Abstract
Liposomes carrying both recombinant glycoprotein Ia/IIa (rGPIa/IIa) and Ibα (rGPIbα) (rGPIa/IIa-Ibα-liposomes) instantaneously and irreversibly adhered to the collagen surface in the presence of soluble von Willebrand factor (VWF) at high shear rates, in marked contrast with translocation of liposomes carrying rGPIbα alone on the VWF surface. In the absence of soluble VWF, the adhesion of rGPIa/IIa-Ibα-liposomes to the collagen surface decreased with increasing shear rates, similar to liposomes carrying rGPIa/IIa alone. While adhesion of liposomes with exofacial rGPIa/IIa and rGPIbα densities of 2.17 × 103 and 1.00 × 104molecules per particle, respectively, was efficient at high shear rates, reduction in rGPIbα density to 5.27 × 103molecules per particle resulted in decreased adhesion even in the presence of soluble VWF. A 50% reduction in the exofacial rGPIa/IIa density resulted in a marked decrease in the adhesive ability of the liposomes at all shear rates tested. The inhibitory effect of antibody against GPIbα (GUR83-35) on liposome adhesion was greater at higher shear rates. Further, the anti-GPIa antibody (Gi9) inhibited liposome adhesion more than GUR83-35 at all shear rates tested. These results suggest that the rGPIa/IIa–collagen interaction dominates the adhesion of rGPIa/IIa-Ibα-liposomes to the collagen surface at low shear rates, while the rGPIa/IIa–collagen and rGPIbα–VWF interaction complements each other, and they synergistically provide the needed functional integration required for liposome adhesion at high shear rates. This study thus has confirmed for the first time the proposed mechanisms of platelet adhesion to the collagen surface under flow conditions using the liposome system.
Introduction
The basic and important platelet functions for primary hemostasis are adhesion and aggregation, and this can be easily understood from the observations that patients with congenital platelet membrane defects such as Bernard-Soulier syndrome or Glanzmann thrombasthenia are deficient in platelet adhesion or aggregation and have severe bleeding tendencies. The contribution of specific platelet receptors or adhesive proteins to platelet adhesion and aggregation onto immobilized collagen under flow conditions is usually studied with monoclonal antibodies or inhibitors specific to particular platelet receptors or adhesive proteins or, also, with blood from patients with congenital bleeding disorders deficient in specific receptors or adhesive proteins. These analyses indicate that initial platelet adhesion depends on the interaction of glycoprotein (GP) Ib/IX/V complexes on platelets with von Willebrand factor (VWF) adsorbed on the collagen surface. This is a rapid but low-affinity interaction, suggesting that it serves to tether platelets, flowing at high speed in the bloodstream, to the collagen surface.1-4 The collagen receptors of the tethered platelets then bind strongly with the collagen surface, activating platelets to form aggregates. This was supported by observations that platelets deficient in one of the collagen receptors failed to adhere and form aggregates on subendothelium or the collagen surface under flow conditions.4,5 GPIa/IIa and GPVI are known to be involved in platelet adhesion under static conditions.6,7 GPIa/IIa (integrin α2β1, VLA2, CD49b/29) is a member of the integrin family of heterodimeric molecules that mediate both cell-to-cell adhesion and adhesion between cells and the extracellular matrix.8 GPIa/IIa is also a major collagen receptor in platelets.9-11 Although GPIa/IIa-mediated adhesion appears to be an essential primary step in collagen–platelet interactions, the functional integration of the distinct adhesion pathways involved in the initiation of platelet adhesion has not yet been defined. To address this issue, we prepared liposomes with covalently bound recombinant GPIa/IIa (rGPIa/IIa)12 and/or recombinant fragments of GPIbα consisting of residues 1 to 302 (rGPIbα)13 and evaluated their interaction with the collagen or VWF surface under flow conditions in the absence of other platelet components. Previously, we reported that liposomes carrying rGPIbα (rGPIbα-liposomes) reversibly interact with the VWF surface under flow conditions, depending on the shear rate and the densities of receptor and matrix, and the interaction is directly related to shear rate.14 The purpose of the present study was to examine how GPIa/IIa and GPIbα contribute to platelet adhesion to the collagen surface under flow conditions in an in vitro reconstituted system, using liposomes carrying both rGPIa/IIa and rGPIbα (rGPIa/IIa-Ibα-liposomes). Our results suggest that rGPIa/IIa and rGPIbα reconstituted into liposomes retain hemostatic functions under flow conditions in vitro, and direct interaction of rGPIa/IIa with the collagen surface dominates the adhesion of rGPIa/IIa-Ibα-liposomes to the collagen surface at low shear rates. At high shear rates, tethering of liposomes through the interaction between rGPIbα and the VWF-adsorbed collagen surface reduces the velocity of liposomes, enabling binding of rGPIa/IIa to the collagen surface. This is the first study to prove the proposed mechanisms of platelet adhesion to the collagen surface involving 2 distinct receptor–ligand pairs with unique properties, GPIa/IIa–collagen and GPIbα–VWF, using reconstituted system, rGPIa/IIa-Ibα-liposomes.
Materials and methods
Materials
N-glutaryl-phosphatidylethanolamine (NGPE), egg phosphatidylcholine (EPC), and N-(lissamine rhodamine B sulfonyl) phosphatidylethanolamine (N-Rh-PE) were purchased from Avanti (Birmingham, AL). Cholesterol (CHO), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI), n-octyl-d-glucopyranoside (OG), bovine serum albumin (BSA), HEPES, and MES were obtained from Sigma Chemical (St Louis, MO). N-hydroxysulfosuccinimide (NHSS) was obtained from Pierce Chemical (Rockford, IL). Mouse monoclonal antibody against GPIbα (purified immunoglobulin G [IgG]), GUR83-35, was made against crude glycocalicin fraction extracted from washed human platelets.15 A mouse anti-GPIa monoclonal antibody, Gi9, and a mouse anti-GPIIa monoclonal antibody, Lia1/2, were purchased from Immunotech (Marseille, France). Sephadex G-25 and Sephadex G-75 were obtained from Pharmacia Biotech (Uppsala, Sweden). The phospholipid-test Wako was from Wako (Osaka, Japan). The F-kit CHO was obtained from Boehringer Mannheim (Mannheim, Germany). Nonidet P-40 was obtained from Nacalai Tesque (Kyoto, Japan). Expression and purification of rGPIbα containing the VWF binding site (residues 1 to 302) were performed as described by Murata et al.13Specific binding of VWF to rGPIbα was assayed by measuring the125I-VWF binding to rGPIbα.13 Preparation of the extracellular domain of rGPIa/IIa in which α2 and β1 chains were covalently bound by disulfide bond was performed according to the following method. Thus, DNA fragments encoding the extracellular domain of GPIa16 and GPIIa17 were amplified by polymerase chain reaction using template complementary DNA obtained from human fibroblast cell line MRC-5 (ATCC CCL 171) and primers. Polymerase chain reaction products were subcloned into the pBlueScriptIISK(+) (Stratagene, La Jolla, CA), and then the fragments of resultant plasmid were introduced into the expression vector pcDLSRa,18respectively. One milligram each of the expression plasmids was mixed with 0.1 mg each of pSV2dhfr (Gibco, New York, NY) and pSV2neo (Gibco), and the mixture was introduced into dihydrofolic acid reductase–deficient CHO cells (ATCC CRL 9096) using lipofection reagent (Gibco). Then the cells were cultured in the nucleic acid–free modified Eagle medium containing 10% fetal bovine serum and 1 mg/mL neomycin (Gibco), and resistant cells were cloned by the limiting dilution method. The rGPIa/IIa-producing CHO clone was cultured using EX-CELL 301 media (JRH Bioscience, Lenexa, KS) without serum. The culture supernatant was collected and concentrated by ultrafiltration. The rGPIa/IIa was purified by collagen Sepharose affinity column chromatography by the method described previously.19 The eluates by 20 mM Tris-HCl (pH 7.5) containing 10 mM ethylenediaminetetraacetic acid and 150 mM NaCl were further purified by gel filtration chromatography (TSKGel3000SW; TOSO, Tokyo, Japan). The purity of the obtained rGPIa/IIa was more than 95% by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Coomassie blue staining. Apparent dissociation constant values for the binding of rGPIa/IIa to collagen was determined using an enzyme-linked immunosorbent assay (ELISA).12 Human VWF was purified from human plasma cryoprecipitate. Purification steps were performed according to the previously described method.20 The VWF preparations used in our experiments had a VWF concentration of 2 mg/mL, specific activity of 200 U/mL ristocetin cofactor activity, and 210 U/mL VWF antigen. Polycarbonate was obtained from Mitsubushi Engineering (Tokyo, Japan). Glass slides were purchased from Corning (New York, NY). All other chemicals were of analytical grade or better.
Preparation of reconstituted blood
Blood drawn from a healthy volunteer was mixed with a 1:10 volume of acid-citrate-dextrose composed of 2.2% (wt/vol) sodium citrate, 0.8% (wt/vol) citric acid, and 2.2% (wt/vol) glucose (ACD). The blood was centrifuged at 100g for 15 minutes at room temperature, and the platelet-rich plasma on top of the erythrocytes was replaced with an equal volume of 0.9% NaCl solution containing 10% (vol/vol) ACD (10% ACD-saline). Red cells were resuspended and centrifuged at 2200g for 10 minutes at room temperature, and the supernatant was replaced with 10%ACD-saline. Each procedure was repeated twice. For perfusion studies, the red cells were reconstituted to 37.5% of the hematocrit (Hct) using 0.9% NaCl solution. The residual platelet count was 1.25 × 104/μL (12.5 × 109/L). The Hct and platelet concentrations were determined using an automated hematology analyzer (SYSMEX, Kobe, Japan).
Preparation of liposomes
Liposomes were prepared by the detergent-dialysis method,21,22 originally developed for the reconstitution of membrane proteins, using the detergent OG. The protein was first conjugated to NGPE in the presence of detergent. The conjugated protein was then mixed with the lipid-detergent mixture, and the incorporation of protein is achieved upon the removal of the detergent by dialysis. Thus, NHSS (0.1 M in H2O) and EDCI (0.25 M in H2O) were added to NGPE solubilized with 2% (wt/vol) OG in 50 mM MES buffer, pH 5.5, and the mixture was incubated for 10 minutes at room temperature. NGPE with an NHSS-activated carboxylic derivative was purified using a Sephadex G-25 column with 50 mM HEPES/0.1% (wt/vol) OG, pH 8.0, and was added to a solution of recombinant protein. The resultant solution was incubated for 12 hours at 4°C with gentle stirring. For rhodamine-labeled liposome preparation,23 a thin film of the lipid mixture containing EPC, CHO, and N-Rh-PE in a molar ratio of 2:1:0.024 was solubilized with OG in 50 mM HEPES/110 mM NaCl buffer, pH 7.4. The resultant solution was mixed vigorously with the NGPE-conjugated protein. The liposomes were then purified using a Sephadex G-75 column, CsCl density gradient centrifugation, and dialysis against 0.9% NaCl. Control liposomes were made with EPC, CHO, and N-Rh-PE in a molar ratio of 2:1:0.024 in the absence of the NGPE-conjugated protein. The liposomes were extruded repeatedly through double-stacked 1.0- and 0.8-μm pore-size polycarbonate membranes (Whatman/Nuclepore, Clifton, NJ) in a high-pressure extrusion cell (Lipex Biomembrane, Vancouver, British Columbia, Canada) as described before24 to produce a final mean diameter range of 800 to 900 nm. Liposomes with different protein-to-lipid ratios were obtained by altering the initial protein-to-lipid ratio. EPC and CHO were quantified using a phospholipid-test Wako and F-kit CHO, respectively. The exofacial densities of rGPIa/IIa and rGPIbα were determined using an ELISA with Integrin β1 EIA Kit (Takara Shuzo, Otsu, Japan) and Glycocalicin EIA Kit (Takara Shuzo), respectively. The exofacial density of rGPIa/IIa was also determined using an ELISA with anti-GPIIa monoclonal antibody, Lia1/2, and horseradish peroxidase–conjugated functional anti-GPIa monoclonal antibody, HRP-Gi9. The amount of rGPIa/IIa or rGPIbα associated with the liposome bilayer was determined using the same method as described above in the presence of 1% (vol/vol) Nonidet P-40. The rGPIa/IIa and rGPIbα solutions were used as standards for measuring receptor density. Absorbance at 492 nm was measured with an Easy Reader EAR 340 (SLT-Lab Instruments, Grodig, Austria). The exofacial densities of rGPIa/IIa determined with Integrin β1 EIA Kit and Lia1/2/HRP-Gi9 system were very consistent within standard deviation. The liposome size was measured with a dynamic light scattering technique using a particle analyzer N4 PLUS (Beckman, Fullerton, CA). The particle numbers of liposomes were calculated based on particle size, EPC concentration, bilayer thickness (15.0 nm), and EPC specific gravity (1.0305).
Preparation of the immobilized collagen surface
Glass slides, 2.5-cm diameter and 0.5-mm thick, were spin-coated with 6% (wt/vol) polycarbonate solution in tetrachloroethane. The glass slides were then incubated with 30 μg/mL porcine tendon acid soluble type I collagen (Nitta Gelatin, Osaka, Japan) in phosphate-buffered saline overnight at 4°C followed by blocking with 1% (wt/vol) BSA in phosphate-buffered saline. After removing excess BSA with 3 sequential phosphate-buffered saline rinses, the glass slides were assembled in the chamber to measure the interaction of the liposomes with the immobilized collagen.
Measurements of the interaction of the liposomes with immobilized collagen
The interaction of rhodamine-labeled liposomes with immobilized collagen was studied using a recirculating chamber, mounted on an epifluorescence microscope, (ECLIPS TE300, Nikon, Tokyo, Japan), using the excitation and emission wavelengths of 550 and 590 nm, respectively. This allowed direct visualization in real time of the liposome interaction with the collagen surface, which was recorded with a videocassette recorder. The flow chamber consisted of upper lid, packing, and glass slide. The upper lid had a depression of 0.030 cm perpendicular to the blood flow and served as part of the roof of the flow chamber that was formed when the upper lid and the glass slide were joined with 4 screws. The packing, hollowed out of a square 1.5 × 1.5 cm, was put between the upper lid and the glass slide, making a flow chamber with a width, length, and depth of 1.5 × 1.5 cm by 0.030 cm. The wall shear rate (γW) is given by the Muggli equation25: γW = 1.03 × 6Q/ab2, where Q is the flow rate (cm3/sec), and a and b are the chamber width and height (cm).
Perfusion studies were performed in the presence of liposomes at a final particle number of 2.5 × 105/μL, Hct 37.5%, platelet count 1.25 × 104/μL (12.5 × 109/L), 2 mM Mg2+, 10 μg/mL soluble VWF, and 37°C. Some experiments were performed in the absence of soluble VWF. Single-frame images of the liposomes interacting with the surface were obtained using the image processor ARGUS-50 (Hamamatsu Photonics, Hamamatsu, Japan). The percentages of surface coverage of liposomes were obtained using the image processor ARGUS-20 (Hamamatsu Photonics). For the inhibition experiments, the liposomes were incubated with 10 μg/mL mouse anti-GPIbα monoclonal antibody, GUR83-35, 10 μg/mL mouse anti-GPIa monoclonal antibody, Gi9, or 10 μg/mL control mouse IgG for 5 minutes at 37°C before perfusion.
Results
Adhesion of rGPIa/IIa-liposomes to the collagen surface under flow conditions
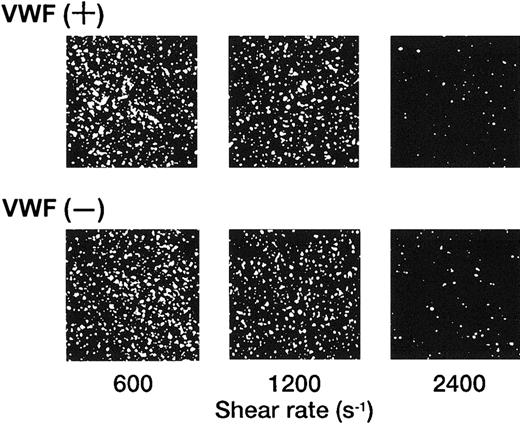
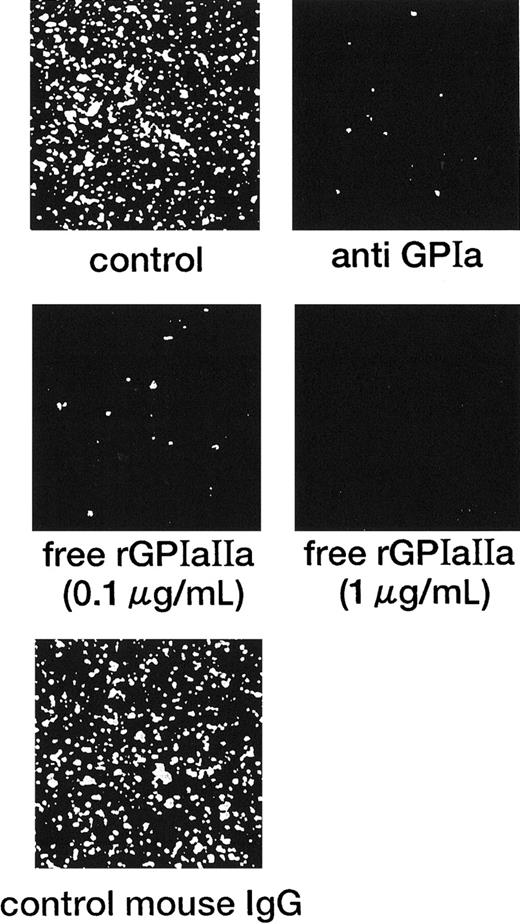
In marked contrast with the translocation of rGPIbα-liposomes on the VWF surface,14 rGPIa/IIa-liposomes instantaneously and irreversibly adhered to the collagen surface. Each single frame shown in Figure 1 was obtained after 3 minutes of perfusion of rGPIa/IIa-liposomes with an exofacial rGPIa/IIa density of 2.22 × 103 molecules per particle on the collagen surface at different shear rates, as indicated. When exposed to shear rates of 600 s−1 for 3 minutes, the percentages of surface coverage of rGPIa/IIa-liposomes were 23.0% ± 2.2% and 23.8% ± 2.0%, in the presence and absence of soluble VWF, respectively. At a shear rate of 2400 s−1, the percentages of surface coverage remarkably decreased to 3.5% ± 0.6% and 3.0% ± 0.6%, in the presence and absence of soluble VWF, respectively. No interaction was observed between rGPIa/IIa-liposomes and the BSA surface at any shear rates tested regardless of whether or not soluble VWF was present (data not shown). The liposome adhesion was abolished by preincubation of the liposomes with the functional anti-GPIa monoclonal antibody, Gi9, or in the presence of free rGPIa/IIa (Figure 2). No effect of control mouse IgG on the liposome adhesion was observed (Figure 2). These results indicate that rGPIa/IIa-liposomes retain a receptor function against immobilized collagen, and the targeting of rGPIa/IIa-liposomes is specific to the collagen surface under flow conditions. Also, the adhesion of rGPIa/IIa-liposomes is more efficient in lower flow environments and is independent of VWF.
Dependence of the interaction of rGPIa/IIa-liposomes with the collagen surface on shear rate.
Images were obtained after 3 minutes of perfusion on the collagen surface at different shear rates, as indicated, in the presence and absence of 10 μg/mL soluble VWF. Liposomes have an exofacial rGPIa/IIa density of 2.22 × 103 molecules per particle.
Dependence of the interaction of rGPIa/IIa-liposomes with the collagen surface on shear rate.
Images were obtained after 3 minutes of perfusion on the collagen surface at different shear rates, as indicated, in the presence and absence of 10 μg/mL soluble VWF. Liposomes have an exofacial rGPIa/IIa density of 2.22 × 103 molecules per particle.
Inhibitory effect of the anti-GPIa monoclonal antibody, Gi9, and free rGPIa/IIa on the interaction of rGPIa/IIa-liposomes with the collagen surface under flow conditions.
Control: images were obtained after 3 minutes of perfusion at a shear rate of 600 s−1. Liposomes have an exofacial rGPIa/IIa density of 2.22 × 103 molecules per particle. Anti-GPIa: experimental conditions were the same as control except that rGPIa/IIa-liposomes were incubated with 10 μg/mL Gi9 for 5 minutes at 37°C before perfusion. Free rGPIa/IIa: experimental conditions were the same as for the control except that 0.1 or 1.0 μg/mL free rGPIa/IIa was present in perfusion solutions. Control mouse IgG: experimental conditions were the same as control except that rGPIa/IIa-liposomes were incubated with 10 μg/mL control mouse IgG for 5 minutes at 37°C before perfusion.
Inhibitory effect of the anti-GPIa monoclonal antibody, Gi9, and free rGPIa/IIa on the interaction of rGPIa/IIa-liposomes with the collagen surface under flow conditions.
Control: images were obtained after 3 minutes of perfusion at a shear rate of 600 s−1. Liposomes have an exofacial rGPIa/IIa density of 2.22 × 103 molecules per particle. Anti-GPIa: experimental conditions were the same as control except that rGPIa/IIa-liposomes were incubated with 10 μg/mL Gi9 for 5 minutes at 37°C before perfusion. Free rGPIa/IIa: experimental conditions were the same as for the control except that 0.1 or 1.0 μg/mL free rGPIa/IIa was present in perfusion solutions. Control mouse IgG: experimental conditions were the same as control except that rGPIa/IIa-liposomes were incubated with 10 μg/mL control mouse IgG for 5 minutes at 37°C before perfusion.
Adhesion of rGPIa/IIa-Ibα-liposomes to the collagen surface under flow conditions
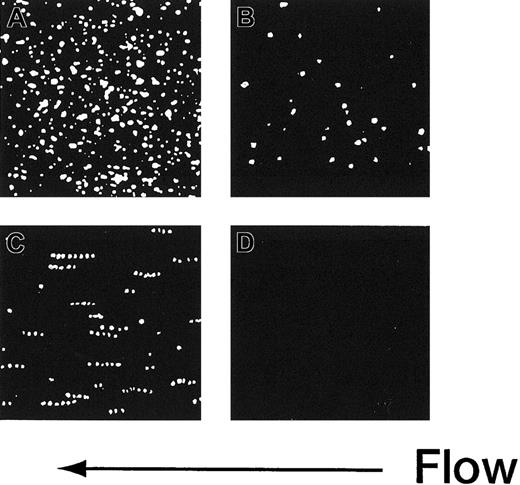
The adhesion of rGPIa/IIa-Ibα-liposomes to the collagen surface was also instantaneous and irreversible. The images shown in Figure 3 are composites created by the superimposition of 30 successive frames, taken at 66-millisecond intervals. In the case of rGPIa/IIa-Ibα- or rGPIa/IIa-liposomes, the fluorescent dots of the liposomes stayed on the collagen surface, representing irreversible adhesion of the liposomes to the surface (Figure 3A,B). Short tracks formed by closely spaced fluorescent dots of rGPIbα-liposomes extending in the direction of flow can be seen, demonstrating transient interaction of rGPIbα-liposomes with VWF adsorbed on the collagen surface (Figure3C). No interaction of control liposomes with the collagen surface was observed (Figure 3D).
Time-lapse analysis of liposome movement on the collagen surface.
Each image is a composite created by the superimposition of 30 successive frames, taken at 66-millisecond intervals, Hct of 37.5%, shear rate of 2400 s−1, platelet count of 1.25 × 104/μL (12.5 × 109/L), 10 μg/mL soluble VWF, and 37°C. (A) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.17 × 103 and 1.00 × 104 molecules per particle, respectively. (B) The rGPIa/IIa-liposomes with exofacial density of rGPIa/IIa at 2.21 × 103 molecules per particle. (C) The rGPIbα-liposomes with exofacial density of rGPIbα at 1.16 × 104 molecules per particle. (D) Control liposomes.
Time-lapse analysis of liposome movement on the collagen surface.
Each image is a composite created by the superimposition of 30 successive frames, taken at 66-millisecond intervals, Hct of 37.5%, shear rate of 2400 s−1, platelet count of 1.25 × 104/μL (12.5 × 109/L), 10 μg/mL soluble VWF, and 37°C. (A) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.17 × 103 and 1.00 × 104 molecules per particle, respectively. (B) The rGPIa/IIa-liposomes with exofacial density of rGPIa/IIa at 2.21 × 103 molecules per particle. (C) The rGPIbα-liposomes with exofacial density of rGPIbα at 1.16 × 104 molecules per particle. (D) Control liposomes.
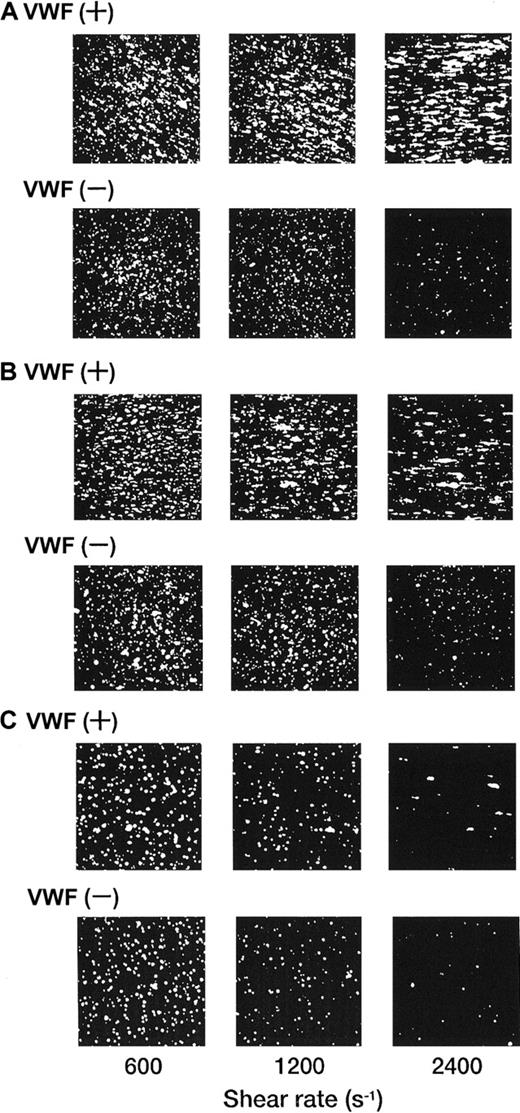
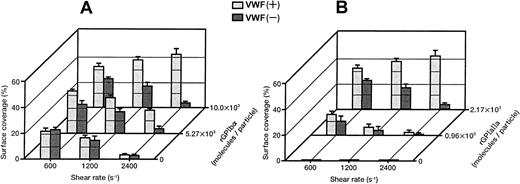
Each single-frame image shown in Figure 4was obtained after 3 minutes of perfusion of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIbα and rGPIa/IIa. The adhesion of rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα of 2.17 × 103 and 1.00 × 104 molecules per particle, respectively, was more efficient at high shear rates in the presence of soluble VWF (Figure 4A). When exposed to shear rates of 600 s−1, the percentages of surface coverage of the liposomes were estimated to be 33.1% ± 2.3% and 23.1% ± 0.8%, in the presence and absence of soluble VWF, respectively. At a shear rate of 2400 s−1, the surface coverage increased to 43.2% ± 3.8% in the presence of soluble VWF. In the absence of soluble VWF, however, the surface coverage decreased to 3.8% ± 0.7%, as observed with rGPIa/IIa-liposomes. The reduction of the exofacial density of rGPIbα by almost 50% (5.27 × 103 molecules per particle), while the exofacial density of rGPIa/IIa was kept constant at approximately 2.00 × 103 molecules per particle, resulted in a decreased surface coverage at high shear rates (Figure4B). The surface coverage decreased from 33.8% ± 0.9% to 19.2% ± 0.8% with an increasing shear rate from 600 to 2400 s−1 in the presence of soluble VWF. In the absence of soluble VWF, the percentages of surface coverage decreased from 24.4% ± 0.9% to 4.6% ± 0.8% with an increasing shear rate from 600 to 2400 s−1 as observed with rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα of 2.17 × 103 and 1.00 × 104molecules per particle, respectively. These results suggest that the interaction of rGPIbα on the liposome surface with the collagen surface is negligible in the absence of soluble VWF. A 50% reduction in the exofacial rGPIa/IIa density resulted in decreased adhesion by the liposomes at all shear rates tested (Figure 4C). The percentages of the surface coverage of the liposomes with exofacial densities of rGPIa/IIa and rGPIbα of 0.96 × 103 and 1.08 × 104 molecules per particle, respectively, decreased from 16.9% ± 2.2% to 3.1% ± 0.9% with increasing shear rates from 600 to 2400 s−1 in the presence of soluble VWF. In the absence of soluble VWF, the surface coverage decreased from 10.8% ± 3.9% to 0.8% ± 0.4%.
Dependence of adhesion of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIa/IIa and rGPIbα on shear rate, in the presence and absence of VWF.
Images were obtained after 3 minutes of perfusion on the collagen surface at different shear rates, as indicated, in the presence and absence of 10 μg/mL soluble VWF. (A) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.17 × 103 and 1.00 × 104 molecules per particle, respectively. (B) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.19 × 103 and 5.27 × 103 molecules per particle, respectively. (C) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 0.96 × 103 and 1.08 × 104 molecules per particle, respectively.
Dependence of adhesion of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIa/IIa and rGPIbα on shear rate, in the presence and absence of VWF.
Images were obtained after 3 minutes of perfusion on the collagen surface at different shear rates, as indicated, in the presence and absence of 10 μg/mL soluble VWF. (A) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.17 × 103 and 1.00 × 104 molecules per particle, respectively. (B) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.19 × 103 and 5.27 × 103 molecules per particle, respectively. (C) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 0.96 × 103 and 1.08 × 104 molecules per particle, respectively.
Surface coverage of the liposomes with different exofacial rGPIbα densities (1.00 × 104, 5.27 × 103, and 0 molecules per particle), and an equivalent exofacial rGPIa/IIa density at approximately 2.00 × 103 molecules per particle, are shown in Figure5A. It is clear that high densities of rGPIbα on the liposome surface and the presence of soluble VWF are required for efficient adhesion to the collagen surface at high shear rates. The surface coverage of the liposomes with different exofacial rGPIa/IIa densities (2.17 × 103, 0.96 × 103, and 0 molecules per particle), while the exofacial rGPIbα density was kept constant at approximately 1.00 × 104 molecules per particle, is shown in Figure5B. The liposomes carrying rGPIbα alone at an exofacial density of 1.16 × 104 molecules per particle never formed a stationary adhesion on the collagen surface, but stopped transiently in the millisecond range on the surface, demonstrating the tethering of the liposomes to VWF adsorbed on the collagen surface (Figure 5B, front row). The duration of contact with the surface was calculated to be less than 33 milliseconds. These results suggest that the rGPIa/IIa–collagen interaction is important not only in lower flow environments, but also at high shear rates, and that rGPIa/IIa and rGPIbα cooperatively contribute to the liposome adhesion, especially at high shear rates.
Percentages of surface coverage of rGPIa/IIa-Ibα-liposomes on the collagen surface.
Dependence on shear rate, densities of rGPIa/IIa and rGPIbα, and VWF. Values are the mean ± SD; n = 6. (A) Percentages of surface coverage of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIbα, as indicated. The exofacial density of rGPIa/IIa was kept constant at 2.19 × 103 ± 0.02 × 103 molecules per particle. (B) Percentages of surface coverage of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIa/IIa, as indicated. The exofacial density of rGPIbα was kept constant at 1.08 × 104 ± 0.08 × 104molecules per particle. The percentages of surface coverage of rGPIbα-liposomes on the collagen surface in the presence and absence of soluble VWF (front row) were 0.07% ± 0.02% at all shear rates tested.
Percentages of surface coverage of rGPIa/IIa-Ibα-liposomes on the collagen surface.
Dependence on shear rate, densities of rGPIa/IIa and rGPIbα, and VWF. Values are the mean ± SD; n = 6. (A) Percentages of surface coverage of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIbα, as indicated. The exofacial density of rGPIa/IIa was kept constant at 2.19 × 103 ± 0.02 × 103 molecules per particle. (B) Percentages of surface coverage of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIa/IIa, as indicated. The exofacial density of rGPIbα was kept constant at 1.08 × 104 ± 0.08 × 104molecules per particle. The percentages of surface coverage of rGPIbα-liposomes on the collagen surface in the presence and absence of soluble VWF (front row) were 0.07% ± 0.02% at all shear rates tested.
Inhibitory effect of anti-rGPIbα or anti-rGPIa antibody on the liposome adhesion to the collagen surface under flow conditions
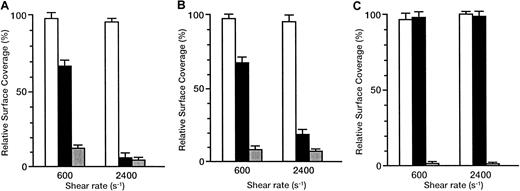
The inhibitory effects of antibodies are shown as the relative surface coverage, that is, the surface coverage in the presence of antibody relative to that in the absence of antibody (Figure6). When the rGPIbα–VWF axis was blocked by the anti-rGPIbα antibody, GUR 83-35, the liposomes still adhered irreversibly to the collagen surface in a shear rate–dependent fashion. The relative surface coverage decreased from 66.2% ± 3.9% to 6.5% ± 2.6% with the shear rate increasing from 600 to 2400 s−1 for the liposomes with exofacial densities of rGPIa/IIa and rGPIbα of 2.17 × 103 and 1.00 × 104 molecules per particle, respectively (Figure6A). The same trend was observed for liposomes with exofacial densities of rGPIa/IIa and rGPIbα of 2.19 × 103 and 5.27 × 103 molecules per particle, respectively (Figure6B), although the inhibitory effects were smaller than those in liposomes with a higher exofacial density of rGPIbα at a shear rate of 2400 s−1 (compare Figures 6A and 6B). No effect of GUR 83-35 was observed for the liposomes carrying rGPIa/IIa alone (Figure6C). These results suggest that the inhibitory effect of GUR 83-35 is greater at high shear rates and the extent of dependence of liposome adhesion on the rGPIbα–VWF interaction is greater at higher shear rates. When the rGPIa/IIa–collagen axis was blocked by the anti-rGPIa antibody, Gi9, the liposome displacement on the surface was observed, as with the rGPIbα-liposomes on the collagen surface. The inhibitory effect of Gi9 was always greater than that of GUR 83-35, especially at low shear rates. No effect of control mouse IgG on the liposome adhesion was observed. These observations indicate that both the rGPIa/IIa–collagen interaction and the tethering of the liposomes by the rGPIbα–VWF interaction are required for liposome adhesion, and they synergistically contribute to stable adhesion of rGPIa/IIa-Ibα-liposomes, especially at high shear rates.
Inhibitory effect of GUR83-35 or Gi9 on the adhesion of rGPIa/IIa-Ibα-liposomes to the collagen surface under flow conditions.
Relative surface coverage of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIa/IIa and rGPIbα, in the presence of specific antibody and 10 μg/mL soluble VWF, are shown. Values are the mean ± SD; n = 6. White bar indicates control mouse; black bar, GUR83-35 (+); grey bar, Gi9(+). (A) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.17 × 103 and 1.00 × 104molecules per particle, respectively. (B) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.19 × 103 and 5.27 × 103 molecules per particle, respectively. (C) The rGPIa/IIa-liposomes with an exofacial density of rGPIa/IIa at 2.22 × 103 molecules per particle.
Inhibitory effect of GUR83-35 or Gi9 on the adhesion of rGPIa/IIa-Ibα-liposomes to the collagen surface under flow conditions.
Relative surface coverage of rGPIa/IIa-Ibα-liposomes with different exofacial densities of rGPIa/IIa and rGPIbα, in the presence of specific antibody and 10 μg/mL soluble VWF, are shown. Values are the mean ± SD; n = 6. White bar indicates control mouse; black bar, GUR83-35 (+); grey bar, Gi9(+). (A) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.17 × 103 and 1.00 × 104molecules per particle, respectively. (B) The rGPIa/IIa-Ibα-liposomes with exofacial densities of rGPIa/IIa and rGPIbα at 2.19 × 103 and 5.27 × 103 molecules per particle, respectively. (C) The rGPIa/IIa-liposomes with an exofacial density of rGPIa/IIa at 2.22 × 103 molecules per particle.
Discussion
The recognition of exposed subendothelial collagen by blood platelets is a key early step in the formation of a hemostatic plug after vascular injury. Many different platelet surface and platelet surface–associated proteins have been proposed as mediators of platelet–collagen adhesion. Santoro has defined a Mg2+-dependent mechanism of platelet adhesion to collagen6 apparently identical to that observed by Shadle and Barondes26 and have isolated a platelet surface Mg2+-dependent heterodimeric collagen-binding complex composed of platelet membrane GPIa and GPIIa.27 When incorporated into liposomes, the purified complex mediated the Mg2+-dependent adhesion of the liposomes to collagen substrates at static conditions.28,29 The rGPIa/IIa used in this study has an activated form and the specific binding to collagen characterized by a dissociation constant of the same order of magnitude as that for the binding of collagen to GPIa/IIa on activated platelets.12 Also, rGPIbα used in this study has an affinity of interaction with VWF characterized by a dissociation constant of the same order of magnitude as that reported previously for the binding of VWF to GPIb-IX on platelets.13,30 31
In the present study, liposomes carrying rGPIa/IIa and rGPIbα were chosen as a model system used to examine the process of initiating the adhesion of platelets under flow conditions. Such proteoliposomes previously prepared by direct hydration followed by freeze–thawing32 are much too small (diameters of 200 nm or less) to be useful for fluorescence microscopy studies. In addition, liposomes with diameters less than 80 nm have a very high binding energy and thus will not undergo adhesion, with adhesion above this critical size, however, increasing with vesicle size.33 We therefore prepared the liposomes carrying both rGPIa/IIa and rGPIbα by detergent dialysis followed by extrusion through polycarbonate membranes to produce a final mean diameter range of 800 to 900 nm, suitable for adhesion studies with fluorescence microscope under flow conditions.
Our results suggest that 2 distinct substrates, collagen and VWF, are required in order to provide the biomechanical properties necessary to mediate stable liposome adhesion, especially at high shear rates. The rGPIa/IIa supports immediate arrest of flowing liposomes onto the collagen surface but works efficiently only at the lower shear rates, presumably because of a relatively slow rate of bond formation with immobilized collagen and a low resistance of the bond to tensile stress. In contrast, the interaction of rGPIbα with immobilized VWF is inherently not sufficient to arrest the liposomes but results in a very marked decrease in velocity of flowing liposomes, relative to the hydrodynamic flow, when surface contact is established.14Moreover, possibly because of a fast bond formation and the high resistance of the bond to tensile stress, this function is efficiently displayed even at higher shear rates. Thus, rGPIa/IIa is essential for the stability of liposome adhesion to the collagen surface. The interaction of rGPIbα with VWF immobilized on the collagen surface, however, is required first to reduce the velocity of the liposomes contacting the surface under high flow conditions, thereby prolonging the time available for the bond formation of rGPIa/IIa with immobilized collagen. When the shear rate is low, the function of VWF is initially limited because of the reduction of the interactions between rGPIbα and immobilized VWF,14 the soluble VWF and the collagen surface, or both, and the function of rGPIa/IIa as a collagen receptor is efficiently displayed.
Our findings now define a unique function for rGPIa/IIa, expressed by its ability to act in concert with the rGPIbα–VWF interaction to promote stable adhesion of rGPIa/IIa-Ibα-liposomes to the collagen surface. The 2 receptors, rGPIa/IIa and rGPIbα, therefore, have complementary roles, and the corresponding adhesive substrates, collagen and VWF, are also complementary in the adhesion of rGPIa/IIa-Ibα-liposomes.
These results contribute to the long-term purpose of our studies, which is to prepare liposome systems that improve primary hemostasis under thrombocytopenic conditions and that are promising agents for the prophylaxis and treatment of bleeding in patients with severe thrombocytopenia. The simplest type of artificial platelets might be particles carrying platelet membrane proteins and/or ligands of the proteins involved in platelet adhesion and aggregation. Based on this idea, some materials have been developed as platelet substitutes, such as erythrocytes with fibrinogen, or RGD peptides, covalently linked to their surfaces,34,35 liposomes bearing more than 15 kinds of platelet membrane proteins (eg, GPIb, GPIIb/IIIa, GPVI) isolated from the platelet membranes with deoxycholate,36 and fibrinogen-coated albumin microparticles.37,38Some of these are reactive with adhesive ligands or with normal platelets in vitro, or are effective in enhancing hemostatic function in thrombocytopenic or thrombocytopathic animals in vivo. Recently, it has been determined that rGPIa/IIa-liposomes have hemostatic activity in vivo.12 However, no platelet substitute has yet been reported to be effective for hemostasis in large clinical studies so far.
In conclusion, we have developed an effective tool for studying adhesive interactions of platelets under flow conditions and proved that 2 distinct receptor–ligand pairs with unique properties, GPIa/IIa–collagen and GPIbα–VWF, complement each other and synergistically provide the needed functional integration required for platelet adhesion under unfavorable shear forces. Furthermore, our results have demonstrated that the liposomes carrying rGPIa/IIa and/or rGPIbα are the potential candidates for platelet substitutes. Development of effective platelet substitutes using liposome system is now underway.
We thank Welfide Corporation (Osaka, Japan) for preparation of rGPIbα and VWF.
Supported by health science research grants for research on advanced medical technology from the Ministry of Health and Welfare, Tokyo, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Takako Nishiya, Department of Internal Medicine, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo, 160-8582, Japan; e-mail: nishiya@med.keio.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal