Abstract
CD40 is present on both normal and neoplastic B-lineage cells. CD40 stimulation of normal B cells has been shown to promote normal growth and differentiation, whereas aggressive histology B lymphomas are growth inhibited. The inhibition of neoplastic B-cell growth is believed to occur via activation-induced cell death in which stimuli that typically promote the growth of normal cells prevent the growth of their neoplastic counterparts. We show here that CD40 stimulation using either a soluble recombinant human CD40 ligand (srhCD40L) or anti-CD40 monoclonal antibody resulted in apoptosis of human Burkitt lymphoma cell lines. Additional studies examining the mechanism of CD40-mediated death revealed an increase in bax messenger RNA with a subsequent increase in Bax protein in the mitochondria of the treated cells. In vitro exposure of the cells to bax antisense oligonucleotides resulted in a significant decline in Bax protein levels and partial protection from CD40-mediated death, indicating that induction of Bax was at least one mechanism underlying this inhibitory effect of CD40 stimulation on lymphomas. When immunodeficient mice bearing Burkitt lymphoma were treated with srhCD40L, significant increases in survival were observed indicating a direct antitumor effect as a result of CD40 stimulation in vivo. Overall, these results demonstrate that CD40 ligation of aggressive histology B-lymphoma cells results in inhibition both in vitro and in vivo and thus may be of potential clinical use in their treatment.
Introduction
CD40 is a 55-kd molecule present on both normal and neoplastic B-lineage cells.1 It is a member of the tumor necrosis factor (TNF)/nerve growth factor (NGF) receptor superfamily, which includes CD30 and CD95 (Apo-1/Fas).2 CD40 stimulation is critical for B-lymphocyte growth, differentiation, and function.1-3 The ligand for CD40 (CD40L, gp39, CD154) is expressed mainly on activated CD4+ T cells.4CD40L is a member of the TNF family and functions in regulating cytokine production in numerous cell types as well as affecting B-cell function.4-7 CD40L has also been engineered to exist as a soluble ligand.8 The soluble recombinant ligand (srCD40L) consists of the extracellular domains of CD40L fused to an amino proximal 30 amino acid-modified leucine zipper motif and has been demonstrated to exert biologic activity in vitro.9Engagement of CD40 by CD40L is believed to result in CD40 trimerization on B cells, ultimately leading to signal transduction.10
CD40 has been shown to be expressed at high levels on a variety of lymphomas including non-Hodgkin lymphoma,11-13 hairy cell leukemia,14 and B-chronic leukocytic leukemia (B-CLL).15 Interestingly, CD40 stimulation of high-grade aggressive lymphomas such as Burkitt lymphoma and Epstein-Barr virus (EBV)–derived lymphomas results in a decrease in proliferation,16 whereas a transient increase in proliferation and survival in vitro is seen with indolent lymphomas such as follicular lymphomas and B-CLL after CD40 stimulation.10,13,14,17 The inhibitory effects of CD40 stimulation in Burkitt lymphoma and EBV lymphomas is presumably mediated through activation-induced cell death (AICD).3,16 18
We and others have shown that some signals of normal cell activation will inhibit the growth of transformed cells.3,16,18 AICD has been reported to occur by cell cycle arrest, apoptosis, or necrosis. Examples of AICD, other than the reported effects of CD40 on aggressive lymphomas, are cell cycle arrest of B-cell lymphomas by anti-CD19 or anti-IgM monoclonal antibodies (mAbs),19 and the CD30-mediated necrosis of anaplastic large-cell lymphomas.20 The mechanism by which CD40 stimulation causes AICD in aggressive lymphomas is not yet known and we therefore wanted to investigate the mechanism underlying these inhibitory effects.
The bcl-2 family of genes plays a crucial role in regulating apoptosis. Bcl-2 represses apoptosis, whereas other members such as bax, bak, and bcl-xS promote cell death.21,22 Bax is a soluble protein present mostly in the cytosol in a variety of tissues. However, on translocation to the mitochondria, it facilitates the formation of ion channels and mediates the release of cytochrome c.21-25 In the current report, we demonstrate that CD40 stimulation increasesbax messenger RNA (mRNA) expression and induces Bax protein in aggressive histology lymphomas. Furthermore, inhibitingbax expression partially protected the lymphomas from death by CD40 stimulation. Thus, CD40 stimulation induces bax and this may contribute to the mechanism by which stimulation of CD40 results in AICD in these tumors.
Materials and methods
Tumor cell lines
Daudi and Raji are B-lymphoma cell lines established in cell culture from patients with Burkitt lymphoma (American Type Culture Collection, Rockville, MD). RL is a B-lymphoma cell line established in cell culture from a patient with diffuse large B-cell lymphoma.3 These lymphomas were kept in culture for no more than 6 weeks before the studies.
Mice
Nonobese diabetic (NOD) severe combined immune deficiency (SCID) mice were bred in our colony (National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, MD) and were not used until 6 to 8 weeks of age. NOD/SCID mice were kept under strictly pathogen-free conditions at all times. The mice were housed in microisolator cages and all food, water, and bedding were autoclaved before use. NOD/SCID mice received trimethoprim/sulfamethoxazole (40 mg trimethoprim and 200 mg sulfamethoxazole/320 mL drinking water) in suspension in their drinking water. (Animal care was provided in accordance with the procedures outlined in the “Guide for the Care and Use of Laboratory Animals” [NIH publication no. 86-23, 1985]).
Cell culture and treatments
The Daudi, Raji, and RL cell lines were cultured in RPMI-1640 medium (Biowhittaker, Walkersville, MD), supplemented with 10% fetal bovine serum (FBS; Gibco, Life Technologies, Grand Island, NY), 1% 200 mM l-glutamine (Life Technologies), 1% penicillin-streptomycin and amphotericin B (Fungizone) mix (Biowhittaker). Media was then filtered through a 0.22 μm filter (Nalgene, Rochester, NY). The cells were cultured at 37°C with 5% CO2. Cell lines were subcultured every 2 to 3 days.
Daudi, Raji, and RL cells were analyzed by an EPICS flow cytometer (Coulter Electronic, Hialeah, FL) for CD40 expression. Cells were counted on a Coulter cell counter (Coulter Electronic), and viability was determined microscopically using a hemacytometer and the trypan blue exclusion method (Life Technologies). Cell preparations were not used unless their viability was greater than 90%.
In vitro proliferation assay
Proliferation of cell lines was measured by a microculture tetrazolium (MTT) assay (Boehringer Mannheim, Mannheim, Germany). In a 96-well flat bottom plate (Corning, Corning, NY), 5 × 104 cells/well were incubated in culture medium containing log dilutions of reagents for 72 hours. The reagents used were soluble recombinant CD40 ligand (srhCD40L, Immunex, Seattle, WA), and anti-CD40 SGN-14 (Seattle Genetics, Bothell, WA) at concentrations of 10 μg/mL decreasing to 1 × 10−3 μg/mL. At the same concentrations, human antibody- or isotype-matched mouse IgG1 control antibody (Pharmingen, San Diego, CA) was also used. After 72 hours of incubation, 100 μg MTT (3-[4,5 dimethylthiozol-2-yl]-2,5-diphenyl tetrazolium bromide; Boehringer Mannheim) was added to each well and then incubated for 4 hours. After the incubation, 100 μL of 10% sodium dodecyl sulfate (SDS) in 0.01 M HCl was added to each well to solubilize the MTT formazan crystals. After incubating overnight, spectrophotometric absorbance at 570 nm was determined using a scanning multiwell spectrophotometer (EI 900, Bio-Tec Instruments, Winooski, VT) to determine proliferation. Cell viability was assessed using the trypan blue exclusion method. Data were plotted on Sigma Plot; and the means and SDs were computed. All experiments were repeated 3 times with a representative experiment being shown.
In situ cell death detection
Daudi cells were treated with 10 μg/mL srhCD40L or anti-CD40 (SGN-14 clone, mouse IgG1) and incubated for 24, 48, and 72 hours. Cell samples were washed 2 times in phosphate-buffered saline (PBS)/1% bovine serum albumin (BSA) at 4°C and adjusted to 1 to 2 × 107 cells/mL. Cell suspension (100 μL/well) was transferred to a V-bottom microtiter plate (Corning). Cells were fixed by using 100 μL/well of a 4% paraformaldehyde solution and incubated on a shaker plate for 30 minutes at room temperature. Following incubation, the plate was centrifuged at 300g for 10 minutes and fixative was removed by gentle tapping. Cells were washed once with 200 μL PBS/BSA and resuspended in permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 minutes on ice. Cells were then washed 2 times with PBS/BSA as described before. A positive control was added by incubating fixed cells with 1 μg/mL DNase I for 10 minutes at room temperature. Following the second wash, cells were resuspended in 50 μL/well TUNEL (TdT-mediated dUTP nick end labeling) reaction mixture or 50 μL Label solution as a negative control (In Situ Cell Death Detection Kit, Fluorescein, Boehringer Mannheim). Cells were incubated for 60 minutes at 37°C in a humidified atmosphere in the dark. Following incubation, the cells were washed 2 more times as described above. Cells were then transferred to a tube with a final volume of 250 to 500 μL in PBS. Cells were analyzed by flow cytometry.
In vitro assessment of apoptosis (annexin V–fluorescein isothiocyanate)
Daudi cells were cultured in the presence and absence of 3 μg/mL srhCD40L for 24 hours. Cells (106/sample) were washed with PBS and centrifuged at 200g for 5 minutes. Cells stained with annexin were resuspended in 100 μL stain solution (20 μL annexin in 1 mL Hepes buffer; Boehringer Mannheim) before incubation for 15 minutes. Immediately prior to analysis by flow cytometry, 300 μL incubation buffer (10 mM Hepes buffer, 40 mM NaCl, 5 mM CaCl2 in NaOH, pH 7.4) was added to each sample.
RNA preparation
Daudi cells were treated with 10 μg/mL srCD40L or anti-CD40 (SGN-14 clone, mouse IgG1) and incubated for 24, 48, and 72 hours. Before RNA extraction, cells were isolated and pelleted to remove culture media. Trizol (Gibco, Life Technologies) was added to each sample tube according to manufacturer's instructions. Following a 5-minute incubation, 20% chloroform (CMS, Houston, TX) by volume to the amount of Trizol used was added to the tube and shaken vigorously for 15 seconds. After a 3-minute incubation, samples were centrifuged at 12 000g for 15 minutes. Fifty percent isopropyl alcohol (Sigma, St Louis, MO) by volume to the amount of Trizol used was added to new tubes. Following centrifugation, the aqueous phase only was removed from the sample tubes and transferred to tubes containing isopropanol. The tubes were mixed gently and allowed to rest for 10 minutes. The samples were then centrifuged at 12 000g for 15 minutes. The supernatant was discarded and the RNA pellet was transferred to a 1.5-mL Eppendorf tube and washed twice with 70% ice-cold ethanol. RNA pellets were dried by vacuum centrifugation, resuspended in DepC water (Quality Biologicals, Gaithersburg, MD), and placed in a 50°C water bath for 5 minutes.
Ribonuclease protection assay
To analyze mRNAs in the bcl-2 family of genes, Pharmingen 45004K or 45014K (transcription/ribonuclease protection assay [RPA] kits) were used unless otherwise noted (Pharmingen). Reagents were brought to room temperature before use. Probe synthesis and electrophoresis was carried out according to manufacturer's instructions. Following electrophoresis, the gel was absorbed to blotting paper (VWR Products, Media, PA) and dried in a vacuum gel dryer at 80°C for 1 hour. The dried gel was placed on a film cassette with x-ray film and developed at −70°C for 1 to 3 days, depending on application. Following development, the gel was placed in a phosphorimager with an intensifying screen for 24 hours before analysis on a scanner (Storm 860, Molecular Dynamics, Sunnyvale, CA) to obtain densitometric analysis. Each experiment was performed 3 times.
Protein isolation and Western blot
Raji and RL cells were treated with 10 μg/mL CD40L or anti-CD40 mAb SGN-14 for 6, 24, 48, and 72 hours and analyzed for Bax using a previously described protocol.23 Lysis of Raji and RL cells following treatment was performed in isotonic buffer (200 mM mannitol/70 mM sucrose/1 mM EDTA/10 mM Hepes, pH 6.9) by Dounce homogenization. Unbroken cells, heavy membranes, and nuclei were pelleted and discarded. The mitochondrial and cytosolic fractions were separated by centrifugation at 18 000g for 10 minutes. The cytosolic fraction (supernatant) was transferred to a clean Eppendorf tube and stored.
For detection of Bax or Erk by Western blot, cell equivalent samples (10 μL aliquots) of the fractions were separated by SDS-polyacrylamide gel electrophoresis on 12% Tris-glycine gels (Novex, San Diego, CA), and transferred to 0.2-μm polyvinylidene difluoride membranes (Novex). Blots were probed with a rabbit polyclonal antiserum specific for the amino terminal of Bax (N20; Santa Cruz Biotechnology, Santa Cruz, CA), or with a rabbit polyclonal antibody specific for Erk (Santa Cruz Biotechnology), followed by the appropriate secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology). The blots were then visualized by enhanced chemiluminescence (Pierce, Rockford, IL) as indicated in the manufacturer's protocol. Each experiment was performed twice.
Antisense experiments
Daudi and Raji cells were initially cultured with Bax antisense (TGCTCCCCGGACCCGTOOFT) (Gibco) or mixed sense (CCGCTGCGCCAGTCCCZFZC) (Gibco) oligonucleotides at 20 μM concentrations overnight in 96-well enzyme-linked immunosorbent assay plates in media containing 5% FBS. The following day, cells were centrifuged and resuspended in media containing 10% FBS, bax antisense, or mixed sense. Cells were treated with either plain media or 10 μg/mL anti-CD40 mAb (SGN-14 clone, mouse IgG1), which we determined provided the optimal inhibitory stimulus. Trypan blue (Gibco) analysis and MTT (Boehringer Mannheim) analysis were performed at 12, 24, and 48 hours to obtain cellular viability. Data were plotted on Sigma Plot, and the mean and SD was calculated.
In vivo experiments
Raji tumor cells (5 × 106) were administered by intravenous (IV) injection. Recipients then received either PBS or 100 μg srhCD40L intraperitoneally (IP) every day for 10 days, totaling 10 injections starting the day after tumor inoculation. Tumor-bearing mice were then monitored for tumor development and progression. Moribund mice were euthanized. Tumors were removed for histologic examination. Parametric (Student t test) analyses were performed to determine if the groups differed significantly (P < .05). Experiments had 10 mice per group and were performed 2 times.
Results
Effects of CD40L and anti-CD40 on human B-cell lymphoma growth
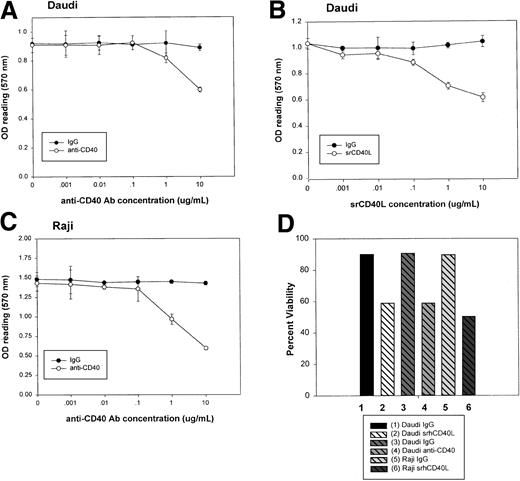
Incubation of the tumor cells with either srhCD40 ligand or an agonist anti-CD40 mAb (SGN-14 clone) significantly (P < .005) inhibited the proliferation and decreased the viability of both Daudi and Raji Burkitt lymphoma cells in vitro (Figure 1). Both cell lines have been previously shown to express CD40.3 Proliferation was assessed using a microculture tetrazolium (MTT) assay. The cells were cultured with log dilutions of either srhCD40L or anti-CD40 SGN-14 at concentrations of 10 μg/mL to 0.001 μg/mL for 72 hours before the addition of MTT. An optimal inhibition of 40% to 60% was seen with 10 μg/mL srhCD40L or antibody compared with an isotype-matched antibody control depending on the cell line used (Figure 1A-C). Viability was tested at 72 hours using the trypan blue exclusion method. A 31.1% to 49.5% decrease in viability was seen in the groups treated with srhCD40L or anti-CD40 mAb (Figure 1D). These results are in agreement with earlier studies showing inhibitory effects of anti-CD40 antibodies on Burkitt lymphoma cells.3 26 These results demonstrate that srhCD40L and an agonist anti-CD40 mAb are capable of inhibiting human B-lymphoma proliferation in vitro.
Effect of CD40 stimulation on Burkitt lymphoma proliferation in vitro.
Daudi (A,B) and Raji (C) cells were incubated either alone or with log dilutions of srhCD40L or CD40 antibody (SGN-14 clone, mouse IgG1) for 72 hours. Measurement of proliferation was performed using a microculture tetrazolium (MTT) assay as described in “Materials and methods.” Data are presented as the mean with SD. Viability of the cells (D) was assessed using the trypan blue exclusion method. Treatment of Daudi and Raji cells with either CD40L or CD40 antibody (at 10 μg/mL concentration) resulted in a significant (P < .001) decrease in proliferation, as well as a decrease in viability in these cells versus untreated cells as indicated by the asterisk.
Effect of CD40 stimulation on Burkitt lymphoma proliferation in vitro.
Daudi (A,B) and Raji (C) cells were incubated either alone or with log dilutions of srhCD40L or CD40 antibody (SGN-14 clone, mouse IgG1) for 72 hours. Measurement of proliferation was performed using a microculture tetrazolium (MTT) assay as described in “Materials and methods.” Data are presented as the mean with SD. Viability of the cells (D) was assessed using the trypan blue exclusion method. Treatment of Daudi and Raji cells with either CD40L or CD40 antibody (at 10 μg/mL concentration) resulted in a significant (P < .001) decrease in proliferation, as well as a decrease in viability in these cells versus untreated cells as indicated by the asterisk.
CD40 stimulation causes apoptosis in human B-lymphoma lines
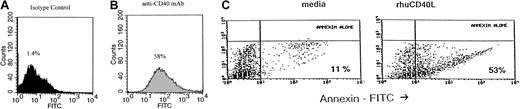
We then investigated whether this inhibitory effect of CD40 stimulation was due to apoptosis. This was assessed by incubating Daudi lymphoma cells with srhCD40L or anti-CD40 and quantifying DNA strand breaks using TUNEL assay or annexin V staining. The cells were cultured with 10 μg/mL anti-CD40 for 24, 48, and 72 hours before staining and flow cytometric analysis. A 5-fold increase in the number of apoptotic Daudi cells occurred after 24 hours' incubation as determined by TUNEL assay (from 1.4% to 58%; Figure 2A). Using annexin staining, the fraction of apoptotic cells increased from 11% to 53% after incubation of Daudi cells with 3 μg/mL srhCD40L (Figure 2C). Similar results were seen with the anti-CD40 mAb (data not shown). These results indicate that CD40 stimulation induces apoptosis in human B lymphomas in vitro.
CD40 stimulation causes apoptosis in Burkitt lymphoma.
Daudi cells were cultured in media in the presence or absence of srhCD40L or anti-CD40 antibody (SGN-14 clone, mouse IgG1). TUNEL (A,B) and annexin V–fluorescein isothiocyanate (FITC; C) stains were performed following incubation. Twenty-four hours after culture initiation, Daudi cells were analyzed for nuclear DNA damage by TUNEL and for phospholipid externalization to the cell surface by annexin and flow cytometry. The results are expressed as the number of cells (percent of total) that have either DNA strand breaks or that bind annexin V-FITC and are therefore undergoing apoptosis. Culture with 10 μg/mL srhCD40L (A) caused a 60% increase in DNA strand breaks compared to an untreated control (from 1.4% to 58%). Dark gray shade (A) is the untreated control group. Light gray shade (B) is the group treated with srhCD40L. Culture with 3 μg/mL srhCD40L (C) induced a 5-fold increase in the number of apoptotic Daudi cells over that observed in the media culture alone (from 11% to 53%).
CD40 stimulation causes apoptosis in Burkitt lymphoma.
Daudi cells were cultured in media in the presence or absence of srhCD40L or anti-CD40 antibody (SGN-14 clone, mouse IgG1). TUNEL (A,B) and annexin V–fluorescein isothiocyanate (FITC; C) stains were performed following incubation. Twenty-four hours after culture initiation, Daudi cells were analyzed for nuclear DNA damage by TUNEL and for phospholipid externalization to the cell surface by annexin and flow cytometry. The results are expressed as the number of cells (percent of total) that have either DNA strand breaks or that bind annexin V-FITC and are therefore undergoing apoptosis. Culture with 10 μg/mL srhCD40L (A) caused a 60% increase in DNA strand breaks compared to an untreated control (from 1.4% to 58%). Dark gray shade (A) is the untreated control group. Light gray shade (B) is the group treated with srhCD40L. Culture with 3 μg/mL srhCD40L (C) induced a 5-fold increase in the number of apoptotic Daudi cells over that observed in the media culture alone (from 11% to 53%).
CD40 stimulation causes an increase in baxmRNA
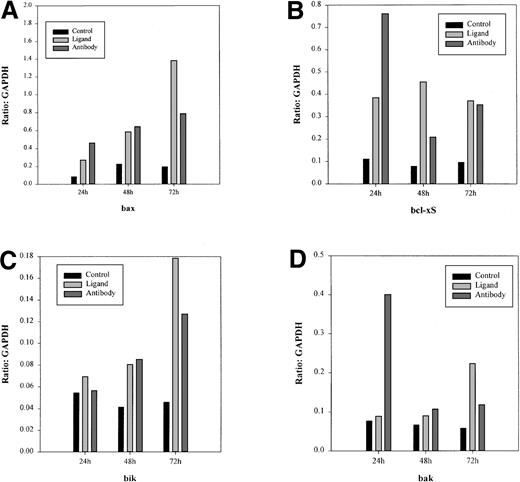
We then determined whether treatment of Daudi cells with srhCD40L or anti-CD40 SGN-14 caused an increase in apoptosis-related genes. The cells were cultured with 10 μg/mL srhCD40L or anti-CD40 SGN-14 for 24, 48, and 72 hours before RNA extraction and analysis by RPA. We used this concentration of antibody and ligand because we have shown that this resulted in optimal growth inhibition of the lymphomas (Figure 1). We observed that there was an increase in baxmRNA as well as increases in mRNA of other proapoptotic genes such asbak, bik, and bcl-xS after CD40 stimulation by either antibody or ligand (Figure3). Interesting, both bif-1and mcl-1, which have been associated with inhibition of apoptosis were also detected. L32 is a control gene, similar to GAPDH. There was an absence of the antiapoptotic genebcl-2 in the untreated as well as treated groups at each time point, in agreement with previous reports on the bcl-2status of these cell lines.34 There was a significant (P < .01) increase in bax mRNA levels in both the srhCD40L and anti-CD40 (SGN-14)–treated groups at 24, 48, and 72 hours compared to an untreated control shown by densitometric analysis of the gels (Figure 4). Thus, CD40 stimulation, by either antibody or soluble ligand, increasesbax mRNA levels in Burkitt lymphoma cell lines.
RPA analysis of apoptotic mRNA after CD40 stimulation.
Daudi cells were cultured in the presence or absence (U) of either 10 μg/mL srhCD40L (L) or 10 μg/mL anti-CD40 SGN-14 (A) for 24, 48, and 72 hours before RNA extraction. RNA samples were analyzed by RPA with probes for the bcl-2 family of genes for changes in mRNA levels. Increases in bax mRNA were seen in each treatment group at each time point compared to an untreated control. Increases in other proapoptotic genes such as bcl-xL/S, bik, and bak were also seen at each time point. These data are representative of 3 experiments.
RPA analysis of apoptotic mRNA after CD40 stimulation.
Daudi cells were cultured in the presence or absence (U) of either 10 μg/mL srhCD40L (L) or 10 μg/mL anti-CD40 SGN-14 (A) for 24, 48, and 72 hours before RNA extraction. RNA samples were analyzed by RPA with probes for the bcl-2 family of genes for changes in mRNA levels. Increases in bax mRNA were seen in each treatment group at each time point compared to an untreated control. Increases in other proapoptotic genes such as bcl-xL/S, bik, and bak were also seen at each time point. These data are representative of 3 experiments.
Densitometric analysis of RPA gels.
Quantitative levels of bax, bak, bik, and bclx-S mRNA levels in Daudi cells were established through densitometric analysis of the RPA gel. The gel was exposed to a intensifying screen for 24 hours before analysis on a scanner. Levels of these genes are expressed in ratios to the housekeeping geneGAPDH. There was an increase in all of these gene transcripts compared to the untreated controls. These data are representative of 3 experiments.
Densitometric analysis of RPA gels.
Quantitative levels of bax, bak, bik, and bclx-S mRNA levels in Daudi cells were established through densitometric analysis of the RPA gel. The gel was exposed to a intensifying screen for 24 hours before analysis on a scanner. Levels of these genes are expressed in ratios to the housekeeping geneGAPDH. There was an increase in all of these gene transcripts compared to the untreated controls. These data are representative of 3 experiments.
Bax protein levels are increased as a result of CD40 stimulation
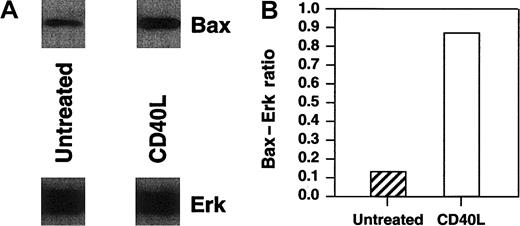
Bax has been previously demonstrated to play a critical role in the death of neoplastic cells.31,32 To correlate levels of corresponding Bax protein with bax transcription, we analyzed Bax protein levels by Western blot analysis. In these experiments, we also assessed the levels of Bax in a diffuse large-cell lymphoma cell line, RL, after CD40 stimulation. We have previously shown that RL cells are inhibited by CD40 stimulation both in vitro and in vivo.3 Protein was extracted from Daudi, Raji, and RL cells after culture with 10 μg/mL srhCD40L or anti-CD40 SGN-14 at various time points. Cell lysates were quantified using Western blot for Bax concentration in both cytosolic and mitochondrial fractions of RL and Daudi cells and mitochondrial fractions of Raji. There was an increase in the levels of Bax protein in RL cytosolic fractions as determined by Western blot and densitometric analysis relative to Erk levels, which was used as a loading control (Figure5A,B). Erk was chosen as our control because, although it is posttranslationally activated by exogenous stimuli, it is expressed constitutively. The actual loading of the gels for Western blots was based on a fixed cell number per lane, whereas there was no adjustment for the amount of protein loaded. Thus, the Erk levels show that there was no major change in the protein content per cell at this time point following CD40 ligation, whereas there was an actual increase in the quantity of Bax present per cell.
Western blot analysis of cytosolic Bax protein in RL cells after CD40 stimulation.
RL cells were cultured in the presence or absence of either 10 μg/mL srhCD40L for 48 hours before protein extraction. Protein was extracted by isotonic lysis. Cell lysates containing the cytosolic fraction were analyzed by Western blot using Erk cytosolic protein as a loading control and densitometric analysis for changes in Bax protein relative to the Erk control levels. There was an increase in Bax protein in the cytosolic fractions of RL cells following CD40 stimulation.
Western blot analysis of cytosolic Bax protein in RL cells after CD40 stimulation.
RL cells were cultured in the presence or absence of either 10 μg/mL srhCD40L for 48 hours before protein extraction. Protein was extracted by isotonic lysis. Cell lysates containing the cytosolic fraction were analyzed by Western blot using Erk cytosolic protein as a loading control and densitometric analysis for changes in Bax protein relative to the Erk control levels. There was an increase in Bax protein in the cytosolic fractions of RL cells following CD40 stimulation.
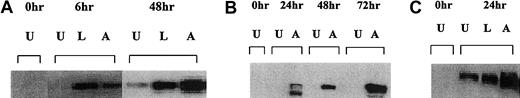
Translocation of Bax into the mitochondria has been shown to be associated for the induction of apoptosis.23 There was no Bax protein present in the mitochondria of Raji or Daudi cells at 0 hours or without CD40 stimulation (Figure6). The Daudi cell line cultured with the anti-CD40 antibody showed a significant level of Bax protein associated with the mitochondria after 6 hours (Figure 6A). Similar results were seen with Raji and RL cells in which the presence of Bax in the mitochondria was detected after 24 hours (Figure 6B,C). Thus, in aggressive histology human B lymphoma cells, CD40 stimulation increased cytosolic Bax levels as well as the amount of Bax protein in the mitochondria and this correlates with the induction of apoptosis observed after treatment.
Western blot analysis of mitochondrial Bax protein in Daudi, Raji, and RL cells after CD40 stimulation.
(A) Daudi cells were cultured in the absence (U) or presence of either 10 μg/mL anti-CD40 antibody (SGN-14 clone, mouse IgG1) (A) or 10 μg/mL srhCD40L (L) for 6 and 48 hours before protein extraction. Protein was extracted by isotonic lysis. Cell lysates using the mitochondrial fractions were analyzed by Western blot for changes in Bax protein. (B) Assessment of Bax protein levels in the mitochondria of Raji cells after anti-CD40 exposure using the SGN-14 antibody and assessed at various time-points. (C) RL cells were cultured in the absence (U) or presence of either 10 μg/mL srhCD40L (L) or 10 μg/mL anti-CD40 SGN-14 (A) for 24 hours. Protein was extracted by isotonic lysis. Cell lysates were analyzed by Western blot for changes in mitochondrial Bax protein. There was an increase in Bax protein after treatment with either ligand or antibody.
Western blot analysis of mitochondrial Bax protein in Daudi, Raji, and RL cells after CD40 stimulation.
(A) Daudi cells were cultured in the absence (U) or presence of either 10 μg/mL anti-CD40 antibody (SGN-14 clone, mouse IgG1) (A) or 10 μg/mL srhCD40L (L) for 6 and 48 hours before protein extraction. Protein was extracted by isotonic lysis. Cell lysates using the mitochondrial fractions were analyzed by Western blot for changes in Bax protein. (B) Assessment of Bax protein levels in the mitochondria of Raji cells after anti-CD40 exposure using the SGN-14 antibody and assessed at various time-points. (C) RL cells were cultured in the absence (U) or presence of either 10 μg/mL srhCD40L (L) or 10 μg/mL anti-CD40 SGN-14 (A) for 24 hours. Protein was extracted by isotonic lysis. Cell lysates were analyzed by Western blot for changes in mitochondrial Bax protein. There was an increase in Bax protein after treatment with either ligand or antibody.
Bax antisense is protective against CD40-mediated cell death of lymphomas
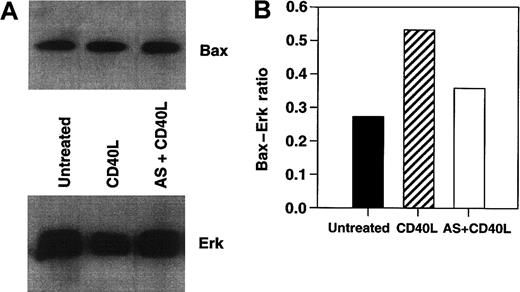
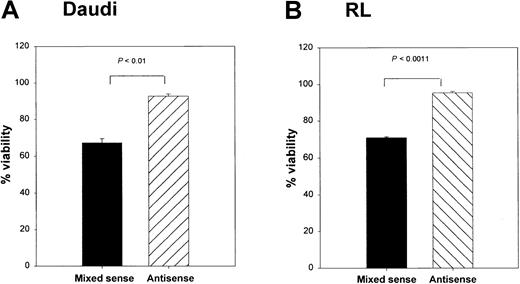
To determine whether Bax was responsible for the CD40-mediated death in these lymphoma lines, we cultured RL cells with srhCD40L alone, or srhCD40L plus bax antisense. A significant reduction in cytosolic Bax protein was observed by Western blot and densitometric analysis after 48 hours of culture with the antisense (Figure 7A,B). In the next series of experiments, Daudi and RL cells treated with thebax antisense were also stimulated with either media control or 10 μg/mL anti-CD40 mAb (SGN-1 clone, mouse IgG1) to determine if the reduction of Bax protein by the antisense could protect the lymphomas from CD40-mediated cell death. We have determined that this concentration of antibody or ligand results in optimal growth inhibition of the lymphomas (Figure 1). Viability of the cells was measured by the trypan blue exclusion method. There was a significant (P < .001) increase in viability in both tumor lines treated with anti-CD40 mAb and the bax antisense versus those that received the mixed sense control and anti-CD40 mAb for 48 hours (Figure 8A,B). The viability of control cells not treated with anti-CD40 mAb remained unchanged with either media control, mixed sense, or bax antisense (data not shown). Thus, inhibiting bax by antisense was capable of at least partially protecting aggressive histology B-cell lymphomas from CD40-mediated death suggesting that at least one of the mechanisms underlying the inhibitory effects of CD40 stimulation was through the induction of Bax.
Effects of
bax antisense of Bax protein levels in lymphoma cell lines. RL cells were cultured with either srhCD40L alone, srhCD40L plus bax antisense, or media control for 48 hours. Effects on total Bax protein levels were then assessed by Western blot. Erk cytosolic protein was included as a loading control. Densitometric analysis was performed to determine the amount of Bax protein reduction from the srhCD40L-treated group relative to the Erk levels, which remained unchanged. Treatment of RL cells with bax antisense causes a reduction of Bax protein following CD40 stimulation.
Effects of
bax antisense of Bax protein levels in lymphoma cell lines. RL cells were cultured with either srhCD40L alone, srhCD40L plus bax antisense, or media control for 48 hours. Effects on total Bax protein levels were then assessed by Western blot. Erk cytosolic protein was included as a loading control. Densitometric analysis was performed to determine the amount of Bax protein reduction from the srhCD40L-treated group relative to the Erk levels, which remained unchanged. Treatment of RL cells with bax antisense causes a reduction of Bax protein following CD40 stimulation.
Effects of
bax antisense on Burkitt lymphoma viability and proliferation. Daudi (A) and RL (B) cells were cultured with either bax antisense or a mixed sense control. In addition, these cells were also cultured with either a media control or 10 μg/mL anti-CD40 (SGN-14 clone, mouse IgG1). Viability was measured by the trypan blue exclusion method. Viability was increased in the antisense-treated groups after 12 hours of CD40 stimulation, whereas the mixed sense groups showed a significant (P < .01) decrease in viability. Maximum effects were seen at 48 hours of treatment as shown above. Data are shown as the mean with SD.
Effects of
bax antisense on Burkitt lymphoma viability and proliferation. Daudi (A) and RL (B) cells were cultured with either bax antisense or a mixed sense control. In addition, these cells were also cultured with either a media control or 10 μg/mL anti-CD40 (SGN-14 clone, mouse IgG1). Viability was measured by the trypan blue exclusion method. Viability was increased in the antisense-treated groups after 12 hours of CD40 stimulation, whereas the mixed sense groups showed a significant (P < .01) decrease in viability. Maximum effects were seen at 48 hours of treatment as shown above. Data are shown as the mean with SD.
Antitumor effects of CD40 stimulation on NOD/SCID mice bearing B lymphomas
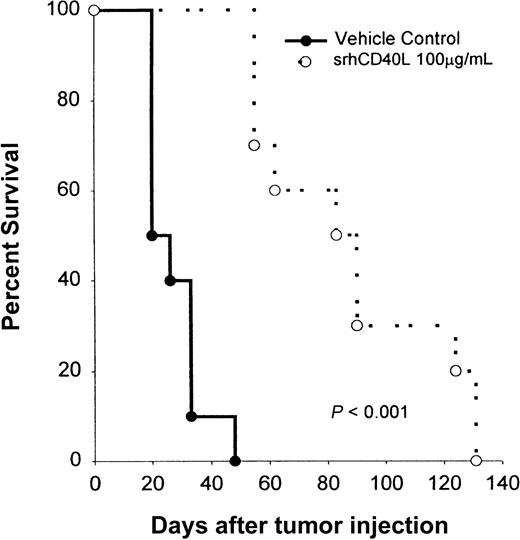
We then investigated whether CD40 stimulation of the tumor alone would be efficacious in treating Burkitt lymphomas in vivo. NOD/SCID mice bearing Raji lymphoma cells were treated with srhCD40L (100 μg/d) every day for 10 IP injections starting on day 1 after tumor injection. Recipient mice received 5 × 106 tumor cells by IV injection. The ligand was used because it has no Fc region and thus cannot mediate antitumor effects by antibody-dependent cell-mediated cytotoxicity (ADCC). Treatment with srhCD40L significantly (P < .005) inhibited tumor growth and promoted survival in these mice (Figure9). The mice treated with srhCD40L had 60% survival 30 days past the control group. No overt toxicity was observed in the mice receiving repeated administration of the ligand. Similar antitumor results were previously observed using antibodies to CD40 in vivo.3 Thus, CD40 stimulation using a recombinant soluble ligand prolongs survival of Burkitt lymphoma-bearing mice in vivo.
Effects of srhCD40L administration on the survival of NOD/SCID mice bearing a Burkitt lymphoma line.
NOD/SCID mice received IV inoculations with 5 × 106 Raji cells on day 0. The following day, mice were treated with either 100 μg srhCD40L or PBS as a vehicle control IP daily for 10 days. There were 10 mice per group and animals were monitored for tumor growth, survival, and morbidity. The results are expressed as the percentage of surviving mice at various days after tumor challenge.
Effects of srhCD40L administration on the survival of NOD/SCID mice bearing a Burkitt lymphoma line.
NOD/SCID mice received IV inoculations with 5 × 106 Raji cells on day 0. The following day, mice were treated with either 100 μg srhCD40L or PBS as a vehicle control IP daily for 10 days. There were 10 mice per group and animals were monitored for tumor growth, survival, and morbidity. The results are expressed as the percentage of surviving mice at various days after tumor challenge.
Discussion
We report here that CD40 stimulation of Burkitt and diffuse large-cell lymphoma cell lines induces apoptosis accompanied by an up-regulation in bax mRNA and Bax protein in the mitochondria; in addition, inhibition of Bax production resulted in partial protection from CD40-mediated death. Previous studies have demonstrated that CD40 stimulation can promote the growth and differentiation of normal B lymphocytes while causing a decrease in aggressive histology B lymphocytes.1 7 This is the first report implicating apoptosis via bax as one of the mechanisms by which CD40 stimulation inhibits aggressive histology B-lymphoma growth.
CD40 cross-linking has been previously demonstrated to have markedly different effects on the growth of B-cell lymphomas, and this has been shown to be dependent on their type and the assay used to determine effects. CD40 stimulation of multiple myeloma cells, for example, has been reported to either promote or inhibit their growth in vitro.27,28 Additionally, it has been demonstrated that CD40 stimulation by CD40L in indolent lymphomas such as follicular lymphoma and B-CLL in the presence of interleukin 4 will promote clonogenic growth of these neoplasms for a short time in vitro.12,13,15,17 Conversely, stimulation of CD40 on aggressive B-cell lymphomas, such as Burkitt, diffuse large-cell, and EBV-derived lymphomas, results in inhibition of proliferation in vitro and production of antitumor effects in vivo.3,16,18Antitumor effects associated with CD40 stimulation of lymphomas have been only shown using antibodies to CD40 up until now. Previous studies using anti-CD40 antibodies have shown in vivo efficacy due in part to ADCC,18 thereby making it difficult to discern whether the antitumor effects were due to the direct effects of CD40 stimulation on the tumor or by ADCC. The antitumor effects reported here with srhCD40L confirm that CD40 stimulation has direct antitumor effects that are independent of ADCC in vivo.
Activation-induced cell death is a process by which a signal that would promote growth in a normal cell will cause death in a transformed cell. This process can involve cell cycle arrest, apoptosis, or necrosis.17,19,20 It was observed that CD40 stimulation induced apoptosis in the cell lines tested. Furthermore, CD40 stimulation caused an increase in 4 proapoptotic gene transcripts:bax, bak, bik, and bclx-S. It will be of interest to ascertain the role of the other proapoptotic molecules in CD40-mediated apoptosis. More quantitative analysis by Western blot was focused on Bax, which was a reasonable candidate in light of reports on its critical role in inducing apoptosis in neoplastic cells after exposure to chemotherapeutic agents.31,32 Bax is of particular interest due to its ability to dimerize with itself on the mitochondrial membrane to facilitate ion release through Bax-induced pores as well as cytochromec release and the induction of caspases.22Little is known about the direct effects of the other genes. Corresponding with the increase in bax mRNA, there was an increase in Bax protein and a significant increase in Bax in the cytosolic fractions of cells treated with srhCD40L. This effect was also seen in the cells treated with anti-CD40 antibody (SGN-14 clone). Blocking the effects of bax through antisense oligonucleotides was able to protect the cells from death as seen by a rise in viability, suggesting that bax is directly involved in CD40-mediated death in these cells. However, the data showed that only partial protection was achieved with the antisense constructs. It is unclear whether this is related to incomplete blocking of Bax or whether other proapoptotic molecules (ie, bik, bak, bclx-S) also play a role in CD40-mediated inhibition.
There are 2 pathways that can trigger a death signal to the apoptotic machinery in a cell. The first is the activation of death receptors such as CD95/Fas/Apo-1 or TNF receptor 1 by binding their respective ligands.30 This, in turn, causes the recruitment of caspase 8 and the subsequent activation of the other effector caspases.21 The second mechanism is death-receptor independent, in which the apoptotic machinery is triggered directly by a death signal, which then leads to cytochrome c release from the mitochondria to the cytosol.25 In the presence of adenosine triphosphate, this causes the recruitment of caspase 3 and the other effector caspases.31 Cytochrome crelease is regulated directly by the members of the bcl-2family of genes. A number of studies have shown a central role of the p53 tumor suppressor gene in facilitating the death signal as in the second mechanism mentioned above.25,32 It has been demonstrated that p53 induces cytochrome c release from the mitochondria, a process solely dependent on the recruitment of Bax from the cytosol.25 However, diffuse large-cell lymphoma lines have also been shown to be inhibited by CD40 stimulation.11 These cells (including the RL cell line) are characterized as having mutations in their p53 gene among other genetic lesions.11 Interestingly, the RL lymphoma has a t(14;18) translocation and an overexpression of Bcl-2 as result.34 The results presented here suggest that CD40-mediated inhibition occurs even during overexpression of Bcl-2. These data indicate that the induction of Bax is independent of p53 and apoptosis induced by CD40 stimulation cannot be compensated by Bcl-2. Overall, these results suggest that CD40 agonists (srCD40L or CD40 mAb) may have potential clinical use for the treatment of aggressive histology lymphomas.
The authors gratefully acknowledge the expert secretarial assistance of Ms Laura Knott and superb technical support by Mr Steve Stull.
Supported in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract no. N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William J. Murphy, Director of Basic Research, SAIC-Frederick, National Cancer Institute at Frederick, Bldg 567, Rm 210, Frederick, MD 21702; e-mail: murphyw@ncifcrf.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal