tool for analyzing the TCR usage of antigen-specific T cells without generating T-cell clones.
Our results indicate the preferential usage of Vβ7 and Vβ8 segments by pathogenic β2GPI-reactive T cells in patients with APS. Although our findings were based on in vitro experiments on a relatively small number of patients, we could propose that the elimination or the inactivation of T cells expressing Vβ7+ or Vβ8+ TCRs may suppress the production of a disease-inducing anti-β2GPI antibody. For this purpose, the unique structures of Vβ7 and Vβ8 could be targets of TCR-based immunotherapy, including anti-TCR Vβ mAb administration and vaccination with Vβ peptides or naked DNA encoding Vβ sequences.15-18 Because regulatory T cells recognizing idiotopes on antigen-specific TCRs represent an important peripheral mechanism for controlling autoreactive T cells,31selective stimulation of an anti-TCR response is a potential strategy for unleashing its powerful immunoregulatory activity.
The efficacy of TCR-based immunotherapy was shown in animal models for various autoimmune diseases,15-18 but this strategy in patients was still under active investigation. For example, encephalitogenic T cells to MBP in the peripheral blood and central nervous system plaques of MS patients with DRB1*1501 were highly restricted and expressed similar Vβ5.2+ or Vβ6.1+ TCR chains.32,33 Based on this evidence, a double-blind, placebo-controlled trial to evaluate clinical and immunologic changes after vaccination with the Vβ5.2 CDR2 peptide in patients with progressive MS was performed.34 A strong T-cell response to the Vβ5.2 sequence was induced in 6 of 17 patients who received the Vβ5.2 peptide, but in none of 6 placebo-injected patients. Responders to the Vβ5.2 peptide had a reduced T-cell response to MBP and remained clinically stable without any side effects during 1 year of therapy, whereas nonresponders had increased MBP responses and progressed clinically. Furthermore, in patients with rheumatoid arthritis, vaccination of a combination of peptides corresponding to the CDR2 sequences of Vβ3, Vβ14, and Vβ17, which are predominantly expressed in synovial fluid and tissue,35 diminished disease activity without any side effects.36 TCR vaccination stimulated regulatory T cells that down-regulate pathogenic T cells without necessarily deleting them.37,38 In this regard, in MS patients, Vβ5.2-specific T-helper 2 cells induced by the Vβ5.2 peptide vaccination directly inhibited MBP-reactive T helper 1 cells through the release of IL-10.34
In summary, we have shown that the set of TCRβ chains of pathogenic β2GPI-reactive T cells in APS patients is highly restricted. TCR-based immunotherapy targeting Vβ7 or Vβ8 is a potential strategy to selectively suppress pathogenic β2GPI-specific T cells, resulting in the inhibition of anti-β2GPI antibody production and the prevention of thrombotic events in patients with APS.
We thank Yuka Okazaki and Kyoko Kimura for their expert technical assistance and Noriko Hattori for helpful discussions.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan; the Keio University Medical Science Fund; and the Japan Intractable Diseases Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masataka Kuwana, Institute for Advanced Medical Research, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: kuwanam@sc.itc.keio.ac.jp.