Abstract
The ashen (ash) mouse, a model for Hermansky-Pudlak syndrome (HPS) and for a subset of patients with Griscelli syndrome, presents with hypopigmentation, prolonged bleeding times, and platelet storage pool deficiency due to a mutation which abrogates expression of the Rab27a protein. Platelets of mice with the ashen mutation on the C3H/HeSnJ inbred strain background have greatly reduced amounts of dense granule components such as serotonin and adenine nucleotides though near-normal numbers of dense granules as enumerated by the dense granule-specific fluorescent dye mepacrine. Thus, essentially normal numbers of platelet dense granules are produced but the granule interiors are abnormal. Collagen-mediated aggregation of mutant platelets is significantly depressed. No abnormalities in the concentrations or secretory rates of 2 other major platelet granules, lysosomes and alpha granules, were apparent. Similarly, no platelet ultrastructural alterations other than those involving dense granules were detected. Therefore, Rab27a regulates the synthesis and secretion of only one major platelet organelle, the dense granule. There were likewise no mutant effects on levels or secretion of lysosomal enzymes of several other tissues. Together with other recent analyses of the ashen mouse, these results suggest a close relationship between platelet dense granules, melanosomes of melanocytes and secretory lysosomes of cytotoxic T lymphocytes, all mediated by Rab27a. Surprisingly, the effects of the ashen mutation on platelet-dense granule components, platelet aggregation, and bleeding times were highly dependent on genetic background. This suggests that bleeding tendencies may likewise vary among patients with Griscelli syndrome and HPS with Rab27a mutations.
Introduction
Hermansky-Pudlak syndrome (HPS; OMIM no. 203300) is a recessively inherited disease that affects the biosynthesis and/or trafficking of the related subcellular organelles platelet-dense granules, melanosomes and lysosomes.1,2 The organellar abnormalities in turn cause platelet dysfunction, hypopigmentation, and, in most cases, defective secretion of lysosomal contents. The clinical consequences of these abnormalities include prolonged bleeding and hemorrhaging, significantly reduced visual acuity, colitis, and fibrotic lung disease. A significant fraction of patients die prematurely in the fourth to fifth decades of life due to fibrotic lung disease.3 Bleeding may be severe; a recent study determined that major bleeding events occurred in 40% of patients studied.3 There is presently no cure for HPS.
There are 4 genetically distinct forms of the disease (HPS1, HPS2, HPS3 and HPS4) that have been molecularly identified. Alterations in a novel Chr 10 gene are responsible for HPS14 whereas mutations in the Chr 5 AP3B1 gene occur in patients with HPS2.5 Mutations in the novel Chr 3 HPS3 gene were recently identified in patients with HPS in central Puerto Rico6 whereas mutations in a novel Chr 22 HPS4 gene were identified in several non-Puerto Rican patients.7 The 79-kd HPS1 protein is largely cytosolic though a portion is weakly membrane-bound.8,9 It is a component of a high-molecular-weight complex though its exact function in vesicle trafficking is unknown. The HPS1 and HPS4 proteins may function in the same pathway of organelle biogenesis.7 TheAP3B1 protein is a subunit of the AP-3 adaptor complex which is well known to regulate vesicle trafficking by capturing membrane proteins destined for lysosomes and melanosomes at the trans-Golgi complex.10-12
HPS is genetically highly heterogeneous not only in humans but also in mice where a large number (at least 15) of mouse hypopigmentation mutants,13,14 including ashen,15 are encoded by distinct genes and appropriately model human HPS. The pale ear and pearl mutants are orthologous models for human HPS1 and HPS2, respectively, whereas the cocoa and light ear mutants are the corresponding mouse models for HPS3 and HPS4.7,16 Other mouse HPS mutants are expected to (1) serve as appropriate models for existing and future human patients with HPS who are thus far molecularly unidentified and (2) allow identifications of proteins critical for the synthesis of specialized mammalian organelles such as platelet-dense granules, melanosomes, and lysosomes. There are 4 other mouse HPS mutants that have been molecularly identified by positional/candidate gene approaches. The mocha, pallid, and ashen genes encode the delta subunit of the AP-3 adaptor complex,17 a novel 25-kd syntaxin 1–interacting protein (pallidin),18 and the Rab27a protein,15respectively. The gunmetal mouse,19,20 which shares features of HPS and the related gray platelet syndrome,21has lowered expression of Rab geranylgeranyl transferase.22 Thus, a common feature of 5 of the 8 mouse HPS genes thus far cloned, including ashen, is that they specify known components of the vesicle trafficking apparatus.
Griscelli syndrome (OMIM no. 214450), like HPS, is a recessively inherited hypopigmentation disorder.23 One subset of patients with Griscelli syndrome exhibits neurologic impairment caused by a mutation in the gene encoding myosin Va.24 The mutation in a second group of patients with Griscelli syndrome, which have widespread immune abnormalities, is, like that of the ashen mouse, in Rab27a.25
Rab proteins, a subclass of low-molecular-weight guanine nucleotide triphosphatases (GTPases), are particularly appropriate candidates for HPS genes since they are well known to regulate vesicle fusion and fission events at specific subcellular sites as vesicles traffic within the cell.26-28 Several Rabs have been identified within platelets including Rab1, Rab3b, Rab4, Rab6, Rab8, Rab11a, Rab27a, and Rab27b.29
The Rab27a mutation30 in ashen (ash) mutant mice on the C3H/HeSnJ strain background is a loss of function mutation that causes hypopigmentation and platelet storage pool deficiency accompanied by prolonged bleeding.15 Further, platelet-dense granules are reduced in number when examined in the electron microscope by the wet-mount procedure. All of these features qualify this ashen mutant as an appropriate model for HPS. However, the potential regulatory role(s) of Rab27a in the synthesis or secretion of other platelet subcellular organelles was not assessed in these studies. Consistent with the immune abnormalities of patients with Griscelli syndrome with Rab27a mutations and with the role of Rabs in vesicle transport, ashen mice have T lymphocyte granule transport defects.31 32 Thus, ashen mice can be considered as a potential model for both HPS and the closely related Griscelli syndrome.
Rab27a is a potentially important regulator of platelet organelles as it is found at high concentrations in platelets and megakaryocytes.29 Nevertheless, the physiologic role(s) of Rab27a and other Rabs in platelets and in particular the exact platelet organelle(s) regulated by individual Rabs remain largely undefined. We now report that Rab27a is critically and specifically important in the ability of dense granules to accumulate and/or retain their contents. Also, we show that the effects of the Rab27a gene on dense granules are highly dependent on genetic background, thus offering a possible explanation for the absence of reports of prolonged bleeding in patients with Griscelli syndrome with Rab27a mutations. Likewise, these studies indicate that problematic bleeding may occur if patients with Griscelli syndrome contain a susceptible genetic background.
Materials and methods
Mice
C3H/HeSnJ ash/ash mice were originally obtained from the Jackson Laboratory and subsequently bred at The National Cancer Institute, Frederick, MD, and the Roswell Park Cancer Institute. Mutant mice were maintained by breeding homozygous males with heterozygous females. The inbred nonagouti (a/a, ash/ash) ashen strain was generated by crossing C3H/HeSn-ash/ash mice to C57BL/6J mice. The F1 progeny were intercrossed and a/a, ash/ash mice were selected and intercrossed for 7 more generations. The a/a, ash/ash F8 mice were backcrossed once to C57BL/6J mice and the stock has been maintained by brother × sister matings for 39 generations (F8N1F39). The presence of the ashen Rab27a mutation was confirmed in mutant mice by appropriate reverse transcription–polymerase chain reaction (RT-PCR) procedures (see below).
Cells and tissues
To isolate platelets, mice were killed by anoxia with CO2, and blood was immediately withdrawn by heart puncture with a 22 g needle attached to a 1 mL syringe containing 0.1 mL 3.8% sodium citrate. Platelets were isolated by differential centrifugation as described.33 36 Mouse tissues were isolated from 6- to 8-week-old animals, washed with phosphate buffered saline (PBS), and stored at −70°C. Tissues were thawed and homogenized with a Polytron homogenizer in proteinase inhibitor cocktail. Postnuclear supernatants were obtained by centrifugation at 500g for 10 minutes.
Antibodies
Rabbit antiserum to human fibrinogen was from Diagnostica Stago (Asnieres, France) and was used at 1000:1 in western blots. Platelet factor 4 (PF4) was measured with a rabbit polyclonal antibody to rat PF434 at 200:1 dilution. Peroxidase-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Labs, West Grove, PA.
Electron microscopy
Platelets were harvested from the peripheral blood of C3H/HeSnJ and C3H/HeSnJ ash/ash mice in the presence of sodium citrate. Platelets were pelleted gently and fixed in 2% paraformaldehyde/1.5% gluteraldehyde. The pellets were osmicated, stained with tannic acid, and embedded in Epon for ultrathin section electron microscopy.
Platelet serotonin assays
Platelets were lysed in 1 mL distilled water and assayed fluorometrically for serotonin according to Crosti and Lucchelli.35
Platelet adenine nucleotides
Platelet adenine nucleotides were determined by a modification of the firefly assay of Holmsen et al.36
Platelet aggregation
Aggregation of platelets was assayed by whole blood aggregometry33 after collection of citrated blood.37 Final concentrations of platelet aggregating agents were 1 and 4 μg/mL collagen. Adenosine triphosphate (ATP) secretion was determined simultaneously with platelet aggregation by coupling with luciferin-luciferase.33
Thrombin-stimulated platelet secretion
Platelets were washed 2 times in PBS containing 2% bovine serum albumin (BSA). Platelets (2 × 108/mL) were treated with 0.6 units thrombin (Sigma Chemical, St Louis, MO) for 3 minutes with constant shaking. The reaction was stopped with 2.5 nmol/mL of Thromstop (American Diagnostics, Greenwich, CT), and platelets were separated from supernatant by differential centrifugation.
Mepacrine uptake
Platelets were incubated with mepacrine and analyzed with a Leitz MPV-2 fluorescent microscope as described.38
Urine and tissue collection
To amplify lysosomal enzyme concentrations in kidney and urine, female mice were treated for 20 days with testosterone.39After induction, mice were placed in metabolism cages at 2 to 4 per cage and urine was collected at 24-hour intervals for 7 days. Mice were killed by anoxia with CO2 and tissues were homogenized and stored frozen.
Immunoblotting
Extracts of fresh platelets were boiled in Laemlli sample buffer with 10% mercaptoethanol. Platelet or tissue protein (30 μg) was electrophoresed on 10% sodium dodecyl sulfate (SDS) polyacrylamide or 8% to 16% gradient (Bio-Rad Laboratories, Hercules, CA) SDS polyacrylamide gels. Separated proteins were electrophoretically transferred to nitrocellulose or polyvinylidenefluoride (PVDF) membranes (Bio-Rad Laboratories). Filters were blocked with 5% nonfat dry milk in PBS for 1 hour, then probed with rabbit primary antibodies in blocking solution for 1.5 hours. The filters were washed, then incubated with a peroxidase-labeled goat anti–rabbit secondary antibody for 1.5 hours. Bands were visualized with the enhanced chemiluminescence (ECL) system (ECL+Plus; Amersham, Piscataway, NJ). Films were scanned and bands quantitated using NIH IMAGE 1.61 software. All blots were exposed to film for several different lengths of time to ensure that the density of bands were within the linear range. Equivalent loading and transfer were verified by India ink staining of blots.
Enzyme assays
β-Glucuronidase and β-galactosidase were assayed with fluorescent methylumbelliferyl substrates.39 Protein was determined with the Bio-Rad protein assay system (Bio-Rad Laboratories).
Genotyping
The ashen genotype15 of all mutant mice was confirmed by RT-PCR analysis of kidney total RNA usingRab27a-specific primers to nucleotides 361-380 and 1070-1051 (Genbank accession no. AF304376), which flank the splice donor site mutation located downstream of exon 4 in the Rab27a gene in ashen mutant mice. PCR products were analyzed on 1% agarose gels stained with ethidium bromide. Wild-type C3H/HeSnJ RNA produces a single band at 710 bp compared with a single band at 945 bp for homozygousash/ash and both bands for heterozygousash/+.
Results
Hematologic parameters
No large abnormalities in numbers of blood cells or other common hematologic parameters were detected in peripheral blood of adult C3H/HeSnJ ash/ash mice (Table1). Similarly, no quantitative or qualitative alterations in cellularity of femoral marrow or spleen were apparent in mutant mice (not shown). These results indicated that, similar to patients with HPS and Griscelli syndrome, quantitative abnormalities of peripheral blood cells are not a feature of the ashen mutation. Because of this result, our analyses shifted to the qualitative abnormalities of ashen platelets.
Hematologic parameters in adult normal C3H/HeSnJ and C3H/HeSnJ ash/ash mice
| Genotype . | WBCs (103/μL) . | Lym (%) . | Mono (%) . | EOS (%) . | N-PMN (%) . | RBCs (× 106/dL) . | HGB (g/dL) . | PLT (103/μL) . |
|---|---|---|---|---|---|---|---|---|
| Normal | 6.0 ± 0.5 | 73 ± 2.5 | 8.3 ± 2.7 | 1.5 ± 0.5 | 17.3 ± 4.8 | 8.7 ± 0.05 | 14.7 ± 0.2 | 710 ± 33 |
| ash/ash | 4.9 ± 2.0 | 77 ± 3.5 | 6.5 ± 1.5 | 1.8 ± 0.8 | 14.8 ± 1.8 | 8.6 ± 0.09 | 14.7 ± 0.4 | 652 ± 6 |
| Genotype . | WBCs (103/μL) . | Lym (%) . | Mono (%) . | EOS (%) . | N-PMN (%) . | RBCs (× 106/dL) . | HGB (g/dL) . | PLT (103/μL) . |
|---|---|---|---|---|---|---|---|---|
| Normal | 6.0 ± 0.5 | 73 ± 2.5 | 8.3 ± 2.7 | 1.5 ± 0.5 | 17.3 ± 4.8 | 8.7 ± 0.05 | 14.7 ± 0.2 | 710 ± 33 |
| ash/ash | 4.9 ± 2.0 | 77 ± 3.5 | 6.5 ± 1.5 | 1.8 ± 0.8 | 14.8 ± 1.8 | 8.6 ± 0.09 | 14.7 ± 0.4 | 652 ± 6 |
All values are mean ± range of 2 mice.
WBCs indicates white blood cells; Lym, lymphocytes; Mono, monocytes; EOS, eosinophils; N-PMN, polymorphonuclear neutrophils; RBCs, red blood cells; HGB, hemoglobin; PLT, platelets.
Platelet-dense granule abnormalities in C3H/HeSnJ ashen mice
Previous studies of ashen mice on the C3H/HeSnJ genetic background15 documented a prolonged bleeding time (> 15 min) accompanied by a deficiency of platelet-dense granules as enumerated by wet-mount electron microscopic examination and serotonin analyses. The decrease in observable platelet-dense granules, together with obvious coat pigment dilution, established ashen as a model2 3 for HPS. The following experiments were designed to further characterize the platelet-dense granule deficiency.
The wet-mount technique detects only granule contents (the high metal content of the dense granule renders it impermeable to the electron beam). Therefore, dense granules may be truly absent or they may be present in normal quantities, but are “empty” (ie, the dense granule membrane is intact, but the granules lack typical dense granule constituents such as serotonin, adenine nucleotides, and metals). To distinguish between these possibilities, dense granules were enumerated (Table 2) by the independent method of visualization with the fluorescent dye mepacrine, which is specifically incorporated into platelet-dense granules.38 40 When fluorescent granules were enumerated in more than 50 C3H/HeSnJash/ash platelets, the number of granules per platelet was only slightly depressed (20%) compared with C3H/HeSnJ +/+ normal platelets, indicating that ashen mice have a near-normal number of dense granules. However, a major qualitative abnormality of the contents of these ashen dense granules was apparent by their nearly complete lack of “flashing” (see “Discussion”) upon prolonged exposure to UV light (Table 2).
Characteristics of dense granules of normal, C3H/HeSnJash/ash and a/a, ash/ash platelets as determined by fluorescence microscopy of mepacrine-labeled platelets
| . | Granules/platelet . | Flashes/platelet . | Flashes/granule . |
|---|---|---|---|
| C3H/HeSnJ +/+ | 5.08 ± .23 (50) | 4.10 ± .25 (64) | 0.81 |
| C3H/HeSnJash/ash | 4.00 ± .25 (61)* | 0.065 ± .03 (61)* | 0.02* |
| C57BL/6J +/+ | 4.96 ± .31 (25) | 3.88 ± .36 (25) | 0.78 |
| a/a, ash/ash | 5.06 ± .29 (49) | 3.61 ± .40 (49) | 0.71 |
| . | Granules/platelet . | Flashes/platelet . | Flashes/granule . |
|---|---|---|---|
| C3H/HeSnJ +/+ | 5.08 ± .23 (50) | 4.10 ± .25 (64) | 0.81 |
| C3H/HeSnJash/ash | 4.00 ± .25 (61)* | 0.065 ± .03 (61)* | 0.02* |
| C57BL/6J +/+ | 4.96 ± .31 (25) | 3.88 ± .36 (25) | 0.78 |
| a/a, ash/ash | 5.06 ± .29 (49) | 3.61 ± .40 (49) | 0.71 |
Values represent the mean ± SEM of determinations on the number of individual platelets in parentheses. These platelets were derived from 3 separate mice for each genotype.
P ≤ .001.
Other important components of platelet-dense granules include the adenine nucleotides ATP and adenosine diphosphate (ADP). ADP released from activated platelets plays a critical role as an agonist in platelet aggregation. The firefly luciferase assay (Table3) revealed significant deficiencies of both ADP and ATP in C3H/HeSnJ ash/ash platelets compared with control C3H/HeSnJ. ADP and ATP are differentially distributed within platelets with the majority of ATP found in the cytoplasmic metabolic pool, whereas in contrast the majority of ADP is stored within the dense granule. The C3H/HeSnJ ash/ash mutant platelets exhibit a relatively greater loss of ADP than ATP, thus increasing the ATP/ADP ratio, indicating a deficiency of the dense granule adenine nucleotide pool.
Platelet-dense granule adenine nucleotides are significantly reduced in C3H/HeSnJ ash/ash mice
| . | ATP (μmol/1011 platelets) . | ADP (μmol/1011 platelets) . | ATP/ADP . |
|---|---|---|---|
| C3H/HeSnJ +/+ | 4.37 ± 0.18 (16) | 2.40 ± 0.15 (16) | 1.82 |
| C3H/HeSnJash/ash | 3.03 ± 0.27 (7)3-150 | 1.08 ± 0.11 (7)3-151 | 2.80 |
| C57BL/6J +/+ | 5.72 ± 0.71 (8) | 3.34 ± 0.29 (8) | 1.77 |
| a/a, ash/ash | 4.80 ± 0.23 (5) | 2.28 ± 0.21 (5) | 2.10 |
| . | ATP (μmol/1011 platelets) . | ADP (μmol/1011 platelets) . | ATP/ADP . |
|---|---|---|---|
| C3H/HeSnJ +/+ | 4.37 ± 0.18 (16) | 2.40 ± 0.15 (16) | 1.82 |
| C3H/HeSnJash/ash | 3.03 ± 0.27 (7)3-150 | 1.08 ± 0.11 (7)3-151 | 2.80 |
| C57BL/6J +/+ | 5.72 ± 0.71 (8) | 3.34 ± 0.29 (8) | 1.77 |
| a/a, ash/ash | 4.80 ± 0.23 (5) | 2.28 ± 0.21 (5) | 2.10 |
Adenosine triphosphate (ATP) and adenosine diphosphate (ADP) were assayed with a modified luciferase assay. Values represent the mean ± SEM of the number of mice in parentheses.
P < .01.
P < .001 (compared with C3H/HeSnJ +/+ controls).
Therefore, by 4 separate platelet analyses (wet-mount electron microscopy, serotonin, mepacrine, and adenine nucleotides) a dense granule deficiency has been demonstrated in C3H/HeSnJ ash/ashmice.
Abnormal platelet aggregation in C3H/HeSnJ ash/ash
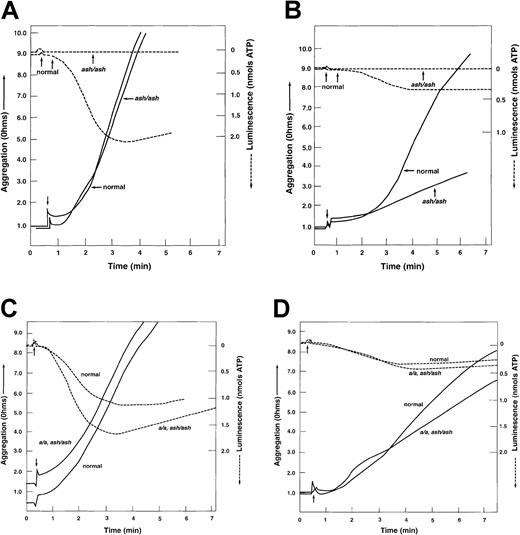
Dense granule deficiencies such as those found in patients with HPS are a form of platelet storage pool deficiency41which in humans typically leads to abnormalities in platelet aggregation, prolonged bleeding times, and problematic hemorrhaging.42 43 The ability of ashen platelets to aggregate was assessed with the agonist collagen. At high (4 μg/mL) collagen levels, no abnormalities in rates of aggregation of C3H/HeSnJ ashen platelets were apparent in the whole blood aggregometer (Figure1A). Nevertheless, a dense granule abnormality was apparent in mutant platelets in that, despite the presence of this very high concentration of agonist, no release of granule ATP occurred. As expected, large amounts of ATP were released from dense granules of normal platelets at this collagen concentration. At low (1 μg/mL) collagen concentrations (Figure 1B) mutant platelets exhibited not only an undetectable release of ATP, but also significantly impaired (about 20% of normal) aggregation rates. Taken together, the above results indicate that prolonged bleeding in ashen mice is caused by defective aggregation of platelets which are deficient in the dense granule storage pool.
Collagen-mediated platelet aggregation and secretion of dense granule ATP is diminished in ashen mutants on the C3H/HeSnJ background but unaffected in ashen mutants on the a/a, ash/ashbackground.
Platelet aggregation was determined by the impedance method in whole blood in response to 4 μg/mL (A,C) and 1 μg/mL (B,D) collagen. A, B: C3H/HeSnJ ash/ash; C, D: a/a, ash/ash. In all cases, “normal” refers to the C3H/HeSnJ strain. ATP release was determined by luminescence methods. The small arrows indicate the time of addition of collagen.
Collagen-mediated platelet aggregation and secretion of dense granule ATP is diminished in ashen mutants on the C3H/HeSnJ background but unaffected in ashen mutants on the a/a, ash/ashbackground.
Platelet aggregation was determined by the impedance method in whole blood in response to 4 μg/mL (A,C) and 1 μg/mL (B,D) collagen. A, B: C3H/HeSnJ ash/ash; C, D: a/a, ash/ash. In all cases, “normal” refers to the C3H/HeSnJ strain. ATP release was determined by luminescence methods. The small arrows indicate the time of addition of collagen.
Platelet alpha granules
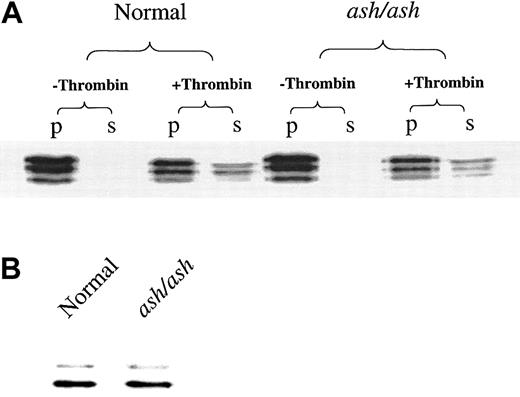
A second important platelet subcellular organelle is the alpha granule, which contains a large number of procoagulant proteins. Immunoblotting with specific antibodies to the alpha granule component fibrinogen revealed the typical triplet banding pattern of fibrinogen at 57 kd to 66 kd.19 The fibrinogen levels in untreated platelets and the degree of thrombin-mediated secretion of fibrinogen were not altered in ashen mice (Figure2). Similarly, normal steady-state levels of another platelet alpha granule component, platelet factor 4, were observed (Figure 2B) in mutant platelets, though the low levels of this component did not allow accurate measurements in secretions.
Platelet concentrations and secretory rates of alpha granule components are unaffected in C3H/HeSnJ ashen mice.
(A) Platelets (30 μg protein) of normal C3H/HeSnJ and C3H/HeSnJash/ash mice were incubated in the absence and presence of thrombin for 3 minutes and immediately separated into pellet (p) and supernatant (s) fractions by centrifugation. All fractions were immunoblotted with specific antibody to fibrinogen. (B) Platelets (30 μg protein) were immunoblotted with specific antibody to platelet factor 4.
Platelet concentrations and secretory rates of alpha granule components are unaffected in C3H/HeSnJ ashen mice.
(A) Platelets (30 μg protein) of normal C3H/HeSnJ and C3H/HeSnJash/ash mice were incubated in the absence and presence of thrombin for 3 minutes and immediately separated into pellet (p) and supernatant (s) fractions by centrifugation. All fractions were immunoblotted with specific antibody to fibrinogen. (B) Platelets (30 μg protein) were immunoblotted with specific antibody to platelet factor 4.
Lysosomal enzyme concentrations and secretory rates
Most other mouse pigment mutants which are models for HPS have alterations in either the contents of lysosomal enzymes, their secretory rate, or both.13 Accordingly, concentrations and secretion rates of lysosomal enzymes were evaluated in 2 tissues, platelets and kidney, of normal and C3H/HeSnJ ashen mice. Platelet steady-state concentrations of β-glucuronidase and β-galactosidase were unaffected in ashen mice. Similarly, steady-state levels of β-glucuronidase and β-galactosidase were not significantly altered in any of 4 mutant tissues including brain, liver, spleen, and kidney (Table 4).
Concentrations of lysosomal enzymes in tissues of normal C3H/HeSnJ +/+ and C3H/HeSnJ ash/ash mice
| . | Units/g . | |
|---|---|---|
| β-glucuronidase . | β-galactosidase . | |
| Brain | ||
| Normal | .117 ± .013 (4) | 2.64 ± .27 (4) |
| ash/ash | .114 ± .010 (4) | 2.60 ± .05 (4) |
| Liver | ||
| Normal | 1.27 ± .10 (7) | 4.49 ± .18 (4) |
| ash/ash | 1.61 ± .10 (7) | 4.23 ± .22 (4) |
| Spleen | ||
| Normal | 6.3 ± .96 (4) | 18.3 ± 2.0 (4) |
| ash/ash | 8.1 ± .12 (4) | 22.3 ± 1.5 (4) |
| Kidney | ||
| Normal | 127 ± 12 (4) | 23 ± 1.4 (4) |
| ash/ash | 110 ± 10 (4) | 20 ± 0.2 (4) |
| Platelets | ||
| Normal | .0134 ± .0007 (3) | .125 ± .006 (3) |
| ash/ahs | .0131 ± .0012 (3) | .110 ± .008 (3) |
| . | Units/g . | |
|---|---|---|
| β-glucuronidase . | β-galactosidase . | |
| Brain | ||
| Normal | .117 ± .013 (4) | 2.64 ± .27 (4) |
| ash/ash | .114 ± .010 (4) | 2.60 ± .05 (4) |
| Liver | ||
| Normal | 1.27 ± .10 (7) | 4.49 ± .18 (4) |
| ash/ash | 1.61 ± .10 (7) | 4.23 ± .22 (4) |
| Spleen | ||
| Normal | 6.3 ± .96 (4) | 18.3 ± 2.0 (4) |
| ash/ash | 8.1 ± .12 (4) | 22.3 ± 1.5 (4) |
| Kidney | ||
| Normal | 127 ± 12 (4) | 23 ± 1.4 (4) |
| ash/ash | 110 ± 10 (4) | 20 ± 0.2 (4) |
| Platelets | ||
| Normal | .0134 ± .0007 (3) | .125 ± .006 (3) |
| ash/ahs | .0131 ± .0012 (3) | .110 ± .008 (3) |
Kidney values are those of mice treated 20 days with testosterone. Other tissues were collected from untreated males. Platelet values are units/109 platelets. Values represent the mean ± SEM of the determinations on the number of individual mice indicated in parentheses.
No mutant effects on rates of thrombin-mediated secretion of these lysosomal enzymes from platelets were apparent (Table5). Another tissue, murine kidney, is a particularly appropriate one to study lysosomal secretion, as massive amounts of lysosomal enzymes are synthesized in kidney proximal tubule cells in testosterone-treated animals and secreted constitutively into urine.44 Rates of testosterone-induced lysosomal enzyme secretion were identical in normal and C3H/HeSnJ ashen mice (Table6).
Thrombin-stimulated secretion of lysosomal enzymes from platelets of normal C3H/HeSnJ +/+ and C3H/HeSnJ ash/ashmutant mice
| . | Secretion (% total) . | |
|---|---|---|
| − Thrombin . | + Thrombin . | |
| C3H/HeSnJ +/+ (6) | ||
| β-glucuronidase | 1.82 ± 0.36 | 24.8 ± 1.8 |
| β-galactosidase | 1.34 ± 0.19 | 25.6 ± 1.9 |
| C3H/HeSnJ ash/ash(6) | ||
| β-glucuronidase | 3.60 ± 0.99 | 30.4 ± 1.9 |
| β-galactosidase | 1.56 ± 0.40 | 28.7 ± 1.0 |
| . | Secretion (% total) . | |
|---|---|---|
| − Thrombin . | + Thrombin . | |
| C3H/HeSnJ +/+ (6) | ||
| β-glucuronidase | 1.82 ± 0.36 | 24.8 ± 1.8 |
| β-galactosidase | 1.34 ± 0.19 | 25.6 ± 1.9 |
| C3H/HeSnJ ash/ash(6) | ||
| β-glucuronidase | 3.60 ± 0.99 | 30.4 ± 1.9 |
| β-galactosidase | 1.56 ± 0.40 | 28.7 ± 1.0 |
Platelets (1 × 109/mL) were treated with buffer or with 0.625 U/mL thrombin for 3 minutes at 37°C. The reaction was terminated with Thromstop. Values represent the mean ± SEM of the individual determinations on the number of mice indicated in parentheses.
Daily secretion of lysosomal enzymes into urine of normal C3H/HeSnJ +/+ and mutant C3H/HeSnJ ash/ash mice
| . | Daily secretion (U/mouse) . | |
|---|---|---|
| β-glucuronidase . | β-galactosidase . | |
| Normal | 23 ± 5.1 (7) | 6.3 ± .37 (7) |
| ash/ash | 21 ± 2.1 (7) | 6.8 ± .54 (7) |
| . | Daily secretion (U/mouse) . | |
|---|---|---|
| β-glucuronidase . | β-galactosidase . | |
| Normal | 23 ± 5.1 (7) | 6.3 ± .37 (7) |
| ash/ash | 21 ± 2.1 (7) | 6.8 ± .54 (7) |
Each metabolism cage contained four mice that had been treated with testosterone for 20 days. Values represent the mean ± SEM of the number of determinations in parentheses.
Thus, among the 3 major platelet subcellular granules, abnormalities are apparent in C3H/HeSnJ ashen mutants only in dense granules.
Platelet ultrastructure in ashen mutants
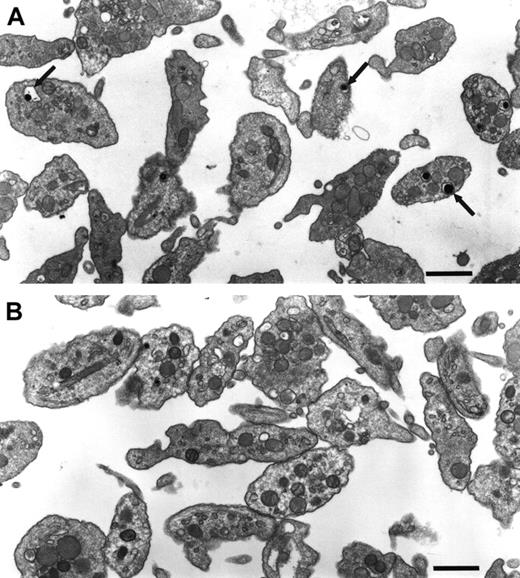
Ultrastructural analyses by electron microscopy of fixed sections of normal and C3H/HeSnJ ashen platelets (Figure3) confirmed a deficiency in ashen platelets of dense granules. Dense granules, ascertained by their “bulls-eye” appearance,45 were readily apparent in normal platelets but were not visible in ashen platelets. Very rarely, much smaller densely stained granules which do not exhibit the typical bulls-eye appearance were visible in ashen platelets. These may represent an aberrant form of mutant dense granule or a precursor form of the normal dense granule that is never fully processed in mutant platelets. Further, the dense granule abnormality was quite specific. No additional abnormalities in either general cellular morphology or in quantity or quality of other subcellular organelles were apparent in mutant platelets.
A deficiency of platelet-dense granules is the only obvious ultrastructural abnormality in C3H/HeSnJ ashen mice.
Normal C3H/HeSnJ platelets (above) and mutant C3H/HeSnJ ash/ashplatelets (below) were examined by electron microscopy. The platelet pellets were osmicated and stained with 190 tannic acid in 0.05 M sodium cacodylate. Electron microscopy grids were then stained with lead citrate. Arrows indicate the typical “bulls eye” appearance of dense granules in normal platelets. Bar is 1 μm.
A deficiency of platelet-dense granules is the only obvious ultrastructural abnormality in C3H/HeSnJ ashen mice.
Normal C3H/HeSnJ platelets (above) and mutant C3H/HeSnJ ash/ashplatelets (below) were examined by electron microscopy. The platelet pellets were osmicated and stained with 190 tannic acid in 0.05 M sodium cacodylate. Electron microscopy grids were then stained with lead citrate. Arrows indicate the typical “bulls eye” appearance of dense granules in normal platelets. Bar is 1 μm.
Effect of genetic background
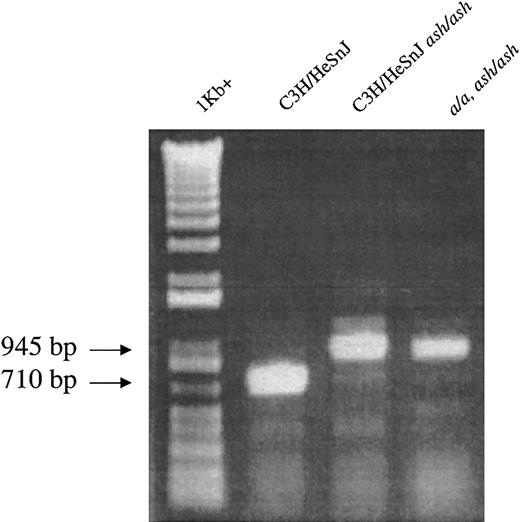
The availability of the ash allele on the a/a, ash/ash mutant inbred strain enabled tests of the effect(s) of genetic background on the platelet phenotypes of mutant mice. These mice have a gray hypopigmented coat color indistinguishable from that of C3H/HeSnJ ash/ash mice. Further, RT-PCR analyses (Figure4) confirmed that the molecular nature of the ash mutation is identical in the C3H/HeSnJash/ash and a/a, ash/ash ashen mutants.
The diagnostic 235-bp insertion within the ashRab27a transcript is found in homozygous form in both C3H/HeSnJash/ash and a/a, ash/ash mutant mice.
RT-PCR amplification of kidney RNA produces a 710-bp product in normal C3H/HeSnJ mice and a 945-bp product in both ashen mutants. The 1 Kb+ size markers (GibcoBRL, Carlsbad, CA) and PCR products were detected by ethidium bromide staining.
The diagnostic 235-bp insertion within the ashRab27a transcript is found in homozygous form in both C3H/HeSnJash/ash and a/a, ash/ash mutant mice.
RT-PCR amplification of kidney RNA produces a 710-bp product in normal C3H/HeSnJ mice and a 945-bp product in both ashen mutants. The 1 Kb+ size markers (GibcoBRL, Carlsbad, CA) and PCR products were detected by ethidium bromide staining.
Surprisingly, however, several measures indicated that, unlike C3H/HeSnJ ash/ash mutants, platelet-dense granules and the general platelet coagulation system of a/a, ash/ash mutants are normal or near normal. For example, whereas platelet-dense granule serotonin levels were somewhat depressed in a/a, ash/ashplatelets compared with their C57BL/6J controls (Table7), the levels in a/a, ash/ashwere far greater than those in C3H/HeSnJ ash/ash mutant platelets. Also, they were not significantly decreased compared with another control strain, C3H/HeSnJ +/+, which has normal bleeding times. Concentrations of the adenine nucleotides, ADP and ATP (Table 3) ofa/a, ash/ash mutant platelets, while nominally lower than control C57BL/6J values, were not significantly different from those control values. The minimal effect on the dense granule interior ina/a, ash/ash mutants was confirmed by incorporation of mepacrine, which revealed a normal platelet-dense granule number and normal UV-induced granule flashing of those granules, in contrast to the near absence of granule flashing in C3H/HeSnJ ash/ashmutant platelets (Table 2). Similarly, normal rates of aggregation and normal quantities of secreted dense granule ATP were apparent when platelets of a/a, ash/ash mutants were treated with either high or low collagen (Figures 1C-D). Finally, only a modest increase in bleeding time to 6.9 minutes from the normal 2.7 minutes was measurable (Table 8) in a/a, ash/ash mice, in contrast to the more than 15-minute bleeding times previously observed15 in C3H/HeSnJ ash/ashmutants.
Platelet serotonin concentrations in normal C3H/HeSnJ and in homozygous ash/ash mice on 2 genetically distinct backgrounds
| . | Serotonin (μg/109 platelets) . |
|---|---|
| C3H/HeSnJ +/+ | 2.81 ± 0.24 (8) |
| C3H/HeSnJash/ash | 0.24 ± 0.11 (8)7-150 |
| C57BL/6/J +/+ | 5.04 ± 0.38 (5) |
| a/a, ash/ash | 2.11 ± 0.13 (7)7-151 |
| . | Serotonin (μg/109 platelets) . |
|---|---|
| C3H/HeSnJ +/+ | 2.81 ± 0.24 (8) |
| C3H/HeSnJash/ash | 0.24 ± 0.11 (8)7-150 |
| C57BL/6/J +/+ | 5.04 ± 0.38 (5) |
| a/a, ash/ash | 2.11 ± 0.13 (7)7-151 |
Values are the mean ± SEM of the number of mice in parentheses.
P ≤ .001 (compared with C3H/HeSnJ +/+).
P ≤ .01 (compared with C57BL/6J control).
Bleeding times of normal C57BL/6J +/+ and a/a, ash/ash mice
| . | Bleeding time (min) . |
|---|---|
| C57BL/6J +/+ | 2.7 ± 0.38 (11) |
| a/a, ash/ash | 6.9 ± 0.49 (11)8-150 |
| . | Bleeding time (min) . |
|---|---|
| C57BL/6J +/+ | 2.7 ± 0.38 (11) |
| a/a, ash/ash | 6.9 ± 0.49 (11)8-150 |
Values represent the mean ± SEM of the number of mice in parentheses.
P ≤ .001.
Discussion
The finding that the ashen mutant phenotype is caused by a mutation in Rab27a15 allows assignment of a physiologic role(s) and in particular exploration of the role of this Rab in the synthesis/processing of platelet granules. The ashen mouse is ideal for these studies since complete loss of Rab27a function in mutant mice was predicted,15 and supported by determinations31,32 46 that Rab27a protein is undetectable in ashen or Griscelli cells by immunomethods. Our results indicate that Rab27a specifically regulates the ability of one of the major platelet organelles, the dense granule, to accumulate and or retain its contents and that this regulation is critically dependent on the contribution of differing background genes.
By several criteria, ashen mice on the C3H/HeSnJ background have significant alterations in contents of platelet-dense granules. Previous studies showed that mutant platelet-dense granules are highly deficient as ascertained by chemical analyses of serotonin and by wet-mount electron microscopy.15 In contrast, the present experiments demonstrated that the numbers of dense granules are only marginally reduced when enumerated by the fluorescent mepacrine technique. Visualization of dense granules by the wet-mount method depends on a normal complement of dense granule components such as calcium, ADP, and serotonin. In contrast, visualization by mepacrine, although specific to dense granules, is not content dependent.47 Together, therefore, the 2 techniques indicate that ashen mice do have a near-normal granule number, but these granules are relatively “empty.” Such an interpretation is consistent with the near total absence of UV-mediated “flashing” of dense granules of mepacrine-labeled ashen platelets. Flashing occurs when previously quenched mepacrine is released from dense granules after damage of the granule membrane by UV light. It is dependent on a normal abundance of internal components of dense granules such as calcium, ADP, ATP, and serotonin.47 The deficiency of flashing in ashen platelets is consistent with previous findings15 of greatly depressed serotonin levels (10% of normal) in ashen platelets by chemical analyses. The deficiency of dense granule contents in C3H/HeSnJ mice is similar to that observed in other mouse HPS mutants13 and certain patients with HPS.43 Dense granule visualization by ultrastructural analyses of fixed sections (Figure 3), which like the wet-mount method is dependent on normal quantities of granule contents, confirmed the ashen dense granule deficiency. A fourth indication of the dense granule content deficiency of ashen mice was the lack of secretion of platelet ATP even at high collagen concentrations (Figure 1A). Finally, direct analyses (Table 3) of adenine nucleotides revealed a significant deficiency of these critical dense granule components, typical of platelet storage pool deficiency. This deficiency of dense granule contents is the likely explanation for the greatly prolonged bleeding times observed15 in C3H/HeSnJ ashen mice.
The reduction in collagen-induced aggregation of C3H/HeSnJ ashen platelets (Figure 1) is similar to that observed in patients with HPS42,43 and in a large number of other mouse HPS models.13 Normal aggregation at high collagen and reduced aggregation at low collagen has been observed for all mouse HPS mutants tested.13
Comparatively little is known of the mechanisms regulating platelet-dense granule synthesis. The dense granule is unlike other platelet secretory granules (lysosomes and alpha granules) in that its contents apparently consist entirely of low-molecular-weight components such as ADP, ATP, serotonin, and metals such as calcium.42Several proteins of dense granule membranes have been identified including GP1b, αIIb/β3, P-selectin, granulophysin, LAMP-1, and LAMP-2,42 but dense granule localization of these proteins is not specific since they are likewise found in the plasma membrane or the membranes of other platelet organelles such as lysosomes and/or alpha granules.42Immunoelectron microscopic analyses, using granulophysin and serotonin as specific dense granule markers,48 suggest that dense granules form in immature megakaryocytes at the same time as alpha granules and that megakaryocyte multivesicular bodies are biosynthetic precursor organelles for both platelet granules.48,49 This implies segregation of dense granule components from other platelet granules within the multivesicular body. Accordingly, the dependence of dense granule synthesis on Rab27a suggests that this Rab acts at the multivesicular body and that its action is specific to dense granules within the multivesicular body. This hypothesis is consistent with a wide body of evidence that multivesicular bodies are important intermediates in vesicle trafficking in the endosomal/lysosomal systems in both lower and higher eukaryotes.50 A related possibility is that dense granules are formed at least in part by the endocytic system since plasma membrane markers have been found within the dense granule membrane.51 A speculative explanation for the empty dense granules in ashen platelets is that, in the absence of Rab27a, dense granules are formed normally but then undergo unregulated “kiss and run” fusion with the platelet plasma membrane. This process would cause loss of granule contents though it would retain the normal dense granule membrane. Similar unregulated transient fusions of another granule, the mast cell granule, with the plasma membrane has in fact been documented in the case of another mouse HPS mutant, ruby-eye.52
Studies on the regulation of vesicle trafficking by Rabs in other systems suggest 2 general possibilities26-28 for the control of dense granule synthesis/trafficking by Rab27a. First, Rab27a may mediate the interaction of members of cytosolic docking complexes with the dense granule or its precursor(s) during granule synthesis. Second, it may form part of a complex of proteins, including molecular motors such as kinesin or myosin, which regulate the translocation of dense granules along the cytoskeleton. The latter possibility is consistent with studies of normal and ashen mice detailing the transfer of melanosomes along the cytoskeleton of melanocytes. Rab 27a is necessary for the recruitment of myosin Va to the surface of melanosomes46,53,54 and their capture at the dendritic tips. A deficiency in this process produces the hypopigmentation typical of ashen mice. A similar vesicle/cytoskeletal requirement may hold for normal secretion of lytic granules from cytotoxic T lymphocytes.31,32 The megakaryocyte cytoskeleton is likewise a critical player in platelet synthesis.55 Whether platelet granules are similarly transported along the cytoskeleton from the megakaryocyte cell body to developing proplatelets is uncertain, but plausible.
Concentrations and secretion rates of platelet alpha granules were normal in platelets of ashen mice. These results suggest that Rab27a is either not required for synthesis and secretion of alpha granules or that other molecules can compensate for its loss. This ashen phenotype is dissimilar to that observed in the gunmetal mouse, which has significant reductions in alpha granule components19 due to reduced prenylation of many platelet Rabs56 caused in turn by a deficiency of Rab geranylgeranyl transferase activity.22 The gunmetal studies indicate that Rab proteins are important in alpha granule synthesis, though they do not define the specific Rab(s). The ashen studies show that Rab27a is either not involved in alpha granule synthesis or that its loss can be compensated for by other Rabs. The gunmetal mouse also exhibits a partial deficiency of platelet-dense granules.19,20 This result is consistent with the present studies on the C3H/HeSnJ ashen mutant since Rab27a is only partially prenylated, and therefore is only partially functional, in the gunmetal mouse.22 In contrast to the thrombocytopenia evident in the gunmetal mouse, ashen mice have normal platelet numbers indicating that Rab27a is not critical for platelet synthesis.
Other mutations that cause abnormalities of alpha granule content and/or function have been described in patients with gray platelet syndrome,21 alpha/delta storage pool deficiency,57 and the Wistar Furth rat.58However, the molecular bases of these inherited syndromes remain unknown. Studies utilizing permeabilized platelets51,59,60have implicated the general membrane fusion protein N-ethylmaleimide–sensitive factor (NSF) as well as the t-SNAREs syntaxin 2,4 and SNAP-23 in alpha granule secretion. Syntaxin 2 and SNAP-2361 regulate dense granule release, while both syntaxin 2 and 4 together with SNAP-23 regulate lysosome release.62 However, the role of Rab27a (or in fact of any Rab) in these important platelet vesicle trafficking processes has not been investigated in in vitro systems.
The normal lysosomal secretory rate in ashen and gunmetal19platelets suggests that Rab proteins are unlikely to control either the levels or secretion of most lysosomal proteins, an exception being the secretory lysosome of cytotoxic T lymphocytes.32 The calcium-sensing proteins, synaptotagmins, have been implicated in regulating lysosomal enzyme secretion from mast cells (synaptotagmins I and II)63 and fibroblasts (synaptotagmin VII).64 The lack of effect of Rab27a on lysosomes combined with obvious effects on melanosomes and platelet-dense granules is consistent with the fact that a wide variety of genetic and biochemical evidence29,51,61,65 66 indicates that these granules exhibit distinct features though they are regulated by overlapping mechanisms.
Although additional experiments are required to define the background gene(s) responsible for the major differences in platelet phenotypes of the 2 ashen mutants studied here, the available experimental results have immediate important implications for (1) platelet-dense granule synthesis and (2) patients with Griscelli syndrome and HPS with abnormalities in Rab27a. Large differences in platelet-dense granule contents and associated bleeding times were observed between the inbred C3H/HeSnJ ash/ash and a/a, ash/ashmutant mice on the C57BL/6J inbred strain background despite the facts that these 2 mutants have identical hypopigmentation and the identical deleterious mutation in Rab27a. C3H/HeSnJ ash/ashmutants exhibit a severe form of platelet dense granule deficiency, while the dense granules of a/a, ash/ashmutants are essentially normal. These results indicate that resistant forms or combinations of background genes (in this case contributed by the C57BL/6J inbred strain background) are able to compensate for a Rab27a deficiency to form normal granules in platelets (but not melanocytes). A corollary is that the ashen mutation on the C57BL/6J background is a more suitable model for the patients with Griscell syndrome thus far described, while the ashen mutation on the C3H/HeSnJ background is a more suitable model for patients with HPS.
Similarly, bleeding in patients with Griscelli syndrome or HPS with abnormal hypopigmentation may or may not be problematic depending on the presence or absence of compensating background genes. No systematic studies of the effects of differing background genes in patients with Griscelli syndrome and HPS have been reported. However, differing modifying genes clearly influence the expression of HPS traits since significant variability in severity of hypopigmentation, ceroid accumulation, and platelet aggregation have long been noted67 among Puerto Rican patients containing the identical mutation in the HPS1 gene. While prolonged bleeding has not yet been reported in patients with Griscelli syndrome with Rab27a mutations, our results suggest that hemorrhaging will be problematic for patients with Griscelli sundrome containing a sensitive genetic background similar to that of C3H/HeSnJ ash/ash mice.
We thank Debra Tabaczynski, Aaron Mammoser, Diane Poslinski, and Mary Kay Ellsworth for excellent technical assistance.
Supported by grants HL51480, HL31698, and EY12104 (R.T.S.), by the Roswell Park Cancer Institute Cancer Center support grant CA 16056, and by the National Cancer Institute, DHHS (N.A.J. and N.G.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard T. Swank, Molecular and Cellular Biology Department, Roswell Park Cancer Institute, Carlton and Elm Streets, Buffalo, NY 14263; e-mail: richard.swank@roswellpark.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal