Abstract
Hepatocyte growth factor (HGF), a heparin-binding factor, is synthesized as a single-chain inactive precursor (pro-HGF), which is converted by proteolysis to an active heterodimer (mature HGF). HGF has pleiotropic activities and has been implicated in the regulation of mitogenesis, motogenesis, and morphogenesis of epithelial and endothelial cells. As polymorphonuclear neutrophils (PMNs) secrete numerous cytokines involved in the modulation of local inflammation, we investigated their ability to produce HGF. We found that HGF was stored in secretory vesicles and in gelatinase/specific granules. This intracellular stock was rapidly mobilized by degranulation when neutrophils were stimulated with phorbol myristate acetate or N-formylmethionyl-leucyl-phenylalanine. Cycloheximide did not affect the release of HGF. Moreover, HGF messenger RNA and protein expression was found in bone marrow myeloid cells, suggesting that HGF synthesis likely occurs during PMN maturation. In mature circulating PMNs, intracellular HGF was in the pro-HGF form, whereas the HGF secreted by degranulation was the mature form. Furthermore, PMNs pretreated with diisopropyl fluorophosphate only released the pro-HGF form, suggesting that PMN-derived serine protease(s) are involved in the proteolytic process. We also obtained evidence that secreted mature HGF binds PMN-derived glycosaminoglycans (probably heparan sulfate). These findings suggest that PMNs infiltrating damaged tissues may modulate local wound healing and repair through the production of HGF, a major mediator of tissue regeneration.
Introduction
Hepatocyte growth factor (HGF), a heparin-binding factor, was originally described as a potent mitogen for the growth of hepatocytes in vitro and was subsequently purified from rat platelets,1 plasma of a patient with fulminant hepatic failure,2 and normal human plasma.3 HGF has mitogenic, motogenic, and morphogenic effects on various epithelial and endothelial cell types.4,5 Many studies have demonstrated that embryogenesis, angiogenesis, hematopoiesis, as well as wound healing and organ regeneration, are critically controlled by HGF.5-7 HGF is secreted as a 92-kd single-chain pro-HGF that requires endoproteolytic processing. This processing is mediated by a serine protease. The HGF activator,8 blood coagulation factor XII,9 urokinase,10 and tissue-type plasminogen activator11 are reported to activate HGF. Processing of HGF results in a bioactive form (mature HGF) consisting of a disulfide-linked 69-kd α chain and a 32-kd β chain.12
The biologic effects of HGF are mediated by the activation of its high-affinity binding site known as the tyrosine kinase receptor c-met.13 Besides c-met, HGF possesses lower-affinity/high-capacity binding sites corresponding to extracellular matrix molecules (glycosaminoglycans or collagen) or to cell surface–associated heparan sulfate14,15; this correspondence creates a molecular reservoir of HGF on the cell surface, whereas HGF transfer to c-met initiates the cellular response.16 The broad range of HGF activities on many physiologic and pathologic processes is reflected by c-met expression in a variety of organs and cell types. The finding that c-met receptor expression is induced by inflammatory cytokines17 suggests that the HGF/c-met system is involved in inflammatory responses to tissue injury.
Polymorphonuclear neutrophils (PMNs), the predominant tissue infiltrating cells during acute inflammatory process, participate in host defenses by generating reactive oxygen species and releasing proteolytic enzymes. Human PMNs can synthesize and secrete many proteins that influence the course of inflammatory responses and contribute to the modulation of local inflammation.18,19After reports that human liver and bone marrow PMNs contain immunoreactive HGF,20 21 we investigated the regulation of HGF production by human blood PMNs, together with the subcellular localization and molecular form of HGF in these cells.
Materials and methods
Purification of blood PMNs and bone marrow myeloid cells
Human PMNs were purified as previously described.18Briefly, blood PMNs from healthy volunteers were isolated in sterile conditions by sedimentation in medium containing 9% Dextran T-500 (Amersham Pharmacia Biotech, Uppsala, Sweden) and 38% Radioselectan (Schering, Lys-lez-Lannoy, France), and the leukocyte suspension was centrifuged on Ficoll-Paque medium (Amersham Pharmacia Biotech). The cell pellet was washed with phosphate-buffered saline (PBS), and erythrocytes were removed by hypotonic lysis. PMNs were further purified by 20-minute incubation with pan antihuman HLA class II–coated magnetic beads (Dynal, Oslo, Norway) to deplete B lymphocytes, activated T lymphocytes, and monocytes. Nonspecific esterase staining always showed less than 0.5% of monocytes, and flow cytometry showed the absence of CD14+, CD3+, and CD19+ cells, confirming the recovery of highly purified PMNs. Two bone marrow samples were obtained from healthy allograft donors. Mononuclear cells had been removed by Ficoll density gradient centrifugation. These depleted samples were sedimented in medium containing 9% Dextran T-500 and 38% Radioselectan. The leukocyte-rich supernatant was centrifuged, and the cell pellet was washed with PBS. Erythrocytes were removed by hypotonic lysis. The distribution of neutrophil precursors was determined by differential cell counting of cytospin preparations from each donor sample, as follows: promyelocytes, 2% and 3%; myelocytes, 11% and 14%; metamyelocytes, 34% and 23%; band and segmented cells, 53% and 60%. In both preparations, myeloblasts, monocytes, and lymphocytes represented less than 1% of cells.
Immunocytochemical staining of HGF
Blood and bone marrow smears from healthy control subjects were air-dried for 24 hours and fixed in 4% formaldehyde in PBS for 20 minutes and permeabilized by incubation in PBS containing 0.1% Triton X-100 at room temperature for 30 minutes.22 Smears were first incubated with normal horse serum for 30 minutes to block nonspecific binding. The primary antibody, an antihuman-HGF monoclonal antibody (12.5 μg/mL) (MAB294, clone 24 612.111; R&D Systems, Abingdon, United Kingdom), was diluted in PBS containing 0.3% gelatin and added for 1 hour at room temperature. The slides were then incubated with a biotinylated antibody and then with alkaline phosphatase–labeled streptavidin according to the manufacturer's instructions (Vectastain ABC kit; Vector Laboratories, Burlingame, United Kingdom). Staining was completed by incubation with a substrate chromogen solution (Fast red substrate solution; DAKO, Carpinteria, CA). After washing, the slides were counterstained in Mayer hematoxylin, mounted, and examined with a light microscope. Positive staining developed as a red-colored reaction product. Smears incubated with a nonspecific mouse immunoglobulin of the same isotype (10 μg/mL; R&D Systems) served as negative controls.
Subcellular fractionation of blood PMNs
Specific and azurophilic granules were purified as previously described.23 Briefly, purified neutrophils (100 × 106) in 5 mL ice-cold relaxation buffer (100 mM KCl, 3 mM NaCl, 1 mM Na2ATP, 3.5 mM MgCl2, 10 mM PIPES, pH 7.2) supplemented with EGTA and antiproteases were pressurized with N2 for 20 minutes at 450 psi with constant stirring in a nitrogen bomb. The cavitate was then collected dropwise into EGTA, pH 7.4, sufficient for a final concentration of 1.25 mM. Nuclei and unbroken cells were pelleted by centrifugation of the cavitate at 400g for 15 minutes. The supernatant was decanted, loaded on top of a 2-layer Percoll gradient (1.05/1.12 g/mL), precooled to 4°C, and centrifuged at 4°C for 30 minutes at 40 000g. Three visible bands were identified: a lower band containing azurophil granules, an intermediate band containing specific and gelatinase granules, and an upper band containing plasma membranes and secretory vesicles. The cytosol remained above the upper band. The different fractions were collected, and the purity of specific and azurophilic granule fractions was assessed by enzyme-linked immunosorbent assay (ELISA) measurement of their respective markers (lactoferrin and myeloperoxidase) in each fraction and in the total cavitate.
Degranulation experiments and purification of PMN-derived HGF
Pure PMNs (107/mL) were resuspended in Hanks balanced salt solution (HBSS with Ca++/Mg2+; Life Technologies, Cergy Pontoise, France). Part of the cells (unstimulated control PMNs) were kept for 20 minutes in medium alone, on ice, or at 37°C; the other part was preincubated at 37°C for 20 minutes with the following degranulating agents: lipopolysaccharide at 1 μg/mL (LPS from Escherichia coli 055:B5; Sigma, St Louis, MO), 10−6 M and 10−8 M N-formylmethionyl-leucyl-phenylalanine (fMLP; Sigma), or phorbol myristate acetate at 100 ng/mL (PMA; Sigma). In some experiments, to ensure total degranulation, PMNs were preincubated with 5 μg/mL cytochalasin B (Sigma) for 5 minutes, then with 10−6 M fMLP for 15 minutes at 37°C. In another set of experiments, to inhibit serine proteases, neutrophils were preincubated with 2 mM diisopropyl fluorophosphate (DFP; Sigma) for 15 minutes at 4°C and then treated as indicated above with cytochalasin B and fMLP. All tubes were then centrifuged, and the cell-free supernatants were stored at −80°C until HGF quantitation and Western blot analysis.
For HGF purification, the cell-free supernatant of PMNs stimulated with cytochalasin B + fMLP was applied to a Hi-Trap heparin affinity column (Amersham Pharmacia Biotech); the starting washing buffer was 0.02 M Tris, 0.2 M NaCl, pH 7.5, and the eluting buffer was 0.02 M Tris, 2 M NaCl, pH 7.5. Each harvested fraction (1 mL) was dialyzed against distilled water and assayed for HGF with an ELISA method.
Blood PMN culture
Pure PMNs (107/mL) were cultured for 24 hours at 37°C with 5% CO2 in 24-well tissue-culture plates (Costar, Cambridge, MA). The culture medium was RPMI 1640 (Sigma) supplemented with 2 mM glutamine and antibiotics. To avoid contaminating HGF and/or protease activities,24 all culture media were free of fetal calf serum. Neutrophils were cultured with or without the following stimulating agents: LPS at 1 μg/mL, fMLP at 10−6 M, PMA at 100 ng/mL, and interleukin-1β (IL-1β) at 10 ng/mL (Immugenex, Los Angeles, CA). To evaluate de novo HGF synthesis, PMNs (107/mL) were preincubated with or without 10 μg/mL cycloheximide (Sigma) for 30 minutes at 37°C and further incubated with the stimulating agents for 24 hours. Cell-free PMN culture supernatants were then collected and stored at −80°C until HGF assay.
HGF, lactoferrin, and myeloperoxidase assays
HGF was quantified by using a commercial ELISA kit (Quantikine; R&D Systems) with a detection limit of 40 pg/mL. As indicated by the manufacturer, both pro-HGF and mature HGF were recognized by this assay. Lactoferrin and myeloperoxidase were quantified by using ELISA kits (Oxis International, Portland, OR; detection limits 1 ng/mL), following the manufacturer's instructions.
Western blot analysis
Purified subcellular fractions of PMN granules, supernatants from cytochalasin B + fMLP–stimulated PMNs, or recombinant human HGF (rh-HGF, a mixture of pro-HGF and mature HGF according to the manufacturer; R&D Systems) were added to 2× Laemmli sample buffer, with or without 2-mercaptoethanol as reducing agent. Proteins were then resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA), the proteins were immunoblotted. Nonspecific sites were blocked by overnight immersion in 10% nonfat dried milk, and the membranes were then probed with a mixture (1:1) of 2 monoclonal anti-HGF antibodies (0.5 μg/mL) both recognizing the α chain (MAB694 clone 24 516.1 and MAB294 clone 24 612.111; R&D Systems) or, in one set of experiments, with a monoclonal antiheparan sulfate antibody (5 μg/mL) (Seikagaku, Tokyo, Japan).25The immunoblots were developed by using an enhanced chemiluminescence method (Amersham Pharmacia Biotech) following the manufacturer's instructions. In selected experiments, before Western blot analysis, supernatants from cytochalasin B + fMLP–stimulated PMNs were incubated in 2 M NaCl, 0.02 M Tris, pH 7.5 for 30 minutes at 37°C to disrupt ionic interactions.26 To check their specificity, the anti-HGF antibodies (5 μg) were preincubated with or without rh-HGF (1 μg) for 1 hour at room temperature before probing the membranes. The intensities of HGF bands (pro-HGF and α chain) decreased when the primary antibodies were preincubated with rh-HGF.
Treatment of pro-HGF from specific granules with serine proteases
Aliquots of purified specific granules (containing 1 ng pro-HGF as determined by Western blot analysis and ELISA) were incubated at 37°C for 10 and 60 minutes with either 0.1 to 100 IU urokinase-type plasminogen activator (uPA)/ng pro-HGF in 20 mM phosphate buffer pH 7.4,11 or with 1 to 100 pmol elastase/ng pro-HGF in HBSS pH 7.5,27 or with 1 to 100 pmol cathepsin G/ng pro-HGF in HBSS pH 7.5.27 Elastase and cathepsin G were kindly provided by M. Chignard from Institut Pasteur-INSERM 285 (Paris, France); uPA was from Hoechst (Paris La Defense, France). After incubation, samples were immediately added to 2× Laemmli sample buffer with 2-mercaptoethanol as reducing agent and analyzed by Western blotting as detailed above.
Reverse transcriptase–polymerase chain reaction
Purified blood PMNs and bone marrow cells prepared as described above were harvested and analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR) for HGF messenger RNA (mRNA) expression. In selected experiments, highly purified PMNs were incubated for 1 hour in 2 mL culture medium with or without 1 μg/mL LPS, 100 ng/mL PMA, 10−6M fMLP, or 10 ng/mL IL-1β as stimulating agents. Total cellular RNA was isolated with Trizol (Life Technologies) according to the manufacturer's instructions. Total RNA (4 μg) was reverse transcribed in reverse transcriptase reaction buffer (10 mM MgCl2, 50 mM KCl, 50 mM Tris-HCl pH 8.3) with 25 μg/mL oligo-dT primer, 1 mM each deoxynucleotide triphosphate (Life Technologies), 1 U/μL Rnasin, and 0.5 U/μL AMV reverse transcriptase (Promega, Madison, WI). Samples were incubated for 5 minutes at 65°C and then at 42°C for 60 minutes. A specific pair of primers designed for HGF (Life Technologies) was then added to each complementary DNA reaction mix; the sense primer was 5′-TCCCCATCGCCATCCCC-3′ and the antisense primer was 5′-CACCATGGCCTCGGCTGG-3′.28 Amplification was performed in a solution containing 2 mM MgCl2, 1× Taq polymerase buffer, 0.2 mM each deoxynucleotide triphosphate, 5% dimethyl sulfoxide (vol/vol), 25 U/mL Taq polymerase AmpliTaq Gold (Perkin Elmer, Foster City, CA), and 0.4 μmol/L specific primer, in an automated thermal cycler (Uno II; Biometra, Maidstone, United Kingdom) at 94°C for 30 seconds, 68°C for 30 seconds, and 72°C for 40 seconds. Amplification was stopped after 35 cycles. The MRC-5 human embryonic lung fibroblast line served as a positive control for HGF gene expression,29 and water was used instead of RNA as a negative control of RT-PCR. The housekeeping gene porphobilinogen deaminase (PBGD) was amplified in each sample to confirm the integrity of RNA and the efficiency of complementary DNA synthesis. The expected PCR products of 749 (HGF) and 338 (PBGD) base pairs were detected by electrophoresis in 2% agarose gel containing ethidium bromide, along with molecular weight standards (Roche Diagnostics, Mannheim, Germany).
Results
PMNs contain an intracellular pool of pro-HGF
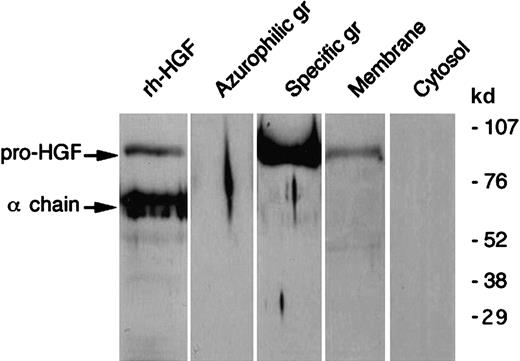
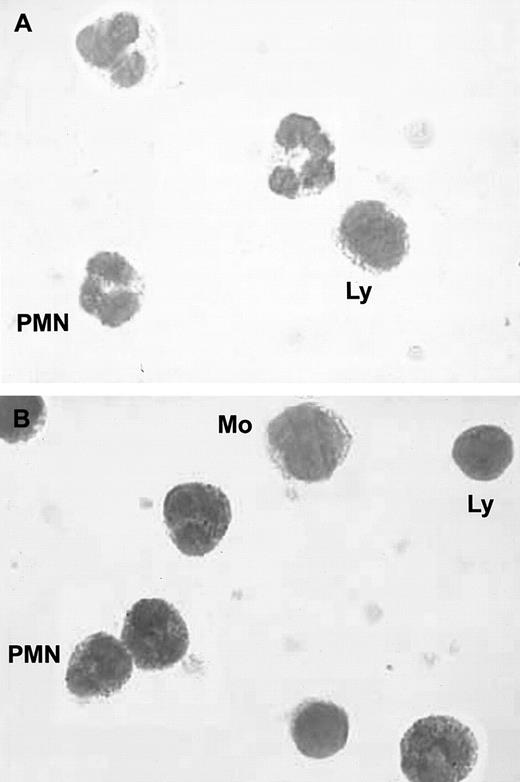
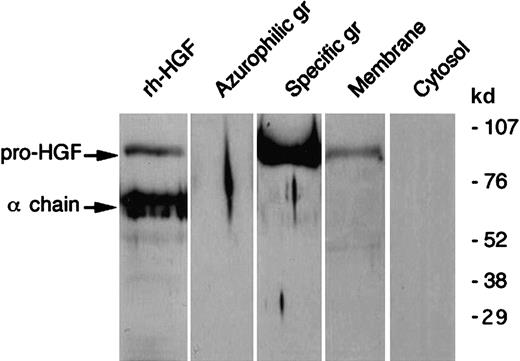
As shown in Figure 1, immunocytochemistry revealed the presence of intracellular HGF in unstimulated blood PMNs. To determine the precise localization of HGF, HGF, lactoferrin, and myeloperoxidase were determined in subcellular fractions. HGF was mainly located in the gelatinase/specific granules fraction and, to a lesser extent, the plasma membrane fraction (Table1); the distribution of HGF (ELISA) matched that of lactoferrin but not that of myeloperoxidase. Western blot analysis confirmed the subcellular localization of HGF (Figure2). We observed a distinct 92-kd band in the gelatinase/specific granules fraction and a weak band in the plasma membrane fraction, both migrating at the level of pro-HGF. HGF was not detected in azurophilic granules or in cytosol fractions. As Western blot analysis was conducted in reducing conditions, these results indicate that HGF was present in PMNs as the pro-HGF single-chain form.
Immunocytochemical staining of blood PMNs.
(A) Negative control; no staining was observed with a control immunoglobulin. (B) Intracellular red staining was observed in PMNs with specific monoclonal anti-HGF antibodies; smears were examined by light microscopy at ×800; (Ly indicates lymphocytes; Mo, monocytes).
Immunocytochemical staining of blood PMNs.
(A) Negative control; no staining was observed with a control immunoglobulin. (B) Intracellular red staining was observed in PMNs with specific monoclonal anti-HGF antibodies; smears were examined by light microscopy at ×800; (Ly indicates lymphocytes; Mo, monocytes).
Western blot analysis of HGF in human neutrophil subcellular fractions.
Human neutrophil subcellular fractions were prepared as described in “Materials and methods”; proteins from each fraction (azurophil and specific granules (gr), membranes and cytosol) were separated by SDS-PAGE in reducing conditions and revealed with antihuman HGF monoclonal antibodies; rh-HGF (a mixture of pro-HGF and mature HGF) was loaded and blotted in parallel. The molecular masses of protein standards are indicated. These data are representative of one typical experiment of 5.
Western blot analysis of HGF in human neutrophil subcellular fractions.
Human neutrophil subcellular fractions were prepared as described in “Materials and methods”; proteins from each fraction (azurophil and specific granules (gr), membranes and cytosol) were separated by SDS-PAGE in reducing conditions and revealed with antihuman HGF monoclonal antibodies; rh-HGF (a mixture of pro-HGF and mature HGF) was loaded and blotted in parallel. The molecular masses of protein standards are indicated. These data are representative of one typical experiment of 5.
PMNs release HGF by degranulation
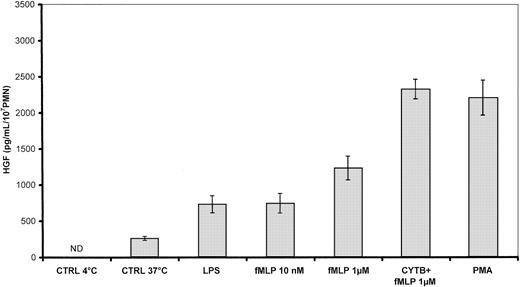
As shown in Figure 3, PMN stimulated with 1 μg/mL LPS, 10−8 and 10−6M fMLP, the combination of cytochalasin B + 10−6 M fMLP, or 100 ng/mL PMA for 20 minutes at 37°C released a large amount of HGF (730 ± 120, 741 ± 142, 1 234 ± 165, 2 322 ± 141, and 2 202 ± 246 pg/mL/107 PMN, respectively) in comparison with unstimulated control cells maintained at 37°C (261 ± 30 pg/mL/107 PMNs) or at 4°C (< 40 pg/mL).
Effect of degranulating agents on HGF secretion by human PMNs.
Secreted HGF was measured in supernatants of PMNs (107/mL) incubated for 20 minutes at 37°C with 1 μg/mL LPS, 100 ng/mL PMA, 10−8 M, and 10−6 M fMLP. When indicated, PMNs were preincubated with 5 μg/mL cytochalasin B (CYTB). Unstimulated control cells (CTRL) were incubated at 4°C or 37°C in the medium alone. Results are expressed as the mean ± SEM of 3 independent experiments. ND indicates not detected (< 40 pg/mL).
Effect of degranulating agents on HGF secretion by human PMNs.
Secreted HGF was measured in supernatants of PMNs (107/mL) incubated for 20 minutes at 37°C with 1 μg/mL LPS, 100 ng/mL PMA, 10−8 M, and 10−6 M fMLP. When indicated, PMNs were preincubated with 5 μg/mL cytochalasin B (CYTB). Unstimulated control cells (CTRL) were incubated at 4°C or 37°C in the medium alone. Results are expressed as the mean ± SEM of 3 independent experiments. ND indicates not detected (< 40 pg/mL).
To investigate the ability of PMNs to synthesize HGF de novo, purified blood PMNs were stimulated, in the presence or absence of cycloheximide, with LPS, PMA, fMLP, or IL-1, which induce cytokine synthesis in PMNs.30 After 24 hours, unstimulated control PMNs cultured in medium alone showed spontaneous HGF release that was not affected by the presence of cycloheximide (Table2). Each stimulating agent slightly increased HGF concentrations in PMN supernatants as compared with unstimulated PMNs. However, whatever the stimulus, cycloheximide treatment did not significantly modify the amounts of HGF detected after 24 hours of stimulation, suggesting that the HGF released in the culture supernatants was mainly derived from degranulation induced by LPS, PMA, fMLP, or IL-1. Similar results were obtained when PMNs were cultured with tumor necrosis factor α (100 U/mL) or granulocyte-macrophage colony-stimulating factor (100 U/mL) (data not shown).
In agreement with the absence of active HGF synthesis, a very low level of HGF mRNA was detected in unstimulated mature PMNs (Figure4), and none of the stimulating agents (LPS, PMA, fMLP, or IL-1) modified the HGF mRNA level compared with unstimulated PMNs (densitometric analysis, in comparison with the housekeeping gene PBGD; data not shown).
RT-PCR analysis of HGF mRNA expression by bone marrow cells and mature PMNs.
Total RNA was extracted from bone marrow (BM) cells (previously depleted from their mononuclear cells) and from mature PMNs (PMN). After reverse transcription, PCR was carried out with specific pairs of primers designed for HGF and PBGD. Unstimulated MRC-5 human embryonic lung fibroblasts served as positive controls (+). The figure indicates the size of amplification products relative to molecular weight standards run in parallel (M) and the negative control (−).
RT-PCR analysis of HGF mRNA expression by bone marrow cells and mature PMNs.
Total RNA was extracted from bone marrow (BM) cells (previously depleted from their mononuclear cells) and from mature PMNs (PMN). After reverse transcription, PCR was carried out with specific pairs of primers designed for HGF and PBGD. Unstimulated MRC-5 human embryonic lung fibroblasts served as positive controls (+). The figure indicates the size of amplification products relative to molecular weight standards run in parallel (M) and the negative control (−).
Expression of HGF in human bone marrow myeloid cells
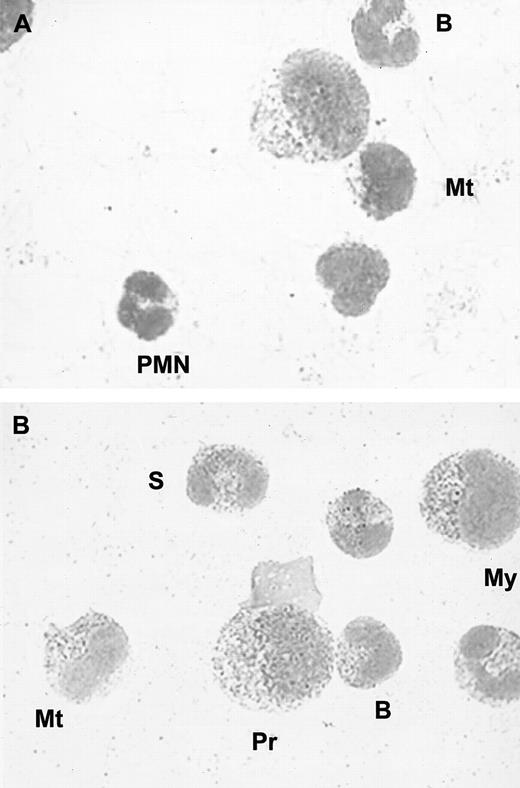
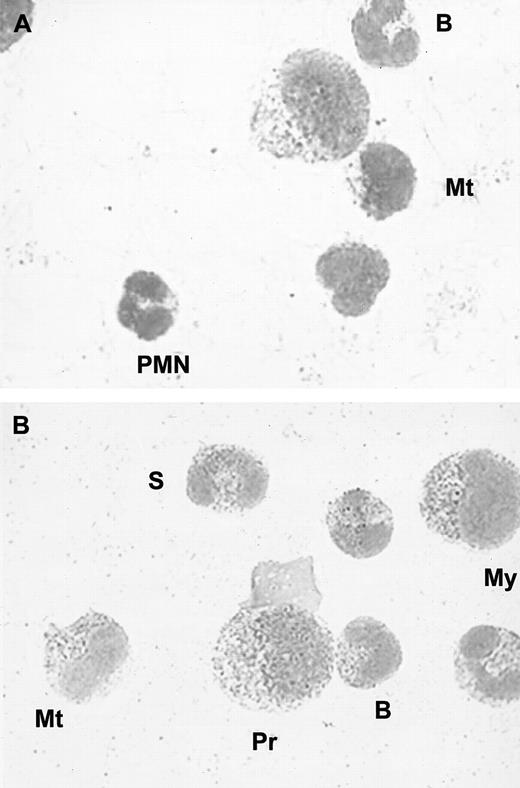
As most of the proteins stored in PMN granules are actively synthesized during granulocyte maturation, we investigated whether HGF protein and mRNA could be detected in bone marrow myeloid cells. We first performed immunocytochemical analysis of normal human bone marrow smears (n = 3). We found strong positive staining in myelocytes, metamyelocytes, band, and segmented cells in all samples. Weak to moderate staining was found in promyelocytes, whereas lymphocytes, monocytes, and megakaryocytes were negative for HGF (Figure5).
Immunocytochemical staining of bone marrow cells.
(A) Negative control; no staining was observed with a control immunoglobulin. (B) Intracellular red staining was observed with specific monoclonal anti-HGF antibodies; (Pr indicates promyelocyte; My, myelocyte; Mt, metamyelocyte; B, band cell; S, segmented cell). Smears were examined by light microscopy at × 800.
Immunocytochemical staining of bone marrow cells.
(A) Negative control; no staining was observed with a control immunoglobulin. (B) Intracellular red staining was observed with specific monoclonal anti-HGF antibodies; (Pr indicates promyelocyte; My, myelocyte; Mt, metamyelocyte; B, band cell; S, segmented cell). Smears were examined by light microscopy at × 800.
Mononuclear cell–depleted bone marrow samples and circulating purified PMNs were subjected to RT-PCR in the same run. HGF mRNA expression was found in bone marrow samples but was only weakly positive in mature circulating PMNs (Figure 4). By using densitometric analysis in comparison with the housekeeping gene PBGD, we found that the relative intensity was higher in bone marrow cells than in mature circulating PMNs (data not shown).
Taken together, these results show that HGF mRNA and protein are present in neutrophil precursors and suggest that HGF synthesis occurs during PMN maturation.
PMNs process pro-HGF to mature HGF in vitro
Western blot analysis in reducing conditions was used to characterize the HGF form released by PMNs into the extracellular medium. As shown in Figure 6, HGF released by cytochalasin B + fMLP–stimulated PMNs was mainly the mature form (presence of the 69-kd α chain). As the processing of pro-HGF to mature HGF involves serine proteases, the same degranulating experiment was conducted with an additional preincubation step of 15 minutes with 2 mM DFP, used to irreversibly inhibit neutrophil serine proteases. HGF secreted by DFP-pretreated PMNs was exclusively in the pro-HGF form, as shown by the sole detection of the 92-kd band by Western blotting in reducing conditions (Figure 6). These results indicated that the pro-HGF contained in PMNs was processed to mature HGF by neutrophil serine protease(s) during degranulation. We, thus, investigated the effects of elastase and cathepsin G, which are the main PMN serine proteases; uPA, which is present in specific granules31 and processes pro-HGF,11 was also tested. As shown in Figure 7, none of these agents were able to catalyze the conversion of pro-HGF from specific granules, whatever the incubation period (from 10 to 60 minutes) or concentration (elastase and cathepsin G, from 1 to 100 pmol/ng pro-HGF; uPA, from 0.1 to 100 IU/ng pro-HGF).
Western blot analysis of HGF released after PMN degranulation: effect of DFP.
PMNs were incubated with 2 mM DFP (DFP+) or without DFP (DFP−) for 15 minutes at 4°C then stimulated with cytochalasin B and fMLP at 37°C; the supernatant was then subjected to Western blot analysis in reducing conditions and revealed with antihuman HGF monoclonal antibodies. These data are representative of one experiment of 3.
Western blot analysis of HGF released after PMN degranulation: effect of DFP.
PMNs were incubated with 2 mM DFP (DFP+) or without DFP (DFP−) for 15 minutes at 4°C then stimulated with cytochalasin B and fMLP at 37°C; the supernatant was then subjected to Western blot analysis in reducing conditions and revealed with antihuman HGF monoclonal antibodies. These data are representative of one experiment of 3.
Effects of serine proteases on pro-HGF from specific granules.
Aliquots of purified specific granules were incubated at 37°C for 10 and 60 minutes with 10 pmol elastase/ng pro-HGF (E10, E60), 10 pmol cathepsin G/ng pro-HGF (C10, C60), or 10 IU uPA/ng pro-HGF (U10, U60); specific granules (gr) incubated in the medium alone for 60 minutes served as control. Proteins were then subjected to Western blot analysis in reducing conditions and revealed with antihuman HGF monoclonal antibodies. These data are representative of one experiment of 3.
Effects of serine proteases on pro-HGF from specific granules.
Aliquots of purified specific granules were incubated at 37°C for 10 and 60 minutes with 10 pmol elastase/ng pro-HGF (E10, E60), 10 pmol cathepsin G/ng pro-HGF (C10, C60), or 10 IU uPA/ng pro-HGF (U10, U60); specific granules (gr) incubated in the medium alone for 60 minutes served as control. Proteins were then subjected to Western blot analysis in reducing conditions and revealed with antihuman HGF monoclonal antibodies. These data are representative of one experiment of 3.
Secreted HGF binds PMN-derived heparan sulfate
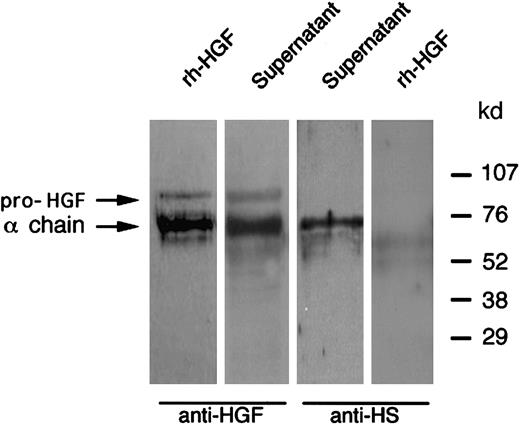
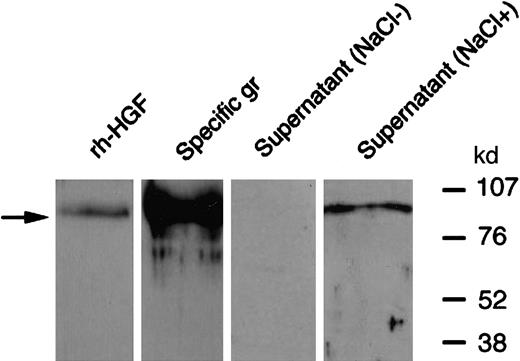
As HGF is a heparin-binding factor,32 we investigated whether HGF secreted by PMNs could link to glycosaminoglycans. First, in an attempt to purify PMN-derived HGF, the cell-free supernatant of cytochalasin B + fMLP–stimulated PMNs was submitted to heparin affinity chromatography. Contrary to control rh-HGF, HGF secreted by PMNs was not retained on the heparin column (ELISA; data not shown), suggesting that HGF binding sites for heparin were not available. Second, contrary to HGF purified from specific granules, Western blot analysis in nonreducing conditions of HGF secreted by PMNs failed to yield a specific HGF band (Figure 8). This latter result suggests that HGF epitopes were inaccessible to anti-HGF antibodies because of the presence of masking substances. As a high salt concentration is commonly used to release HGF from polyanionic substances such as glycosaminoglycans,26 the supernatant of cytochalasin B + fMLP–stimulated PMNs was then incubated with 2 M NaCl. Western blot analysis revealed a 92-kd band in nonreducing conditions (Figure 8). This finding suggested that HGF secreted by PMNs binds glycosaminoglycans. As heparan sulfate is known to interact with HGF,32 we performed Western blot analysis of PMN-derived HGF with antiheparan sulfate monoclonal antibodies.25 A specific 69-kd band migrating at the level of the HGF α chain was revealed, whereas no band was found with rh-HGF (Figure 9). Taken together, our results suggest that HGF secreted by PMNs binds a PMN-derived heparinlike substance (likely heparan sulfate).
Western blot analysis of PMN-derived HGF: effect of NaCl pretreatment.
PMNs were stimulated with cytochalasin B and fMLP at 37°C; the supernatant was then incubated for 30 minutes at 37°C with 2 M NaCl (NaCl+) or without NaCl (NaCl−) and then subjected to Western blot analysis in nonreducing conditions and revealed with antihuman HGF monoclonal antibodies; rh-HGF and HGF derived from purified specific granules (gr) were loaded and blotted in parallel. Note that both pro-HGF and mature HGF yielded a 92-kd band (arrow) in nonreducing conditions. These data are representative of one experiment of 3.
Western blot analysis of PMN-derived HGF: effect of NaCl pretreatment.
PMNs were stimulated with cytochalasin B and fMLP at 37°C; the supernatant was then incubated for 30 minutes at 37°C with 2 M NaCl (NaCl+) or without NaCl (NaCl−) and then subjected to Western blot analysis in nonreducing conditions and revealed with antihuman HGF monoclonal antibodies; rh-HGF and HGF derived from purified specific granules (gr) were loaded and blotted in parallel. Note that both pro-HGF and mature HGF yielded a 92-kd band (arrow) in nonreducing conditions. These data are representative of one experiment of 3.
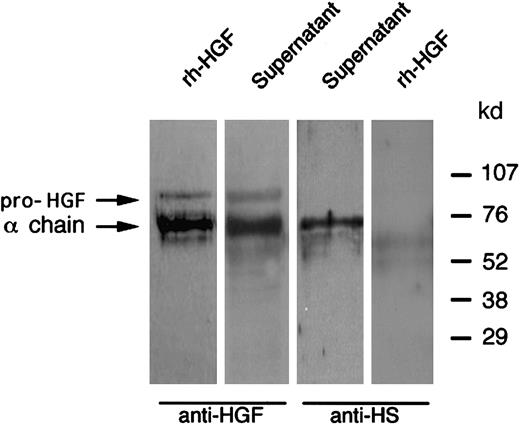
Western blot analysis of HGF released after PMN degranulation: colocalization with neutrophil-derived heparan sulfate.
PMNs were stimulated with cytochalasin B and fMLP at 37°C; the supernatant was then subjected to Western blot analysis in reducing conditions and revealed with either antihuman HGF (anti-HGF) or antiheparan sulfate (anti-HS) monoclonal antibodies, as indicated; rh-HGF was loaded and blotted in parallel. These data are representative of one experiment of 3.
Western blot analysis of HGF released after PMN degranulation: colocalization with neutrophil-derived heparan sulfate.
PMNs were stimulated with cytochalasin B and fMLP at 37°C; the supernatant was then subjected to Western blot analysis in reducing conditions and revealed with either antihuman HGF (anti-HGF) or antiheparan sulfate (anti-HS) monoclonal antibodies, as indicated; rh-HGF was loaded and blotted in parallel. These data are representative of one experiment of 3.
Discussion
PMNs synthesize inflammatory cytokines and growth factors,30 but there are few reports of preformed intracellular stocks of either cytokines18 or growth factors.33 Our results demonstrate that both secretory vesicles and gelatinase/specific granules of human blood PMNs contain a mobilizable stock of pro-HGF that is proteolytically processed to mature HGF by neutrophil serine protease(s) during degranulation. Furthermore, this mature HGF bound neutrophil-derived polyanionic substances (likely heparan sulfate).
The bulk of HGF was found in the gelatinase/specific granules fraction and colocalized with the lactoferrin-containing fraction. The efficiency of fractionation was confirmed by measuring the reference markers of specific and azurophil granules (lactoferrin and myeloperoxidase, respectively) in each fraction. Interestingly, vascular endothelial growth factor, another heparin-binding factor, has also been found in specific granules.33,34 Intracellular HGF was also present in the plasma membrane and/or secretory vesicles, constituting a readily mobilizable store. This localization demonstrated by the results in Table 1 and in Figure 1 was confirmed by the pattern of HGF release obtained with 10−8 M fMLP (Figure 3), a concentration known to mobilize the exocytosis of secretory vesicles.35 36 Altogether, our results argue for a dual subcellular localization of HGF, in both secretory vesicles and gelatinase/specific granules.
In addition to releasing numerous preformed mediators into the extracellular medium, PMNs can synthesize cytokines de novo in certain conditions.30 For instance, we have previously demonstrated that PMNs can release oncostatin M through a 2-step mechanism involving the release of a preformed stock followed by de novo protein synthesis.18 In the present study we found no evidence of active HGF synthesis by human blood PMNs, as cycloheximide did not affect HGF levels after 24 hours of stimulation. Therefore, our results show that acute HGF release by mature circulating PMNs does not depend on ongoing or activated synthesis but rather on the release of HGF stored in PMNs. Most of the proteins stored in PMN granules are actively synthesized during granulocyte maturation; indeed, we found that HGF mRNA was expressed by bone marrow cells at a higher level than in circulating PMNs. The timing of granule formation is well documented; specific granules along with their protein contents are mainly formed at the myelocyte and metamyelocyte stages.37,38 As expected, our immunocytochemistry experiments showed that HGF+ cells are essentially myelocytes, metamyelocytes, and segmented cells. Weak to moderate staining was found in promyelocytes. This latter result could be explained by a continuum during precursor maturation.38Inaba et al39 have shown that the human promyelocytic leukemia cell line HL-60 produces HGF and that dibutyryl cAMP (a granulocyte inducer) stimulates HGF release. In addition, Majka et al40 have shown that highly purified human CD34+ cells express HGF transcripts and produce HGF. Altogether, our results suggest that the bulk of HGF synthesis occurs during PMN maturation, along with most other granule constituents.
As shown by Western blot analysis in reducing conditions, HGF was present as the pro-HGF form in both gelatinase/specific granules and plasma membrane fractions; this mobilizable intracellular pool of HGF was released as the mature disulfide-linked heterodimer into the extracellular medium, as shown by the presence of the 69-kd α chain. These results suggest that HGF is processed to its mature form during PMN degranulation. Furthermore, proteolysis of pro-HGF to mature HGF was completely inhibited in DFP-treated PMNs, suggesting that PMN-derived serine protease(s) is/are involved in this process. Indeed, serine proteases have been shown to convert pro-HGF to mature HGF,8-11,41 and the active form of HGF has been found exclusively in injured tissues.42 In response to a trigger such as tissue injury, it is conceivable that locally activated PMNs release pro-HGF along with serine protease(s) and that neutrophils infiltrating damaged tissues participate in the local maturation of HGF required for its biologic activities.43 Urokinase, a protease known to be present in PMN-specific granules31 and to process pro-HGF,10 failed to exhibit any converting activity in our experimental conditions. These results are in agreement with those of Mizuno et al41 who found no in vitro properties for plasminogen activators such as urokinase and tissue-type plasminogen activator and with those of Shimomura et al9who reported that the converting activities of these agents were more than 1000 times lower than that of the HGF activator. We also investigated the effects of elastase and cathepsin G, the main serine proteases of PMNs, used over a range of relevant concentrations,27 neither agent had any effect on the processing of pro-HGF. Therefore, the nature of the HGF-converting enzyme(s) in PMNs remains to be determined.
In addition to its specific signaling receptor c-met, HGF interacts with glycosaminoglycans, especially heparan sulfate.32 We infer that HGF secreted by PMNs into the extracellular medium binds PMN-derived heparan sulfate. Indeed, PMNs contain sulfated glycosaminoglycans (eg, heparan sulfate) in their azurophil granules,44 which may bind to HGF during the degranulating process. Antiheparan sulfate antibodies revealed the existence of a 69-kd band comigrating with the HGF α chain. This finding is in keeping with reports that HGF binds heparan sulfate by the N-terminal hairpin loop and the second kringle domain, both of which are located on the HGF α chain.26 Unexpectedly, the apparent molecular mass of HGF bound to heparan sulfate was not modified. However, sulfated oligosaccharides as small as hexasaccharides are known to bind to HGF with high affinity.34 Heparan sulfate potentiates HGF biologic activities; for instance, highly sulfated oligosaccharides increase HGF binding to the c-met receptor, as well as c-met dimerization and subsequent activation.34
These results have functional implications for the role of PMNs in wound repair. First, the existence of a mobilizable stock of HGF would allow PMNs to respond rapidly to changes in the extracellular environment. PMNs represent the majority of infiltrating cells in injured tissues, at least in the early phases, and may, therefore, be an important source of HGF, a critical factor for tissue repair and regeneration. Indeed, HGF acts as a mitogen and morphogen for various epithelial cells and has been shown to play an essential part in liver45 and lung7 regeneration after damage. HGF may exert its protective action by stimulating proliferation and angiogenesis46 and by inhibiting apoptosis.47Various effects of HGF on peripheral blood cells have been described: HGF primes neutrophil oxidative response,48 triggers PMN transmigration,49 and stimulates T-cell adhesion and migration,50 as well as monocyte cytokine production (eg, IL-6) and monocyte motility and invasiveness.51,52 In monocytes, c-met expression is up-regulated in conditions mimicking inflammation (stimulation with IL-1 or LPS),52 53 making these cells accessible to the functional effects of HGF. Therefore, the autocrine/paracrine effects of HGF may play a role in the regulation of inflammatory process.
Finally, PMN-derived metalloproteases (eg, MMP-8 and MMP-9) can mobilize growth factors, including HGF, tethered to extracellular matrix proteoglycans and collagen,14 15 further supporting the role of PMNs in tissue repair.
In conclusion, our findings point to a new role of PMNs in tissue repair, through the release of HGF.
We thank A. Barnier, F. Hochedez, S. Laribe, V. Leçon-Malas, and J. Moreau for expert technical assistance and C. Poüs and J. B. Stern for helpful discussions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Monique Dehoux, Laboratoire de Biochimie and INSERM U408, Hôpital Bichat, 46 rue Henri Huchard, 75877 Paris Cedex 18, France; e-mail: monique.dehoux@bch.ap-hop-paris.fr.