Abstract
Tissue factor–induced blood coagulation was studied in 20 individuals, for varying periods of time during 54 months, in contact pathway–inhibited whole blood at 37°C and evaluated in terms of the activation of various substrates. After quenching over time with inhibitors, the soluble phases were analyzed for thrombin–antithrombin III (TAT) complex formation, prothrombin fragments, platelet activation (osteonectin release), factor Va generation, fibrinopeptide (FP) A and FPB release, and factor XIII activation. TAT complex formation, for 35 experiments, showed an initiation phase (up to 4.6 ± 0.6 minutes) in which thrombin was generated at an average rate of 0.93 ± 0.3 nM/min catalyzed by about 1.3 pM prothrombinase yielding approximately 26 nM thrombin. During a subsequent propagation phase, thrombin was generated at a rate of 83.9 ± 3.8 nM/min by about 120 pM prothrombinase, reaching ultimate levels of 851 ± 53 nM. Clot time, determined subjectively, occurred at 4.7 ± 0.2 minutes and correlated with the inception of the propagation phase. The thrombin concentrations associated with the transitions to rapid product formation are 510 ± 180 pM for platelet activation (1.9 ± 0.2 minutes), 840 ± 280 pM for factor XIII activation and factor Va generation (2.2 ± 0.6 minutes), 1.3 ± 0.4 nM for FPA release (2.5 ± 0.7 minutes), 1.7 ± 0.5 nM for FPB release and prethrombin 2 (2.8 ± 0.8 minutes), 7.0 ± 2.2 nM for thrombin B chain (3.6 ± 0.2 minutes), and 26 ± 6.2 nM for the propagation phase of TAT formation (4.6 ± 0.6 minutes). These results illustrate that the initial activation of thrombin substrates occurs during the initiation phase at less than 2 nM thrombin (0.2%). Most thrombin (96%) is formed well after clotting occurs.
Introduction
Blood coagulation is best described as a complex, threshold-limited intertwined set of processes that includes physical, cellular, and biochemical events. Each of the subprocesses leading to thrombin generation can be operationally described by initiation, propagation, and termination phases.1 When the vascular endothelium is damaged, blood factor VIIa comes in contact with exposed/expressed tissue factor (TF) and forms the vitamin K–dependent extrinsic tenase complex, which activates factor X and factor IX.2 Factor Xa on a membrane activates a small amount of prothrombin to thrombin, which activates factor V and factor VIII to their respective active products. During this initiation phase of thrombin generation, the subnanomolar amounts of thrombin produced amplify thrombin production by platelet, factor V, and factor VIII activation, begin to cleave fibrinogen, and activate the protransglutaminase factor XIII. Vigorous thrombin generation occurs during a propagation phase when the kinetically efficient intrinsic tenase (factor IXa–factor VIIIa, Ca++, membrane) activates increased levels of factor X generating the major burst of prothrombinase and thrombin. Thrombin generation is attenuated and terminated by a collection of stoichiometric and enzymatic inhibitors (antithrombin III, tissue factor pathway inhibitor, and the protein C pathway).
Prothrombin is converted to the serine protease α-thrombin, composed of an A′-chain (residues 285-320) and a B chain (residues 321-579). In purified systems, the formation of α-thrombin by prothrombinase occurs via cleavage at Arg320 yielding meizothrombin, which is subsequently cleaved at Arg271 and processed by autolytic cleavage at Arg284.3,4 The enzyme thrombin has long been recognized for its multiple functions in blood coagulation including the proteolytic processes of converting fibrinogen to fibrin (release of fibrinopeptides A [FPA] and B [FPB]),5 activating platelets,6 factor V,7 factor VIII,8 factor XI,9 factor XIII,10 protein C,11 and the thrombin-activatable fibrinolysis inhibitor (TAFI).12These activation processes are initiated at various stages of the dynamic process of thrombin generation. Thrombin possesses at least 4 distinct binding sites for substrates, inhibitors, cofactors, and Na+. The Na+ binding site appears to help determine whether thrombin acts as a procoagulant and recognizes fibrinogen as a substrate (presence of sodium ions) or acts as an anticoagulant and recognizes protein C as a substrate (absence of sodium ions). The other 3 sites, exosite I, exosite II, and the active site recognize a variety of molecules and account for the diverse functions of thrombin.13
The present study was undertaken to evaluate the enzymatic selection and activation of thrombin-sensitive substrates during the dynamic process of TF-initiated coagulation of whole blood.
Patients, materials, and methods
Materials
HEPES, Tris-HCl, EDTA, TFA, bovine brain phosphatidyl serine (PS), and egg yolk phosphatidyl choline (PC) were purchased from Sigma Chemical (St Louis, MO). High-performance liquid chromatography (HPLC) grade H2O and CH3CN were purchased from Fisher Scientific (Pittsburgh, PA). Benzamidine-HCl was purchased from Aldrich (Milwaukee, WI). Recombinant TF was a generous gift from Drs Roger Lundblad and Shu-Len Liu (Hyland Division, Baxter Healthcare, Duarte, CA) and was relipidated in PCPS (25% PS, 75% PC) vesicles by a previously described protocol.14,15 Corn trypsin inhibitor (CTI) was prepared as described.16d-Phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (FPRck) was a generous gift from Dr Richard Jenny (Haematologic Technologies, Essex Junction, VT). Burro α-prethrombin 1 antibody, which recognizes prothrombin, prethrombin 1, prethrombin 2, prothrombin fragment 2, and α-thrombin B chain was prepared at the division of Hematology Research, Mayo Clinic (Rochester, MN).17 Murine monoclonal α-FVaLC#9, which recognizes FVa light chain, was prepared as described.18Rabbit polyclonal α-fXIII (D4679) was a gift from Drs Paul Bishop and Gerry Lasser (Zymogenetics, Seattle, WA). Horseradish peroxidase–labeled goat antirabbit, antimouse, and antihorse IgG antibodies were purchased from Southern Biotechnology (Birmingham, AL). Enzyme-linked immunosorbent assay (ELISA) kits were used to estimate thrombin–antithrombin III (TAT) complex formation (Behring, Westwood, MA), platelet osteonectin release (a gift from Dr Richard Jenny), and FPA generation (Asserachrom FPA; Diagnostica Stago/American Bioproducts, Parsippany, NJ).
Patients
Eleven men and 9 women were recruited and advised according to a protocol approved by the University of Vermont Human Studies Committee. Healthy individuals, mean age 34 ± 12 years, were selected so as to exclude donors with a personal history of thrombosis/hemorrhage or regular aspirin or drug use. Some individuals were studied multiple times over the course of 54 months, to bring the total number of experiments to 42. Contemporaneous coagulation profiles were performed by Fletcher Allen Hematology Clinic (Burlington, VT) for each experiment. All individuals' profiles were normal: prothrombin time (PT; 12.4 ± 0.9 seconds), activated partial thromboplastin time (aPTT; 27.0 ± 2.5 seconds), fibrinogen (2.5 ± 0.4 mg/mL), and platelet count (2.5 ± 0.6 × t 108 platelets/mL).
Whole blood coagulation
Blood was collected by venipuncture at the Clinical Research Center, Fletcher Allen Health Care (Burlington, VT) and aliquoted (1 mL) into tubes containing CTI (50-100 μg/mL) and TF (nominally 12.5-25 pM; functionally ∼5 pM) relipidated in PCPS (1:2000 protein/lipid) as previously described.16,19 20 The TF concentration was changed during the course of the 54 months due to different preparation methods. Based on a functional assay (see below) the concentration of active TF used in whole blood was ∼5 pM. The TF/PCPS concentrations used were chosen to give clot times in the range of 4 to 5 minutes. Samples were quenched at intervals over the course of 20 minutes with a cocktail of inhibitors: 50 mM EDTA and 20 mM benzamidine-HCl in HEPES-buffered saline (HBS), pH 7.4, and 10 μL 10 mM FPRck in 10 mM HCl. The zero time point contained the inhibitors prior to the addition of blood. A control tube, containing CTI and no TF, was used each time. Clot time was determined visually (2 observers). After quenching, samples were centrifuged for 15 minutes at 2000 rpm and clot material separated from the solution phase. Solid and solution phases were stored at −80°C for further analysis.
Determination of active TF in TF/PCPS preparations
Relipidated TF (TF/PCPS: 200 pM/400 nM) and varying concentrations of factor VIIa (0-600 pM) were incubated at 37°C in HBS/2 mM CaCl2/0.1% polyethylene glycol (PEG) containing 10 μM PCPS for 10 minutes (total volume = 250 μL). This was followed by the addition of 170 nM factor X. Aliquots (20 μL) were removed every 30 seconds for 5 minutes and quenched into 160 μL HBS containing 20 mM EDTA. The rate of substrate hydrolysis was observed on addition of 20 μL 2 mM Spectrozyme Xa. Factor Xa generation rate was evaluated from a standard curve using serial dilutions of purified factor Xa. The concentration of active TF was evaluated using a Scatchard plot (factor VIIa bound versus bound/free ratio) assuming 1:1 stoichiometry for TF and factor VIIa.
Immunoassays and HPLC
The ELISAs for TAT, osteonectin, and FPA were performed according to the manufacturers' protocols in duplicate or triplicate using a minimum of 5 standards as previously described.16The operational sensitivity of the TAT ELISA is about 40 pM. Results were analyzed on a Vmax microtiter plate reader equipped with Softmax version 2.35 (both from Molecular Devices, Menlo Park, CA).
The FPA analyses were also performed using HPLC methods. FPA and FPB were isolated and quantitated using reverse-phase (C18 Bakerbond, 4.6 × 250 mm) HPLC methodology.20 Peptide samples were eluted by using linear gradients of H2O/CH3CN/0.05% TFA. The peptides were identified by matrix-assisted laser desorption ionization time of flight mass spectrometry (linear model, PE Applied Biosystems). Data presented for FPA analysis are obtained from the combination of ELISA and HPLC quantitation.
Measure of free thrombin
The rate of FPA generation used to estimate active thrombin concentration was calculated using a modified Michaelis Menten equation that accounts for competitive inhibition as described by Rand et al19 and shown below:
Rates were calculated from the slope of the FPA versus time curve (Figure 2) for the first 7 minutes of production up to about 80% released. The constants Km and kcat for human α-thrombin and fibrinogen are 7.2 μM and 84/s.21 For product inhibition, KI = Km is equal to the free inhibitory product, FPA concentration at time t. [S] is the concentration of free substrate (total fibrinogen-FPA) at time t.
Western analysis
Sodium dodecyl sulfate-polyacrylamide gel electrophoreses (SDS-PAGE) was performed using a modified Laemmeli procedure.19 22 Factor XIIIAa, factor Va light chain, α-thrombin B chain, and a prethrombin 2-like product were analyzed by 5% to 15% SDS-PAGE under either nonreducing or reducing conditions as specified. The actual sequence of the prethrombin 2–like product from whole blood has not been identified, but migrates along with the purified and characterized prethrombin 2 standard. We will refer to this as “prethrombin 2” in the text. The proteins from the electrophorogram were transferred to nitrocellulose membranes using a semidry method followed by blotting with the primary antibodies rabbit α-factor XIII (7.5 μg/mL), mouse α-factor Va#9 (7.5 μg/mL), and burro α-prethrombin 1 (7.5 μg/mL). Secondary horseradish peroxidase–conjugated goat α-rabbit, α-mouse, and α-horse antibodies were used at dilutions of 1:12 000, 1:15 000, and 1:7500, respectively. Time courses of factor XIII activation, factor Va light chain generation, α-thrombin B chain, and prethrombin 2 formation were analyzed and quantified by densitometry of immunoblots on a Hewlett-Packard Scanjet 4C/T (Hewlett-Packard, Palo Alto, CA). Concentrations were determined from horizontal band density comparison of standard protein dilutions. Relative concentrations were determined by normalizing the data with regard to the maximum.
Results
TAT complex formation and prothrombinase concentration
Whole blood from 35 experiments (16 individuals, 10 men and 6 women) was studied over the course of 54 months. The time course of TAT complex formation (♦, TAT [nM] versus time [minute]) is shown as the mean ± SEM with a clot time of 4.7 ± 0.2 minutes (Figures1 and 3A). Complex formation proceeded initially at a rate of 0.93 ± 0.3 nM/min for a duration of 4.6 ± 0.6 minutes. Subsequently, thrombin formation proceeded at a rate of 83.9 ± 3.8 nM/min and reached a maximum level of 851 ± 53 nM (Table 1).
Comparison of TAT complex formation and platelet activation.
Clot time was calculated to be 4.7 ± 0.2 minutes for the group studied. Data are shown as the mean ± SEM. The TAT (♦) composite represents 35 experiments and has an initiation phase duration up to 4.6 ± 0.6 minutes with an increasing rate of propagation of 83.9 ± 3.8 nM/min. The detected maximum level reached was 851 ± 53 nM TAT. Osteonectin (○) released from activated α-granules of platelets (from 18 experiments) showed an initial baseline in whole blood of 6.9 ± 1.2 nM. The propagation phase began at 1.9 ± 0.2 minutes and increased to 44.6 ± 6.7 nM at a rate of 6.8 ± 0.4 nM/min.
Comparison of TAT complex formation and platelet activation.
Clot time was calculated to be 4.7 ± 0.2 minutes for the group studied. Data are shown as the mean ± SEM. The TAT (♦) composite represents 35 experiments and has an initiation phase duration up to 4.6 ± 0.6 minutes with an increasing rate of propagation of 83.9 ± 3.8 nM/min. The detected maximum level reached was 851 ± 53 nM TAT. Osteonectin (○) released from activated α-granules of platelets (from 18 experiments) showed an initial baseline in whole blood of 6.9 ± 1.2 nM. The propagation phase began at 1.9 ± 0.2 minutes and increased to 44.6 ± 6.7 nM at a rate of 6.8 ± 0.4 nM/min.
Thrombin formation and thrombin substrates
| Substrate . | Initiation phase duration (min) . | Thrombin concentration at the inception point (nM) . | Thrombin levels at the inception point (%) . | Rate of propagation (nM/min) . | Maximum levels of products (nM) . | Product generated (%) . |
|---|---|---|---|---|---|---|
| Osteonectin | 1.9 ± 0.2 | 0.51 ± 0.18 | 0.06 | 6.8 ± 0.4 | 44.6 ± 6.7 | 77 |
| FXIIIa | 2.2 ± 0.6 | 0.84 ± 0.28 | 0.099 | 10.3 ± 0.9 | 56.3 ± 3.4 | 62 |
| (11.4 ± 1.0%/min) | (62.5 ± 3.8%) | |||||
| FV | 2.2 ± 0.4 | 0.84 ± 0.28 | 0.099 | 4.5 ± 1.3 | 12.3 ± 2.1 | 62 |
| (22.4 ± 6.3%/min) | (61.7 ± 10.4%) | |||||
| FPA | 2.5 ± 0.7 | 1.3 ± 0.4 | 0.15 | 2 700 ± 200 | 15 300 ± 1 300 | 100 |
| FPB | 2.8 ± 0.8 | 1.7 ± 0.5 | 0.20 | 1 200 ± 100 | 5 800 ± 600 | 39 |
| Thrombin B chain | 3.6 ± 0.2 | 7.0 ± 2.2 | 0.82 | 56.9 ± 2.1 | 494 ± 66 | 35 |
| TAT | 4.6 ± 0.6 | 26 ± 6.2 | 3.1 | 83.9 ± 3.8 | 851 ± 53 | 61 |
| Substrate . | Initiation phase duration (min) . | Thrombin concentration at the inception point (nM) . | Thrombin levels at the inception point (%) . | Rate of propagation (nM/min) . | Maximum levels of products (nM) . | Product generated (%) . |
|---|---|---|---|---|---|---|
| Osteonectin | 1.9 ± 0.2 | 0.51 ± 0.18 | 0.06 | 6.8 ± 0.4 | 44.6 ± 6.7 | 77 |
| FXIIIa | 2.2 ± 0.6 | 0.84 ± 0.28 | 0.099 | 10.3 ± 0.9 | 56.3 ± 3.4 | 62 |
| (11.4 ± 1.0%/min) | (62.5 ± 3.8%) | |||||
| FV | 2.2 ± 0.4 | 0.84 ± 0.28 | 0.099 | 4.5 ± 1.3 | 12.3 ± 2.1 | 62 |
| (22.4 ± 6.3%/min) | (61.7 ± 10.4%) | |||||
| FPA | 2.5 ± 0.7 | 1.3 ± 0.4 | 0.15 | 2 700 ± 200 | 15 300 ± 1 300 | 100 |
| FPB | 2.8 ± 0.8 | 1.7 ± 0.5 | 0.20 | 1 200 ± 100 | 5 800 ± 600 | 39 |
| Thrombin B chain | 3.6 ± 0.2 | 7.0 ± 2.2 | 0.82 | 56.9 ± 2.1 | 494 ± 66 | 35 |
| TAT | 4.6 ± 0.6 | 26 ± 6.2 | 3.1 | 83.9 ± 3.8 | 851 ± 53 | 61 |
Prothrombinase concentrations were estimated from the rate of thrombin generation using a modified form of the Michaelis-Menten equation ([E]t = ν(Km + [S])/kcat[S]) as previously described.19The values substituted into the equation were a rate (ν) of either 0.93 ± 0.3 nM/min (initial rate) or 83.9 ± 3.8 nM/min (propagation rate), Km = 0.82 μM, [S] = 1.4 μM, and kcat = 19 s−1. During the initial phase of thrombin generation, product formation was catalyzed by prothrombinase levels of about 1.3 pM. Thrombin generation subsequently increased, during the propagation phase, produced by prothrombinase levels of 120 pM. These values are similar to those previously reported for a single individual (7 pM and 155 pM) by Rand et al.19
Platelet activation
Release of α-granule osteonectin from activated platelets was analyzed in quenched whole blood samples in 18 experiments from 10 individuals (5 men/5 women). Osteonectin (○) release is shown in Figure 1 as the mean ± SEM of osteonectin versus time with a baseline value of 6.9 ± 1.2 nM. By 1.9 ± 0.2 minutes platelet release occurred at a rate of 6.8 ± 0.4 nM/min, with maximum levels of osteonectin release of 44.6 ± 6.7 nM. The initial baseline and maximum level reached are consistent with published results of osteonectin levels present in plasma prior to and on platelet activation.23
Fibrinopeptide release
The cumulative results for FPA release (26 experiments; 13 individuals, 6 men/7 women) are shown in Figure2 as the mean ± SEM. Rapid FPA release (⋄) was observed at 2.5 ± 0.7 minutes and increased to maximum levels of 15.3 ± 1.3 μM by 8 minutes at a rate of 2.7 ± 0.2 μM/min.
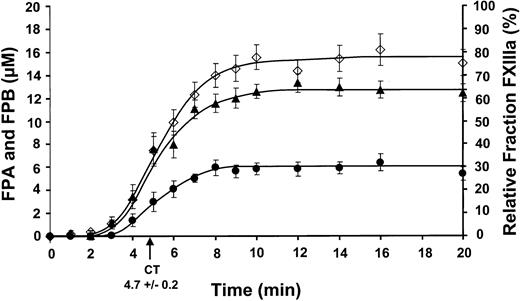
Comparison of FPA, FPB, and factor XIII activation.
Clot time from all experiments was calculated to be 4.7 ± 0.2 minutes. Data are shown as the mean ± SEM. FPA (⋄) and FPB (●) release (24 and 9 experiments, respectively) are shown as μM versus time (minute). FPA release occurs prior to FPB release and occurs at 2.5 ± 0.7 minutes with a rate of propagation of 2.7 ± 0.2 μM/min. FPB release occurs at 2.8 ± 0.8 minutes with a rate of propagation of 1.2 ± 0.1 μM/min. Maximum FPB release is about 39% compared to FPA release. Factor XIII activation (▴) is shown as a relative fraction of activated factor XIII present at each time point, determined from densitometric analysis. Factor XIII activation occurs prior to FPA release at 2.5 ± 0.7 minutes and increases at a rate of 11.4% ± 1.0%/min.
Comparison of FPA, FPB, and factor XIII activation.
Clot time from all experiments was calculated to be 4.7 ± 0.2 minutes. Data are shown as the mean ± SEM. FPA (⋄) and FPB (●) release (24 and 9 experiments, respectively) are shown as μM versus time (minute). FPA release occurs prior to FPB release and occurs at 2.5 ± 0.7 minutes with a rate of propagation of 2.7 ± 0.2 μM/min. FPB release occurs at 2.8 ± 0.8 minutes with a rate of propagation of 1.2 ± 0.1 μM/min. Maximum FPB release is about 39% compared to FPA release. Factor XIII activation (▴) is shown as a relative fraction of activated factor XIII present at each time point, determined from densitometric analysis. Factor XIII activation occurs prior to FPA release at 2.5 ± 0.7 minutes and increases at a rate of 11.4% ± 1.0%/min.
The release of FPB from fibrinogen/fibrin is initially seen followed by the cleavage of the COOH terminal arginine (des-Arg FPB) by an unidentified carboxypeptidase B-like enzyme (most likely TAFI).20 The sum of FPB and des-Arg FPB from 9 experiments (7 individuals, 4 men/3 women) detected by HPLC are shown (●) in Figure 2. FPB release became rapid at 2.8 ± 0.8 minutes and was produced at a maximum rate of 1.2 ± 0.1 μM/min. The maximum levels achieved (5.8 ± 0.6 μM, 8 minutes) was about 39% that of FPA, consistent with values reported previously.20 The majority of FPB remains as Bβ in the fibrinogen/fibrin clot.20
Factor XIII activation
Factor XIII concentrations detected by Western immunoblots for 9 experiments (6 individuals, 4 men/2 women) is plotted in Figure 2(▴). Factor XIIIa is detected slightly prior to FPA release, occurring at 2.2 ± 0.6 minutes. Factor XIIIa is generated at a rate of 11.4% ± 1.0%/min (∼10.3 ± 0.9 nM/min, based on a mean concentration of 90 nM) reaching maximum levels of 62.5% ± 3.8% (∼56.3 ± 3.4 nM).
Factor Va generation
The concentration of factor Va light chain was determined from 5 experiments (4 individuals, 2 men/2 women). Light chain was detected coincidental with factor XIIIa at 2.2 ± 0.4 minutes. The concentration of the light chain increased at a rate of 22.4% ± 6.3%/min (∼4.5 ± 1.3 nM/min, based on a mean concentration of 20 nM). Maximum product level present by 20 minutes was determined to be about 62% (∼12 nM).
Prethrombin 2 and thrombin B chain formation
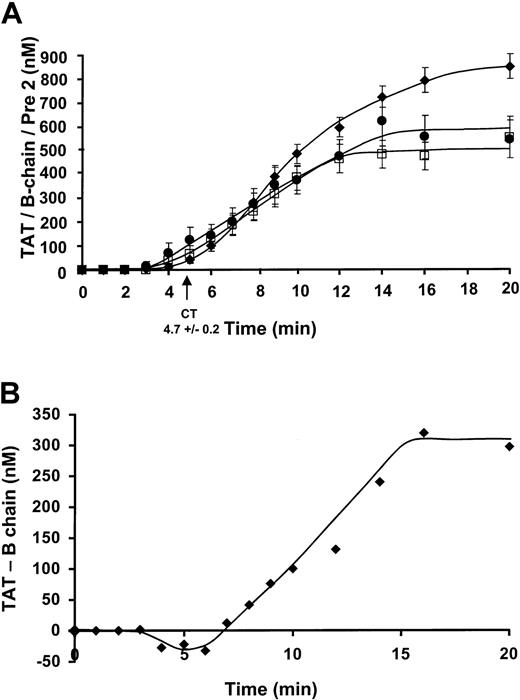
The concentration of prothrombin fragments, α-thrombin B chain (□), and prethrombin 2 (●) were detected by Western immunoblots (Figure 3A) with quantitation by comparison to a standard density curve derived from known amounts of purified standard. Data were collected from 8 experiments (7 individuals, 4 men/3 women) for the prothrombin fragments. The prethrombin 2 was first detected at 2.8 ± 0.8 minutes and increased to maximum levels of 549 ± 86 nM at a rate of 52.1 ± 2.0 nM/min. α-thrombin B chain formation was first detected after a slightly longer period, at 3.6 ± 0.2 minutes and increased at a comparable rate of 56.9 ± 2.1 nM/min, reaching maximum levels of 494 ± 66 nM by 20 minutes. TAT complex formation (⧫) reaches maximum levels of 851 ± 53 nM. This number corresponds to approximately 61% of prothrombin conversion to thrombin.
Comparison of a prethrombin 2, thrombin B chain, TAT complex formation, and the quantitation of covalently bound thrombin.
(A) Data were collected from 8 experiments for the prothrombin fragments and 35 experiments for TAT formation. The prethrombin 2 (●) has an initiation phase duration of 2.8 minutes and increases at a rate of propagation of 52.1 ± 2.0 nM/min. Maximum levels attained are 549 ± 86 nM. α-thrombin B chain (□) formation has a slightly longer initiation phase duration of 3.6 ± 0.2 minutes and increases at a comparable rate of 56.9 ± 2.1 nM/min, reaching maximum levels of 494 ± 66 nM by 20 minutes. The TAT (⧫) composite has an initiation phase duration up to 4.6 ± 0.6 minutes with an increasing rate of propagation of 83.9 ± 3.8 nM/min. The detected maximum level reached was 851 ± 53 nM TAT. (B) TAT complex formation (ELISA)–thrombin B chain (immunoblot) is shown (⧫, nM) versus time over the course of 20 minutes.
Comparison of a prethrombin 2, thrombin B chain, TAT complex formation, and the quantitation of covalently bound thrombin.
(A) Data were collected from 8 experiments for the prothrombin fragments and 35 experiments for TAT formation. The prethrombin 2 (●) has an initiation phase duration of 2.8 minutes and increases at a rate of propagation of 52.1 ± 2.0 nM/min. Maximum levels attained are 549 ± 86 nM. α-thrombin B chain (□) formation has a slightly longer initiation phase duration of 3.6 ± 0.2 minutes and increases at a comparable rate of 56.9 ± 2.1 nM/min, reaching maximum levels of 494 ± 66 nM by 20 minutes. The TAT (⧫) composite has an initiation phase duration up to 4.6 ± 0.6 minutes with an increasing rate of propagation of 83.9 ± 3.8 nM/min. The detected maximum level reached was 851 ± 53 nM TAT. (B) TAT complex formation (ELISA)–thrombin B chain (immunoblot) is shown (⧫, nM) versus time over the course of 20 minutes.
The 3 principal species of thrombin are irreversibly inhibited, reversibly inhibited, and free thrombin. The major depot for thrombin product is TAT and the significant amount of B chain seen in SDS electropherograms must come from dissociation of TAT. Therefore, the immunochemically determined ELISA of TAT complex formation in the native state at the time of quenching can be expressed as TATTOTAL = TATIRREVERSIBLE + TATREVERSIBLE (Figure 3A). The Western immunoblot detection of thrombin B chain after denaturation is then derived from TATREVERSIBLE broken down under SDS conditions and free thrombin where the amount of free thrombin is negligible (Figure 4). The amount of TATIRREVERSIBLE can then be calculated from TATTOTAL-thrombin B chain, assuming all of the reversible complex is dissociated. The amount of irreversible complex formed is about 308 nM by 20 minutes (Figure 3B). The negative values seen prior to 7 minutes probably represent excess thrombin.
Thrombin formation and thrombin substrates.
Early TAT complex formation from time 0 to 6 minutes is illustrated on a semilogarithmic scale along with thrombin generation calculated from the rate of FPA generation. The concentration of TAT required for the transition to rapid product formation for osteonectin (OSN, platelet activation), factor XIII activation (fXIIIa), FPA release, FPB release, and TAT propagation was calculated to be 0.51 ± 0.18 nM, 0.84 ± 0.28 nM, 1.3 ± 0.4 nM, 1.7 ± 0.5 nM, and 26 ± 6.2 nM, respectively. An extrapolation of the propagation phase for each substrate showed an initiation phase duration of 1.9 ± 0.2 minutes for platelet activation, 2.2 ± 0.6 minutes for factor XIII activation, 2.5 ± 0.7 minutes for FPA release, 2.8 ± 0.8 minutes for FPB release, and 4.6 ± 0.6 minutes for the onset of TAT propagation phase. The percent of TAT present at the point of activation is shown in parenthesis. Thrombin concentration calculated from the rate of FPA generation is shown as a dashed line.
Thrombin formation and thrombin substrates.
Early TAT complex formation from time 0 to 6 minutes is illustrated on a semilogarithmic scale along with thrombin generation calculated from the rate of FPA generation. The concentration of TAT required for the transition to rapid product formation for osteonectin (OSN, platelet activation), factor XIII activation (fXIIIa), FPA release, FPB release, and TAT propagation was calculated to be 0.51 ± 0.18 nM, 0.84 ± 0.28 nM, 1.3 ± 0.4 nM, 1.7 ± 0.5 nM, and 26 ± 6.2 nM, respectively. An extrapolation of the propagation phase for each substrate showed an initiation phase duration of 1.9 ± 0.2 minutes for platelet activation, 2.2 ± 0.6 minutes for factor XIII activation, 2.5 ± 0.7 minutes for FPA release, 2.8 ± 0.8 minutes for FPB release, and 4.6 ± 0.6 minutes for the onset of TAT propagation phase. The percent of TAT present at the point of activation is shown in parenthesis. Thrombin concentration calculated from the rate of FPA generation is shown as a dashed line.
Thrombin product formation
The thrombin concentrations extant at the inception of major product formation for each of the substrates, the prothrombin fragments, prethrombin 2, and α-thrombin B chain are presented in Figure 4. These values calculated from the first 6 minutes of TAT generation are shown on a logarithmic scale of TAT (nM) versus time (minute) with the percent of the maximum TAT observed shown in parentheses. Platelet activation based on osteonectin release is first observed at 1.9 ± 0.2 minutes and 510 ± 180 pM TAT (arrow in Figure 4, OSN). Rapid activation of factor XIII (2.2 ± 0.6 minutes) and factor Va (2.2 ± 0.4 minutes) occur next at 840 ± 280 pM TAT. The rapid releases of fibrinopeptides, FPA and FPB, are observed at 2.5 ± 0.7 minutes and 2.8 ± 0.8 minutes, respectively, corresponding to TAT concentrations of 1.3 ± 0.4 nM or 1.7 ± 0.5 nM. Prethrombin 2 is observed simultaneously with FPB at 2.8 ± 0.8 minutes at 1.7 ± 0.5 nM TAT. Thrombin B chain is observed in the denatured samples at 3.6 ± 0.2 minutes when 7.0 ± 2.2 nM TAT complex is present. Rapid thrombin generation signaling the transition to the propagation phase occurs at 4.6 ± 0.6 minutes corresponding to 26 ± 6.2 nM TAT. The thrombin concentrations associated with activation of its substrates are less than 1% of maximum thrombin observed (as TAT).
Active thrombin estimated from the rate of FPA generation during the initiation phase of the reaction is presented in Figure 4 as a dashed line. At clot time, the thrombin concentration calculated from FPA release is approximately 2 nM and remains fairly constant.
The maximum concentration and product yields are shown in Table 1. On average about 61% of prothrombin is converted to thrombin (0.851 μM). Similar yields are observed for factor XIII and factor V. Although 15.3 μM FPA is released, only 5.8 μM FPB is released (∼39%).
Discussion
These data describe the concentrations of thrombin associated with the onsets of rapid activation for several processes. Relatively minute concentrations of thrombin are generated during the initiation phase of blood coagulation, primarily due to the factor Xa generated from the extrinsic tenase complex. These levels, in the range of 0.51 to 2 nM are sufficient for the initiation of rapid activation of platelets, factor XIII, factor V, and fibrin formation. All of these processes occur prior to the major burst of thrombin generation during the propagation phase of the reaction.
The small amount of thrombin generated (26 ± 6.2 nM) at approximately clot time (4.6 ± 0.6 minutes) corresponds to a prothrombinase concentration of only about 1.3 pM. Platelet activation (∼0.5 nM thrombin) is followed by factor XIII and factor V activation (∼0.8 nM thrombin), both of which precede fibrinopeptide release (∼1.5 nM thrombin). The advent of formation of major catalysts is signaled by the detection of appreciable amounts of prothrombin activation products by about 4 minutes. At clot time, approximately 4.7 minutes, 25% to 50% of the procoagulant substrates are cleaved and 60% of platelets are activated. These activation processes occur with less than 1% of the total amount of thrombin ultimately produced. This value, calculated from TAT concentration, is an overestimate of the free thrombin available. The amount of free thrombin available at any given time in the reaction is somewhere between that estimated from the rate of FPA generation and the amount of reversible thrombin from the TAT complex. Thrombin calculated from the rate of FPA generation shows about 2 nM thrombin is present at clot time. Because only 2 nM thrombin is involved in fibrinogen cleavage, the amount of free thrombin is somewhere between that and the approximate 540 nM thrombin in reversible complex with antithrombin III.
The prothrombin product prethrombin 2 is not predicted by analysis of prothrombin activation in vitro. In our studies in whole blood the prothrombin fragment prethrombin 2 is detected by Western immunoblotting and plotted as concentration versus time in Figure 3A. This product has previously been detected in whole blood using the same antibody.19 In purified systems, generation by factor Xa-membrane prethrombin 2 (272-579) is a precursor to α-thrombin. Cleavage of prethrombin 2 by factor Xa at Arg320 yields the 2-chain thrombin. Possibilities for its formation in blood include cleavage of prothrombin at Arg271 by factor Xa in complex with platelet membrane, cleavage at Arg284 by thrombin or altered rates of prothrombin cleavage at Arg271 versus Arg320 by prothrombinase in blood.
The data presented here are consistent with our earlier report of the simultaneous event of Aα-chain cleavage with activation of factor XIII and with cross-linking preceding cleavage of the Bβ-chain.20
In summary, these results show that it is during the initiation phase of thrombin generation that is crucial to the activation of the procoagulant substrates. When a clot is visually seen about 25% to 60% of the reactions have already occurred and about 96% of thrombin is still being generated. The reason for the overabundance of thrombin generation after clot time has yet to be elucidated.
We the authors would like to thank Heather Kovich and Shyla L. Tessmer for their technical assistance and Dr Thomas Orfeo for assistance in the preparation of the manuscript.
Supported in part by Program Project Grant no. HL 46703 (Project 1) from the National Institutes of Health (to K.G.M.) and by Training Grant no. PHST32HL07594-15 from the US Public Health Service (to K.E.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth G. Mann, Department of Biochemistry, 89 Beaumont Ave, University of Vermont, Given Building, Room C401, Burlington, VT 05405; e-mail: kmann@zoo.uvm.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal