Using a perfusion chamber and confocal laser scanning microscopy, we analyzed the interplay of von Willebrand factor (VWF) and fibrinogen during thrombus growth on a collagen surface under physiologic high shear rate conditions. During initial thrombogenesis, platelet thrombi were constructed totally by VWF, not by fibrinogen. Fibrinogen accumulated predominantly inside the growing thrombi as a function of time, whereas the thrombus surfaces directly exposed to flow were occupied constantly by VWF throughout the observation period. In perfusion of afibrinogenemia (AF) blood lacking both plasma and platelet fibrinogen, the final height and volume of thrombi were significantly reduced compared with controls, albeit the area of surface coverage was normal. The impaired thrombus growth in AF was only partially corrected by the addition of purified fibrinogen to AF blood, whereas the addition of purified VWF to blood of severe von Willebrand disease (VWD) completely normalized the defective thrombus growth in this disease. Thus, the initial 2-dimensional thrombus expansion involves only VWF, whereas the time-dependent accumulation of fibrinogen, released from activated platelets, acts as a core adhesive ligand, increasing thrombus strength and height and resulting in 3-dimensional thrombus development against rapid blood flow.
Introduction
Mural thrombus formation to repair damaged vessel walls is essential for the arrest of bleeding1,2 and also triggers fatal intravascular thrombosis such as myocardial infarction or stroke.3,4 At the initial stage of thrombus formation, platelets respond quickly to changes in the vessel wall, adhering to extracellular matrices at sites of rupture or alteration. Platelets adhering to the thrombogenic surfaces then aggregate to generate a platelet plug.2 These events occur in vivo under blood flow conditions, so that in vitro experimental systems used to study the molecular mechanisms involved in platelet thrombogenesis must also take blood flow into consideration.5 Indeed, recent studies using a perfusion chamber that simulates physiologic blood flow conditions have drastically revised the concept of initial platelet adhesion under high shear rate conditions; that concept differs significantly from the classic adhesion theory derived from results of assays under static conditions.6-9 Platelet adhesion under high shear represents a 2-step event that involves initial “platelet rolling,” which is mediated by transient interaction of platelet membrane glycoprotein (GP) Ib-IX-V complex with surface-immobilized von Willebrand factor (VWF). This is followed by “firm platelet adhesion” on a thrombogenic surface, which occurs through stable binding of platelet integrin αIIbβ3 or various collagen receptors to surface-immobilized VWF or collagens in vascular subendothelium.6-9
In addition to the essential function of VWF in initial platelet adhesion, the interaction of VWF with platelet GP Ibα plays a pivotal role in subsequent platelet aggregation, leading to spatial thrombus growth under high shear flow.10,11 Indeed, our recent study with afibrinogenemia (AF) blood, which lacks both plasma and platelet fibrinogen, showed that VWF, through its binding to integrin αIIbβ3, mediated thrombus development to an extent comparable to that in normal controls.12 However, compared with normal thrombi, AF thrombi appeared to be loosely packed and fragile when blood flow was very fast.12,13 Thus, the adhesive functions of both VWF and fibrinogen were assumed to be indispensable for proper thrombus growth under high flow conditions.12-17 However, the precise molecular mechanisms underlying the interplay of adhesive proteins in spatial thrombus growth during high shear flow remain poorly defined.
In this work, we analyzed in detail the process of thrombus growth on a collagen surface under flow, focusing on the interplay of VWF and fibrinogen. We show that the 3-dimensional development of mural thrombi is precisely orchestrated by the distinct and phase-specific concerted actions of VWF and fibrinogen under high shear rate conditions.
Patients, materials, and methods
Materials
The specific thrombin inhibitor argatroban (MD-805) was supplied by Mitsubishi Welpharma (Tokyo, Japan); type I acid-insoluble collagen fibrils from bovine Achilles tendon and the fluorescent dye mepacrine (quinacrine dihydrochloride) were purchased from Sigma-Aldrich (Tokyo, Japan); FluoroLink mAb Cy2 and Cy3 labeling kits were obtained from Amersham Pharmacia Biotech (Tokyo, Japan). Human native VWF containing the highest molecular weight multimers as judged by sodium dodecyl sulfate (SDS)–1.5% agarose gel electrophoresis18was purified from cryoprecipitates as described.19-21 Purified human fibrinogen (99.8% coagulability; lot no. L0717) was purchased from Hematologic Technologies (Essex Junction, VT). Anti-VWF monoclonal antibody LJ-2.2.9 was a kind gift from Z. M. Ruggeri (The Scripps Research Institute, La Jolla, CA). This antibody recognizes the carboxy-terminal region of the VWF subunit and does not affect any adhesive function of VWF.19,21,22 Antifibrinogen monoclonal antibody IF-1 recognizes the fibrinogen conformation in the presence of calcium ions.23 Both monoclonal antibodies were used as F(ab′)2 fragments prepared by pepsin digestion of the purified IgG at low pH and collection of the flow-through fractions in a protein A–sepharose (Pharmacia-LKB Japan, Tokyo, Japan) column.19 22
Patient profiles
The profiles of the patient with type 3 VWD and the patient with congenital AF have been described12 (VWD type 3 and Af-1 in Table 1 of reference 12). Briefly, the VWD patient had no detectable VWF antigen in plasma or platelets and exhibited complete lack of ristocetin-induced platelet aggregation, with normal ranges of platelet counts and plasma fibrinogen. The AF patient had undetectable fibrinogen levels in plasma and platelets and showed complete lack of platelet aggregation by adenosine diphosphate or collagen, with normal platelet counts and plasma VWF antigen levels. Bleeding times in both patients were markedly prolonged (longer than 20 minutes). Included in this study as controls were 5 nonsmoking healthy volunteers who had not taken any medications in the previous 2 weeks and whose platelet counts were between 200-350 × 103/μL. All blood donors, including the patients, showed no significant anemia, with hematocrit values always greater than 35%.
Blood collection and platelet labeling
After informed consent was obtained, blood was collected from patients and healthy volunteers using argatroban (final concentration, 240 μM) as an anticoagulant.11,12,24 At this argatroban concentration, no thrombin generation was assessed using the chromogenic substrate S-2238 (KabiVitrum AB, Stockholm, Sweden), and no visible fibrin clot formation was detected for at least 2 hours after blood collection.12 Anticoagulated whole blood was kept at 37°C and used in perfusion studies within 30 minutes of blood collection. In some experiments, the fluorescent dye mepacrine was added to the blood prior to perfusion to label platelets (final concentration, 10 mM), allowing visualization of platelet-surface interactions by epifluorescence videomicroscopy.11,12 24
Fluorescence labeling of monoclonal antibodies
The F(ab′)2 fractions of anti-VWF or anti–fibrinogen IgG were concentrated to 1-3 mg/mL, dialyzed with 20 nmol/L phosphate-buffered saline (PBS, pH 7.35), and fluorescence labeled using FluoroLink mAb Cy2 or Cy3 labeling kit according to the manufacturer's protocol. The Cy2 and Cy3 reagents produce a green and orange signal, respectively. These fluorescence-labeled F(ab′)2 fractions were stored at 4°C until used.
Flow chamber and epifluorescence videomicroscopy
Type I collagen-coated glass coverslips were prepared as described11,12,24 and placed in a parallel plate flow chamber (rectangular type; flow path of 1.9-mm width, 31-mm length, and 0.1-mm height).25,26 The chamber was assembled and mounted on a microscope (BX60; Olympus, Tokyo, Japan) equipped with epifluorescent illumination (BX-FLA; Olympus) and a charge-coupled device (CCD) camera system (U-VPT-N; Olympus) as described.11,12,24,25 Whole blood containing mepacrine-labeled platelets was aspirated through the chamber by a syringe pump (Model CFV-3200, Nihon Kohden, Tokyo, Japan) at a constant flow rate of 0.285 mL per minute, producing a wall shear rate of 1500 s−1 at 37°C in a thermostatic air bath (Model UI-50, Iuchi, Osaka, Japan). This wall shear rate is considered a physiologically relevant high shear flow.6,9,11,12 Where indicated, perfusion was also performed with a typical low wall shear rate of 300 s−1. The process of platelet thrombogenesis was recorded with a Hi-8 videocassette recorder (VL-HL1; Sharp, Osaka, Japan) with a time resolution of 0.033 seconds.11 12
Assessment of surface coverage and size of thrombi
Platelet thrombi generated on a collagen-coated surface were fixed at several time points (1, 3, 5, and 7 minutes after initial platelet-surface interaction) by paraformaldehyde. The fixation of thrombi was performed so that the sample whole blood was sharply switched to the fixation buffer (0.1 M PBS containing 4% paraformaldehyde, pH 7.4) at several time points. The fixation buffer was continuously perfused with the same flow rate, in which whole blood was gradually replaced by the fixation buffer at the collagen-coated surface in a chamber. The perfusion of fixation buffer was continued for 10 minutes at 37°C, and the entire fixation process in perfusion of blood containing mepacrine-labeled platelets was observed in real time by epifluorescence microscopy to confirm fixing of the generated platelet thrombi without collapse or peel-off from the collagen surface. After fixation, the perfusion chamber was disassembled, and a coverslip was rinsed 3 times with PBS, mounted in Dako fluorescent mounting medium (DAKO; Carpinteria, CA) as an antifade medium, and viewed with a confocal laser scanning microscope (CLSM; MRC-600, Nippon Bio-Rad Laboratories, Tokyo, Japan). Mepacrine fluorescence corresponding to platelets was examined at an excitation wavelength of 488 nm with a barrier filter at 500 nm. Specimens were viewed at 1-μm intervals from the collagen surface to a height of 60 μm from the surface. After background subtraction, each image was digitized with the standard imaging analysis software (“histogram” function) of the MRC600 system and subjected to computer-assisted analysis with an image processing application (Win ROOF; Mitani, Fukui, Japan). This program was used to calculate the percentage of the area covered by adhering platelets in a defined area (surface coverage) after setting the threshold value and binarization of each image.24 25Each thrombus height and volume in a frame was also calculated based on images with a 1-μm interval from the collagen surface with the assistance of Win ROOF software (Mitani, Fukui, Japan).
Immunohistochemical staining of adhesive proteins
In experiments using platelets not labeled with mepacrine, a coverslip fixed with paraformaldehyde at several time points during perfusion was double-stained with 100 μL of a solution mixture of Cy3-labeled anti-VWF and Cy2-labeled antifibrinogen F(ab′)2(each at 0.24 μg/mL) for 2 hours at 37°C and viewed with CLSM. In some experiments using mepacrine-labeled platelets, a fixed coverslip was incubated with 100 μL of Cy3-labeled anti-VWF or antifibrinogen F(ab′)2 solution (each at 0.24 μg/mL) for 2 hours at 37°C before CLSM analysis. These experimental conditions for immunohistochemical staining were determined in preliminary experiments that confirmed the sufficient infiltration of fluorescence-labeled antibodies into thrombi; that is, the portions most distant from the outside surface were stained.
Evaluation of VWF and fibrinogen distribution within thrombi
Sections immunostained as described above were rinsed 3 times with PBS, mounted, and viewed with CLSM at an excitation wavelength of 488 nm with a barrier filter at 500 nm for Cy2 (green) and mepacrine fluorescence, and at an excitation wavelength of 529 nm with a barrier filter at 550 nm for Cy3 fluorescence (orange). The Cy2 (or mepacrine) and Cy3 images were obtained at 1-μm intervals simultaneously for the 2 excitation channels and were filtered to diminish background. Images were then projected onto the 2-dimensional plane simultaneously and merged for evaluation of the distribution of fibrinogen and VWF or the distribution of platelets and adhesive proteins within thrombi. In both Cy2 and Cy3 digitized images, the fluorescence intensity in a frame was evaluated from 0 (background value) to 255 pixel value (sites with the highest intensity) in the CLSM system. Thus, merged images of portions stained with both green and orange fluorescence basically showed the color of the higher pixel value in the merged images. When both pixel values were nearly equal, the merged image of that portion showed a yellowish color. In some experiments where thrombi were double-stained with anti-VWF and antifibrinogen antibodies, each VWF or fibrinogen deposition within individual thrombi was quantified as VWF- or fibrinogen-associated thrombus volume based on images with a 1-μm interval from the collagen surface with the assistance of Win ROOF software.
Results
Generation of thrombi on a collagen surface by perfusion under low or high shear rate with blood from healthy donors, an AF patient, and a type 3 VWD patient
When normal control blood was perfused, the area covered by platelets adhering to a collagen surface (surface coverage) increased as a function of perfusion time under both low and high shear rate conditions (Figure 1). The time-dependent increase in the surface coverage by platelets from blood of the AF or VWD patient was comparable to that of healthy controls under low shear rate conditions, whereas almost no platelet-surface interaction was observed during VWD blood perfusion under high shear rate conditions (Figure 1), and the final height of AF thrombi was about half that of control thrombi, although surface coverage was comparable, in high shear rate conditions (Figure2). These results suggest that fibrinogen does not play a major role in the 2-dimensional expansion of mural thrombi but is required for normal 3-dimensional thrombus development under high shear rate conditions.
Time-course changes in surface coverage by thrombi generated during perfusion of blood from normal controls, an AF patient, or a type 3 VWD patient.
A collagen-coated coverslip was fixed at several time points (1, 3, 5, and 7 minutes after initial platelet-surface interaction) during perfusion, and platelet thrombi labeled with mepacrine on a coverslip were evaluated by CLSM. The percentage of the area covered by thrombi (surface coverage) in a defined area (211 × 317 μm) within a frame was calculated at a height of 2 μm from the collagen surface. In control thrombi, each data point represents the average (± SD) of 15 areas examined (5 areas randomly selected in 3 independent perfusions of blood from 3 individual donors, respectively). In AF or VWD thrombi, each data point represents the average (± SD) of 10 areas examined (5 areas randomly selected in each of 2 separate perfusions). Under high shear rate (1500 s−1), the surface coverage of AF thrombi increased as a function of perfusion time to an extent comparable to that of controls, whereas almost no platelet-surface interactions were observed in the perfusion of VWD blood. In contrast, the surface coverage of AF or VWD thrombi was comparable to that of healthy controls throughout the observation periods under low shear rate conditions (300 s−1).
Time-course changes in surface coverage by thrombi generated during perfusion of blood from normal controls, an AF patient, or a type 3 VWD patient.
A collagen-coated coverslip was fixed at several time points (1, 3, 5, and 7 minutes after initial platelet-surface interaction) during perfusion, and platelet thrombi labeled with mepacrine on a coverslip were evaluated by CLSM. The percentage of the area covered by thrombi (surface coverage) in a defined area (211 × 317 μm) within a frame was calculated at a height of 2 μm from the collagen surface. In control thrombi, each data point represents the average (± SD) of 15 areas examined (5 areas randomly selected in 3 independent perfusions of blood from 3 individual donors, respectively). In AF or VWD thrombi, each data point represents the average (± SD) of 10 areas examined (5 areas randomly selected in each of 2 separate perfusions). Under high shear rate (1500 s−1), the surface coverage of AF thrombi increased as a function of perfusion time to an extent comparable to that of controls, whereas almost no platelet-surface interactions were observed in the perfusion of VWD blood. In contrast, the surface coverage of AF or VWD thrombi was comparable to that of healthy controls throughout the observation periods under low shear rate conditions (300 s−1).
Relationship between surface coverage and maximum height of thrombi generated under high shear rate conditions from blood of normal controls, an AF patient, or a type 3 VWD patient.
Experimental conditions were as described in the Figure 1 legend, except that only a high shear rate (1500 s−1) was applied. Each data point represents the surface coverage and the maximum height of thrombi generated at 7 minutes of perfusion. Randomly selected 15 or 10 defined areas (211 × 317 μm each, see Figure 1 legend) were examined in normal controls or patients, respectively. Maximum thrombus height was based on the highest thrombus (distance from the collagen surface) within a defined area. The average (±SD) surface coverage and maximum height of thrombi in controls and in the AF patient were also indicated. The asterisk indicates statistically significant differences from control (P < .01). Note that the heights of AF thrombi were about half that of healthy controls, whereas surface coverages were comparable to normal.
Relationship between surface coverage and maximum height of thrombi generated under high shear rate conditions from blood of normal controls, an AF patient, or a type 3 VWD patient.
Experimental conditions were as described in the Figure 1 legend, except that only a high shear rate (1500 s−1) was applied. Each data point represents the surface coverage and the maximum height of thrombi generated at 7 minutes of perfusion. Randomly selected 15 or 10 defined areas (211 × 317 μm each, see Figure 1 legend) were examined in normal controls or patients, respectively. Maximum thrombus height was based on the highest thrombus (distance from the collagen surface) within a defined area. The average (±SD) surface coverage and maximum height of thrombi in controls and in the AF patient were also indicated. The asterisk indicates statistically significant differences from control (P < .01). Note that the heights of AF thrombi were about half that of healthy controls, whereas surface coverages were comparable to normal.
Addition of purified VWF to VWD blood completely normalized the defective thrombus growth under high shear conditions, whereas addition of fibrinogen to AF blood only partially reversed the defective thrombus formation (Figure 3), suggesting that platelet fibrinogen, in addition to plasma fibrinogen, plays a significant role in thrombus generation under high shear rate conditions.
Effect of addition of fibrinogen or VWF to AF or VWD blood on thrombus volume generated under high shear rate conditions.
Whole blood containing mepacrine-labeled platelets from a VWD or AF patient was perfused over a collagen surface for 7 minutes under high shear rate (1500 s−1) with or without addition of the respective deficient protein to blood prior to perfusion. Data represent the average (+ SD) total thrombus volume in a defined area (15 or 10 defined areas in control or patients, respectively, as described in Figure 1 legend). One-way factorial ANOVA and Scheffe method were used for analysis of variance and for comparisons with controls, respectively, with assistance of Stat View computer software (Abacus Concepts, Berkeley, CA). Asterisks indicate statistically significant differences from controls (P < .01). Statistical analyses demonstrated that the reduced thrombus volume in AF blood was only partially corrected by addition of purified fibrinogen (final concentration, 2.0 or 4.0 mg/mL) to AF blood, whereas the addition of purified VWF (20 μg/mL) to type 3 VWD blood completely normalized the reduced thrombus volume.
Effect of addition of fibrinogen or VWF to AF or VWD blood on thrombus volume generated under high shear rate conditions.
Whole blood containing mepacrine-labeled platelets from a VWD or AF patient was perfused over a collagen surface for 7 minutes under high shear rate (1500 s−1) with or without addition of the respective deficient protein to blood prior to perfusion. Data represent the average (+ SD) total thrombus volume in a defined area (15 or 10 defined areas in control or patients, respectively, as described in Figure 1 legend). One-way factorial ANOVA and Scheffe method were used for analysis of variance and for comparisons with controls, respectively, with assistance of Stat View computer software (Abacus Concepts, Berkeley, CA). Asterisks indicate statistically significant differences from controls (P < .01). Statistical analyses demonstrated that the reduced thrombus volume in AF blood was only partially corrected by addition of purified fibrinogen (final concentration, 2.0 or 4.0 mg/mL) to AF blood, whereas the addition of purified VWF (20 μg/mL) to type 3 VWD blood completely normalized the reduced thrombus volume.
Distribution of VWF and fibrinogen in thrombi generated under high shear rate conditions
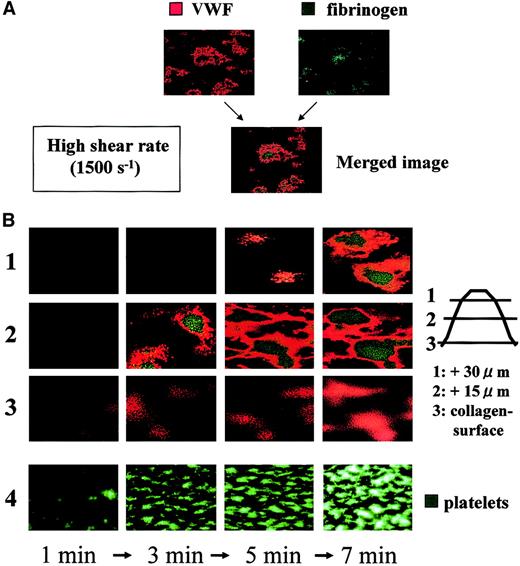
Time-course analysis during the initial phase of thrombogenesis indicated that mural thrombi, which were less than 15 μm in height on the collagen surface, were basically constructed by VWF (Figure4). As thrombi grew, fibrinogen became distributed predominantly in the inner area of thrombi as a function of time, whereas the outer areas of thrombi, including those adjacent to the collagen surface, were occupied by VWF at any time point examined (Figure 4). Indeed, detailed dissection of thrombi generated under high shear identified VWF as the adhesive protein that constructs the outside frame of thrombi, and fibrinogen as the core adhesive ligand present in the inner portions of thrombi (Figure5). Both the horizontal and longitudinal views of AF thrombi confirmed that they were totally constructed by VWF and a bit flatter than control thrombi (Table1; Figure 5).
Temporal changes in distribution of VWF and fibrinogen in thrombi generated under high shear rate conditions.
Whole blood without mepacrine labeling from healthy controls was perfused over a collagen surface under a high shear rate (1500 s−1). Thrombi generated at several time points (1, 3, 5, and 7 minutes of perfusion) were fixed, double-stained with Cy3-labeled anti-VWF and Cy2-labeled antifibrinogen antibody, and viewed by CLSM. (A) Merged CLSM images were obtained by the superimposition of 2 images of the identical portion and slice of thrombi; orange or green colors indicate VWF and fibrinogen, respectively, within thrombi. (B) CLSM images displayed in rows 1, 2, and 3 (left) are cross-sections at a height of 30, 15, and 0 μm from the collagen surface, respectively (right; original magnifications, × 400). Images are representative of 3 independent perfusions using blood from 3 individual donors. Images in row 4 taken at time points corresponding to those of the upper rows in a real-time observation with epifluorescence microscopy are displayed as a reference, in which platelet thrombi are visualized by mepacrine green fluorescence (original magnification, × 200). Note the gradual accumulation of fibrinogen at the inner areas of thrombi, as they grow, whereas the outer areas, including the portions adjacent to the collagen surface, are constantly occupied by VWF.
Temporal changes in distribution of VWF and fibrinogen in thrombi generated under high shear rate conditions.
Whole blood without mepacrine labeling from healthy controls was perfused over a collagen surface under a high shear rate (1500 s−1). Thrombi generated at several time points (1, 3, 5, and 7 minutes of perfusion) were fixed, double-stained with Cy3-labeled anti-VWF and Cy2-labeled antifibrinogen antibody, and viewed by CLSM. (A) Merged CLSM images were obtained by the superimposition of 2 images of the identical portion and slice of thrombi; orange or green colors indicate VWF and fibrinogen, respectively, within thrombi. (B) CLSM images displayed in rows 1, 2, and 3 (left) are cross-sections at a height of 30, 15, and 0 μm from the collagen surface, respectively (right; original magnifications, × 400). Images are representative of 3 independent perfusions using blood from 3 individual donors. Images in row 4 taken at time points corresponding to those of the upper rows in a real-time observation with epifluorescence microscopy are displayed as a reference, in which platelet thrombi are visualized by mepacrine green fluorescence (original magnification, × 200). Note the gradual accumulation of fibrinogen at the inner areas of thrombi, as they grow, whereas the outer areas, including the portions adjacent to the collagen surface, are constantly occupied by VWF.
Analysis of thrombi generated during perfusion of blood from normal controls or an AF patient under high shear rate conditions.
Experimental conditions were as described in the Figure 4 legend, except that AF blood was also perfused. Top images (original magnifications, × 400) are horizontal views, obtained at a height of 15 μm from the collagen surface, of thrombi generated after 7-minute perfusion of blood from a healthy control or from a patient with AF under a high shear rate (1500 s−1); bottom images are the corresponding longitudinal views, synthesized using imaging analysis software of the CLSM system based on overall horizontal slices at the identical positions with 1-μm intervals. The VWF- or fibrinogen-associated volume within each normal or AF thrombus displayed in the upper panels was also indicated. In the healthy control, fibrinogen is distributed at the inner or core areas of thrombi, whereas VWF is distributed predominantly in the outer areas. In contrast, only VWF appears in the AF thrombi, which appear flatter than control thrombi.
Analysis of thrombi generated during perfusion of blood from normal controls or an AF patient under high shear rate conditions.
Experimental conditions were as described in the Figure 4 legend, except that AF blood was also perfused. Top images (original magnifications, × 400) are horizontal views, obtained at a height of 15 μm from the collagen surface, of thrombi generated after 7-minute perfusion of blood from a healthy control or from a patient with AF under a high shear rate (1500 s−1); bottom images are the corresponding longitudinal views, synthesized using imaging analysis software of the CLSM system based on overall horizontal slices at the identical positions with 1-μm intervals. The VWF- or fibrinogen-associated volume within each normal or AF thrombus displayed in the upper panels was also indicated. In the healthy control, fibrinogen is distributed at the inner or core areas of thrombi, whereas VWF is distributed predominantly in the outer areas. In contrast, only VWF appears in the AF thrombi, which appear flatter than control thrombi.
Quantitative analysis of VWF and fibrinogen deposition within thrombi generated under high shear rate conditions from blood of a healthy control or an AF patient
| . | Control . | AF . |
|---|---|---|
| VWF | 3.5 ± 1.2 × 103 | 2.0 ± 0.7 × 103 (μm3) |
| Fibrinogen | 1.3 ± 0.4 × 103 | 0 |
| . | Control . | AF . |
|---|---|---|
| VWF | 3.5 ± 1.2 × 103 | 2.0 ± 0.7 × 103 (μm3) |
| Fibrinogen | 1.3 ± 0.4 × 103 | 0 |
Experimental conditions were identical to those described in the Figure 5 legend (perfusion of whole blood without mepacrine labeling from healthy controls or an AF patient with a shear rate of 1500 s−1 for 7 minutes). Thrombi generated were fixed, double-stained with Cy3-labeled anti-VWF and Cy2-labeled antifibrinogen antibody, and viewed by CLSM. VWF- or fibrinogen-associated volume of individual thrombi was calculated based on images with a 1-μm interval from the collagen surface. Data represent the average ± SD of 5 individual thrombi randomly selected in a defined area (211 × 317 μm) in each single perfusion of blood from a control or patient, respectively.
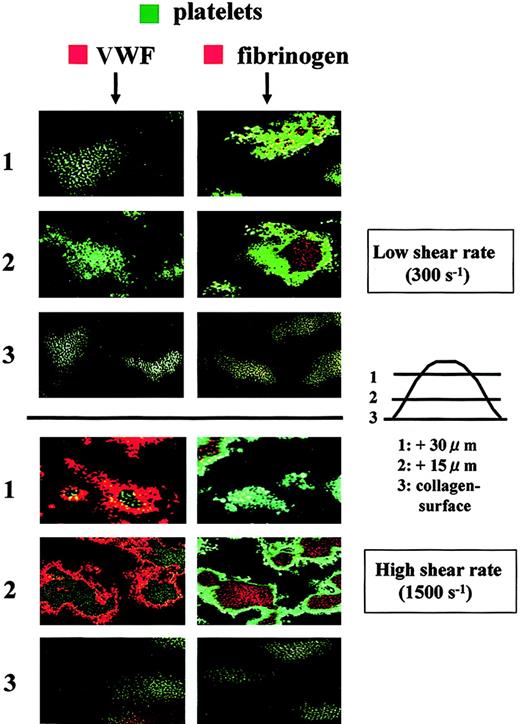
The distribution of adhesive proteins in thrombi was also examined in relationship to the platelet component. Under high shear rate conditions, VWF appeared to wrap the platelet component, whereas fibrinogen was wrapped by the platelet component (Figure6). These observations provide further confirmation that VWF predominantly resides at the outer areas of thrombi and that fibrinogen is limited to the inner areas of thrombi. Interestingly, only fibrinogen was stained in thrombi generated under low shear rate conditions, and VWF was invisible even at the portions adjacent to the collagen surface (Figure 6), suggesting that VWF does not play a major role in platelet thrombogenesis under low shear rate conditions. Instead, the direct interaction of platelet collagen receptors with a collagen surface appears to be critical for initial platelet adhesion to the surface under flow conditions where platelets flow slowly.7,9,27 28
Distribution of adhesive proteins and platelets in thrombi generated in perfusion of normal control blood under a low or high shear rate condition.
Whole blood from a healthy control containing mepacrine-labeled platelets was perfused over a collagen surface with a low (300 s−1) or high (1500 s−1) shear rate for 7 minutes. Fixed thrombi on coverslips were stained with a Cy3-labeled anti-VWF or antifibrinogen antibody and analyzed by CLSM. Merged CLSM images were obtained by the superimposition of 2 images at the identical portion and slice of thrombi; green indicates platelets in thrombi; orange indicates VWF (left panels) and fibrinogen (right panels). CLSM images displayed in rows 1, 2, and 3 are cross-sections at a height of 30, 15, and 0 μm from the collagen surface, respectively (original magnifications, × 400). Images are representative of 5 independent perfusions using blood from 5 individual donors. Under high shear rate conditions, VWF is predominantly distributed at the outer areas of thrombi, whereas fibrinogen is present at the inner areas. In contrast, only fibrinogen appears in thrombi under low shear rate conditions.
Distribution of adhesive proteins and platelets in thrombi generated in perfusion of normal control blood under a low or high shear rate condition.
Whole blood from a healthy control containing mepacrine-labeled platelets was perfused over a collagen surface with a low (300 s−1) or high (1500 s−1) shear rate for 7 minutes. Fixed thrombi on coverslips were stained with a Cy3-labeled anti-VWF or antifibrinogen antibody and analyzed by CLSM. Merged CLSM images were obtained by the superimposition of 2 images at the identical portion and slice of thrombi; green indicates platelets in thrombi; orange indicates VWF (left panels) and fibrinogen (right panels). CLSM images displayed in rows 1, 2, and 3 are cross-sections at a height of 30, 15, and 0 μm from the collagen surface, respectively (original magnifications, × 400). Images are representative of 5 independent perfusions using blood from 5 individual donors. Under high shear rate conditions, VWF is predominantly distributed at the outer areas of thrombi, whereas fibrinogen is present at the inner areas. In contrast, only fibrinogen appears in thrombi under low shear rate conditions.
Discussion
Mural thrombus growth on a thrombogenic surface is a consequence of platelet aggregation in which adhesive proteins of at least a divalent structure mediate the platelet-platelet engagement as a molecular glue. In a classic platelet aggregometer, platelet aggregation stimulated by exogenous agonists is achieved basically by the binding of fibrinogen to the activated integrin αIIbβ3, whereas the binding of VWF to GP Ibα and αIIbβ3 establishes platelet aggregation induced by high shear stress in a cone-and-plate type viscometer.5,29 Spatial growth of mural thrombi on a thrombogenic surface under whole blood flow conditions, relevant for both in vivo hemostasis and arterial thrombosis, is in part a solid-phase event and thus might differ from the platelet aggregation generated in a platelet-rich plasma solution under closed stirring experimental conditions.15-17
Our results identify the distinct phase-specific roles of VWF and fibrinogen in the overall process of mural thrombus growth under high shear rate conditions. At the initial phase in which thrombi are not yet spatially large, VWF alone mediates the 2-dimensional thrombus expansion, involving the initial platelet adhesion, cohesion of adhering platelets, and subsequent second- (or oligo-) layer platelet adhesion to platelets adhering and cohering to the surface. Under rheological conditions of high shear rates, when platelets flow at a relatively high speed, these events can be initiated only by the VWF-GP Ibα interaction, which is thought to have an extremely high association rate among all the ligand-receptor interactions involved in platelet thrombogenesis. Indeed, VWF immobilized to the surface can make the initial contact with flowing platelets, and VWF in plasma can do so with platelets already adhering to the surface through its interaction with platelet GP Ibα.10,15-17 Thus, the VWF-GP Ibα interaction, although insufficient in establishing initial-phase thrombus expansion presumably due to its high dissociation rate, does give an advantage to the RGD (Arg-Gly-Asp) sequence in the VWF subunit, leading to the subsequent stable binding to activated integrin αIIbβ3, in a manner analogous to that of firm adhesion of individual platelets to the surface.15-17 In contrast, fibrinogen appears to play no role at this phase because its ability to bind αIIbβ3 cannot compete with that of VWF and its 2-hit mechanism under fast blood flow conditions. Thus, the fundamental construction of mural thrombi under high shear is established by the VWF binding to GP Ibα and αIIbβ3, with essentially no contribution from fibrinogen during the initial 2-dimensional expansion of thrombi.
Through the adhesive functions of VWF, thrombi grow on the surface, increasing their height and volume as a function of time. However in 3-dimensional thrombus development, it is important to reconsider the rheological circumstances, especially at local areas around growing thrombi. As thrombi grow and become spatially bulky, the local wall shear rates in the microenvironment might become considerably higher than initially estimated, because the growing thrombi increasingly limit the flow path height. Furthermore, the resistant forces created by blood flow in the direction to collapse thrombi might be drastically increased at the lateral sites of growing thrombi with which larger cells such as erythrocytes or leukocytes might directly collide. This resistance force, in parallel with the shear stress, could be greater at the upper portions (close to the center of vessel lumens in vivo) of growing thrombi than that at the bottom portions (close to the vessel walls in vivo). Under such conditions, platelet thrombi solely constructed by VWF cannot maintain their height and volume and collapse due to elevated rheological forces.12 In contrast to the outer areas of thrombi exposed directly to flow, areas deep inside thrombi might effectively encounter a blood flow so slow and indirect that the rheological resistance force is apparently reduced.
In this regard, the gradually increased binding of fibrinogen to αIIbβ3 in the developing thrombus is thought to provide sufficient strength to withstand rheological resistance forces. Although the binding ability of fibrinogen for αIIbβ3 is apparently inferior to VWF under fast blood flow, fibrinogen once bound to αIIbβ3 is assumed to pack thrombi more tightly, presumably due to its lower dissociation rate for αIIbβ3 than that of VWF binding to αIIbβ3. Indeed, the molar excess of fibrinogen over VWF could favor αIIbβ3 under slow blood flow, as demonstrated by the generation of thrombi totally constructed by fibrinogen without VWF under low shear rate conditions (Figure 6). This binding characteristic of fibrinogen may explain, compared with the outer portions of thrombi, the apparently reduced amount of VWF deposition at the center of thrombi finally formed under high shear (Figures 4 and 5). In addition to a simple development of fibrinogen binding to αIIbβ3, fibrinogen at the center core of thrombi under slow blood flow might gradually replace VWF that initially constructed thrombi under fast blood flow. Thus, fibrinogen might gradually accumulate from an initial area deep inside thrombi, where the effective blood flow may be slowest. In this way, fibrinogen accumulating within thrombi acts as a core adhesive ligand, contributing to the increase in thrombus height and volume and resulting in 3-dimensional development of mural thrombi under high shear rate conditions. In this regard, Remijn et al13reported that AF thrombi generated on a collagen surface were larger than normal thrombi in a similar flow experiment. At present, the basis for these discrepant findings remains uncertain, but may rest in the respective different experimental situations. In addition to the difference in extent of fibrinogen deficiency in each patient, our approach, a time-point observation, could reflect the final thrombus size that comprehensively includes thrombus growth and concomitant collapse under increasing rheological resistance forces, perhaps resulting in the difference between their data and ours.
Fibrinogen released from activated platelets, especially the local release from deep inside thrombi, is likely to be advantageous in this regard, as compared with flowing fibrinogen inherently present in the normal bloodstream. Thus, platelet fibrinogen is assumed to contribute significantly to the elevation of local concentrations of this protein at a stage when fibrinogen plays a major role, as suggested by the fact of the incomplete correction of impaired thrombus development in AF after addition of purified fibrinogen to AF blood (Figure 3). In contrast to the case of VWD, in which platelet VWF might be less important than plasma VWF, infusion of platelet concentrates, in addition to fibrinogen products, in AF patients might be useful in the hemostatic management of the life-threatening bleeding in these patients.
The precise time-course dissection of growing thrombi in the present study clearly illustrates that the proper spatial growth of mural thrombi under high shear is precisely conducted by the distinct and phase-specific concerted functions of 2 major adhesive proteins. Although the molecular mechanisms of mural thrombogenesis in vivo may be more complex, involving other adhesive proteins such as fibronectin and vitronectin,30 the current study might provide the groundwork for therapeutic strategies that target the functions of VWF and fibrinogen to counter fatal arterial thrombosis formed under high shear rate conditions.
We thank M. Hoffman for editorial assistance.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-02-0508.
Supported by grant nos. 11670780 and 13671074 from the Ministry of Education, Science, and Culture of Japan (M.S.).
Part of this work was presented at the meeting of the American Society of Hematology, San Francisco, CA, December 1-5, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mitsuhiko Sugimoto, Department of Pediatrics, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan; e-mail: sugi-ped@naramed-u.ac.jp.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal