At sites of inflammation and in normal immune surveillance, chemokines direct leukocyte migration across the endothelium. Many cell types that are extravascular can produce chemokines, and for these mediators to directly elicit leukocyte migration from the blood, they would need to reach the luminal surface of the endothelium. This article reviews the evidence that endothelial cells are active in transcytosing chemokines to their luminal surfaces, where they are presented to leukocytes. The endothelial binding sites that transport and present chemokines include glycosaminoglycans (GAGs) and possibly the Duffy antigen/receptor for chemokines (DARC). The binding residues on chemokines that interact with GAGs are discussed, as are the carbohydrate structures on GAGs that bind these cytokines. The expression of particular GAG structures by endothelial cells may lend selectivity to the type of chemokine presented in a given tissue, thereby contributing to selective leukocyte recruitment. At the luminal surface of the endothelium, chemokines are preferentially presented to blood leukocytes on the tips of microvillous processes. Similarly, certain adhesion molecules and chemokine receptors are also preferentially distributed on leukocyte and endothelial microvilli, and evidence suggests an important role for these structures in creating the necessary surface topography for leukocyte migration. Finally, the mechanisms of chemokine transcytosis and presentation by endothelial cells are incorporated into the current model of chemokine-driven leukocyte extravasation.
Introduction
A central feature of inflammatory diseases is the migration of leukocytes from the circulation, across the endothelium and the basement membrane, and into the affected tissue. This mechanism of extravasation is induced by chemokines (chemoattractant cytokines), which are a family of proinflammatory mediators produced at the inflammatory site.1,2 As part of the migration process, circulating leukocytes must first adhere to the luminal surface of the endothelium. According to the current paradigm, this interaction involves the sequential engagement of leukocyte and endothelial adhesion molecules. First, selectins and their carbohydrate counterligands mediate leukocyte tethering and rolling. Then, leukocyte integrins and their ligands, including immunoglobulinlike intercellular adhesion molecules, mediate firm leukocyte adhesion.3Chemokines play a role in firm adhesion by activating integrins on the leukocyte cell surface.4 5 The leukocytes are directed by chemoattractant gradients to migrate across the endothelium, and through the extracellular matrix into the tissue.
The intent of this review is to focus on the endothelium and its role in transcytosing and presenting chemokines to blood leukocytes, resulting in leukocyte extravasation. The molecular nature of the endothelial binding sites that are proposed to transport and present chemokines are discussed. The mechanisms of chemokine transcytosis and presentation by endothelial cells are then fitted into the current model of how leukocytes emigrate into tissues at sites of inflammation.
Chemokine-binding sites on the endothelium
It has been traditionally held that chemoattractants stimulate directional leukocyte migration (ie, chemotaxis) by soluble gradients. However, it has been suggested that soluble chemokine gradients are unlikely to exist at the luminal endothelial surface, where chemokines would be washed away by the blood.6,7 In addition, soluble chemokines could activate leukocytes in the circulation prior to their selectin-mediated adhesion to the endothelium, resulting in loss of subsequent interaction with the endothelium and emigration. Therefore, it has been proposed that chemokines could act in a bound form, immobilized on the luminal endothelial surface where they exert their proadhesive and migratory effects on blood leukocytes.6 7
Experimental evidence for the hypothesis that chemokines can act in a bound form has come from the findings that specific and saturable chemokine-binding sites exist in situ on the venular endothelium of human skin and synovium, and immobilized interleukin-8 (IL-8) (also designated CXC ligand–8 [CXCL-8]) (Table1) attracts leukocytes in vitro.8,9 These sites are multispecific since a CXC chemokine, such as IL-8, can be displaced by a CC chemokine, such as RANTES, suggesting that the sites are not classic chemokine receptors but may be other binding proteins or carbohydrates (as discussed below). Electron microscopy has shown that when IL-8 and RANTES are injected into the skin, they are bound at the abluminal surface of the endothelium, internalized into caveolae (plasmalemmal vesicles), and transported transcellularly to the luminal surface.10 Here the chemokines are presented on the external aspect of the membrane to blood leukocytes. The endothelial binding sites that function in IL-8 transcytosis and presentation interact with the C-terminus of the chemokine, since a C-terminal truncation of IL-8 is neither significantly transcytosed nor presented. Using intact IL-8, we found approximately 10 times more immunolabel at the luminal endothelial surface compared with the truncated chemokine, suggesting that the majority of the chemokine is presented to leukocytes in a bound, rather than free, form. The functional significance of the chemokine transport and presentation is evident since C-terminally truncated IL-8 shows reduced chemotactic activity in vivo and in vitro.10 Similar transport and presentation mechanisms have been shown for ELC in the high endothelial venules of lymphoid tissue,11 suggesting that these mechanisms function in normal immune surveillance as well as inflammation.
Chemokines mentioned in this review together with their alternative names, according to a recent classification system, and their receptors
| Traditional name . | Alternative designation . | Chemokine receptor . |
|---|---|---|
| CXC family of chemokines | ||
| GRO/MGSA | CXCL1 | CXCR2 > CXCR1 |
| IL-8 | CXCL8 | CXCR1, CXCR2 |
| IP-10 | CXCL10 | CXCR3 |
| MIG | CXCL9 | CXCR3 |
| NAP-2 | CXCL7 | CXCR2 |
| PF4 | CXCL4 | Unknown |
| SDF-1 | CXCL12 | CXCR4 |
| CC family of chemokines | ||
| ELC | CCL19 | CCR7 |
| I-309 | CCL1 | CCR8 |
| MCP-1 | CCL2 | CCR2 |
| MIP-1α | CCL3 | CCR1, CCR5 |
| MIP-1β | CCL4 | CCR5 |
| RANTES | CCL5 | CCR1, CCR3, CCR5 |
| C family of chemokines | ||
| Lymphotactin | XCL1 | XCR1 |
| CX3C family of chemokines | ||
| Fractalkine | CX3CL1 | CX3CR1 |
| Traditional name . | Alternative designation . | Chemokine receptor . |
|---|---|---|
| CXC family of chemokines | ||
| GRO/MGSA | CXCL1 | CXCR2 > CXCR1 |
| IL-8 | CXCL8 | CXCR1, CXCR2 |
| IP-10 | CXCL10 | CXCR3 |
| MIG | CXCL9 | CXCR3 |
| NAP-2 | CXCL7 | CXCR2 |
| PF4 | CXCL4 | Unknown |
| SDF-1 | CXCL12 | CXCR4 |
| CC family of chemokines | ||
| ELC | CCL19 | CCR7 |
| I-309 | CCL1 | CCR8 |
| MCP-1 | CCL2 | CCR2 |
| MIP-1α | CCL3 | CCR1, CCR5 |
| MIP-1β | CCL4 | CCR5 |
| RANTES | CCL5 | CCR1, CCR3, CCR5 |
| C family of chemokines | ||
| Lymphotactin | XCL1 | XCR1 |
| CX3C family of chemokines | ||
| Fractalkine | CX3CL1 | CX3CR1 |
The current classification system is described by Zlotnik and Yoshie.107
GRO indicates growth-regulated gene product; MGSA, melanoma growth-stimulatory activity; IP, interferon-inducible protein; MIG, monokine-induced by interferon γ; NAP, neutrophil-activating protein; PF, platelet factor; SDF, stromal cell-derived factor; ELC, EBII-ligand chemokine; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normal T cell expressed and secreted; and CXCR, CXC receptor.
Many of the cell types that produce chemokines are extravascular. Thus, abluminal-to-luminal chemokine transcytosis and presentation by endothelial cells provide a posting mechanism, enabling the chemokines to reach the blood-endothelial interface and stimulate leukocyte emigration.12 Candidate endothelial molecules involved in chemokine transport and presentation include glycosaminoglycans (GAGs) and the Duffy antigen/receptor for chemokines (DARC), and these are further discussed below. Endothelial cells themselves can produce chemokines, in which case these mediators may be pesented to leukocytes at the endothelial cell surface but not transcytosed. One particular chemokine produced by the endothelium is fractalkine, which carries the chemokine domain on top of an extended mucinlike stalk.13It exists as a membrane-anchored form and a shed soluble form and can induce both adhesion and emigration of leukocytes.
The molecular nature of the chemokine transporters/presenters: glycosaminoglycans
GAGs are polysaccharides with a high negative charge that is due to sulfate and carboxyl groups and are usually attached to core proteins to form proteoglycans. Since chemokines are largely basic molecules, they exhibit electrostatic interactions with GAGs, especially heparin and heparan sulfate.14 The main GAG expressed by endothelial cells is heparan sulfate. Heparan sulfate proteoglycans compose 50% to 90% of total endothelial proteoglycans.15 Some of these proteoglycans, such as syndecan, glypican, and CD44, are membrane-associated glycoproteins while others, such as perlecan, are found in the basement membrane. The dissociation constant (Kd) of the GAG-chemokine interaction has been variously reported in the low nanomolar and mid-micromolar range. Recent studies using isothermal fluorescence titration show that IL-8 as a monomer binds to heparan sulfate with aKd below 5 nM whereas theKd of dimeric IL-8 is in the mid-micromolar range.16 Thus, the strength of the interaction can depend on the oligomerization state of the chemokine. In addition to GAGs, other negatively charged glycans containing sialic acid and mannose have recently been reported to bind the chemokine RANTES.17
Functional aspects of GAG-chemokine interactions
There is an increasing body of evidence suggesting that GAGs bind and present chemokines. For example, MIP-1β, when immobilized to proteoglycans, induces T-cell adhesion to integrin ligands.18 IL-8 and RANTES bind to heparan sulfate in the extracellular matrix, and these bound chemokines are then capable of stimulating leukocyte adhesion and transendothelial migration.19-21 In the case of MCP-1, the importance of chemokine interaction with cell-surface GAGs for transcellular migration was demonstrated by the almost complete absence of leukocyte chemotaxis across monolayers of GAG-deficient mutant cells.22 Further studies have shown that GAGs on the endothelial cell surface immobilize and enhance local concentrations of chemokines, promoting the presentation of these cytokines to their G protein–coupled signaling receptors.14,23 In addition, the interaction allows for the formation of immobilized gradients (haptotactic gradients) to direct the migration of the leukocyte from the blood out into the tissue.24 Recently it was shown that heparan sulfate or heparin prevents IL-8 from unfolding, thereby indicating a role for GAGs in IL-8 stability.16 It was postulated that in vivo this could result in prolonged IL-8 activity and preventing chemokine proteolytic degradation.
Whereas chemokines immobilized to GAGs show enhanced biological activity, the converse is true for soluble chemokine-GAG complexes. Soluble GAGs inhibit the binding of chemokines to cell membranes containing the chemokine receptors CXCR1, CXCR2, and CCR1 and inhibit chemokine-induced calcium flux in neutrophils.14
For the chemokine MIP-1β, chemotaxis assays have shown that GAG interaction is not an absolute requirement for functional interaction of the chemokine with its receptor.25,26 Other work, however, has shown that cell-surface GAGs enhance the activity of low concentrations of MIP-1β and MIP-1α by a mechanism that appears to involve sequestration onto the cell surface.27
Molecular structures involved in chemokine-GAG interactions
For several chemokines, such as IL-8, PF4, SDF, and MCP-1, the C-terminal α-helix is a major region involved in binding GAGs.28-32 This has been shown by the use of C-terminal truncations and mutagenesis of basic residues, leading to the reduction of chemokine binding to heparin. Interestingly, a C-terminal truncation of IL-8 exhibits reduced transcytosis and presentation by endothelial cells in skin, suggesting a role for GAGs in these mechanisms.10 The basic residues in the loop structures away from the C-terminal helices of SDF-1, PF4, and RANTES are also involved in binding GAGs.33-35 In addition, a recent report suggests an involvement of the N terminus of RANTES.17 MIP-1α binds GAGs, despite being an acidic molecule, and the heparin-binding site localizes to 2 or 3 basic residues in the loops outside the C-terminal α-helix.25 36
In the case of IL-8, PF4, SDF-1, and MCP-1, the GAG-binding site is spatially distinct from the residues that interact with the signaling receptor on the leukocyte. This would support a presentation mechanism in which the chemokines bound to endothelial GAGs would be available for interaction with receptors on the leukocyte cell surface. An exception is MIP-1α since its GAG-binding site is the same as that required for binding to its signaling receptor.25
There have been some studies on the GAG sequences that are involved in chemokine binding. A specific domain of heparan sulfate that binds IL-8 has been found; the domain consists of a block that is approximately 6 monosaccharides in length.37 These blocks areN-sulfated and may be separated by unsulfated sequences of up to about 14 monosaccharides. IL-8 binding was reported to correlate with the occurrence of the di-O-sulfated disaccharide IdceA(2-OSO3)-GlcNSO3(6-OSO3). SDF-1 also binds to a heparan sulfate sequence consisting of 6 monosaccharides in length, and PF4 binds to a 9-kDa fragment of the GAG that is enriched in N-sulfated disaccharides and iduronate 2-O-sulfate residues.34 38 Thus, the structure and spacing of the sulfated domains are important variables in GAG-chemokine interactions.
Selective binding of chemokines to GAGs
Chemokines exhibit wide variation in their affinity for heparin, with the order as follows: RANTES, MCP-1, IL-8, MIP-1α. The weaker binding of MIP-1α relates to its overall negative charge, contrasting with other chemokines, which are highly basic.14 Chemokines show selectivity in their strength of interaction with GAGs. For RANTES, the order is heparin, dermatan sulfate, heparan sulfate, chondroitin sulfate whereas for MCP-1 and IL-8 the order is heparin, heparan sulfate, chondroitin sulfate or dermatan sulfate (in this last listing, the final 2, chondroitin sulfate and dermatan sulfate, are equivalent). These differences can in part be explained by the negative charge density of the GAG, but cannot be completely explained by this since dermatan sulfate and chondroitin sulfate have similar levels of sulfation; however, RANTES shows far higher affinity to the former GAG than the latter. Further evidence of selective binding has come from affinity coelectrophoresis experiments.39 IL-8 and GRO were shown to bind preferentially to a fraction of heparin, whereas PF4 or NAP-2 did not show the same binding preference. Furthermore, selectivity is apparent at the cell surface. IP-10 binds to a specific and saturable cell-surface heparan sulfate–binding site on endothelial and other cells.40 This site is shared with PF4 but not with IL-8, MCP-1, RANTES, MIP-1α, or MIP-1β.
Heparan sulfate is highly heterogeneous in structure, and endothelial cells have the capacity to express this molecule on their cell surfaces with subtle variations among different vascular beds. For example, porcine endothelial cells from veins and arteries have been shown to synthesize heparan sulfate chains that differ in charge density and sulfation pattern.41 Although there were no differences in size or chain length, heparan sulfate from aorta was more highly charged, with increased sulfation and more clustering of N-sulfated portions of the chains. In the microvasculature of human skin and synovium where leukocyte transmigration occurs, chemokine-binding sites are expressed only on venular endothelial cells, not on arterioles, suggesting that chemokine-binding motifs on heparan sulfate may be differentially expressed in these 2 vascular sites.8,10 Recent evidence shows that there are differences in the sulfation patterns of heparan sulfate from human bone marrow and human umbilical vein endothelial cells. There was moreO-sulfation of the N-sulfated domains in heparan sulfate from the endothelial cells of bone marrow compared with those from umbilical veins.42 Binding experiments showed that bone marrow endothelial cells bound more SDF-1 per cell than human umbilical vein endothelial cells, and binding was inhibited byO-sulfated heparin and less by N-sulfated heparin. Therefore, it was postulated that highly sulfated domains in heparan sulfate from bone marrow endothelial cells contribute to tissue specificity where endothelial cells present SDF-1 to hematopoietic progenitor cells, resulting in their transmigration. Little is known about the control mechanisms that lead to the regulated diversity of heparan sulfate structures expressed in different cells and tissues. One reason may be the selective expression of isoforms of enzymes involved in heparan sulfate synthesis. Most tissues expressN-deacetylase/N-sulfotransferase-1 (NDST-1) and NDST-2, whereas heparin-producing mast cells show a predominance of NDST-2.43 It remains to be found out if the expression of such enzyme isoforms varies between endothelial cells in different vascular beds.
Although the main GAG expressed by endothelial cells is heparan sulfate, these cells also express chondroitin and dermatan sulfate, and the proportions of these GAGs can change between different endothelial cells. For example, human aortic endothelial cells synthesize mainly heparan sulfate with small amounts of chondroitin sulfate.44,45 Human umbilical vein endothelial cells also produce mainly heparan sulfate, but they also synthesize more chondroitin and dermatan sulfate than aortic endothelial cells.46 In addition, the major GAG in basement membranes is heparan sulfate. However, this can vary since mouse bone marrow venous sinusoids show unusually abundant chondroitin sulfate proteoglycan and an absence of heparan sulfate proteoglycan.47
Altered expression in the type of blood vessel GAG has been observed in several pathological and physiological situations, such as atherosclerosis, inflammatory bowel disease, and wound healing.48,49 For example, in the intima of atherosclerotic human aortas, there is a decrease in the proportion of heparan sulfate and an increase in the proportions of chondoitin and dermatan sulfate, which are produced by smooth muscle cells.44 50 These changes in GAG composition occur in the extracellular matrix of the blood vessel wall and could alter the nature of haptotactic chemokine gradients, thereby influencing leukocyte migration into the tissue.
In summary, endothelial cells demonstrate flexibility in terms of the expression of the type of GAG and the fine structure of their carbohydrate chains in different tissues. Variations in GAG carbohydrate sequences and positioning of negatively charged groups, together with amino acid sequence differences among chemokines, contribute to the selectivity of the chemokine-GAG interactions. This raises the intriguing possibility that GAG substructures at the endothelial cell surface participate in determining the types of chemokines transcytosed and presented in a given inflammatory site and hence lend selectivity to the types or subsets of leukocytes recruited. Furthermore, differential expression of GAGs in the extracellular matrix of the blood vessel wall could modify chemokine gradients and further influence leukocyte migration.
The Duffy antigen/receptor for chemokines (DARC)
This protein was originally described on red blood cells and is the site where the malaria parasite, Plasmodium vivax,invades erythrocytes. The protein also occurs on several other cell types, including the endothelial cells of postcapillary venules in kidney, lung, thyroid, and spleen, but not on the endothelial cells of arterioles and arteries.51 52 Thus, there is selectivity in DARC expression between endothelial cells, occurring in the segment of the circulation where leukocyte extravasation takes place.
DARC has a serpentine structure with 7 transmembrane domains, like other chemokine receptors, yet is not G protein coupled and has no known signaling mechanism.53 It exhibits broad specificity, binding members of both CC and CXC classes of chemokines, except MIP-1α and MIP-1β, and the C-chemokine lymphotactin.54,55 IL-8, MGSA, RANTES, and MCP-1 show high-affinity binding to DARC, with a Kd of approximately 5 nM.54,56 Binding studies in inflamed and normal human tissues have shown that DARC expressed by the venular endothelium binds chemokines in situ.8,9 This has been shown by using blocking antibodies to DARC and by the demonstration that the chemokine-binding profile to venules is similar to that of DARC since IL-8 binding can be displaced by excess RANTES, indicating a multispecific site, and there is lack of MIP-1α binding.8 9
The function of DARC is yet to be fully defined. However, recent gene-deletion studies in mice suggest that the protein has a functional role in inflammation since these animals show altered leukocyte recruitment compared with wild type when given the inflammatory stimuli lipopolysaccharide (LPS) and thioglycollate, yet in all other respects they are normal.57,58 DARC may function in chemokine endocytosis/transcytosis in endothelial cells. This hypothesis is based on the observations that endothelial DARC localizes to caveolae,51 which are associated with vesicular transport. In addition, use of DARC transfectants shows that the protein binds and internalizes chemokines.56 When IL-8 or RANTES is injected into the skin, these chemokines localize to caveolae of endothelial cells, where they are transported to the luminal surface and presented.10 Since DARC also localizes to endothelial caveolae, these data further suggest a role for the protein in chemokine transcytosis. The DARC-binding region of the chemokine MGSA is different from that involved in the interaction with CXCR2.59 This finding, together with the observation that DARC occurs at the endothelial cell surface,51 implies that the protein could potentially act as a chemokine-presenting molecule. However, there is evidence that chemokines bound to DARC on the red blood cell surface do not activate neutrophils in terms of calcium flux.60 Therefore, other molecules, such as GAGs, may be more important in presentation. Further functions have been proposed for DARC. It could act as a decoy receptor or as a signal-transducing receptor with an, as yet, unknown signaling mechanism.52,60 61 Thus, in summary, DARC could potentially act as chemokine transporter although further work is required to clarify its function in the endothelium.
G protein–coupled chemokine receptors
Cultured endothelial cells have been shown to express a variety of G protein–coupled signaling chemokine receptors, including CCR2 and CCR8 and CXCR1, CXCR2, CXCR3, and CXCR4,62-66 although the expression of endothelial CXCR1, CXCR2, and CXCR3 in vitro is variable depending on the cell source and culture conditions.10,67-69 In addition, the in situ expression of CXCR2 and CCR2 has been shown on endothelial cells in human skin and inflamed tissue.63 70
The expression of these chemokine receptors on endothelial cells leads to signal transduction and biological responses. Such responses include shape changes, cytoskeletal rearrangements, and cell division. Some chemokines are angiogenic (eg, IL-8, MCP-1, and I-309) and others angiostatic (eg, IP-10 and MIG), and activation of their respective chemokine receptors results in the stimulation or inhibition of endothelial cell proliferation and chemotaxis. Other endothelial responses to chemokines include IL-8–stimulated increase in vascular permeability in vitro and in vivo and edema formation in vivo.71-73
Topographical distribution of chemokines, chemokine receptors, and adhesion molecules on endothelial cells and leukocytes
The surfaces of endothelial cells and leukocytes are not smooth, but contain numerous microvillous processes. These projections have been shown to harbor the preferential distribution of certain adhesion molecules, chemokines, and chemokine receptors, and this distribution may be functionally important in leukocyte extravasation, as has especially been shown for selectin adhesion molecules.
On the surface of leukocytes, immunoelectron microscopic studies have shown that L-selectin, P-selectin glycoprotein ligand-1 (PSGL-1), and the integrins α4β7 and α4β1 are concentrated on microvilli.74-79 Eighty percent of PSGL-1 label is localized to the tips of neutrophil microvilli.79L-selectin, PSGL-1, and α4β7 and α4β1 integrins, together with their counterligands, can mediate the formation of initial cell contacts between the leukocyte and the endothelium (termed tethering) and subsequent rolling. Less is known about the distribution of counterligands for leukocyte adhesion molecules on the endothelial cell surface. MECA-79, an antibody that recognizes the counterligands for L-selectin, localizes to the microvilli of endothelial cells.80 Similarly, the sialomucin CD34, one of the counterligands for L-selectin, is concentrated on endothelial microvilli.81 82
The functional significance of the microvillous distribution of molecules involved in leukocyte extravasation has been shown for L-selectin. Von Andrian et al 83 constructed chimeras containing the transmembrane and intracellular domains of CD44 and the functional extracellular domain of L-selectin. This chimera targeted the extracellular domain of L-selectin away from leukocyte microvilli to the planar cell body. When intact L-selectin was used, it localized to microvilli and dramatically mediated initial contact formation of leukocytes to its ligand under flow. However, the chimera exhibited little ability to initiate tethering to its ligand. These studies have been confirmed in vivo by means of intravital microscopy, which showed that microvillous expression of the L-selectin ectodomain is important for leukocyte tethering in peripheral lymph nodes.84 In another study, Finger et al85demonstrated that disruption of leukocyte microvilli using cytochalasin B or hypotonic swelling can result in nearly complete inhibition of tethering. Taken together, these studies show that presentation of L-selectin by microvilli is functionally important in the earliest part of the adhesion cascade.
At the luminal cell surface of the endothelium, chemokines are presented to blood leukocytes during the early stages of endothelial-leukocyte interactions.10 Using immunoelectron microscopy, we found that the chemokine IL-8 is preferentially distributed on the microvillous processess of endothelial cells where immunoreactivity is 10 times higher than on the planar cell surface, and 2 out of 3 microvilli harbor the chemokine. This suggests that the chemokine-binding sites, such as GAGs and potentially DARC, are concentrated on these endothelial protrusions. Interestingly, the chemokine receptors CCR2, CCR5, and CXCR4 occur predominantly on the microvilli of macrophages and T cells.86 Thus, the microvillous localization of chemokines and their receptors may be spatially important during the activation stage of leukocyte emigration.
During the subsequent arrest and firm adhesion of the leukocyte, a role for microvilli is less implicated. The β2 integrins are excluded from leukocyte microvilli but are distributed on the planar cell surface.75,76,83,87 These molecules cannot initiate cell-cell contact under physiological flow 88 but are specialized for firm leukocyte adhesion to the endothelial cell surface, once they have been activated by chemokines.89,90Recruitment to the planar cell body may ensure this later role of β2 integrins in the multistep adhesion process by precluding its availability at sites of initial contact. The distribution of the integrin ligands intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on the endothelial cell membrane has been shown in mice.91 ICAM-1 occurs both on the smooth surface of the plasma membrane and on microvilli, and VCAM-1 shows a uniform distribution on the smooth surface of the endothelium. On cultured human dermal endothelial cells, ICAM-1 has been shown to localize to microvillous cell protrusions.92
One reason for the preferential distribution of chemokines, chemokine receptors, and certain adhesion molecules on microvilli is that these structures may be the first points of contact between blood leukocytes and the endothelium, and thus are spatially the most effective site to cluster ligand and receptor pairs. Another reason relates to the glycocalyx. This negatively charged structure, which coats leukocytes and endothelial cells,93,94 could create electrostatic repulsion between the 2 cell types and inhibit cell contact. In the presence of microvilli, the surface area making contact is limited to the outermost cell periphery, where electrostatic repulsion would be least, thereby creating a contact-promoting environment.83
The model
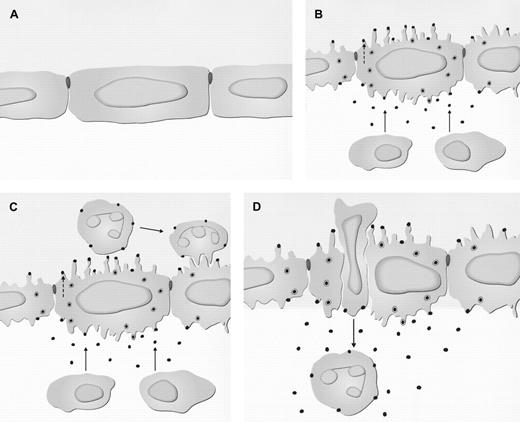
In consideration of some recent ultrastructural reports and the data presented in the previous sections, the current model of chemokine-driven leukocyte transmigration can be extended (Figure1).
Subcellular events in the endothelium during chemokine-directed leukocyte extravasation.
(A) Noninflamed tissue. (B) Inflamed tissue. Release of chemokines from extravascular cells in the tissue occurs (→), and there is wrinkling of the endothelial cell surface. Chemokines are taken up at the abluminal surface of the endothelium and transcytosed in caveolae. This process involves binding to glycosaminoglycans (GAGs) and/or the Duffy receptor. At the luminal surface, chemokines are released and bound preferentially on the tips of projections. These mediators may also be produced and released directly by endothelial cells, in which case they are also bound at the luminal surface but not transcytosed (- - ->). (C) Chemokines bound at the luminal endothelial cell surface build up in concentration. They do this sufficiently to bind to and activate the signaling receptors on the leukocyte cell surface, leading to activation of integrins and firm attachment. (D) Leukocyte migration occurs either transcellularly through a pore in the endothelial cell or through the intercellular junction, following a chemokine gradient bound to GAGs and/or the Duffy receptor. The cell then enters the basement membrane and continues migration along a chemokine gradient that is soluble or immobilized to the extracellular matrix.
Subcellular events in the endothelium during chemokine-directed leukocyte extravasation.
(A) Noninflamed tissue. (B) Inflamed tissue. Release of chemokines from extravascular cells in the tissue occurs (→), and there is wrinkling of the endothelial cell surface. Chemokines are taken up at the abluminal surface of the endothelium and transcytosed in caveolae. This process involves binding to glycosaminoglycans (GAGs) and/or the Duffy receptor. At the luminal surface, chemokines are released and bound preferentially on the tips of projections. These mediators may also be produced and released directly by endothelial cells, in which case they are also bound at the luminal surface but not transcytosed (- - ->). (C) Chemokines bound at the luminal endothelial cell surface build up in concentration. They do this sufficiently to bind to and activate the signaling receptors on the leukocyte cell surface, leading to activation of integrins and firm attachment. (D) Leukocyte migration occurs either transcellularly through a pore in the endothelial cell or through the intercellular junction, following a chemokine gradient bound to GAGs and/or the Duffy receptor. The cell then enters the basement membrane and continues migration along a chemokine gradient that is soluble or immobilized to the extracellular matrix.
One of the earliest ultrastructural changes that occurs when a chemoattractant N-formyl-methionyl-leucyl-phenylalanine (FMLP) or chemokine (IL-8) is injected into the skin is wrinkling of the endothelial plasma membrane, increasing the number of cell-surface projections.1,95,96 This activation occurs as early as 5 minutes after injection and before neutrophil margination and transmigration. The cellular mechanisms causing this wrinkling are not known but could be mediated by chemoattractant receptors and cytoskeletal changes. IL-8 can be detected in the endothelium by immunoelectron microscopy and electron microscopic autoradiography at the abluminal surface, intracellularly, and at the luminal surface.10 The amount of endothelial IL-8 builds up with time from 0 to 120 minutes, with most neutrophil transmigration occurring at 60 and 120 minutes. The chemokine reaches the luminal endothelial surface by abluminal-to-luminal transcytosis, traversing the cell in caveolae. These subcellular structures have been envisaged as forming discrete vesicles that shuttle across the cell or interconnected clusters of vesicles and vacuoles that form transendothelial pores (the vesiculo-vacuolar organelles).97,98 Following fusion of the caveolae with the membrane at the luminal surface, chemokines are preferentially presented on the tips of endothelial projections, creating the spatial organization to maximize the presentation of chemokines in the rolling phase of leukocyte emigration. The buildup of chemokine at the luminal surface of the endothelium is proposed to occur by chemokine immobilization mediated by interactions with cell surface GAG.14 The chemokines interact with the G protein–coupled chemokine receptors on the leukocyte cell surface, resulting in activation of integrins and firm attachment to the endothelium.
Following firm attachment, the leukocyte migrates from the blood across the endothelial barrier. Two possibilities exist concerning the route of migration: transcellular migration across the endothelium or intercellular movement through open junctions between endothelial cells. When FMLP is injected into the guinea pig skin, examination of serial sections by electron microscopy and computer-assisted 3-dimensional reconstruction shows that neutrophils follow the transendothelial route, unrelated to interendothelial junctions.96,99 Earlier studies have also suggested that neutrophils, monocytes, and eosinophils migrate transcellularly in response to the mediators IL-8, NAP, IL-1, C5a, leukotriene B4 (LTB4), and other inflammatory stimuli, but were incapable of proving this because serial sections were not used to generate 3-dimensional information.95,100-102 Other in vivo studies employing serial sections and electron microscopy have indicated that leukocytes can take the intercellular pathway of extravasation.103,104 Therefore, it is probable that leukocytes can take the transcellular or intercellular migratory route, the exact pathway depending on factors such as the type of leukocyte, inflammatory stimulus, tissue, and animal. The in vitro route of leukocyte transmigraton across the endothelium is more clear, with intercellular traffic through endothelial junctions being the accepted mechanism.105
When IL-8 is administered in rabbit and human skin, electron microscopic examination reveals neutrophil extravasation consistent with the transcellular path.95 Over the time course of neutrophil recruitment, the injected IL-8 localizes to caveolae and larger vesicles in the endothelial cytoplasm, which increase in abundance following chemokine administration.10,106 The intercellular junctions are ultrastructurally intact, and no IL-8 label is associated with these structures. Thus, both the transport of IL-8 and the leukocyte migration are transcellular. The former occurs in an abluminal-to-luminal direction and the latter in a luminal-to-abluminal direction. Thus, chemokine bound to GAG or DARC would provide a haptotactic transcellular gradient giving the leukocyte the directional cue to take the transcellular route. When adherent neutrophils migrate transcellularly, they extend projections (pseudopods) into the endothelial cell and then migrate through endothelial pores.96 During this process, it is likely that they encounter immobilized chemokines, which would give them further directional information. Once through the endothelium, the leukocytes encounter further chemokine, in immobilized or soluble form, in the extracellular matrix and on the surface of pericytes, giving them cues to cross the blood vessel wall and move out into the extravascular space.10 95
The views expressed in this publication are those of the authors.
Supported by funding from the Arthritis Research Campaign, Smiths' Charity, Wellcome Trust, Droitwich Medical Trust, and Institute of Orthopaedics, Robert Jones and Agnes Hunt Orthopaedic Hospital, United Kingdom. This work was undertaken by the Robert Jones and Agnes Hunt Orthopaedic and District Hospital National Health Service (NHS) Trust, which receives a portion of its funding from the NHS Executive.
References
Author notes
Jim Middleton, Endocube, Laboratoire de Biologie Vasculaire-IPBS-CNRS, 205 route de Narbonne, 31077 Toulouse, France; e-mail: jim.middleton@ipbs.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal