Graft-versus-host disease in its acute (aGvHD) or chronic form (cGvHD) remains the most important posttransplantation factor influencing outcome after allogeneic hematopoietic stem cell transplantation (HSCT). It increases transplantation-related mortality (TRM) but reduces risk of relapse. The net effect of these 2 discordant effects determines survival. In view of current interests to exploit graft-versus-leukemia (GVL) effects, we analyzed 4174 HLA-identical sibling transplantations for chronic myeloid leukemia in first chronic phase, depending on the presence or absence and severity of GvHD with a landmark analysis. During the first 100 days, only aGvHD grades III and IV had an impact on TRM. During the time period day 100 to 3 years increasing severity of aGvHD is associated with increased TRM and decreased relapse incidence (RI) with hazard ratios (HRs) for TRM as follows: grade 0, HR = 1.0; grade I, HR = 1.52 (1.19-1.96); grade II, HR = 2.48 (1.95-3.14); grade III, HR = 5.76 (4.44-7.48); grade IV, HR = 14.7 (10.9-19.9) and likewise for RI: grade I versus 0, HR = 0.94 (0.76-1.16); grade II, HR = 0.60 (0.46-0.77); grade III, HR = 0.48 (0.29-0.81); grade IV, HR = 0.14 (0.02-0.99). Beyond 3 years, TRM and RI are determined by cGvHD. Limited cGvHD reduces RI to the same extent as extensive cGvHD but has no impact on TRM and, hence, results in best survival with an HR = 0.48 (0.32-0.71). aGvHD grade I has the highest likelihood of subsequent limited cGvHD, which results in cumulative incidence estimates of survival at 10 years being best for patients with initial aGvHD grade I: survival at 10 years grade 0 = 59%, I = 63%, II = 56%, III = 26%, IV = not applicable. These data clarify the role of GvHD in posttransplantation outcome. Considerations for long-term outcome are essential when short-term data of interventions on GvHD are analyzed.
Introduction
Acute and chronic graft-versus-host disease (aGvHD and cGvHD, respectively) remain the major impediment for successful application of allogeneic hematopoietic stem cell transplantation (HSCT). They are the main cause of death and the leading contributing factor for morbidity and impaired quality of life after transplantation.1-9 Multiple studies have documented a clear correlation between GvHD in its acute or chronic form and transplantation-related mortality (TRM) with worse outcomes for moderate and severe aGvHD.10-17 Vice versa, GvHD exerts a powerful graft-versus-leukemia (GVL) effect, recognized already by the 1960s18,19 in animal and clinical studies and documented clearly in its clinical effects on preventing relapse by Weiden et al10 in 1979. These 2 opposite effects on TRM and relapse incidence (RI) are nonsymmetrical and earlier studies suggested an overall beneficial effect on survival of mild aGvHD. Such low-level aGvHD should not affect TRM but already provides protection against relapse.16,17 Based on relatively short observation periods, these data have fostered concepts to stimulate low-level GvHD to enhance GVL effects. The latter forms the cornerstone of modern reduced-intensity conditioning approaches and donor lymphocyte infusions (DLIs).20-26 In view of the broad implications and based on recent observations that best long-term survival for patients with chronic myeloid leukemia (CML) is seen in recipients of twin transplants,27 reassessment of the postulated advantage of mild aGvHD is warranted.
We therefore examined a large uniform cohort of patients treated with an allogeneic transplant from an HLA-identical sibling donor for a single disease at the same stage, that is, CML in first chronic phase. We looked at the 4 main end points,28 TRM, RI, relapse-free survival (RFS), and overall survival, depending on aGvHD grade as defined by the traditional Seattle criteria29 and adjusted for the previously described pretransplantation risk factors of age, donor-recipient sex combination, and time from diagnosis to transplantation.30These data confirm a clear correlation between GvHD and outcome after HSCT. Different effects are observed during different time intervals after transplantation. Competing risks of GvHD and GVL depend on the presence and severity of GvHD. Effects are nonproportional and with different magnitudes over time.
Using a landmark analysis approach, separating analyses for each time period and integrating known pretransplantation risk factors into the analysis, we tried to understand the balance between GvHD and GVL over time. Results show that benefits of GVL (as analyzed by reduced RI) compared to negative effects (as analyzed by increased TRM) are restricted to grade I aGvHD and limited cGvHD. It remains a challenge to make use of this limited and narrow window of opportunity.
Patients and methods
Study design and data collection
This retrospective analysis is based on data of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Data on donors and recipients were collected by questionnaires or an electronic data management system between 1979 and 2000 and updated annually. Participating teams are supposed to submit all consecutive cases, although for logistic reasons this is not the case for all teams throughout the whole observation period 1979-2000. Participating institutions are required to have protocols approved by their Institutional Review Board and informed consent from their patients. Since 1996, EBMT teams are annually subjected to a possible audit program. They are listed in “.”
Grading of GvHD and analysis of outcome
The EBMT uses the traditional Seattle criteria for aGvHD29 with grades 0, I, II, III, and IV and the criteria for cGvHD grading as absent, limited, or extensive. Participating teams are asked to enter the data accordingly. Analyzed outcomes concentrated on TRM, RI, RFS, and survival (SURV), as previously defined. For relapse, only hematologic relapse was taken into consideration. Patients once classified as relapsed remained so even if they entered a second remission after treatment with DLI.
Patients
The present analysis was restricted to patients with CML, receiving a transplant from an HLA-identical sibling donor in first chronic phase of their disease. Only first and fully documented transplantations were analyzed. Patients with other donors receiving transplants at another stage of disease or receiving second transplants were excluded. Information was required on patient age and sex, donor age and sex, donor histocompatibility, stem cell source, diagnosis, date of diagnosis, disease stage at time of transplantation, date of transplantation, and outcome.
Data on a total of 4174 patients were analyzed. They are summarized in Table 1. To adjust for the long observation period and the changes in transplantation technology, they were split into 3 time periods: 1979-1993, 1993-1996, and 1996-2000. There were more male patients (58%) and they were between 1 and 63 years of age (median, 36 years). Age distribution of donors and recipients was very close; 8% were younger than the age of 20 years, 37% between 20 and 40 years of age, and 35% were older than 40 years of age. Age clearly increased over time. Donor age followed the same pattern as patient age with a median of 35 years and a range from 1 to 74 years.
Description of patients with known aGvHD grade
| Factors influencing GvHD . | No. of patients (%) . | |||
|---|---|---|---|---|
| Before 1993 . | 1993-1996 . | After 1996 . | Total . | |
| All patients | 1879 (45) | 1260 (30) | 1035 (25) | 4174 (100) |
| Sex | ||||
| Male | 1095 (58) | 734 (58) | 602 (58) | 2431 (58) |
| Female | 784 (42) | 526 (42) | 433 (42) | 1743 (42) |
| Age, y | ||||
| Younger than 20 | 163 (9) | 85 (7) | 80 (8) | 328 (8) |
| 20-40 | 1241 (66) | 652 (52) | 498 (48) | 2391 (57) |
| Older than 40 | 475 (25) | 523 (41) | 457 (44) | 1455 (35) |
| Donor-recipient sex combination | ||||
| Donor (F) + recipient (M) | 470 (25) | 310 (25) | 273 (26) | 1053 (25) |
| Other | 1409 (75) | 950 (75) | 762 (74) | 3121 (75) |
| Interval between diagnosis and transplantation | ||||
| Shorter than 12 mo | 931 (50) | 771 (61) | 705 (68) | 2407 (58) |
| 12 mo or longer | 948 (50) | 489 (39) | 330 (32) | 1767 (42) |
| aGvHD prevention | ||||
| T-cell depletion | 691 (52) | 173 (24) | 110 (14) | 974 (34) |
| Cyclo alone | 130 (10) | 53 (7) | 17 (2) | 200 (7) |
| Cyclo + mtx | 487 (36) | 451 (61) | 364 (46) | 1302 (45) |
| Other | 32 (2) | 56 (8) | 307 (38) | 395 (14) |
| Source of stem cells | ||||
| BM | 1879 (100) | 1132 (90) | 618 (60) | 3629 (87) |
| PB | 0 (0) | 128 (10) | 417 (40) | 545 (13) |
| Factors influencing GvHD . | No. of patients (%) . | |||
|---|---|---|---|---|
| Before 1993 . | 1993-1996 . | After 1996 . | Total . | |
| All patients | 1879 (45) | 1260 (30) | 1035 (25) | 4174 (100) |
| Sex | ||||
| Male | 1095 (58) | 734 (58) | 602 (58) | 2431 (58) |
| Female | 784 (42) | 526 (42) | 433 (42) | 1743 (42) |
| Age, y | ||||
| Younger than 20 | 163 (9) | 85 (7) | 80 (8) | 328 (8) |
| 20-40 | 1241 (66) | 652 (52) | 498 (48) | 2391 (57) |
| Older than 40 | 475 (25) | 523 (41) | 457 (44) | 1455 (35) |
| Donor-recipient sex combination | ||||
| Donor (F) + recipient (M) | 470 (25) | 310 (25) | 273 (26) | 1053 (25) |
| Other | 1409 (75) | 950 (75) | 762 (74) | 3121 (75) |
| Interval between diagnosis and transplantation | ||||
| Shorter than 12 mo | 931 (50) | 771 (61) | 705 (68) | 2407 (58) |
| 12 mo or longer | 948 (50) | 489 (39) | 330 (32) | 1767 (42) |
| aGvHD prevention | ||||
| T-cell depletion | 691 (52) | 173 (24) | 110 (14) | 974 (34) |
| Cyclo alone | 130 (10) | 53 (7) | 17 (2) | 200 (7) |
| Cyclo + mtx | 487 (36) | 451 (61) | 364 (46) | 1302 (45) |
| Other | 32 (2) | 56 (8) | 307 (38) | 395 (14) |
| Source of stem cells | ||||
| BM | 1879 (100) | 1132 (90) | 618 (60) | 3629 (87) |
| PB | 0 (0) | 128 (10) | 417 (40) | 545 (13) |
Cyclo indicates cyclosporin A; mtx, methotrexate; BM, bone marrow; and PB, peripheral blood.
For one fourth of the transplantations a female donor was used for a male recipient. In view of the higher percentage of male patients, this proportion is lower than expected, probably because a male donor is the preferred choice for a male recipient, if more than one donor is available.30 Time from diagnosis to transplantation was less than 12 months for 58% of the patients. The method used to prevent GvHD varied over time. T-cell depletion was more frequently used in the earlier years, with other methods, including, for example, tacrolimus-based regimens, used in more recent years. Bone marrow was the selected stem cell source for 87% of all transplants. Peripheral blood was introduced only in 1993.31
Statistical analysis
Patient selection, definitions, and subgroup analyses.
First, patients without any missing covariates were compared with all remaining patients in the EBMT CLWP database. Using this dichotomy (“no-missings” versus “some-missings”), the association with pretransplantation risk factors and all outcome variables was investigated. No clinically or statistically significant associations were found. The subgroup of patients in the multivariate models can be supposed to be representative for the total of all patients receiving transplants.
Calendar year influences survival32 and other outcome measures and can introduce a confounding effect and sometimes severe nonproportionality in survival models through interaction with risk factors of interest. We therefore investigated next whether the effect of aGvHD and cGvHD, as expressed by the hazard ratio (HR), was modified by the variable calendar year. This was not the case. We concluded that incorporating calendar year into all survival models was sufficient to cope with its confounding effect and we could use all patients receiving transplants from 1979 to 1999.
Definitions for aGvHD and cGvHD were taken as reported in the data sheet. Both can occur only after a given point in time by their very definition (day 21 for aGvHD and day 100 for cGvHD). These selected time points are arbitrary. A patient can die before day 21 of severe aGvHD. To assess the effect of cutoff points, all analyses were repeated with other time points, for example, day 30. There were no differences in the effects of GvHD on outcome when very early mortality was excluded. Hence, all patients were included for the final analysis.
Pretransplantation factors and changes over time.
Discrete pretransplantation risk factors were analyzed on their effect on incidence (grade 0 versus I-IV for acute; absent versus limited or extensive cGvHD) and severity (grade 0 versus I versus II versus III versus IV; absent versus limited versus extensive) of acute and chronic GvHD in cross-tabulations and associations were tested for with the χ2 test. Because of the changes over time, data were analyzed for each time period.
To adapt for changes over time, 2 risk factors, “source of stem cells” and “prevention of aGvHD” were treated in a different way from the other risk factors (sex, age, sex mismatch, interval-diagnosis transplantation, and calendar year). Since peripheral blood as a source of stem cells was introduced only after 1993, inclusion of this variable in the multivariate analyses restricts the analyses to only part of the data. Therefore all analyses were first carried out without “source of stem cells” and then the final models were evaluated by using transplantations after 1993 to monitor changes in all estimated risk factors due to the inclusion of “source” into the model. “Source” did not change the significance or magnitude nor did it alter significantly the impact of GvHD on outcome in the multivariate analysis. The final analyses are therefore presented without incorporating stem cell source.
“Prevention of aGvHD” was classified into 4 groups. The same strategy was followed. Analyses were in this case restricted to patients with grade I aGvHD. All models used to base conclusions on, without exception, were extended with this 4-group variable. All effect sizes and P values were monitored for possible confounding effects. “Prevention of aGvHD” did not generate any confounding effect. Hence, the final models do not contain this variable “prevention” because it makes the interpretation easier and it does not modify the conclusions.
Estimation of outcome.
To estimate overall outcome, we used a Kaplan-Meier approach. To analyze the associations with the risk factors of interest, aGvHD, and cGvHD and to adjust for various pretransplantation risk factors, the proportional hazard model (Cox model) was used. In each situation the proportionality assumption was verified both graphically and by introducing time as a (time-dependent) covariate and testing for a significant interaction with the risk factors under study. Whenever severe deviation from the proportionality assumption was found, we reverted to a stratified Cox model.
We used RI as an outcome in the usual framework of the Cox model although for a proper and clinically interpretable estimate of the actual RI (ie, the height of the survival curve for a particular group of patients), a cumulative incidence model would be more appropriate. Still, the estimates of the HRs and the relative differences between groups in the Cox model are valid and correct. Because our interests are directed toward possible mechanisms and effects of risk factors on outcome, the (stratified) Cox model remains the model of choice in this situation.
In each model interaction terms were tested for a possibly better model fit. Despite this stratified specification of part of the models, no interaction terms reached statistical significance. They are therefore absent in the final models.
Age was used as a continuous covariate. Deviations from linearity were tested for but none were found, partly due again to the stratified specification.
The main outcomes, indicated as TRM, RI, RFS, and SURV, were analyzed with the usual definitions. For each of the grades I or higher, relative risks with respect to grade 0 were assessed and estimated using the HRs. The terms relative risk (RR) and hazard ratio (HR) were used interchangeably. Risks were analyzed for patients with or without limited or extensive aGvHD. Adjustments are made for the covariables sex, age, donor-recipient sex combination, year of HSCT, and interval from diagnosis to transplantation.
Landmark analysis.
Due to severe nonproportionality it is impossible to grasp the influence of GvHD in one Cox model allowing for estimation of all effects of interest over time. A simple and clinically relevant solution is offered by the landmark analysis approach. In this approach the follow-up time is divided into 2 or more periods of interest. Patient survival is described with the standard techniques conditional on the patient being alive at the start of the interval. This approach has 2 advantages. It deals with severe nonproportionality because the model is fitted to a more restricted time period. Second, interpretation of the HRs becomes easier because the effect of a risk factor is allowed to be entirely different in different time periods. By choosing the time periods in a clinically relevant way, a clearer picture emerges. Three separate sets of analyses were therefore performed: period 1 from day 0 to day 100; period 2 from day 100 until year 3; and period 3 from year 3 until last follow-up (around 15 years). The first cutoff point was chosen because of the very definition of cGvHD. The second cutoff point was chosen on the basis of the data: all models were fulfilling the proportionality assumption conditional on being alive at year 3. This facilitates long-term interpretation of the data. A substantially earlier cutoff point than year 3 would necessitate the use of stratified models and many interaction terms.
Results
Main end points
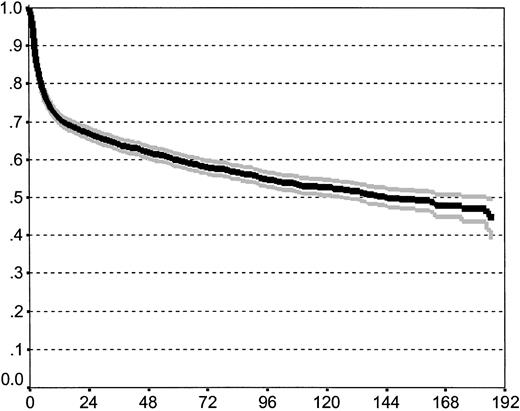
At the time of analysis, 2606 patients (63%) of the initial 4174 patients were alive with a median follow-up of 43 months (range, 0-233 months); 1568 patients (37%) had died, 1292 (82%) of TRM and 276 (18%) of recurrent disease. A relapse occurred in 666 patients (16%). Of these, 276 (41%) had died and 390 were still alive despite their relapse.33 Overall, survival probability at 15 years for the whole cohort is 43% (the CI being 37%-49%; Figure1).
Overall survival of the study population including 95% confidence band (estimated by Kaplan-Meier method).
Overall survival of the study population including 95% confidence band (estimated by Kaplan-Meier method).
Of the initial cohort, 3402 patients (82%) were available for analysis from day 100 onward. Of the 772 not being available at day 100, 610 had died without relapse, 7 died with a relapse, and 155 were lost to follow-up (among whom 1 with a relapse). Of these 3402 patients, 951 (28%) eventually died after day 100, 682 (72%) of TRM and 269 (28%) of relapse.
From year 3 onward, 1651 patients were available. This represents 40% of the initial cohort and 49% of the day-100 cohort. Of these, 212 patients eventually died in our observation period; 73 died of TRM (34%) and 139 (66%) of relapse.
Incidence and severity of GvHD
Incidence of aGvHD.
A total of 1512 patients never had any signs of aGvHD. This corresponds to 36.2% and is close to a previous analysis by the EBMT in which 35.7% of patients were found to have grade 0 aGvHD.16 A total of 2662 patients (63.8%) developed aGvHD grades I to IV. To give an estimate, the individual grades are listed in Table2 according to sex, age, donor-recipient sex combination, time interval, aGvHD prevention method, and stem cell source for the time period 1993-1996.
Incidence of aGvHD in period 1993-1996
| Risk factor . | aGvHD, row % . | ||||
|---|---|---|---|---|---|
| 0 . | I . | II . | III . | IV . | |
| All patients | 33 | 27 | 25 | 9 | 6 |
| Sex | |||||
| Male | 33 | 28 | 24 | 9 | 6 |
| Female | 34 | 25 | 25 | 9 | 7 |
| Age, y*,† | |||||
| Younger than 20 | 42 | 26 | 13 | 11 | 8 |
| 20-40 | 35 | 26 | 25 | 9 | 5 |
| Older than 40 | 29 | 27 | 26 | 10 | 8 |
| Donor-recipient sex combination | |||||
| Donor (F) + recipient (M) | 32 | 28 | 24 | 10 | 6 |
| Other | 33 | 27 | 25 | 9 | 6 |
| Interval between diagnosis and transplantation*,† | |||||
| Shorter than 12 mo | 37 | 26 | 22 | 9 | 6 |
| 12 mo or longer | 27 | 28 | 29 | 9 | 7 |
| aGvHD prevention* | |||||
| T-cell depletion | 32 | 28 | 24 | 11 | 5 |
| Cyclo alone | 21 | 41 | 19 | 11 | 8 |
| Cyclo + mtx | 33 | 28 | 23 | 10 | 6 |
| Other | 62 | 13 | 13 | 5 | 7 |
| Source of stem cells | |||||
| BM | 33 | 27 | 25 | 9 | 6 |
| PB | 30 | 28 | 23 | 11 | 8 |
| Risk factor . | aGvHD, row % . | ||||
|---|---|---|---|---|---|
| 0 . | I . | II . | III . | IV . | |
| All patients | 33 | 27 | 25 | 9 | 6 |
| Sex | |||||
| Male | 33 | 28 | 24 | 9 | 6 |
| Female | 34 | 25 | 25 | 9 | 7 |
| Age, y*,† | |||||
| Younger than 20 | 42 | 26 | 13 | 11 | 8 |
| 20-40 | 35 | 26 | 25 | 9 | 5 |
| Older than 40 | 29 | 27 | 26 | 10 | 8 |
| Donor-recipient sex combination | |||||
| Donor (F) + recipient (M) | 32 | 28 | 24 | 10 | 6 |
| Other | 33 | 27 | 25 | 9 | 6 |
| Interval between diagnosis and transplantation*,† | |||||
| Shorter than 12 mo | 37 | 26 | 22 | 9 | 6 |
| 12 mo or longer | 27 | 28 | 29 | 9 | 7 |
| aGvHD prevention* | |||||
| T-cell depletion | 32 | 28 | 24 | 11 | 5 |
| Cyclo alone | 21 | 41 | 19 | 11 | 8 |
| Cyclo + mtx | 33 | 28 | 23 | 10 | 6 |
| Other | 62 | 13 | 13 | 5 | 7 |
| Source of stem cells | |||||
| BM | 33 | 27 | 25 | 9 | 6 |
| PB | 30 | 28 | 23 | 11 | 8 |
Abbreviations are explained in Table 1.
Indicates that the χ2 test for the comparison of the incidence of aGvHD (yes vs no) has a P < .05.
Indicates that the χ2 test for a linear trend over the grades 0 to IV (severity) comparing the risk factor levels is significant at the .05 level.
Factors influencing incidence and severity of aGvHD.
Younger patients had less frequent and less severe aGvHD than older patients. Sex of donor and recipient had no influence. It is of interest to note that patients receiving a transplant within 1 year from diagnosis had less frequent and less severe aGvHD. The GvHD prevention method did influence incidence of aGvHD with more aGvHD in patients given cyclosporin A alone. Stem cell source had no impact on aGvHD.
Incidence of cGvHD.
Date of onset of cGvHD was not available in the data set. Therefore, no probabilities could be estimated and analysis is restricted to presence or absence of cGvHD at any time beyond day 100 (by definition). A total 1534 patients were analyzable for cGvHD at 3 years. Of these, 723 (47.1%) never had any cGvHD; 811 (52.9%) had cGvHD, 478 (31.2%) had limited and 333 (21.7%) extensive cGvHD. To give an estimate of risk factors, incidence and severity of cGvHD are given for patients receiving transplants between 1985 and 1996 (Table3).
Incidence of cGvHD in period 1993-1996
| Risk factor . | cGvHD, row % . | ||
|---|---|---|---|
| No . | Limited . | Extensive . | |
| All patients | 52 | 25 | 23 |
| Sex3-150,3-151 | |||
| Male | 48 | 25 | 27 |
| Female | 58 | 24 | 18 |
| Age, y3-150,3-151 | |||
| Younger than 20 | 68 | 21 | 11 |
| 20-40 | 51 | 25 | 24 |
| Older than 40 | 50 | 25 | 25 |
| Donor-recipient sex combination3-150,3-151 | |||
| Donor (F) + recipient (M) | 41 | 26 | 33 |
| Other | 56 | 24 | 20 |
| Interval between diagnosis and transplantation | |||
| Shorter than 12 mo | 52 | 23 | 25 |
| 12 mo or longer | 52 | 26 | 22 |
| aGvHD prevention3-150,3-151 | |||
| T-cell depletion | 51 | 28 | 21 |
| Cyclo alone | 52 | 20 | 28 |
| Cyclo + mtx | 55 | 25 | 20 |
| Other | 28 | 22 | 50 |
| Source of stem cells3-150,3-151 | |||
| BM | 54 | 25 | 21 |
| PB | 36 | 24 | 40 |
| aGvHD3-150,3-151 | |||
| Grade 0 | 69 | 15 | 16 |
| Grade I | 46 | 36 | 18 |
| Grade II | 34 | 31 | 35 |
| Grade III | 40 | 18 | 42 |
| Grade IV | 75 | 5 | 20 |
| Risk factor . | cGvHD, row % . | ||
|---|---|---|---|
| No . | Limited . | Extensive . | |
| All patients | 52 | 25 | 23 |
| Sex3-150,3-151 | |||
| Male | 48 | 25 | 27 |
| Female | 58 | 24 | 18 |
| Age, y3-150,3-151 | |||
| Younger than 20 | 68 | 21 | 11 |
| 20-40 | 51 | 25 | 24 |
| Older than 40 | 50 | 25 | 25 |
| Donor-recipient sex combination3-150,3-151 | |||
| Donor (F) + recipient (M) | 41 | 26 | 33 |
| Other | 56 | 24 | 20 |
| Interval between diagnosis and transplantation | |||
| Shorter than 12 mo | 52 | 23 | 25 |
| 12 mo or longer | 52 | 26 | 22 |
| aGvHD prevention3-150,3-151 | |||
| T-cell depletion | 51 | 28 | 21 |
| Cyclo alone | 52 | 20 | 28 |
| Cyclo + mtx | 55 | 25 | 20 |
| Other | 28 | 22 | 50 |
| Source of stem cells3-150,3-151 | |||
| BM | 54 | 25 | 21 |
| PB | 36 | 24 | 40 |
| aGvHD3-150,3-151 | |||
| Grade 0 | 69 | 15 | 16 |
| Grade I | 46 | 36 | 18 |
| Grade II | 34 | 31 | 35 |
| Grade III | 40 | 18 | 42 |
| Grade IV | 75 | 5 | 20 |
Abbreviations are explained in Table 1.
Indicates that the χ2 test for the comparison of the incidence of cGvHD (yes versus no) has a P < .05.
Indicates that the χ2 test for a linear trend over the grades from no to extensive (severity) comparing the risk factor levels is significant at the .05 level.
Factors influencing incidence and severity of cGvHD.
Similarly to aGvHD incidence, cGvHD was more frequent and more severe in older patients. A marked effect was observed in the donor-recipient sex combination. Male recipients of a female transplant had a higher risk of developing cGvHD (51%) than other patients (43%) and were more likely to develop extensive cGvHD (27% versus 19%). Time interval and year of transplantation had no systematic impact but GvHD prevention did influence cGvHD. Clearly, also, patients with peripheral blood transplantations had more frequent and more severe cGvHD.
Incidence and severity showed a clear correlation with previous aGvHD grade. A higher aGvHD grade was associated with more frequent and more increasingly severe cGvHD. This holds true up to grades II to III. In contrast, patients surviving grade IV aGvHD probably present a special category and are less likely to proceed to severe cGvHD.
Influence of GvHD on outcome
Overall influence of aGvHD and pretransplantation risk factors.
In a univariate analysis aGvHD has an impact on all end points according to aGvHD grade, as listed in Table4. There is increasing TRM with increasing aGvHD grade at any time point and decreasing RI. SURV and RFS are accordingly best for grade I aGvHD if the pretransplantation risk factors are considered (Table 5). Effects are different during 3 distinct time periods.
Cumulative incidence estimates for overall survival, RI, TRM, and RFS
| aGvHD grade . | No. of patients at risk for SURV . | SURV4-150 . | RI4-151 . | TRM4-151 . | RFS . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial . | d 100 . | 3 y . | 10 y . | 15 y . | d 100 . | 3 y . | 10 y . | 15 y . | d 100 . | 3 y . | 10 y . | 15 y . | d 100 . | 3 y . | 10 y . | 15 y . | d 100 . | 3 y . | 10 y . | 15 y . | |
| 0 | 1512 | 1279 | 691 | 197 | 73 | 0.88 | 0.74 | 0.59 | 0.54 | 0.01 | 0.20 | 0.31 | 0.32 | 0.12 | 0.21 | 0.23 | 0.26 | 0.87 | 0.59 | 0.46 | 0.42 |
| I | 1084 | 967 | 516 | 113 | — | 0.93 | 0.74 | 0.63 | — | 0.01 | 0.18 | 0.29 | — | 0.07 | 0.21 | 0.24 | — | 0.92 | 0.61 | 0.47 | — |
| II | 922 | 811 | 366 | 117 | — | 0.90 | 0.64 | 0.56 | — | 0.01 | 0.11 | 0.20 | — | 0.10 | 0.32 | 0.35 | — | 0.89 | 0.57 | 0.45 | — |
| III | 359 | 267 | 86 | 23 | — | 0.75 | 0.37 | 0.26 | — | 0.01 | 0.06 | 0.08 | — | 0.24 | 0.60 | 0.67 | — | 0.75 | 0.34 | 0.25 | — |
| IV | 297 | 107 | 19 | — | — | 0.36 | 0.10 | — | — | 0.01 | 0.02 | — | — | 0.63 | 0.89 | — | — | 0.36 | 0.09 | — | — |
| Total | 4174 | 3414 | 1660 | 403 | 59 | 0.85 | 0.64 | 0.53 | 0.47 | 0.01 | 0.15 | 0.24 | 0.24 | 0.15 | 0.32 | 0.35 | 0.39 | 0.84 | 0.53 | 0.41 | 0.37 |
| aGvHD grade . | No. of patients at risk for SURV . | SURV4-150 . | RI4-151 . | TRM4-151 . | RFS . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial . | d 100 . | 3 y . | 10 y . | 15 y . | d 100 . | 3 y . | 10 y . | 15 y . | d 100 . | 3 y . | 10 y . | 15 y . | d 100 . | 3 y . | 10 y . | 15 y . | d 100 . | 3 y . | 10 y . | 15 y . | |
| 0 | 1512 | 1279 | 691 | 197 | 73 | 0.88 | 0.74 | 0.59 | 0.54 | 0.01 | 0.20 | 0.31 | 0.32 | 0.12 | 0.21 | 0.23 | 0.26 | 0.87 | 0.59 | 0.46 | 0.42 |
| I | 1084 | 967 | 516 | 113 | — | 0.93 | 0.74 | 0.63 | — | 0.01 | 0.18 | 0.29 | — | 0.07 | 0.21 | 0.24 | — | 0.92 | 0.61 | 0.47 | — |
| II | 922 | 811 | 366 | 117 | — | 0.90 | 0.64 | 0.56 | — | 0.01 | 0.11 | 0.20 | — | 0.10 | 0.32 | 0.35 | — | 0.89 | 0.57 | 0.45 | — |
| III | 359 | 267 | 86 | 23 | — | 0.75 | 0.37 | 0.26 | — | 0.01 | 0.06 | 0.08 | — | 0.24 | 0.60 | 0.67 | — | 0.75 | 0.34 | 0.25 | — |
| IV | 297 | 107 | 19 | — | — | 0.36 | 0.10 | — | — | 0.01 | 0.02 | — | — | 0.63 | 0.89 | — | — | 0.36 | 0.09 | — | — |
| Total | 4174 | 3414 | 1660 | 403 | 59 | 0.85 | 0.64 | 0.53 | 0.47 | 0.01 | 0.15 | 0.24 | 0.24 | 0.15 | 0.32 | 0.35 | 0.39 | 0.84 | 0.53 | 0.41 | 0.37 |
The CI and Kaplan-Meier estimates coincide for overall survival and RFS.
The RI and TRM add up to the complement of RFS as estimated by the Kaplan-Meier curve.
— indicates not applicable.
Unadjusted overall Kaplan-Meier estimates.
Unadjusted cumulative incidence estimates.
HRs for TRM and RI depending on time interval
| Risk factor . | TRM . | RI . | TRM . | RI . | ||
|---|---|---|---|---|---|---|
| direction . | HR . | CI . | HR . | CI . | ||
| From transplantation until d 100 | ||||||
| aGvHD | ||||||
| Grade 0 (reference) | (1) | (1) | ||||
| Grade I | ↓ 5-153 | ↓5-152 | 0.58 | (0.44-0.76) | 0.47 | (0.18-1.19) |
| Grade II | ↓ 5-153 | ↓5-152 | 0.76 | (0.53-0.38) | 0.44 | (0.16-1.21) |
| Grade III | ↑ 5-151 | ↓ 5-152 | 2.09 | (1.6-2.7) | 0.53 | (0.12-2.32) |
| Grade IV | ↑ 5-151 | ↑5-150 | 6.55 | (5.3-8.1) | 1.31 | (0.38-4.54) |
| Age, per year | ↑ 5-151 | ↑ 5-150 | 1.02 | (1.01-1.03) | 1.00 | (0.97-1.04) |
| Sex, female vs male | ↓ 5-152 | ↑5-151 | 0.99 | (0.82-1.19) | 7.02 | (1.62-30.4) |
| Mismatch, female donor + male recipient versus rest | ↓ 5-152 | ↑5-151 | 0.97 | (0.79-1.19) | 8.73 | (1.97-38.8) |
| Calendar year, per year | ↓ 5-153 | ↑5-150 | 0.93 | (0.91-0.95) | 1.04 | (0.96-1.12) |
| Interval between diagnosis and transplantation, shorter than 12 mo versus 12 mo or longer | ↑ 5-151 | ↑ 5-150 | 1.4 | (1.2-1.7) | 1.30 | (0.64-2.64) |
| From d 100 until 3 y | ||||||
| aGvHD | ||||||
| Grade 0 (reference) | (1) | (1) | ||||
| Grade I | ↑ 5-151 | ↓ 5-153 | 1.52 | (1.19-1.96) | 0.94 | (0.76-1.16) |
| Grade II | ↑ 5-151 | ↓5-153 | 2.48 | (1.95-3.14) | 0.60 | (0.46-0.77) |
| Grade III | ↑ 5-151 | ↓ 5-153 | 5.76 | (4.44-7.48) | 0.48 | (0.29-0.81) |
| Grade IV | ↑ 5-151 | ↓5-153 | 14.7 | (10.9-19.9) | 0.14 | (0.02-0.99) |
| Age, per year | ↑ 5-151 | ↑ 5-151 | 1.03 | (1.02-1.04) | 1.01 | (1.00-1.02) |
| Sex, female versus male | ↑ 5-150 | ↓5-152 | 1.01 | (0.83-1.23) | 0.85 | (0.69-1.05) |
| Mismatch, female donor + male recipient versus rest | ↑ 5-151 | ↓5-152 | 1.36 | (1.11-1.66) | 0.98 | (0.77-1.25) |
| Calendar year, per year | ↓ 5-152 | ↓5-153 | 0.98 | (0.97-1.00) | 0.98 | (0.96-1.00) |
| Interval between diagnosis and transplantation, longer than 12 mo versus 12 mo or longer | ↑ 5-150 | ↑ 5-150 | 1.07 | (0.91-1.26) | 1.00 | (0.83-1.21) |
| From 3 y until 15 y | ||||||
| aGvHD | ||||||
| Grade 0 (reference) | (1) | (1) | ||||
| Grade I | ↓ 5-152 | ↑ 5-150 | 0.75 | (0.35-1.59) | 1.07 | (0.74-1.54) |
| Grade II | ↑ 5-150 | ↑5-150 | 1.03 | (0.52-2.06) | 1.05 | (0.68-1.61) |
| Grades III-IV | ↑ 5-151 | ↓5-152 | 2.94 | (1.45-5.95) | 0.34 | (0.11-1.09) |
| Age, per year | ↑ 5-151 | ↑ 5-150 | 1.04 | (1.01-1.07) | 1.01 | (0.99-1.03) |
| Sex, female versus male | ↓ 5-152 | ↓5-152 | 0.70 | (0.36-1.36) | 0.96 | (0.69-1.35) |
| Mismatch, female donor + male recipient versus rest | ↑ 5-150 | ↓5-153 | 1.72 | (0.93-3.21) | 0.50 | (0.30-0.83) |
| Calendar year, per year | ↓ 5-152 | ↓5-152 | 0.98 | (0.90-1.07) | 0.98 | (0.94-1.03) |
| Interval between diagnosis and transplantation, longer than 12 mo versus 12 mo or longer | ↑ 5-150 | ↓5-152 | 1.19 | (0.71-1.99) | 0.88 | (0.64-1.21) |
| cGvHD | ||||||
| 0 (reference) | (1) | (1) | ||||
| Limited | ↓5-152 | ↓ 5-153 | 0.90 | (0.4-2.1) | 0.54 | (0.34-0.79) |
| Extensive | ↑ 5-151 | ↓5-153 | 3.4 | (1.7-6.8) | 0.43 | (0.26-0.70) |
| Risk factor . | TRM . | RI . | TRM . | RI . | ||
|---|---|---|---|---|---|---|
| direction . | HR . | CI . | HR . | CI . | ||
| From transplantation until d 100 | ||||||
| aGvHD | ||||||
| Grade 0 (reference) | (1) | (1) | ||||
| Grade I | ↓ 5-153 | ↓5-152 | 0.58 | (0.44-0.76) | 0.47 | (0.18-1.19) |
| Grade II | ↓ 5-153 | ↓5-152 | 0.76 | (0.53-0.38) | 0.44 | (0.16-1.21) |
| Grade III | ↑ 5-151 | ↓ 5-152 | 2.09 | (1.6-2.7) | 0.53 | (0.12-2.32) |
| Grade IV | ↑ 5-151 | ↑5-150 | 6.55 | (5.3-8.1) | 1.31 | (0.38-4.54) |
| Age, per year | ↑ 5-151 | ↑ 5-150 | 1.02 | (1.01-1.03) | 1.00 | (0.97-1.04) |
| Sex, female vs male | ↓ 5-152 | ↑5-151 | 0.99 | (0.82-1.19) | 7.02 | (1.62-30.4) |
| Mismatch, female donor + male recipient versus rest | ↓ 5-152 | ↑5-151 | 0.97 | (0.79-1.19) | 8.73 | (1.97-38.8) |
| Calendar year, per year | ↓ 5-153 | ↑5-150 | 0.93 | (0.91-0.95) | 1.04 | (0.96-1.12) |
| Interval between diagnosis and transplantation, shorter than 12 mo versus 12 mo or longer | ↑ 5-151 | ↑ 5-150 | 1.4 | (1.2-1.7) | 1.30 | (0.64-2.64) |
| From d 100 until 3 y | ||||||
| aGvHD | ||||||
| Grade 0 (reference) | (1) | (1) | ||||
| Grade I | ↑ 5-151 | ↓ 5-153 | 1.52 | (1.19-1.96) | 0.94 | (0.76-1.16) |
| Grade II | ↑ 5-151 | ↓5-153 | 2.48 | (1.95-3.14) | 0.60 | (0.46-0.77) |
| Grade III | ↑ 5-151 | ↓ 5-153 | 5.76 | (4.44-7.48) | 0.48 | (0.29-0.81) |
| Grade IV | ↑ 5-151 | ↓5-153 | 14.7 | (10.9-19.9) | 0.14 | (0.02-0.99) |
| Age, per year | ↑ 5-151 | ↑ 5-151 | 1.03 | (1.02-1.04) | 1.01 | (1.00-1.02) |
| Sex, female versus male | ↑ 5-150 | ↓5-152 | 1.01 | (0.83-1.23) | 0.85 | (0.69-1.05) |
| Mismatch, female donor + male recipient versus rest | ↑ 5-151 | ↓5-152 | 1.36 | (1.11-1.66) | 0.98 | (0.77-1.25) |
| Calendar year, per year | ↓ 5-152 | ↓5-153 | 0.98 | (0.97-1.00) | 0.98 | (0.96-1.00) |
| Interval between diagnosis and transplantation, longer than 12 mo versus 12 mo or longer | ↑ 5-150 | ↑ 5-150 | 1.07 | (0.91-1.26) | 1.00 | (0.83-1.21) |
| From 3 y until 15 y | ||||||
| aGvHD | ||||||
| Grade 0 (reference) | (1) | (1) | ||||
| Grade I | ↓ 5-152 | ↑ 5-150 | 0.75 | (0.35-1.59) | 1.07 | (0.74-1.54) |
| Grade II | ↑ 5-150 | ↑5-150 | 1.03 | (0.52-2.06) | 1.05 | (0.68-1.61) |
| Grades III-IV | ↑ 5-151 | ↓5-152 | 2.94 | (1.45-5.95) | 0.34 | (0.11-1.09) |
| Age, per year | ↑ 5-151 | ↑ 5-150 | 1.04 | (1.01-1.07) | 1.01 | (0.99-1.03) |
| Sex, female versus male | ↓ 5-152 | ↓5-152 | 0.70 | (0.36-1.36) | 0.96 | (0.69-1.35) |
| Mismatch, female donor + male recipient versus rest | ↑ 5-150 | ↓5-153 | 1.72 | (0.93-3.21) | 0.50 | (0.30-0.83) |
| Calendar year, per year | ↓ 5-152 | ↓5-152 | 0.98 | (0.90-1.07) | 0.98 | (0.94-1.03) |
| Interval between diagnosis and transplantation, longer than 12 mo versus 12 mo or longer | ↑ 5-150 | ↓5-152 | 1.19 | (0.71-1.99) | 0.88 | (0.64-1.21) |
| cGvHD | ||||||
| 0 (reference) | (1) | (1) | ||||
| Limited | ↓5-152 | ↓ 5-153 | 0.90 | (0.4-2.1) | 0.54 | (0.34-0.79) |
| Extensive | ↑ 5-151 | ↓5-153 | 3.4 | (1.7-6.8) | 0.43 | (0.26-0.70) |
Cox models including recipient sex, sex-mismatch, age, calendar year, interval between diagnosis and transplantation, and aGvHD (and cGvHD after 3 years). Directions of effects always given, regardless of significance; ↑ indicates increasing hazard; ↓, decreasing hazard. Nonsignificant HRs are indicated with * and ‡.
Increasing hazard on outcome (P > .05).
Increasing hazard on outcome (P ≤ .05).
Decreasing hazard on outcome (P > .05).
Decreasing hazard on outcome (P ≤ .05).
Time interval from transplantation to day 100.
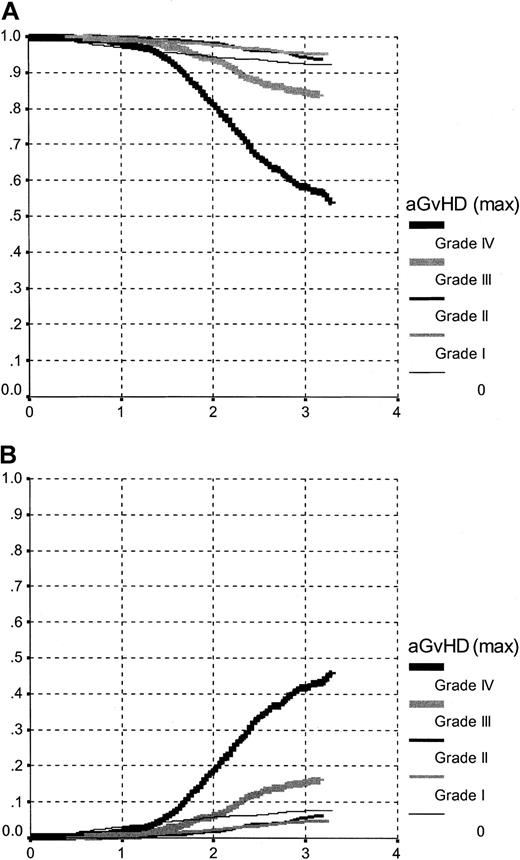
Survival within the first 100 days is highly influenced by aGvHD grade (P < .00; Figure 2A and Table 5). The influence of aGvHD on TRM differs for the individual aGvHD grades and is nonproportional (Figure 2B). There is no difference between grades aGvHD I and II but a clear increase in TRM risk with grades III and IV. Grade 0 aGvHD follows a third pattern, in part influenced by those patients dying early without having a chance to develop aGvHD. As a result, adjusted survival within the first 100 days is best and identical for aGvHD grades I and II, intermediate for grade 0, and increasingly worse for grades III and IV.
Overall survival and TRM.
Overall survival (A) and TRM (B) between transplantation and day 100, by aGvHD grading. The curves represent an estimate of the survival function, based on a (stratified) Cox model, evaluated for a patient with the following characteristics: age, 35 years; year of transplantation, 1995; sex, male; interval-diagnosis transplantation: less than 12 months; sex-mismatch, no.
Overall survival and TRM.
Overall survival (A) and TRM (B) between transplantation and day 100, by aGvHD grading. The curves represent an estimate of the survival function, based on a (stratified) Cox model, evaluated for a patient with the following characteristics: age, 35 years; year of transplantation, 1995; sex, male; interval-diagnosis transplantation: less than 12 months; sex-mismatch, no.
The TRM in addition is influenced by age with an RR of 1.02 (1.01-1.03) per year, an RR of 0.93 (0.91-0.95) per calendar year, and an RR of 1.4 (1.2-1.7) for the interval between diagnosis and transplantation.
For the RI, there were too few relapses within this time interval for a meaningful analysis.
Time interval from day 100 to 3 years.
For patients alive at day 100, aGvHD grade had the strongest impact on survival with decreasing survival probabilities for increasing aGvHD grade (P < .01; Figure 3A and Table 5). There was an almost linear increase in TRM (Figure 3B) and decrease in RI (Figure 3C) with increasing aGvHD grade. Still, the increasing negative impact on TRM with increasing aGvHD cannot be matched by the reduced relapse rate. As a net result, adjusted survival is best within this time period for grade 0 aGvHD.
Overall survival, TRM, and RI for intermediate period.
Overall survival (A), TRM (B), and RI (C) between day 100 and 3 years after transplantation, by aGvHD grading. The curves represent an estimate of the survival function, based on a (stratified) Cox model, evaluated for a patient with the following characteristics: age, 35 years; year of transplantation, 1995; sex, male; interval-diagnosis transplantation, less than 12 months; sex-mismatch, no; conditional on surviving at least up to day 100.
Overall survival, TRM, and RI for intermediate period.
Overall survival (A), TRM (B), and RI (C) between day 100 and 3 years after transplantation, by aGvHD grading. The curves represent an estimate of the survival function, based on a (stratified) Cox model, evaluated for a patient with the following characteristics: age, 35 years; year of transplantation, 1995; sex, male; interval-diagnosis transplantation, less than 12 months; sex-mismatch, no; conditional on surviving at least up to day 100.
Age remains the strongest factor with an impact on TRM during this time period. Year of transplantation and interval from diagnosis to transplantation lose their influence. Donor-recipient sex mismatch still has influence.
Time interval from 3 years onward.
Even after 3 years, aGvHD retained an impact on outcome (Table 5). The situation, however, becomes more complex. TRM was still influenced by aGvHD grade. The effect of aGvHD, however, was overshadowed by presence or absence of cGvHD (Figure 4A). TRM was similar for patients with no cGvHD or limited cGvHD (Figure 4B). In contrast, RI was similar and lower in patients with limited or extensive cGvHD (Figure 4C). Because the preventive effect on relapse of limited cGvHD was as strong as that of extensive cGvHD, the sum of both competing events did translate in an advantage for patients with limited cGvHD. Because patients with aGvHD grade I had the highest likelihood of developing limited cGvHD, survival became best beyond 3 years for patients with initial aGvHD grade I.
Long-term overall survival, TRM, and RI.
Overall survival (A), TRM (B), and RI (C) between 3 years and 15 years after transplantation, by cGvHD grading. The curves represent an estimate of the survival function, based on a (stratified) Cox model, evaluated for a patient with the following characteristics: age, 35 years; year of transplantation, 1995; sex, male; interval-diagnosis transplantation, less than 12 months; sex-mismatch, no; andno acute GvHD. Conditional on surviving at least up to year 3.
Long-term overall survival, TRM, and RI.
Overall survival (A), TRM (B), and RI (C) between 3 years and 15 years after transplantation, by cGvHD grading. The curves represent an estimate of the survival function, based on a (stratified) Cox model, evaluated for a patient with the following characteristics: age, 35 years; year of transplantation, 1995; sex, male; interval-diagnosis transplantation, less than 12 months; sex-mismatch, no; andno acute GvHD. Conditional on surviving at least up to year 3.
Age retained its impact on TRM even beyond 3 years. Moreover, impact of donor-recipient sex combination becomes manifest on both TRM and RI. Year of transplantation and time interval no longer have an influence on outcome.
Discussion
These data clarify the role of aGvHD and cGvHD on outcome of patients receiving transplants for CML in first chronic phase. aGvHD has an impact throughout the whole course up to 15 years after transplantation. The effects are divergent on TRM and RI but the positive effect of GVL on reduced relapse rate is counterbalanced by increased TRM. These effects are observed most pronouncedly during the time period from day 100 to 3 years. In the first 3 months, other effects of early mortality and minimal influence of mild aGvHD on TRM mask the role of aGvHD and limit its influence to the severe forms, grades III and IV. Beyond 3 years, the effects of aGvHD remain with the same divergent results on TRM and RI but are overshadowed by the specific effects of cGvHD. The confounding effects are explained by the fact that not all patients without aGvHD stay free of cGvHD and not all patients with severe aGvHD go on to cGvHD.2-4 In addition, effects of cGvHD have the same divergent effects on TRM and RI. However, in contrast to aGvHD, patients with limited cGvHD profit from reduced RI without detrimental effects on TRM compared to patients with no cGvHD. The net result ends in improved survival for patients with limited cGvHD compared both to patients with no or extensive cGvHD.
The results became clear for 2 main reasons. First, we restricted the analysis to a large homogeneous group, patients with CML in first chronic phase of their disease and receiving a transplant from an HLA-identical sibling. Second, we introduced a landmark analysis. This novel approach for GvHD analyses has additional advantages. In the first place it can deal with the severe nonproportionality of individual risk factors because the model is fitted to a more restricted time period. Secondly a “causal” or mechanistic interpretation of the HRs becomes easier. The effect of a risk factor is allowed to be entirely different within the separate time periods. By choosing the time periods in a clinically relevant way, a clearer picture emerges. In contrast, to evaluate overall survival, a simple Kaplan-Meier approach is most appropriate. The reader should bear in mind that, using a landmark analysis, the interpretation of the effect of the various risk factors in periods 2 and 3 is not the usual one; the analysis is conditional on being alive at the start of the period and hence adequately describes the influence of, for instance, aGvHD during that period. However, the associated HRs do not have any reasonable interpretation at, for example, the moment of transplantation itself because by then it is still unknown who will actually survive period 1.
These data were obtained in patients with CML. Generalizations to other diseases need some cautions. CML is considered to be most sensitive to the effects of GVL, based on the results with DLIs.25,26Benefits of GVL, hence, beneficial effects of GvHD might be less pronounced in other disease categories. Reduction of acute and chronic GvHD remains a major goal in allogeneic HSCT. T-cell depletion can effectively eliminate a GvHD. To what extent this goal is achieved at the expense of lost GVL effects has remained a matter of debate for many years.34-36 To answer this question was not part of this study. It remains to be settled in prospective controlled studies. Similarly, in this retrospective analysis we cannot assess effects of immunomodulations, for example, increasing or decreasing immunosuppression on outcome.
The present data confirm and clarify the role of the well-known pretransplantation risk factors.30 37-41 Age retains its influence on TRM through all time periods without influence on RI. Sex itself is of no influence by itself, but female donors induce more TRM in male recipients. Their effects are explained by the incidence of extensive cGvHD. Clearly, it confirms the previous notion of the male H-Y antigen as a histocompatibility antigen that develops only over time. Of interest is the separation of the effects of time interval on TRM. Clearly, it does so by increasing effects on severity of aGvHD; the mechanisms remain speculative. Stem cell source has an impact on incidence and severity of cGvHD; it has no independent effect on the influence of GvHD, once it is established, on outcome.
The present results confirm the antagonistic effects of GvHD, detrimental through its increase of TRM, beneficial through its impact on RI.10,11,16 They show that the window of opportunity to influence outcome through possible manipulation of aGvHD is very narrow and that slight changes in the relative proportions of grade I and grade II aGvHD can be associated with major changes in long-term outcome due to their association with cGvHD. The latter exerts its effects over a long time. Observation within the early period is insufficient to draw definitive conclusions. Although only severe aGvHD has an impact on mortality within the first 100 days, aGvHD, even very mild as grade I, has a definitive impact on TRM as clarified during the time period day 100 to 3 years. There is no beneficial aGvHD, there is only a benefit in the isolated situation of limited cGvHD. This observation is confirmed for patients with CML in chronic phase receiving transplants. It remains open how far these data will be changed by integration of imatinib mesylate41,42 and by the use of DLIs. Similarly, extrapolations to other donor types43 and other diseases must be made very carefully. Still, results of this study need to be considered in all attempts to manipulate or induce GvHD effects.
List of participating centers for the AcGvHD study. Algeria, Alger, R. Hamladji, Centre Pierre et Marie Curie (703); Argentina, Buenos Aires, L. Feldman, Antartida Hospital Privado (742); Australia, Melbourne, A. Schwarer, Alfred Hospital (595); Australia, Perth, R. Hermann, Royal Perth Hospital (710); Austria, Graz, W. Linkesch, Karl Franzens University Graz (308); Austria, Innsbruck, G. Gastl, University Hospital Innsbruck (271); Austria, Vienna, H. Greinix, AKH Vienna (227); Belarus, Minsk, A. Uss, Hospital No. 9 (801); Belgium, Antwerp, W. Schroyens, Universiteit Zentrum Antwerpen (UZA) (648); Belgium, Brugge, D. Selleslag, A. Z. Sint-Jan (506); Belgium, Brussels, D. Bron, Institut Jules Bordet (215); Belgium, Brussels, A. Ferrant, Cliniques Universitaires St Luc (234); Belgium, Brussels, R. Schots, University Hospital VUB (630); Belgium, Charleroi, M. Andre, Centre Hospitalier Notre Dame Royal de Flamande (CHNDRF) (349); Belgium, Gent, L. Noens, University Hospital Gent (744); Belgium, Leuven, M. Boogaerts, University Hospital Gasthuisberg (209); Belgium, Liege, Y. Beguin, University of Liege (726); Brazil, Campinas, C. de Souza, Cidade Universitaria “Zeferino Vaz” (374); Croatia, Zagreb, B. Labar, University Hospital Centre-Rebro (302); Czech Republic, Brno, J. Vorlicek, University Hospital Brno (597); Czech Republic, Pilsen, V. Koza, Charles University Hospital (718); Czech Republic, Prague, A. Vitek, Institute of Hematology and Blood Transfusion (656); Denmark, Copenhagen, N. Jacobsen, Rigshospitalet (206); Estonia, Tartu, H. Everaus, Tartu University Clinics (746); Finland, Helsinki, U. Pihkala, Children's Hospital University of Helsinki (219); Finland, Helsinki, T. Ruutu, Helsinki University Central Hospital (515); Finland, Turku, K. Remes, Turku University Central Hospital (225); France, Angers, M. Boasson, Centre Hospitalier Regional Universitaire (CHRU) (650); France, Besancon, J. Cahn, Hopital Jean Minjoz (233); France, Caen, O. Reman, Centre Hospitalier Universitaire (CHU) de Caen (251); France, Clermont-Ferrand, J. Bay, Centre Jean Perrin (273); France, Clermont-Ferrand, P. Travade, Hotel Dieu/pediatrie (589); France, Creteil, C. Cordonnier, Hopital Henri Mondor (252); France, Dijon, H. Guy, Hopital le Bocage (667); France, Grenoble, J. Sotto, Hopital A.Michallon (270); France, Lille, J. Jouet, Hopital Claude Huriez (277); France, Lyon, M. Michallet, Hopital E. Herriot (671); France, Marseille, D. Blaise, Institut Paoli Calmettes (230); France, Montpellier, J. Rossi, University Hospital (926); France, Nancy, P. Lederlin, CHRU Nancy (249); France, Nantes, J. Harousseau, Hotel Dieu (253); France, Nice, N. Gratecos, Hopital de l'Arget (523); France, Paris, A. Fischer, Hopital Necker (201); France, Paris, E. Gluckman, Hopital St Louis (207); France, Paris, N. Gorin, Hopital St Antoine (213); France, Paris, B. Rio, Hotel Dieu (222); France, Paris, G. Andreu, Pitie-Salpetriere (262); France, Pessac, J. Reiffers, CHU-Bordeaux/Hopital Haut-Leveque (267); France, Poitiers, F. Guilhot, Hopital La Miletrie (264); France, Rennes, E. le Gall, CHRU de Rennes-Hopital Sud Clinique Medicale Infantile (661); France, Rouen, H. Tilly, Centre Henri Becquerel (941); France, Saint-Etienne, D. Guyotat, CHRU de Saint-Etienne Hopital Nord (250); France, Strasbourg, P. Dufour, Hopital de Hautepierre (672); France, Toulouse, M. Attal, Hopital de Purpan (624); France, Vandoeuvre, D. Sommelet, CHU de Nancy Hopitaux de Brabois-Hopital d'Enfants (676); France, Villejuif, D. Machover, Hopital Paul Brousse (567); France, Villejuif, J. Bourhis, Institut Gustave Roussy (666); Germany, Berlin, W. Siegert, Charite-Virchow Klinikum d. Humboldt-Un (293); Germany, Berlin, R. Arnold, Charite (807); Germany, Dresden, G. Ehninger, Universitatsklinikum Dresden (808); Germany, Dusseldorf, R. Haas, Heinrich Heine Universitat (390); Germany, Erlangen, M. Gramtzki, Universitat Erlangen (809); Germany, Essen, U. Schaefer, University Hospital Essen (259); Germany, Frankfurt, D. Hoelzer, Universitat Frankfurt (297); Germany, Freiburg, J. Finke, University of Freiburg (810); Germany, Hamburg, A. Zander, University Hospital Eppendorf (614); Germany, Hannover, B. Hertenstein, Medical School of Hannover (295); Germany, Homburg, D. Beelen, University of Saarland (785); Germany, Idar-Oberstein, A. Fauser, Klinik fur K. M. T. und Hamato-Onkologie (592); Germany, Jena, H. Sayer, Friedrich Schiller Universitat Jena (533) Germany, Jena, F. Zintl, University of Jena (750); Germany, Kiel, N. Schmitz, Christian-Albrechts-University (256); Germany, Leipzig, D. Niederwieser, University of Leipzig (389); Germany, Mainz, K. Kolbe, Johannes-Gutenberg-University (786); Germany, Marburg, A. Neubauer, Philipps-Universitat Marburg (645); Germany, Munchen, H. Kolb, Klinikum Grosshadern (513); Germany, Munster, J. Kienast, University of Munster (680); Germany, Nurnberg, H. Wandt, Klinikum Nurnberg (625); Germany, Regensburg, R. Andreesen, University Regensburg (787); Germany, Rostock, M. Freund, Universitat Rostock (585); Germany, Tubingen, L. Kanz, Medizinische Klinik (223); Germany, Ulm, L. Bergmann, Universitat Ulm (204); Germany, Wiesbaden, R. Schwerdtfeger, Deutsche Klinik fur Diagnostik (311); Greece, Athens, N. Harhalakis, Evangelismos Hospital (622); Greece, Athens, G. Karianakis, Diagnostic and Therapeutic Centre of Athens (643); Greece, Athens, S. Grafakos, St Sophia Children's Hospital (752); Greece, Exokhi (Thessaloniki), A. Fassas, The G. Papanicolaou General Hospital of Thessaloniki (561); Hungary, Budapest, K. Paloczi, National Institute of Hematology and Immunology (504); Hungary, Budapest, T. Masszi, St Laszlo Hospital (739); Iran, Teheran, A. Ghavamzadeh, Shariati Hospital (633); Ireland, Dublin, S. McCann, St James Hospital Trinity College (257); Israel, Haifa, J. Rowe, Rambam Medical Centre (345); Israel, Jerusalem, S. Slavin, Hadassah University Hospital (258); Israel, Petach-Tikva, I. Yaniv, Schneider Children's Medical Centre of Israel (755); Italy, Bergamo, T. Barbui, Ospedale Bergamo (658); Italy, Bologna, S. Tura, Hospital San Orsola (240); Italy, Bologna, A. Pession, University of Bologna (790); Italy, Brescia, T. Izzi, Spedali Civili–Brescia (288); Italy, Cagliari, G. Broccia, Ospedale Oncologico (791); Italy, Cagliari, L. Contu, University of Cagliari (811); Italy, Catania, R. Giustolisi, University of Catania (792); Italy, Cremona, S. Morandi, Centro Trapianti Midollo Osseo II (226); Italy, Firenze, A. Bosi, Ospedale di Careggi (304); Italy, Genova, A. Bacigalupo, Ospedale San Martino (217); Italy, Genova, G. Dini, Insitute G.Gaslini (274); Italy, Milano, G. Lambertenghi Deliliers, Ospedale Maggiore di Milano (265); Italy, Milano, E. Morra, Ospedale di Niguarda Ca'Granda (294); Italy, Milano, C. Bordignon, Isitituto Scientifico H. S. Raffaele (813); Italy, Monza, C. Uderzo, Ospedale San Gerardo/Pediatrica (279); Italy, Monza, E. Pogliani, Ospedale San Gerardo (544); Italy, Napoli, B. Rotoli, University of Napoli (766); Italy, Padova, C. Messina, Clinica Oncoematologia Pediatrica e Centro Leucemie Infantili (285); Italy, Palermo, R. Scime, Ospedale V. Cervello (392); Italy, Palermo, G. Mariani, University of Palermo (814); Italy, Parma, V. Rizzoli, University of Parma (245); Italy, Pavia, E. Alessandrino, Policlinico San Matteo Istituto Regionale e Centro Clinico di Sangre (IRCCS) (286); Italy, Pavia, F. Locatelli, Policlinico San Matteo IRCCS/Pediatrica (557); Italy, Perugia, M. Martelli, University of Perugia (794); Italy, Pesaro, G. Lucarelli, Pesaro Hospital (529); Italy, Pescara, P. di Bartolomeo, Ospedale Civile (248); Italy, Pisa, M. Petrini, University of Pisa (795); Italy, Reggio Calabria, P. Iacopino, Azienda Ospedaliera “Bianchi-Melacrino-Morelli” (587) Italy, Rome, W. Arcese, University “La Sapienza” (232); Italy, Rome, I. Majolino, Ospedale San Camillo (287); Italy, Rome, G. Leone, Universita Cattolica S. Cuore (307); Italy, Rome, S. Amadori, University Tor Vergata, St Eugenio Hospital (756); Italy, Taranto, P. Mazza, Hospedale Nord (332); Italy, Torino, M. Falda, Azienda Ospedaliera S. Giovanni (231); Italy, Udine, R. Fanin, Udine University Hospital (705); Italy, Verona, F. Benedetti, University of Verona (623); Italy, Vicenza, F. Rodeghiero, S. Bortolo Hospital (797); The Netherlands, Amsterdam, H. van den Berg, Academic Medical Centre (247); The Netherlands, Amsterdam, G. Ossenkoppele, Free University Hospital Amsterdam (588); The Netherlands, Leiden, R. Willemze, Leiden University Hospital (203); The Netherlands, Maastricht, H. Schouten, University Hospital Maastricht (565); The Netherlands, Nijmegen, A. Schattenberg, University Medical Centre St Radboud (237); The Netherlands, Rotterdam, J. Cornelissen, Daniel den Hoed Cancer Centre/AZR (246); The Netherlands, Utrecht, L. Verdonck, University Medical Centre Utrecht (239); Norway, Oslo, L. Brinch, Rikshospitalet National Hospital (235); Poland, Gdansk, A. Hellmann, Medical University of Gdansk (799); Poland, Katowice, J. Holowiecki, Silesian Medical Academy (677); Poland, Krakow, A. Skotnicki, Jagiellonian University (553); Poland, Poznan, J. Wachowiak, K. Marcinkowski University of Medical Sciences (641); Poland, Poznan, J. Hansz, K. Marcinkowski University (730); Poland, Wroclaw, A. Lange, K. Dluski Hospital and Institute of Immunology and Experimental Therapy (538); Portugal, Lisboa, M. Abecasis, Instituto Portogues Oncologia (300); Portugal, Porto, P. Pimentel, Instituto Portogues Oncologia Centro do Porto (291); Russia, St Petersburg, K. R. Abdulkadirov, Russian Institute of Technology (724); Saudi Arabia, Riyadh, S. Bazarbashi, King Faisal Specialist Hospital and Research Centre (397); Saudi Arabia, Riyadh, A. Alabdulaaly, Riyadh Armed Forces Hospital (818); Slovakian Republic, Banska Bystrica, K. Mocikova, Roosevelt Hospital (333); Slovakian Republic, Bratislava, J. Lakota, National Cancer Institute (610); Yugoslavia, Belgrade, M. Malesevic, Military Medical Academy (582); Yugoslavia, Novi Sad, D. Pejin, Clinic of Hematology (655); Slovenia, Ljubljana, J. Pretnar, University Medical Center (640); South Africa, Cape Town, N. Novitzky, University of Cape Town (UCT) Medical School (512); South Africa, Cape Town, P. Jacobs, Searl Lab–Cellular & Molecular Biology Constantiaberg Medi-Clinic (772); Spain, Barcelona, E. Montserrat, Hospital Clinic (214); Spain, Barcelona, J. Sierra, Hospital Santa Creu I Sant Pau (260); Spain, Barcelona, J. Ortega, Hospital M. Infantil Vall d'Hebron (527); Spain, Barcelona, A. Julia, R G Vall d'Hebron (583); Spain, Barcelona, A. Granena, Institut Catala d'Oncologia (759); Spain, Cordoba, A. Torres Gomez, Cordoba Hospital–Reina Sofia (238); Spain, Granada, J. de Pablos Gallego, Hospital University Virgen de las Nieves (559); Spain, La Coruna, J. Torres, Hospital Juan Canalejo (361); Spain, Madrid, J.Fernandez-Ranada, Hospital de la Princesa (236); Spain, Madrid, M. Fernandez, Clinica Puerta de Hierro (728); Spain, Madrid, L. Madero, Hospital Nino Jesus de Madrid (732); Spain, Madrid, A. Martinez, Hospital Infantil La Paz (734); Spain, Malaga, J. Maldonado Eloy-Garcia, Hospital Regional de Malaga (576); Spain, Murcia, J. Moraleda, Hospital Morales Meseguer (735); Spain, Oviedo, D. Carrera Fernandez, Hospital Covadonga (642); Spain, Palma de Mallorca, J. Besalduch, Hospital Son Dureta (722); Spain, Salamanca, D. Caballero, Hospital Clinico (727); Spain, San Sebastian, R. Lasa Isasti, Hospital Aranzazu (598); Spain, Santander, A. Iriondo, Hospital Universitario ‘Marques de Valdecilla’ (242); Spain, Sevilla, J. Rodriguez-Fernandez, Hospital ‘Virgen del Rocio’ (769); Spain, Valencia, J. Garcia-Conde, Hospital Clinico Universitario (282); Spain, Valencia, M. Sanz, Hospital Universitario La Fe (663); Sweden, Goeteborg, M. Brune, Sahlgrenska University Hospital (715); Sweden, Huddinge, P. Ljungman, Huddinge University Hospital (212); Sweden, Linkoping, G. Juliusson, University Hospital Linkoping (740); Sweden, Lund, S. Lenhoff, University Hospital Lund (283); Sweden, Umea, A. Wahlin, Umea University Hospital (731); Sweden, Uppsala, B. Simonsson, University Hospital Uppsala (266); Switzerland, Basel, A. Gratwohl, Kantonsspital (202); Switzerland, Geneva, B. Chapuis, Hopital Cantonal Universitaire (261); Switzerland, Zurich, U. Schanz, University Hospital Zurich (208); Turkey, Ankara, E. Kansu, Hacettepe University (292); Turkey, Ankara, A. Yalcin, Gulhane Military Medical Academy (372); Turkey, Ankara, H. Kok, Ankara University-Ibni Sina Hospital (617); Turkey, Istanbul, M. Bayik, Marmara Universitesi Hastanesi (714); Turkey, Istanbul, S. Besisik-Kalayoglu, University of Istanbul (760); Turkey, Istanbul, B. Ferhanoglu, Cerrahpasa Medical School (761); Turkey, Istanbul, G. Gedikoglu, Our Children Leukemia Foundation/Istanbul University (762); United Kingdom, Birmingham, D. Milligan, Birmingham Heartlands Hospital (284); United Kingdom, Birmingham, C. Craddock, University Hospital National Health Service (NHS) trust/Queen Elizabeth (QE) Medical Center Edgbaston (387); United Kingdom, Bristol, B. Bradley, University of Bristol/Department of Tranplantation Sciences (386); United Kingdom, Cambridge, R. Marcus, Addenbrookes Hospital (566); United Kingdom, Cardiff, A. Burnett, College of Medicine/University of Wales (303); United Kingdom, Clydebank, D. Spence, HCI International Medical Centre (317); United Kingdom, Edinburgh, J. Davies, Western General Hospital (228); United Kingdom, Glasgow, I. Franklin, Glasgow Royal Infirmary (244); United Kingdom, Glasgow, B. Gibson, Royal Hospital for Sick Children (707); United Kingdom, Liverpool, R. Clark, Royal Liverpool University Hospital (501); United Kingdom, London, J. Apperley, Hammersmith Imperial College School of Medicine (205); United Kingdom, London, H. Prentice, Royal Free Hospital and School of Medicine (216); United Kingdom, London, A. Goldstone, University College London Hospital (224); United Kingdom, London, P. Gravett, the London Clinic (263); United Kingdom, London, J. Marsh, St George's Hospital, Medical School (593); United Kingdom, London, S. Schey, Guy's Hospital (721); United Kingdom, London, G. Mufti, GKT School of Medicine (763); United Kingdom, London, A. Newland, St Batholomew's and the Royal London Hospital (768); United Kingdom, Newcastle-upon-Tyne, S. Proctor, Royal Victoria Infirmary (276); United Kingdom, Nottingham, N. Russell, Nottingham City Hospital (717); United Kingdom, Oxford, T. Littlewood, The Oxford Radcliffe Hospital (255); United Kingdom, Pendlebury, A. Will, Royal Manchester Children's Hospital (521); United Kingdom, Plymouth, M. Hamon, Plymouth Hospitals NHS Trust (823); United Kingdom, Poole, A. Bell, Poole Hospital NHS Trust (580); United Kingdom, Sheffield, E. Vandenberghe, Royal Hallamshire Hosptial (778); United Kingdom, Sutton, R. Powles, Royal Marsden Hospital (218).
A complete list of the members of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation appears in the “.”
Supported in part by the Swiss National Foundation no. 32-52756.97 and in part by the contributions of the EBMT corporate members: AMGEN Europe, Gilead Sciences, Nexell International, Pharmacia Corporation, Chugai-Aventis, Fresenius HemoCare, SangStat, Schering AG, Gambro BCT, GlaxoSmithKline, and Wyeth-Lederle.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alois Gratwohl, Division of Hematology, Department of Internal Medicine, Kantonsspital Basel, CH-4031 Basel, Switzerland; e-mail: hematology@uhbs.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal