Abstract
Langerhans cells (LCs) are immature dendritic cells (DCs) that capture antigen in peripheral tissues and migrate to draining lymph nodes, where they reside in the paracortex as interdigitating dendritic cells (IDCs). We studied the effects of simian immunodeficiency virus (SIV) on LCs and IDCs during different stages of infection in monkeys. LCs isolated from monkeys with acute SIV infection or acquired immunodeficiency syndrome (AIDS) underwent normal maturation in vitro, including a switch in chemokine receptor expression from CCR5 to CXCR4 and CCR7. LCs migrated normally from skin in response to contact sensitization in monkeys with acute SIV infection. In contrast, LC migration from skin was markedly impaired during AIDS, associated with a reduction in antigen-bearing DCs in draining lymph nodes. Lymph node IDCs were increased in proportion during acute SIV infection and had an activated phenotype, whereas during AIDS IDCs had significantly lower expression of CD40 and the activation marker CD83. IDCs from monkeys with AIDS were refractory to stimulation with CD40L, demonstrating a functional consequence of decreased CD40 expression. SIV-infected DCs were not identified in lymph nodes or skin of monkeys with AIDS, suggesting an indirect effect of infection on DC populations in vivo. These data indicate that DCs are mobilized to lymph nodes during acute SIV infection, but that during AIDS this process is suppressed, with LC migration and IDC activation being impaired. We conclude that disruption of DC homeostasis may play a role in immunopathology induced by human immunodeficiency virus and suggest that therapeutic strategies targeting DCs may have limited efficacy during AIDS.
Introduction
Dendritic cells (DCs) perform a principal role in the generation of immunity to infectious agents.1 Immature DCs, such as Langerhans cells (LCs), are strategically positioned in peripheral epithelial tissues where they function to internalize and process antigens. Recognition of inflammatory mediators induces a program of DC maturation, including a switch in chemokine receptor expression and a change in function to a potent antigen-presenting cell (APC). Immature DCs express CC chemokine receptors CCR1, CCR2, CCR5, and CCR6, which bind inflammatory chemokines that recruit DCs to inflamed tissues.2,3 On maturation DCs express CCR7, which confers responsiveness to the lymph node–homing chemokines CCL19 (macrophage inflammatory protein 3β) and CCL21 (6 Ckine), and allows DCs to localize to the T-cell–rich paracortex of the lymph node.3-5 Within the lymph node, these mature DCs are referred to as interdigitating dendritic cells (IDCs).6IDCs are characterized by expression of the activation marker CD83 and by high-level expression of major histocompatibility complex (MHC) class II and the T-cell costimulatory molecules CD40 and CD86.6 7
The DC interactions with microbes, in particular human immunodeficiency virus (HIV), have been the subject of intense research. Studies in the rhesus macaque/simian immunodeficiency virus (SIV) model demonstrate that DCs can serve as the initial targets of viral infection and can route captured virions to draining lymph nodes.8,9Clustering of DCs with T cells facilitates HIV replication,10-12 potentially through the actions of DC-specific, ICAM-3–grabbing nonintegrin (DC-SIGN), which captures HIV virions at the DC surface via viral gp120.13 Although the role of DCs in HIV transmission has been relatively well elucidated, the effect of HIV infection on DC function has been less well defined. Loss and dysfunction of circulating DCs during HIV infection have been reported, including both CD11c+ myeloid and CD11c− plasmacytoid subsets.14-17 However, few studies have focused on the effects of HIV infection on DCs in the lymph node, the primary site of both DC/T-cell interactions and virus replication.1,18 One study reported that DCs in spleens of HIV+ patients express lower levels of the activation marker CD83 relative to uninfected controls, suggesting that DCs residing in lymphoid tissues are affected by HIV infection.19 In addition, to our knowledge, no studies of the effects of HIV infection on DC trafficking, a fundamental DC function, have been reported. LC density and the capacity of LCs to stimulate recall T-cell responses appear to be normal during acquired immunodeficiency syndrome (AIDS).20-22 However, studies in the murine Rauscher leukemia virus model showed that infected mice have impaired LC responsiveness to contact sensitization, indicating a possible role for altered DC migration in viral pathogenesis.23
To determine whether disruption of normal DC homeostasis in skin and lymph nodes could play a role in the immunopathogenesis of HIV infection, we studied DC populations in the rhesus macaque/SIV model. Our data suggest that DCs are recruited to lymph nodes during acute SIV infection, but that during AIDS, the capacity for epidermal LCs to migrate from skin and to reside as activated IDCs in lymph nodes is significantly impaired.
Materials and methods
Animals
Adult rhesus macaques (Macaca mulatta) of both sexes were used. Monkeys were infected as part of other studies with SIV/DeltaB670, a primary SIV isolate.24 Twelve animals infected with SIV were killed at 2 weeks after infection and categorized as having acute SIV infection. Seven SIV-infected animals were allowed to progress to AIDS. Seven animals were used as SIV naive controls. Animals were housed and all in vivo experiments were performed at the University of Pittsburgh Primate Facility for Infectious Disease Research using protocols approved by the institutional animal care and use committee.
Epidermal cell suspensions
Epidermal cell suspensions were generated from freshly harvested skin as described25 and maintained on ice for immunostaining. Alternatively, cells were cultured in complete medium (RPMI 1640 containing 10% fetal calf serum [Biowhittaker, Walkersville, MD], l-glutamine, sodium pyruvate, nonessential amino acids, penicillin-streptomycin, 10 mM Hepes [Life Technologies, Grand Island, NY], and 2-mercaptoethanol [Sigma, St Louis, MO]) with and without soluble, trimeric CD40L (3 μg/mL, provided by Immunex, Seattle, WA) for 48 hours prior to immunostaining.
Contact sensitization and quantification of LC migration
The method of contact sensitization was adapted from a previous report.26 Fluorescein isothiocyanate (FITC, Sigma) was dissolved in a 1:4.5:4.5 solution of dimethyl sulfoxide-acetone-dibutyl phthalate at 100 mg/mL, and 30 μL was applied to the inguinal region of anesthetized monkeys. Skin biopsies from the painted and contralateral regions were collected 24 to 48 hours later for generation of epidermal sheets, as described.27 Quantification of LC migration following contact sensitization was determined as described.27
Lymph node processing
Single-cell suspensions of lymph node tissues were generated using digestion with collagenase D as previously described.28 For experiments using CD40L, lymph node cells were cultured in complete medium with and without CD40L for 16 hours before immunostaining. Additionally, tissues were prepared for in situ hybridization and immunohistochemistry, as described below.
Flow cytometric analysis
For immunostaining of epidermal suspensions, cells were incubated with pure monoclonal antibody (mAb) to CCR7 (a gift from Lijun Wu, Leukosite, Cambridge, MA), CD1a (SK9; BD Pharmingen, San Diego, CA), or CCR5 (183; gift from Frank Mortari, R & D Systems, Minneapolis, MN) followed by goat antimouse antibody conjugated to FITC (Biosource, Camarillo, CA). Cells were then labeled with phycoerythrin (PE)–conjugated mAb to CD86 (FUN-1; BD Pharmingen), CD83 (HB15a; Coulter-Immunotech, Miami, FL), CXCR4 (12G5) or CD80 (BB1; both from BD Pharmingen), and biotinylated mAb to HLA-DR, followed by streptavidin-Cy5 (Amersham Pharmacia, Piscataway, NJ). Lymph node cells from animals that had been treated with FITC were labeled with mAb to CD83-PE and biotinylated CD86, followed by streptavidin-Cy5. For analysis of IDCs, lymph node cells were incubated with FcR-Blocking Reagent (Miltenyi Biotec, Auburn, CA) before staining with pure mAb specific for human CD4 (M-T477; BD Pharmingen), CD1a, HLA-DR, CD86, HLA-A, -B, -C (B9.12.1), CD40 (mAb89; both from Coulter-Immunotech), DC-SIGN (DC4; National Institutes of Health AIDS Research and Reference Reagent Program, Rockville, MD), or CCR7, followed by goat antimouse antibody conjugated to allophycocyanin (Molecular Probes, Eugene, OR). Alternatively, an allophycocyanin-conjugated mAb to CD11c (S-HCL-3; BD Pharmingen) was used. Cells were blocked with normal mouse serum, stained with mAb to CD20-FITC (2H7; BD Pharmingen) and CD83-PE, and fixed in 1% paraformaldehyde (PFA). For detection of p55, cells were fixed in 4% formaldehyde in hypertonic phosphate-buffered saline and permeabilized with 0.1% saponin, then stained with a pure mAb specific for p55 (55K-2; Dako, Carpinteria, CA), followed by secondary antibody. Cells were then labeled with mAb to HLA-DR-FITC or CD20-FITC and CD83-PE. Samples were analyzed on a FACSCaliber flow cytometer (Becton Dickinson, Mountain View, CA) using CellQuest software (Becton Dickinson).
Cloning of monkey CD83
Messenger RNA (mRNA) was isolated using the PolyATract System 1000 (Promega, Madison, WI) from lymph node single-cell suspensions following 48 hours of culture in the presence of CD40L (3 μg/mL). Amplification of monkey CD83 was achieved using the Access reverse transcription–polymerase chain reaction (RT-PCR) system (Promega) with primers designed from the human CD83 sequence. The forward primer, CD83F.FL, was 5′-GCGCCTCGAGGCACCATGTCGCGCGGCCTCCAG-3′, and the reverse primer, CD83R.FL, was 5′-GCGCCTCGAGCTACCAGTTCTGTCTTGTG-3′. RT-PCR was performed using a thermocycler (Progene; Techne, Princeton, NJ) with 1 cycle of 48°C for 45 minutes, 1 cycle of 94°C for 2 minutes, 40 cycles of 94°C for 30 seconds, 55°C for 1 minute, and 68°C for 2 minutes, then 1 cycle of 68°C for 7 minutes. RT-PCR products were analyzed on a 1% agarose gel. The appropriate band was gel-purified using the QIAEX II gel extraction kit (Qiagen, Valencia, CA) and TA-cloned into the pGEM-T vector (Promega). The cloned product was sequenced using an automated sequencer (ABI 3700 DNA Analyzer; Applied Biosystems, Foster City, CA) and confirmed to be monkey CD83 based on homology with the human CD83 sequence.
In situ hybridization and immunohistochemistry
In situ hybridization alone or in conjunction with immunohistochemistry was performed as described.29 For detection of SIV mRNA, riboprobes were derived from the SIVmacBK28 molecular clone spanning portions of the gag, pol, env, andnef genes. For detection of CD83 mRNA, riboprobes were generated from the cloned monkey CD83 plasmid described above. Tissues were prepared for immunohistochemistry as described.30Cryosectioned tissues were fixed in cold 2% PFA, pretreated with hydrogen peroxide, and blocked with 5% normal goat serum. Sections were incubated with mAb to CD83 or p55 overnight, followed by biotinylated goat antimouse antibody (Biosource), and then streptavidin-peroxidase (VectorStain Elite ABC kit, Vector Laboratories, Burlingame, CA). Ab staining was detected using diaminobenzidine substrate (Vector Laboratories). Tissues were counterstained with hematoxylin (Fisher Scientific, Pittsburgh, PA).
Statistical analysis
Comparisons between normal monkeys and monkeys with acute SIV infection, and between normal monkeys and monkeys with AIDS, were done by comparing means using a Student t test. Data are presented in the text as mean ± SEM.
Results
LCs from monkeys with AIDS undergo normal maturation in vitro
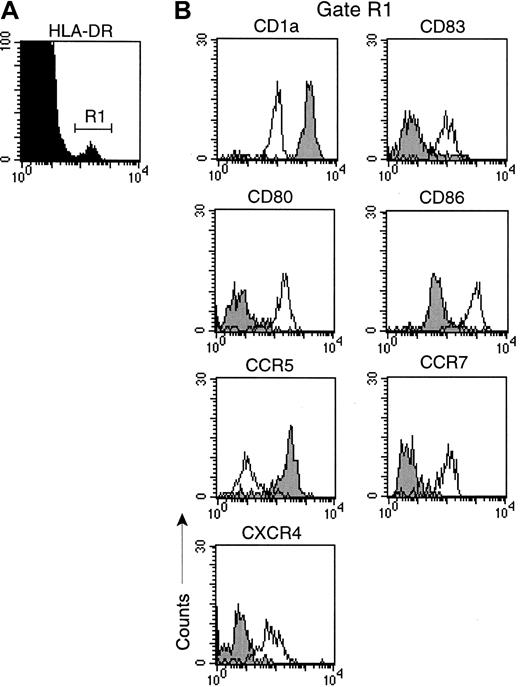
To begin to evaluate the effects of SIV infection on LCs, we generated epidermal cell suspensions from normal monkeys, and monkeys with acute SIV infection or AIDS, and did a phenotypic analysis of LCs. Cells were gated for expression of HLA-DR (Figure1A), and the expression of a panel of surface molecules was determined. Freshly isolated LCs from all animals expressed high levels of HLA-DR and CD1a, but lacked expression of CD83 (Figure 1A,B). LCs lacked expression of CD80, but expressed moderate levels of CD86. Consistent with the phenotype of an immature DC, LCs expressed high levels of CCR5, and lacked expression of CCR7 and CXCR4 (Figure 1B). To determine if LCs could mature in response to maturation stimuli in vitro, we cultured cell suspensions with and without CD40L, which results in rapid maturation of DC.28 In response to culture with CD40L, LCs from all monkeys, regardless of disease status, underwent a pronounced phenotypic maturation (Figure 1B). Mature LCs had reduced levels of CD1a and were positive for the activation marker CD83. Expression of CD80 and CD86 costimulatory molecules was markedly up-regulated (Figure 1B). Finally, the chemokine receptor profile switched to a loss of CCR5 and a gain in expression of CCR7 and CXCR4, consistent with DC maturation.3 Culturing DCs in the absence of CD40L generated cells with an intermediate phenotype (data not shown). These data indicate that LCs isolated from the skin of monkeys with acute SIV infection or AIDS have a normal ability to respond to maturation stimuli in vitro.
LCs from monkeys with AIDS undergo normal maturation in vitro.
Single-cell suspensions were generated from skin of a monkey with AIDS and labeled with mAb before and after 48 hours of culture with CD40L (3 μg/mL). (A) Cells were gated for expression of HLA-DR to identify LCs (R1). (B) Expression of surface antigens on freshly isolated LCs (closed histograms) and cultured LCs (open histograms). Representative histograms of 3 separate experiments using different animals are shown. Similar results were obtained with samples from healthy monkeys and monkeys with acute SIV infection.
LCs from monkeys with AIDS undergo normal maturation in vitro.
Single-cell suspensions were generated from skin of a monkey with AIDS and labeled with mAb before and after 48 hours of culture with CD40L (3 μg/mL). (A) Cells were gated for expression of HLA-DR to identify LCs (R1). (B) Expression of surface antigens on freshly isolated LCs (closed histograms) and cultured LCs (open histograms). Representative histograms of 3 separate experiments using different animals are shown. Similar results were obtained with samples from healthy monkeys and monkeys with acute SIV infection.
LCs fail to migrate from skin in response to contact sensitization in monkeys with AIDS
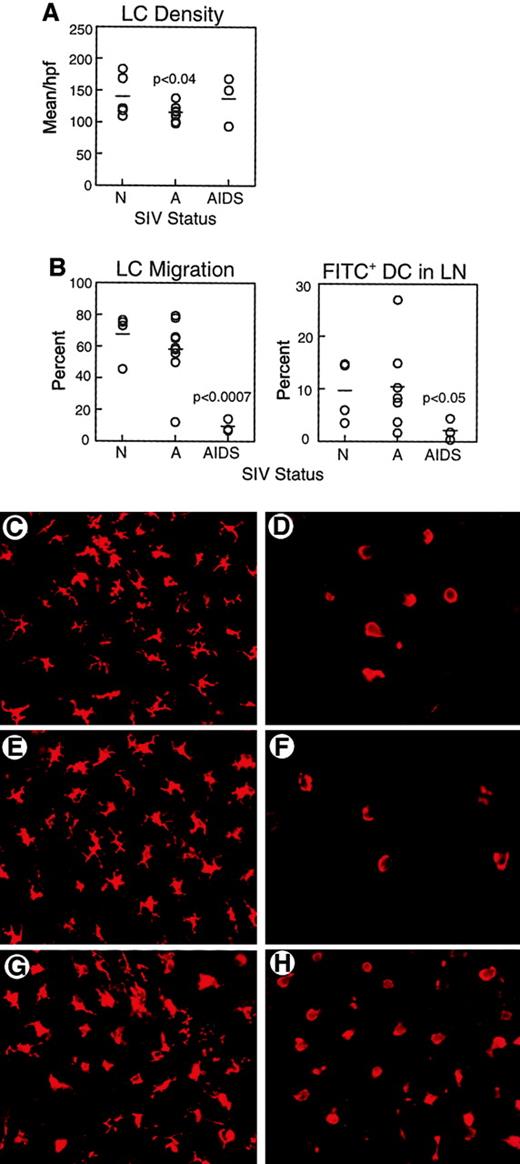
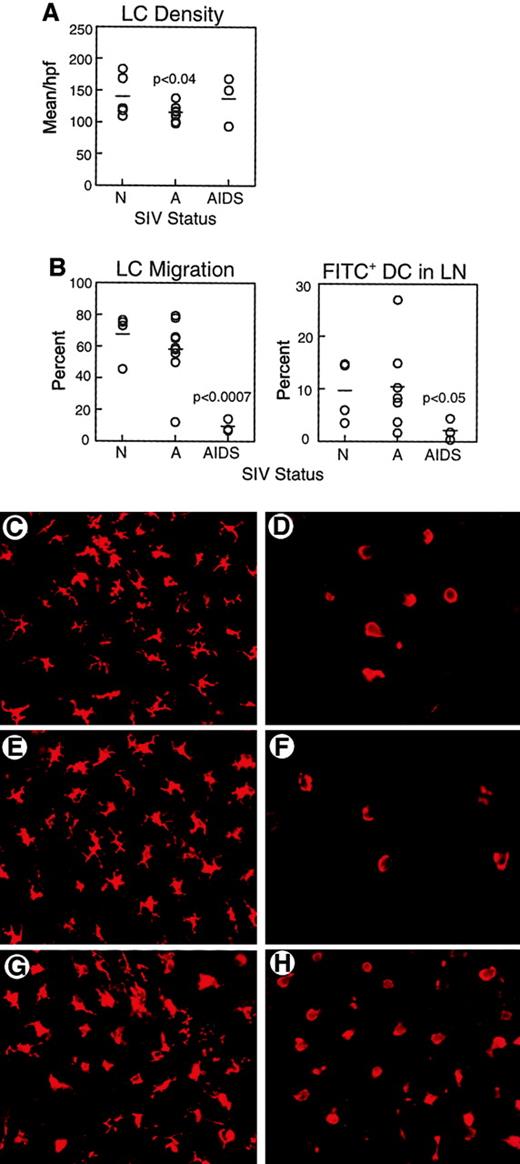
We next sought to determine if there were any effects of SIV infection on LC function in vivo. Epidermal sheets were separated from skin biopsies and labeled with mAb to HLA-DR to identify LCs. The density of LCs in the skin of monkeys with AIDS (137.0 ± 23.0 HLA-DR+ cells/high-power field [hpf]) was similar to that of normal animals (139.0 ± 17.0 HLA-DR+ cells/hpf, Figure 2A,C,G), consistent with previous reports.22 However, LC density in skin of monkeys with acute SIV infection was significantly lower than normal (114.8 ± 4.0 HLA-DR+ cells/hpf,P < .04, Figure 2A,E). To evaluate the functional capacity for LCs to migrate, we used the FITC contact sensitization model. In the mouse, application of FITC to the skin results in a rapid exodus of LCs from skin with a peak appearance of FITC+ DCs in the lymph node at 24 hours.26 We labeled epidermal sheets harvested 24 to 48 hours after sensitization with mAb to HLA-DR to detect LCs. In response to contact sensitization, the majority of LCs migrated from skin of normal animals (67.5% ± 7.4%, Figure2B,D) and from skin of monkeys acutely infected with SIV (58.0% ± 6.6%, Figure 2B,F). Similar to normal monkeys and monkeys with acute SIV infection, LCs in monkeys with AIDS underwent a morphologic change after contact sensitization, losing the characteristic stellate morphology (Figure 2H). However, the proportion of LCs migrating from skin in monkeys with AIDS was significantly reduced to 9.4% ± 2.3% (P < .0007, Figure 2B,H). Migration did not appear to be delayed because the density of LCs in the skin was similar at 24 and 48 hours after sensitization (data not shown). To determine whether impaired LC migration resulted in a reduction in DCs localizing to draining lymph nodes, we harvested inguinal lymph nodes 24 to 48 hours after application of FITC and labeled cell suspensions with mAb to CD86 and CD83 to detect DCs.28 The proportion of FITC+ DCs in draining lymph nodes after FITC painting was similar in normal monkeys (9.6% ± 2.9%) and monkeys with acute SIV infection (10.4% ± 3.2%, Figure 2B). However, the proportion of DCs that were FITC+ in monkeys with AIDS was significantly reduced as compared to naive animals (2.2% ± 1.2%, P < .05, Figure 2B). These data demonstrate that DCs from monkeys with AIDS have a defect in their ability to traffic from peripheral tissues to draining lymph nodes.
Impaired migration of LCs from skin to lymph nodes in response to contact sensitization in monkeys with AIDS.
(A) Epidermal sheets were isolated from skin biopsies from normal, healthy monkeys (N, n = 5), and monkeys with acute SIV infection (A, n = 9) or AIDS (n = 3) and labeled with mAb to HLA-DR to identify LCs. Mean LC density was calculated from 12 high-power fields. LC density is decreased in acute SIV infection as compared to normal animals (P < .04). (B) FITC was applied to inguinal skin and 24 to 48 hours later skin and draining lymph nodes (LN) were harvested to determine LC migration and the proportion of FITC+ DCs in lymph nodes. On the left is shown the proportion of LCs migrating from FITC-painted skin as compared to control contralateral skin in healthy monkeys (n = 4), and monkeys with acute SIV infection (n = 9) or AIDS (n = 3). LC migration in monkeys with AIDS is reduced as compared to normal animals (P < .0007). On the right, FITC+ DCs as a proportion of all CD86+ CD83+ lymph node DCs in healthy monkeys (n = 4) and monkeys with acute SIV infection (n = 7) or AIDS (n = 3) are shown. The proportion of FITC+ DCs in lymph nodes of monkeys with AIDS is lower than that in healthy animals (P < .05). Each circle represents a different animal, and horizontal bars represent the mean. (C-H) Representative images of HLA-DR+ LCs in epidermal sheets of unpainted control skin (C,E,G) and FITC-sensitized skin (D,F,H) from healthy monkeys (C-D), and monkeys with acute SIV infection (E-F) or AIDS (G-H). Original magnification × 400.
Impaired migration of LCs from skin to lymph nodes in response to contact sensitization in monkeys with AIDS.
(A) Epidermal sheets were isolated from skin biopsies from normal, healthy monkeys (N, n = 5), and monkeys with acute SIV infection (A, n = 9) or AIDS (n = 3) and labeled with mAb to HLA-DR to identify LCs. Mean LC density was calculated from 12 high-power fields. LC density is decreased in acute SIV infection as compared to normal animals (P < .04). (B) FITC was applied to inguinal skin and 24 to 48 hours later skin and draining lymph nodes (LN) were harvested to determine LC migration and the proportion of FITC+ DCs in lymph nodes. On the left is shown the proportion of LCs migrating from FITC-painted skin as compared to control contralateral skin in healthy monkeys (n = 4), and monkeys with acute SIV infection (n = 9) or AIDS (n = 3). LC migration in monkeys with AIDS is reduced as compared to normal animals (P < .0007). On the right, FITC+ DCs as a proportion of all CD86+ CD83+ lymph node DCs in healthy monkeys (n = 4) and monkeys with acute SIV infection (n = 7) or AIDS (n = 3) are shown. The proportion of FITC+ DCs in lymph nodes of monkeys with AIDS is lower than that in healthy animals (P < .05). Each circle represents a different animal, and horizontal bars represent the mean. (C-H) Representative images of HLA-DR+ LCs in epidermal sheets of unpainted control skin (C,E,G) and FITC-sensitized skin (D,F,H) from healthy monkeys (C-D), and monkeys with acute SIV infection (E-F) or AIDS (G-H). Original magnification × 400.
Phenotype of IDCs in normal rhesus monkey lymph node
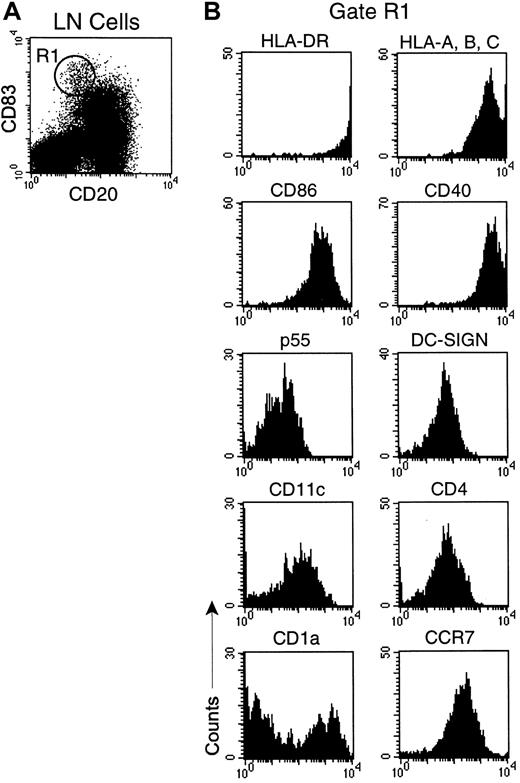
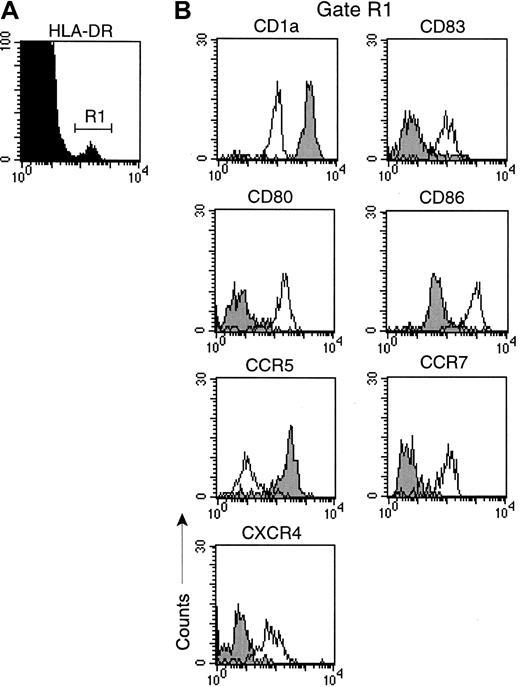
Given the defect in LC migration in monkeys with AIDS, we next determined if there were changes to lymph node IDCs during SIV infection. We characterized IDCs in lymph nodes of healthy monkeys by flow cytometry using CD83 as a lineage marker because it is uniformly expressed on IDCs in humans and monkeys.28,31 To delineate DCs from activated CD83+ B cells31 we double-labeled cells with mAb to CD20. A distinct population of DCs was identified that expressed high levels of CD83, but was CD20− (Figure 3A). CD83+ DCs expressed uniform and high levels of MHC class I and II, and the costimulatory molecules CD40 and CD86, consistent with professional APC (Figure 3B). Additionally, CD83+ DCs were positive for the DC-specific molecules p55 (fascin)32 and DC-SIGN (Figure 4B),33 and lacked expression of lineage markers CD14, CD3, and CD16 (data not shown). CD83+ DCs uniformly expressed the lymph node–homing chemokine receptor CCR7 and were CD4+ and CD11c+ (Figure 3B), but lacked expression of the plasmacytoid marker CD123 (data not shown). Interestingly, CD83+ lymph node DCs were heterogenous for expression of CD1a, indicating that there are phenotypic subsets of CD83+DCs in the monkey lymph node (Figure 3B). By in situ hybridization and immunohistochemistry, CD83+ DC resided in the T-cell–rich paracortex of lymph nodes (Figure 4C,F,I). These data indicate that high-level expression of CD83, and lack of expression of CD20, defines a mature and activated IDC in the normal monkey lymph node.
CD83 expression identifies activated IDCs in lymph nodes of healthy monkeys.
Lymph node single-cell suspensions were stained with mAbs to CD20 and CD83 and a panel of cell surface markers. (A) Dot plot of CD83 versus CD20. IDCs (R1) express high levels of CD83 and lack expression of CD20. (B) Phenotype of CD83+ IDCs gated on R1. Fluorescence due to isotype control mAb was limited to the first decade. Data are representative of 12 experiments.
CD83 expression identifies activated IDCs in lymph nodes of healthy monkeys.
Lymph node single-cell suspensions were stained with mAbs to CD20 and CD83 and a panel of cell surface markers. (A) Dot plot of CD83 versus CD20. IDCs (R1) express high levels of CD83 and lack expression of CD20. (B) Phenotype of CD83+ IDCs gated on R1. Fluorescence due to isotype control mAb was limited to the first decade. Data are representative of 12 experiments.
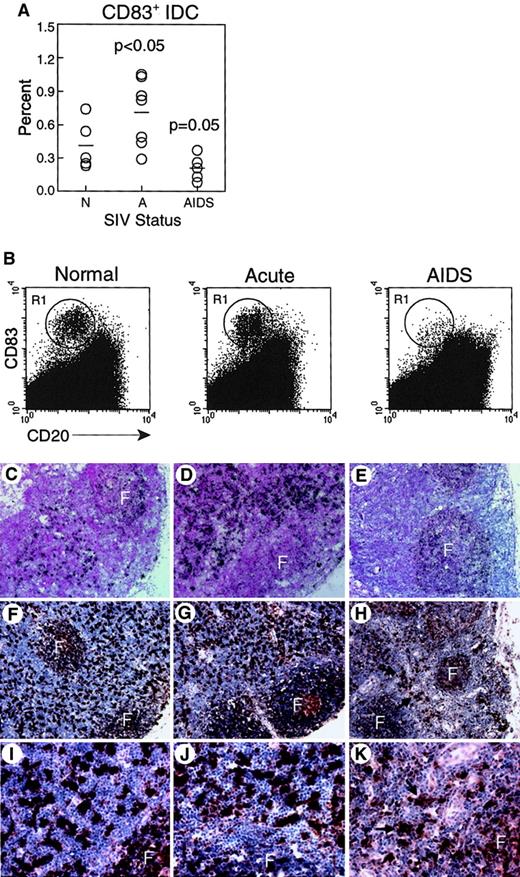
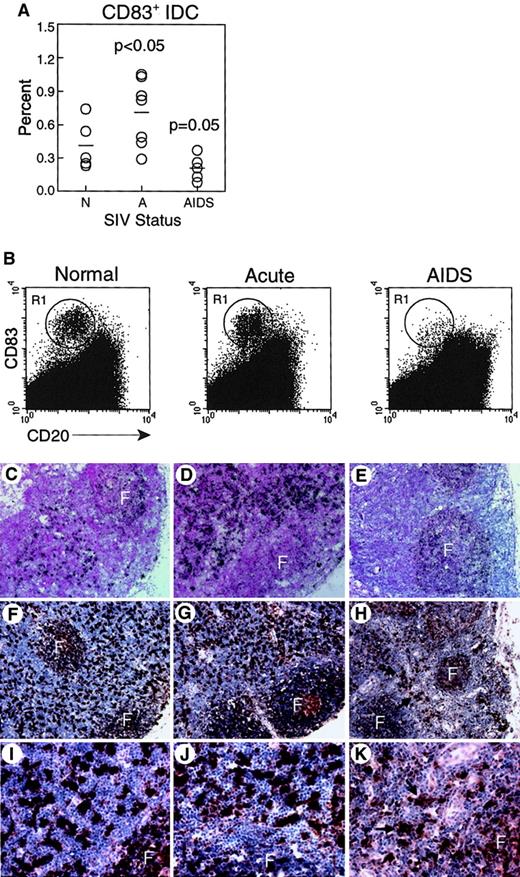
CD83+ IDC in lymph nodes are increased during acute SIV infection but decreased during AIDS.
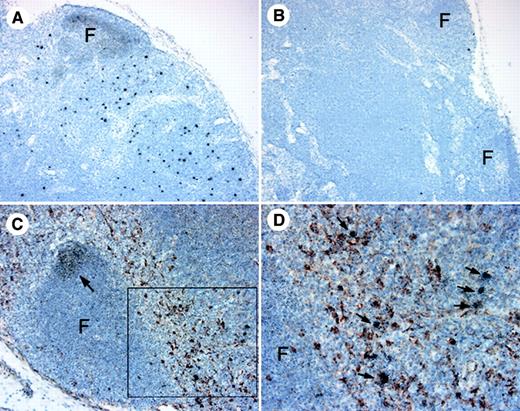
(A-B) Lymph node cell suspensions were labeled and gated for expression of CD83 and lack of CD20 to identify IDCs. (A) Proportion of CD83+ IDCs in unseparated lymph nodes from normal, healthy monkeys (N, n = 5), and monkeys with acute SIV infection (A, n = 7), or AIDS (n = 5). IDCs are increased in acute SIV infection (P < .05) and decreased during AIDS (P = .05) as compared to normal, healthy animals. Each circle represents a different animal, and horizontal bars represent the mean. (B) Representative dot plots of lymph node cells from healthy monkeys and monkeys with acute SIV infection or AIDS; 400 000 events are shown to highlight IDCs (R1). (C-K) CD83 expression in lymph nodes was analyzed using in situ hybridization for CD83 RNA (C-E) and immunohistochemistry for CD83 protein (F-K) in healthy monkeys (C,F,I), and monkeys with acute SIV infection (D,G,J), or AIDS (E,H,K). Infrequent CD83+ IDCs in monkeys with AIDS are highlighted by arrows. F indicates follicle. Ab staining was detected using streptavidin-peroxidase with diaminobenzidine substrate. Tissues were counterstained with eosin (C-E) or hematoxylin (F-K). Original magnification × 100 (C-E), × 200 (F-H), × 600 (I-K).
CD83+ IDC in lymph nodes are increased during acute SIV infection but decreased during AIDS.
(A-B) Lymph node cell suspensions were labeled and gated for expression of CD83 and lack of CD20 to identify IDCs. (A) Proportion of CD83+ IDCs in unseparated lymph nodes from normal, healthy monkeys (N, n = 5), and monkeys with acute SIV infection (A, n = 7), or AIDS (n = 5). IDCs are increased in acute SIV infection (P < .05) and decreased during AIDS (P = .05) as compared to normal, healthy animals. Each circle represents a different animal, and horizontal bars represent the mean. (B) Representative dot plots of lymph node cells from healthy monkeys and monkeys with acute SIV infection or AIDS; 400 000 events are shown to highlight IDCs (R1). (C-K) CD83 expression in lymph nodes was analyzed using in situ hybridization for CD83 RNA (C-E) and immunohistochemistry for CD83 protein (F-K) in healthy monkeys (C,F,I), and monkeys with acute SIV infection (D,G,J), or AIDS (E,H,K). Infrequent CD83+ IDCs in monkeys with AIDS are highlighted by arrows. F indicates follicle. Ab staining was detected using streptavidin-peroxidase with diaminobenzidine substrate. Tissues were counterstained with eosin (C-E) or hematoxylin (F-K). Original magnification × 100 (C-E), × 200 (F-H), × 600 (I-K).
Contrasting effects of SIV infection on CD83+ IDCs depending on stage of infection
We next compared lymph node IDCs from healthy monkeys and monkeys with acute SIV infection or AIDS. We found a significant increase in the proportion of activated IDCs in monkeys with acute SIV infection (0.72% ± 0.12%) relative to uninfected animals (0.41% ± 0.10%, P < .05, Figure 4A,B). In contrast, monkeys with AIDS had a trend toward a loss of activated IDCs in lymph nodes as compared to naive controls, although this was not statistically significant (0.21% ± 0.05%, P = .05, Figure 4A,B). To analyze the effect of SIV infection on IDCs in more detail, we did tissue-based analysis of CD83+ cells. CD83 in situ hybridization in healthy monkeys revealed strong reactivity in the paracortex and dim reactivity in the follicles, consistent with staining of IDCs and B cells, respectively (Figure 4C). Intense staining of IDCs in the paracortex was seen in animals acutely infected with SIV (Figure 4D). In contrast, in monkeys with AIDS, minimal CD83 reactivity was seen in the paracortex, whereas CD83 expression was readily detected associated with B-cell follicles (Figure 4E). Similarly, by immunohistochemistry, an abundance of large, CD83+ cells was detected in the paracortex of normal monkeys and monkeys with acute SIV infection (Figure 4F,G,I,J). However, in the animals with AIDS, the paracortex contained few CD83+ IDCs (Figure 4H,K). Results from these experiments suggest that IDCs are either depleted from the lymph nodes during AIDS or are present at a lower activation state with decreased expression of CD83.
IDCs from monkeys with AIDS have reduced expression of CD40 and fail to respond to CD40L
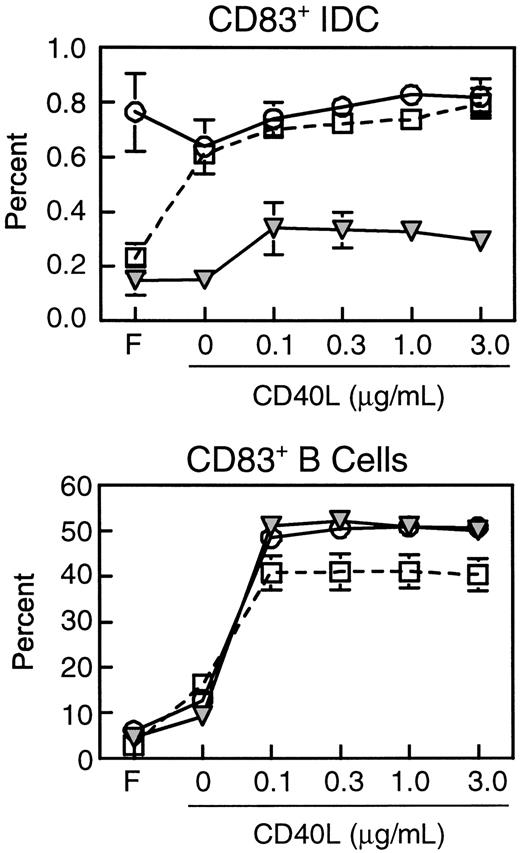
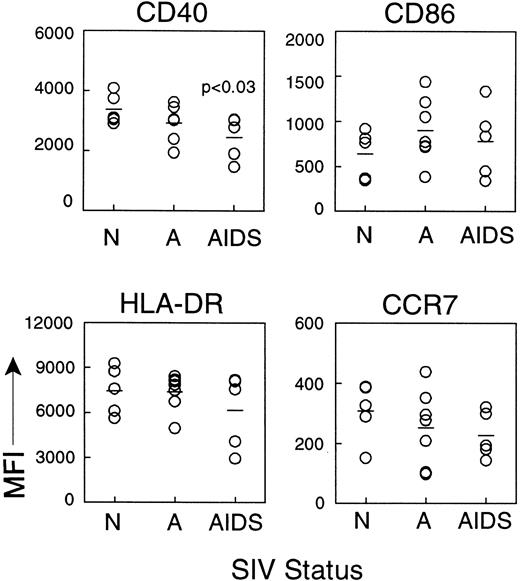
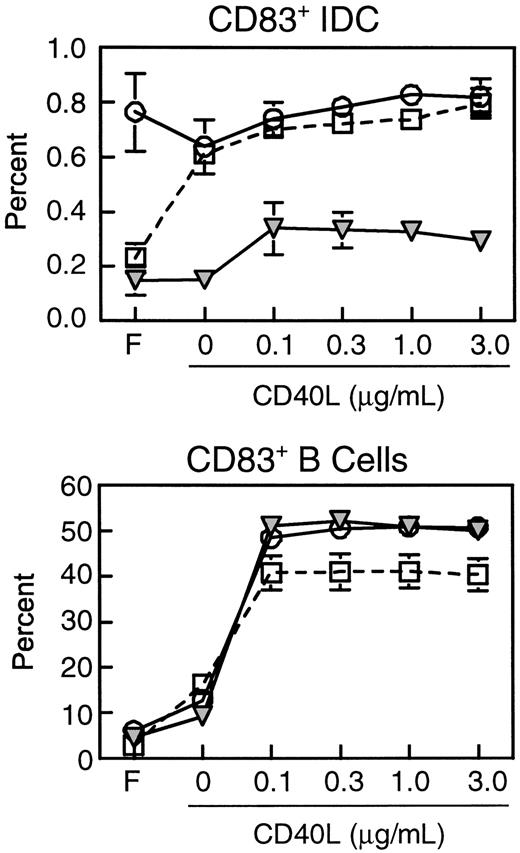
We next compared the expression of costimulatory and MHC class II molecules on CD83+ IDCs during different stages of SIV infection. Expression of CD40, HLA-DR, CD86, and CCR7 on IDCs was similar in healthy monkeys and monkeys with acute SIV infection (Figure5). However, IDCs from lymph nodes of monkeys with AIDS had significantly lower mean expression of the costimulatory molecule CD40 (2447.2 ± 321.2) as compared to IDCs from healthy monkeys (3371.8 ± 227.6, P < .03, Figure5). This indicated that the CD83+ IDCs in monkeys with AIDS are present in a lower state of activation than in healthy monkeys. Expression of HLA-DR and CD86 was not significantly reduced (Figure 5). Importantly, expression of CCR7 was normal in monkeys with AIDS (Figure5), indicating that IDCs that were present in the lymph nodes had the appropriate chemokine receptor expression for localization to the paracortex, consistent with the immunohistochemistry data (Figure 4). The lower expression of CD40 on IDCs of monkeys with AIDS suggested that these cells may have a suboptimal response to CD40L expressed by activated T cells in the paracortex. To test this directly, we cultured lymph node cell suspensions in CD40L and studied the activation of IDCs as measured by CD83 expression. The proportion of CD83+IDCs from lymph nodes of healthy monkeys increased in response to overnight culture and to addition of CD40L, indicating an increase in the proportion of activated IDCs (Figure6). The proportion of lymph node IDCs expressing CD83 in monkeys acutely infected with SIV was high in fresh samples (Figure 6), consistent with our earlier findings (Figure 4). Interestingly, this proportion did not increase in response to CD40L treatment, indicating that IDCs in this group of animals may be maximally activated. In contrast, the proportion of CD83+IDCs from lymph nodes of monkeys with AIDS was similar to that of normal monkeys in fresh samples but did not increase with overnight culture, and only marginally increased in response to CD40L (Figure 6). The proportion of B cells expressing CD83 increased markedly in all animals regardless of disease status, indicating that B cells in animals with AIDS have a normal response to activation stimuli (Figure6). These results indicate that the lower level of CD40 on IDCs during AIDS has functional consequences impairing the activation of IDCs.
IDCs from lymph nodes of monkeys with AIDS have reduced expression of CD40.
Lymph node cell suspensions from normal, healthy animals (N, n = 5) and monkeys with acute SIV infection (A, n = 7) or AIDS (n = 5) were labeled and gated on expression of CD83 and lack of CD20 to identify IDCs, and the mean fluorescence intensity (MFI) of a panel of cell surface markers was determined. CD40 expression on IDCs from monkeys with AIDS is reduced as compared to normal animals (P < .03). Each circle represents a different animal, and horizontal bars represent the mean.
IDCs from lymph nodes of monkeys with AIDS have reduced expression of CD40.
Lymph node cell suspensions from normal, healthy animals (N, n = 5) and monkeys with acute SIV infection (A, n = 7) or AIDS (n = 5) were labeled and gated on expression of CD83 and lack of CD20 to identify IDCs, and the mean fluorescence intensity (MFI) of a panel of cell surface markers was determined. CD40 expression on IDCs from monkeys with AIDS is reduced as compared to normal animals (P < .03). Each circle represents a different animal, and horizontal bars represent the mean.
Lymph node IDCs but not B cells from monkeys with AIDS are refractory to CD40 ligation.
Lymph node cell suspensions were analyzed before or following overnight culture with or without CD40L (3 μg/mL) and stained for expression of CD83 and CD20. The proportions of IDCs (CD83+CD20−, top) and activated B cells (CD83+CD20+, bottom) from freshly isolated lymph nodes (F), or lymph node cells cultured with or without CD40L from healthy monkeys (open squares, n = 3), and monkeys with acute SIV infection (open circles, n = 3) or AIDS (filled triangles, n = 3) are shown. The data represent mean ± SEM.
Lymph node IDCs but not B cells from monkeys with AIDS are refractory to CD40 ligation.
Lymph node cell suspensions were analyzed before or following overnight culture with or without CD40L (3 μg/mL) and stained for expression of CD83 and CD20. The proportions of IDCs (CD83+CD20−, top) and activated B cells (CD83+CD20+, bottom) from freshly isolated lymph nodes (F), or lymph node cells cultured with or without CD40L from healthy monkeys (open squares, n = 3), and monkeys with acute SIV infection (open circles, n = 3) or AIDS (filled triangles, n = 3) are shown. The data represent mean ± SEM.
IDCs from monkeys with AIDS are less activated than those from healthy animals
To further address the possibility of differential activation of IDCs during SIV infection, we labeled lymph node cell suspensions with mAb to HLA-DR and p55, molecules constitutively expressed by IDCs in the lymph node.32 34 We then examined IDCs for expression of the activation marker CD83. Immunofluorescence labeling of lymph node cells from healthy animals revealed a discrete population of HLA-DRhi p55+ cells (Figure7A), representing 0.26% ± 0.06% of all lymph node cells (Figure 7B), approximating the proportion of IDCs identified by staining with CD83. A similar proportion of HLA-DRhi p55+ DCs was seen in lymph nodes of animals with acute SIV infection (0.32 ± 0.05, Figure 7B). As in our previous experiments using mAb to CD83 and CD20, the proportion of IDCs in the lymph nodes of animals with AIDS as determined by HLA-DR/p55 staining was not statistically different from that of healthy animals (0.13% ± 0.03%, P = .05, Figure 7B). The mean expression of CD83 on HLA-DRhi p55+ IDC in lymph nodes of helathy monkeys (314.0 ± 17.2) and monkeys with acute SIV infection (335.9 ± 51.0) was similar. However, the expression of CD83 on IDCs of monkeys with AIDS was significantly reduced as compared to that in healthy animals (205.5 ± 12.8, P < .0005, Figure 7B). To confirm these findings, we did in situ hybridization analyses using antisense probes to CD83 and then double-labeled sections with mAb to p55. The vast majority of IDCs in the lymph node paracortex of healthy monkeys double-labeled with p55 and CD83, indicating that IDCs are predominantly activated in healthy animals (Figure 7C). Similarly, p55+ IDCs in the lymph nodes of monkeys with acute SIV infection were CD83+ (Figure 7D). In contrast, p55+ IDCs lacking expression of CD83 predominated in the lymph nodes of monkeys with AIDS (Figure 7E). Together, these data indicate that there is not an absolute loss of IDCs from lymph nodes during AIDS, but that resident IDCs have a poorly activated phenotype and are functionally impaired.
IDCs from lymph nodes of monkeys with AIDS have markedly reduced expression of CD83.
(A-B) Lymph node cells were stained with mAb to HLA-DR and p55 to identify IDCs. (A) Representative dot plots of cells from a healthy monkey stained with mAb to HLA-DR and either control IgG (left) or p55 (right), showing the HLA-DRhi p55+ IDC (R1). (B) On the left the proportion of HLA-DRhi p55+IDCs in unseparated lymph node cells from normal, healthy monkeys (N, n = 5) and monkeys with acute SIV infection (A, n = 5) or AIDS (n = 5) is shown. IDCs are reduced in lymph nodes of monkeys with AIDS relative to normal animals (P = .05). On the right, the expression of CD83 on the HLA-DRhip55+ IDCs is shown. Expression of CD83 is reduced in monkeys with AIDS compared to normal animals (P < .0005). Each circle represents a different animal, and horizontal bars represent the mean. (C-E) Lymph node sections from a healthy monkey (C) and monkeys with acute SIV infection (D) or AIDS (E) were labeled by in situ hybridization for CD83 RNA (black) and immunohistochemistry for p55 protein (brown). Double-labeled IDCs in lymph nodes of the healthy monkey and monkey with acute SIV infection are highlighted by arrows. Ab staining was detected using streptavidin-peroxidase with diaminobenzidine substrate. Tissues were counterstained with hematoxylin. Original magnification × 600.
IDCs from lymph nodes of monkeys with AIDS have markedly reduced expression of CD83.
(A-B) Lymph node cells were stained with mAb to HLA-DR and p55 to identify IDCs. (A) Representative dot plots of cells from a healthy monkey stained with mAb to HLA-DR and either control IgG (left) or p55 (right), showing the HLA-DRhi p55+ IDC (R1). (B) On the left the proportion of HLA-DRhi p55+IDCs in unseparated lymph node cells from normal, healthy monkeys (N, n = 5) and monkeys with acute SIV infection (A, n = 5) or AIDS (n = 5) is shown. IDCs are reduced in lymph nodes of monkeys with AIDS relative to normal animals (P = .05). On the right, the expression of CD83 on the HLA-DRhip55+ IDCs is shown. Expression of CD83 is reduced in monkeys with AIDS compared to normal animals (P < .0005). Each circle represents a different animal, and horizontal bars represent the mean. (C-E) Lymph node sections from a healthy monkey (C) and monkeys with acute SIV infection (D) or AIDS (E) were labeled by in situ hybridization for CD83 RNA (black) and immunohistochemistry for p55 protein (brown). Double-labeled IDCs in lymph nodes of the healthy monkey and monkey with acute SIV infection are highlighted by arrows. Ab staining was detected using streptavidin-peroxidase with diaminobenzidine substrate. Tissues were counterstained with hematoxylin. Original magnification × 600.
Impaired migration and activation of DCs in AIDS are indirect results of SIV infection
We next sought to determine whether the impaired DC migration and activation observed during AIDS was the direct result of SIV infection. In situ hybridization analysis of lymph nodes from SIV-infected monkeys with AIDS using antisense probes for SIV RNA revealed substantial reactivity in the paracortex (Figure8A). Additionally, we found diffuse labeling in the follicles, likely representing free virus captured by follicular DCs and B cells.18 Control sense probes did not react with the tissues (Figure 8B). Double labels of SIV RNA and p55 mAb were used to determine the relationship between SIV-infected cells and IDCs. p55+ DCs and SIV-infected cells colocalized in the paracortex, but we were not able to detect cells that were SIV+ and p55+, indicating that DCs in the sections examined were not productively infected (Figure 8C,D). The close proximity of IDCs and SIV+ cells in the paracortex is consistent with infected cells being T cells.9,18 35 No SIV+ cells could be identified in skin sections by in situ hybridization in animals acutely infected with SIV or with AIDS (data not shown). These data indicate that SIV infection alters IDC activation and LC migration in vivo by an indirect mechanism.
IDCs are closely associated with SIV-infected cells in the lymph node paracortex.
(A-B) In situ hybridization for SIV RNA using antisense (A) and control (B) sense probes on a lymph node of a monkey with AIDS. (C) In situ hybridization for SIV RNA (black) was combined with immunohistochemistry for p55 protein (brown) to identify IDCs. Arrow identifies SIV RNA in a follicle. (D) Higher power image of the boxed area in panel C highlighting p55+ IDCs in close proximity to SIV-infected cells (arrows). F indicates follicle. Ab staining was detected using streptavidin-peroxidase with diaminobenzidine substrate. Tissues were counterstained with hematoxylin. Original magnification × 100 (A,B), × 200 (C), and × 400 (D).
IDCs are closely associated with SIV-infected cells in the lymph node paracortex.
(A-B) In situ hybridization for SIV RNA using antisense (A) and control (B) sense probes on a lymph node of a monkey with AIDS. (C) In situ hybridization for SIV RNA (black) was combined with immunohistochemistry for p55 protein (brown) to identify IDCs. Arrow identifies SIV RNA in a follicle. (D) Higher power image of the boxed area in panel C highlighting p55+ IDCs in close proximity to SIV-infected cells (arrows). F indicates follicle. Ab staining was detected using streptavidin-peroxidase with diaminobenzidine substrate. Tissues were counterstained with hematoxylin. Original magnification × 100 (A,B), × 200 (C), and × 400 (D).
Discussion
In this study, we characterized the effects of SIV on DC populations in monkeys at different stages of infection. We focused on LCs, which represent immature antigen-capturing DCs within the periphery, and IDCs, the mature antigen-presenting counterpart in secondary lymphoid tissues. We found that the capacity for immature DCs to migrate from peripheral tissues and localize in draining lymph nodes was significantly impaired during AIDS. Moreover, IDCs in monkeys with AIDS were poorly activated in situ and were refractory to activation in vitro. These results suggest that there is a systemic defect in the normal homeostasis of immunostimulatory DCs during AIDS.
The LCs from SIV-infected monkeys had a characteristic immature phenotype when freshly isolated from skin and responded to in vitro stimuli by up-regulating costimulatory and activation molecules and undergoing a switch in chemokine receptor expression. Despite the normal response to maturation stimuli, and a normal density of LCs in skin, there was a significant impairment in LC migration in monkeys with AIDS, associated with a decreased proportion of antigen-bearing DCs in lymph nodes. These data indicate that there was no inherent defect in the capacity of LCs to mature and suggest that the microenvironment of the skin may be important in suppressing LC migration. The lack of migration was not due to a lack of constitutive chemokines, because in situ hybridization analyses revealed normal levels of CCL19 and CCL21 in lymph node paracortical regions (data not shown). Using the same model of FITC sensitization in the mouse, Gabrilovich et al showed that infection with Rauscher leukemia virus, an immunosuppressive retrovirus, can similarly suppress LC migration from skin.23 These workers suggested that the block in LC migration was due to a down-regulation of adhesion molecule expression, although this was minimal.23 In the murine model of schistosomiasis, the presence of Schistosoma mansoniparasites within the epidermis impedes tumor necrosis factor α–triggered migration of LCs through production of prostaglandins.36 In HIV infection, virus-infected cells could potentially impair LC migration from skin through the effects of viral gp120 on immature DCs.37 However, we were not able to detect virus-infected cells in the skin of monkeys with AIDS, consistent with reports in the HIV literature,38 39 and a direct role of SIV in impairing LC migration seems unlikely.
The finding of decreased LC migration from skin, together with a decrease in antigen-bearing DCs in draining lymph nodes, prompted a thorough characterization of lymph node IDCs during SIV infection. Our results indicate that SIV infection has contrasting effects on IDCs depending on the stage of infection. During acute SIV infection, there was an increase in the proportion of CD83+ IDCs in lymph nodes as compared to naive controls, associated with reduced LC density in skin, suggesting mobilization of DCs to lymph nodes. This was supported by intense staining of the paracortex with antisense probes to CD83 RNA. The increased intensity of staining in tissues was not the result of an increase in CD83 expression on individual IDCs, because expression of CD83 on HLA-DRhi p55+ cells was not increased above that of normal monkeys. The recruitment of DCs to lymph nodes may reflect the normal innate immune response to the acute phase of a systemic viral infection, which would serve to maximize stimulation of antigen-specific T cells. Although the proportion of IDCs in lymph nodes of monkeys with AIDS was not statistically different from that of healthy monkeys, there was a significant down-regulation of activation and costimulatory molecules on IDCs in this group. Notably, IDC expression of CD83 was virtually absent in tissues and was decreased by more than 30% as measured by flow cytometry. CD83 was recently characterized as an adhesion molecule of the Siglec (sialic acid-binding Ig-like lectin) family, suggesting a role in intercellular communication.40 Our findings are in agreement with those of a previous study in HIV-infected patients, which indicated that HLA-DR+ lineage− DCs of spleens did not up-regulate CD83 expression following overnight culture, in contrast to normal donors.19 Although the expression of HLA-DR and CD86 on IDCs was not altered during AIDS, the reduction in CD40 expression had clear functional consequences, as determined by a suppressed activation response to CD40L treatment. This finding is particularly significant given that the majority of IDCs in the lymph node during AIDS is nonactivated, and therefore should readily respond to stimulation through CD40L. It is likely that the changes observed in lymph node DCs result at least in part by reduced migration of LCs from the periphery, given that antigen-exposed LCs localize to the lymph node paracortex as activated IDCs.
The mechanism whereby SIV infection impairs LC migration and IDC activation is currently not known. Infection of DCs with several pathogens, including Trypanosoma cruzi and herpes simplex virus, has been shown to directly inhibit DC maturation.41,42 Productive infection of DCs with HIV has been reported by several investigators43,44; however, the proportion of infected DCs in tissues during AIDS is relatively minor,9,35,45 as we found in our study. Hence, direct infection of DCs with SIV is unlikely to explain the broad effects on epidermal and lymph node DCs seen during AIDS. Numerous SIV-infected cells, presumed to be T cells, were closely associated with IDCs in lymph nodes, and consequently direct contact between infected cells and DCs could affect DC activation. Indeed, adherence of Plasmodium falciparum–infected erythrocytes to DCs has been shown to directly impair DC maturation and immunostimulatory function.46 However, such interaction could not account for the profound disruption to LC migration, given the lack of SIV-infected cells in the skin. In a model of measles virus infection in mice, viral proteins, in the absence of viral replication, suppressed DC function by binding to various cell surface receptors.47 However, although the surface glycoprotein gp120 of HIV interacts with several receptors on DC, there is no indication that gp120 directly impairs DC function, other than inducing DC chemotaxis.37 A more plausible scenario to explain the observed defect in DC homeostasis during AIDS may be the effect of immunosuppressive cytokines. Up-regulation of interleukin 10 (IL-10) production by HIV-infected monocytes has been demonstrated48 and been proposed to play an important indirect role in HIV immunopathogenesis.49 IL-10 treatment of cultured DCs strongly reduces their immunostimulatory function, associated with inhibition of expression of CD83.50Importantly, monkeys with AIDS have been shown to have increased levels of IL-10 RNA in lymph nodes as compared to controls,51supporting a role for IL-10 in suppressing IDC activation. Moreover, IL-10 knockout mice have enhanced migration of epidermal LCs from skin in response to contact sensitization,52 suggesting that increased levels of IL-10 in AIDS may impair LC migration in vivo. To address this hypothesis further, we are currently characterizing the cytokine milieu in the skin of monkeys with AIDS.
The defects to DC populations in skin and lymph nodes described here could play a role in the immunopathogenesis of AIDS in a number of important ways. LCs present in epithelial tissues may encounter invading opportunistic pathogens normally, but antigen-loaded DCs will not leave the epithelium appropriately for trafficking to draining lymph nodes. Hence, the number of IDCs available for stimulation of antigen-specific T cells could be greatly reduced. The decreased activation state of IDCs and the lack of responsiveness to CD40L could also significantly impair T-cell stimulation. Activation through CD40 by T-helper cells is essential to condition DCs for effective priming of cytotoxic T cells.53-55 The lack of CD4 T cells in lymph nodes during AIDS18 would only exacerbate this defect by providing less opportunity for CD40 ligation on IDCs. The findings also have broader implications for the immunotherapy of AIDS, particularly those strategies that target DCs in situ, such as cutaneous immunization with DNA.56 DNA vaccines have shown promise in protecting monkeys against mucosal virus challenge.57,58 Given our findings, this approach may not be optimal for therapeutic vaccination during AIDS because transfected DCs may not migrate from skin or be activated appropriately in draining lymph nodes. It will be critical to determine if the disruption to DC homeostasis is corrected by treatment with antiretroviral drugs, which will likely be part of any immunotherapeutic regimen for AIDS. Importantly, the destruction to the follicular DC network in lymph nodes noted during AIDS in humans is reversed by therapy with antiretroviral drugs.59
We would like to thank Larry A. Harshyne for his valuable comments; Dawn C. McClemens for monkey samples; Beth Fallert for advice with in situ hybridization experiments; Ian Bell and Todd Schaefer for help with the CD83 cloning strategy; Simon C. Watkins for image analysis; Lijun Wu for providing Ab to CCR7; and Immunex for providing CD40L. mAb to DC-SIGN was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health, from F. Baribaud, S. Pohlmann, J. A. Hoxie, and R. W. Doms.
Supported by National Institutes of Health grants AI43664 and RR00119 to S.M.B.-B., AI43916 to L.D.F. Jr, and HL62056 to T.A.R. A.T.L. is a fellow of the Dermatology Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Simon M. Barratt-Boyes, Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, 15261; e-mail: smbb+@pitt.edu.