Various cytokines have been shown to protect cells from p53-dependent apoptosis. To investigate the mechanism underlying cytokine-mediated survival, we used a Friend virus–transformed erythroleukemia cell line that expresses a temperature-sensitive p53 allele. These cells express the spleen focus-forming virus-encoded envelope glycoprotein gp55 that allows the cells to proliferate in the absence of erythropoietin (EPO). These cells respond to p53 activation at 32°C by undergoing G1 cell cycle arrest and apoptosis. In the presence of EPO, p53 activation leads only to prolonged but viable G1 arrest. These findings indicate that EPO functions as a survival factor and that gp55/EPO receptor signaling is distinct from EPO/EPO receptor signaling. We demonstrate that p53-dependent apoptosis results in mitochondrial damage as shown by loss of mitochondrial membrane potential, increase in intracellular calcium, and release of mitochondrial cytochrome c into the cytosol. EPO prevented all of these changes including the subsequent activation of caspases. We identify an intrinsic phosphatidylinositol-3′-OH kinase/protein kinase B (PI3′K/PKB)–dependent survival pathway that is constitutively active in these cells. This survival pathway limits p53-dependent apoptosis. We propose that EPO promotes survival through a distinct pathway that is dependent on JAK2 but independent of STAT5 and PI3′K.
Introduction
The p53 tumor suppressor gene plays a fundamental role in promoting apoptosis in response to abnormal proliferative signals and stress including DNA damage.1 Loss of p53-mediated apoptosis results in the survival of oncogene-expressing cells undergoing inappropriate cell growth and in the survival of cells carrying mutations and carcinogenic lesions. Failure to eliminate such cells has been shown to accelerate tumorigenesis.2 The evasion of apoptosis through genetic or epigenetic mechanisms that target death and survival pathways is considered to be a hallmark of cancer cells.3
The cellular decision to undergo apoptosis is governed by the integration of survival and death signals. Growth factors can inhibit apoptosis through increased expression of prosurvival genes and through posttranslational modification and inactivation of proapoptotic proteins. One of the most widely studied cytokines capable of promoting survival in hematopoietic cells is erythropoietin (EPO). The binding of EPO to its receptor (EPO-R) activates multiple signaling pathways that ultimately control the survival, proliferation, and development of immature erythroid cells (for a review, see Wojchowski et al4). EPO-dependent tyrosine phosphorylation of the receptor, which lacks intrinsic kinase activity, and many of its associated signaling molecules is mediated by the cytoplasmic tyrosine kinase JAK2. EPO signaling has been shown to rescue committed erythroid progenitors from undergoing apoptosis during normal erythropoiesis.5,6 The function of EPO as a survival factor has been confirmed using EPO-dependent erythroid cells in which EPO deprivation results in apoptosis.7-9 Various myeloid and lymphoid cell lines have been engineered to express wild-type or mutant forms of the EPO-R and in these cells, EPO-R signaling has also been shown to prevent apoptosis in response to cytokine withdrawal10,11 or irradiation.12
In Epo−/− andEpo-R−/− mice, a block in definitive erythropoiesis leads to embryonic lethality at day 13 caused by a severe anemia.13 Fetal livers fromEpo−/− andEpoR−/− embryos were found to contain increased numbers of nucleated cells undergoing apoptosis. Studies with JAK2-deficient mice indicated that JAK2 is essential for definitive erythropoiesis,14,15 consistent with a primary role of JAK2 in EPO signaling. Furthermore, disruption of JAK2 function through expression of dominant-negative (DN) JAK2 mutants indicated an essential role of JAK2 in EPO-mediated inhibition of apoptosis.11
Erythropoietin signaling, through the activation of JAK2, plays an essential role in regulating the expression of Bcl-XL, a Bcl-2 family member with antiapoptotic function (for a review, see Ihle16). The precise mechanism including the possible involvement of STAT5 in regulating Bcl-XL transcription remains controversial.16-19
Survival-promoting cytokines have been shown to suppress the function of the proapoptotic protein BAD by inducing phosphorylation at 2 critical sites, Ser112 and Ser136. The protein kinase Akt/protein kinase B (Akt/PKB), acting downstream of the phosphatidylinositol-3′-OH kinase (PI3′K) signaling pathway, and RSK1 and RSK2 acting downstream of the mitogen-activated protein kinase (MAPK) pathway, phosphorylate and inactivate BAD.20-24 Both of these pathways are activated when EPO binds to its receptor.4
We have studied the role of EPO in preventing p53-dependent apoptosis in Friend virus–transformed murine erythroleukemia cells. The DP16.1 cell line was established from erythroid cells present in the spleen of a DBA/2J mouse infected with the polycythemia-inducing strain of the Friend virus complex consisting of spleen focus-forming virus (SFFVP) and Friend murine leukemia virus.25DP16.1 cells express the SFFVP-encoded env-related glycoprotein gp55, which binds to the EPO-R and mimics the activation of the receptor with EPO.26 DP16.1 cells proliferate in culture independently of added EPO likely as the result of constitutive EPO-R activation. DP16.1 cells are null for p53, permitting the generation of a DP16.1/p53ts subline carrying a temperature-sensitive (ts) p53 transgene. The p53ts protein contains valine instead of alanine at amino acid position 135 and behaves as a mutant polypeptide at 37°C and as a wild-type polypeptide at 32°C.27DP16.1/p53ts cells grow well at 37°C but can be induced to undergo p53-dependent apoptosis at 32°C when the p53ts protein assumes its wild-type conformation. Using this experimental model, we showed previously that addition of EPO to the culture medium blocked p53-dependent apoptosis.28,29 Hence, constitutive signaling through the EPO-R by gp55 appears to be different from signaling mediated by EPO/EPO-R interaction. DP16.1/p53ts cells provide a useful model to investigate the role of EPO in promoting survival because neither proliferation nor maturation is observed when these cells are cultured at 32°C in the presence of EPO. In the presence of EPO, these cells remain viable in a reversible growth-arrested state.29 Another advantage of this model is that one can be certain that the apoptotic response is a direct consequence of p53 activity. Other studies have also reported on the ability of specific cytokines to block p53-dependent death.12 30-32
Here we demonstrate that activation of JAK2 but not STAT5 is required for EPO-mediated inhibition of p53-dependent apoptosis. Neither PI3′K nor nuclear factor-κB (NFκB), components of 2 major cell survival pathways, is involved in mediating the inhibitory effect of EPO on apoptosis. EPO acts prior to caspase activation and blocks early events associated with p53-dependent apoptosis including the release of cytochrome c from the mitochondria, the decrease in the mitochondrial membrane potential, and the increase in free calcium. These results support a model in which p53-dependent apoptotic signals and EPO-directed survival signals are directed to the mitochondria where cell fate is determined by the balance of these antagonizing forces. In addition, we demonstrate that the PI3′K/protein kinase B (PKB) signaling pathway is constitutively activated in DP16.1 cells and that this survival pathway restricts p53-dependent apoptosis.
Materials and methods
Cell culture
The DP16.1/p53ts cell line28 was established from the Friend virus–transformed mouse erythroleukemia cell line DP16.125 after electroporation of a plasmid containing the temperature-sensitive (ts) mouse p53 A135V allele27 and pSV2neo, which carries the neomycin-resistance gene. Cells were maintained in α minimum essential medium (α-MEM) supplemented with 10% fetal calf serum (FCS). Cell viability was assessed routinely by the exclusion of trypan blue. The PI3′K inhibitors, wortmannin and LY294002 (Calbiochem, San Diego, CA), were stored at −20°C as small concentrated aliquots and added directly to the cell culture in inhibition experiments. Recombinant human EPO was used at a concentration of 1 U/mL.
Cell cycle analysis
Cells were incubated with 10 μM bromodeoxyuridine (BrdU) in α-MEM for 30 minutes, fixed in cold 70% ethanol, and stored at −20°C until use. Then, 2 × 106 cells were rehydrated by washing twice in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (PBS-BSA), resuspended in 500 μL PBS containing 500 μg RNase A and incubated at 37°C for 10 minutes. Cells were centrifuged at 1500 rpm for 5 minutes and the pellet was resuspended in 500 μL 3 N HCl/0.5% Tween 20. After incubation at 37°C for 30 minutes, cells were washed twice in 0.1 M sodium tetraborate (pH 8.5) containing 0.5% BSA and twice in PBS-BSA. Cells were resuspended in 200 μL PBS containing 20 μL fluorescein isothiocyanate (FITC)–conjugated anti-BrdU antibody, 0.1% Tween 20, and 1% BSA and incubated at room temperature for 30 minutes. After 2 washes, cells were finally resuspended in PBS containing propidium iodide (PI; 10 μg/mL) and 0.1% BSA. FITC and PI fluorescences were measured by flow cytometry using an EPICS XL (Beckman Coulter, Fullerton, CA) flow cytometer and data analysis was done using WinMDI software.
Western blotting
Cells were lysed directly in protein sample buffer (2% sodium dodecyl sulfate [SDS], 25 mM Tris [tris(hydroxymethyl)aminomethane]–HCl [pH 6.8], 10% glycerol, 0.1 M dithiothreitol [DTT], 1 mM sodium vanadate, 50 mM sodium fluoride) and boiled for 10 minutes. Protein concentration was estimated by the Sigma Protein Assay (Sigma-Aldrich, St Louis, MO). Total protein (80 μg) in the presence of 0.1% bromophenol blue was loaded onto a polyacrylamide gel containing SDS, subjected to electrophoresis, transferred onto polyvinylidene difluoride (PVDF) membranes, and incubated with the appropriate antibodies. The phospho-STAT5 antibody recognizes the tyrosine-phosphorylated form of STAT5 (Tyr694).33 The phospho-STAT1 antibody was from Zymed Laboratories (San Francisco, CA). The phospho-PKB antibody recognizes the serine-phosphorylated form of PKB (Ser473; New England Biolabs, Beverly, MA). The phospho-BAD monoclonal antibody recognizes the serine-phosphorylated form of BAD (Ser112; Cell Signaling Technology, Beverly, MA). Antibodies directed to poly(ADP-ribose) polymerase (clone 2C-10, gift of G. Poirier, CHUL Research Centre, Ste-Foy, QC, Canada), Bcl-XL (BD Biosciences Pharmingen, San Diego, CA), Bax (Santa Cruz Biotechnology, Santa Cruz, CA), retinoblastoma protein (pRB; BD Biosciences Pharmingen), cytochromec (BD Biosciences Pharmingen), PKB (New England Biolabs), STAT1 and STAT3 (BD Biosciences Pharmingen), BAD (Cell Signaling Technology), and β-actin (Sigma-Aldrich) were used in this study. Equal protein loading of each sample was verified by Coomassie blue staining of the polyacrylamide gel after transfer or by reprobing of the membranes with a β-actin antibody.
Immunoprecipitation and Western blotting for detection of JAK and STAT phosphorylation
Cells (2 × 107) were lysed on ice for 30 minutes in 500 μL lysis buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP40, 1 mM sodium vanadate, 50 mM sodium fluoride, 100 μg/mL Pefabloc, 1 μg/mL each of pepstatin A, aprotinin, and leupeptin). Cell debris was removed by centrifugation at 12 000 rpm for 15 minutes at 4°C. The supernatants (1-2 mg protein) were incubated with antibodies (5 μg) specific for JAK1 or JAK2 (Upstate Biotechnology, Lake Placid, NY) or STAT3 (Zymed Laboratories) for 60 minutes at 4°C and then with 50 μL protein A–Sepharose beads (Amersham Biosciences, Piscataway, NJ) for a further 60 minutes at 4°C. The beads were collected by centrifugation; samples were boiled for 5 minutes and proteins were separated on a 7.5% polyacrylamide gel containing SDS prior to transfer to a PVDF membrane. Blots were incubated with antibodies and immune complexes were visualized with chemiluminescence reagents (Perkin Elmer Life Sciences, Boston, MA). A phosphotyrosine-specific antibody PY20 (Santa Cruz Biotechnology) was used to detect phosphorylated JAK1 and JAK2 proteins, and 4G10 (Upstate Biotechnology) was used to detect phosphorylated STAT3. The blots were reprobed with JAK1-, JAK2-, or STAT3-specific antibodies to reveal total JAK1, JAK2, or STAT3 protein, respectively.
Two-dimensional gel electrophoresis
The DP16.1/p53ts cells were lysed in 8 M urea, 4% 3[3-cholaminopropyl diethyl-ammonio]-1-propane sulfonate (CHAPS), 40 mM Tris base, and 100 mM DTT supplemented with protease and phosphatase inhibitors. Protein extracts were passed through a QIAshredder to reduce viscosity and separated by 2-dimensional gel electrophoresis. Immobilized pH gradient (IPG) strips (linear pH 3-10) were used for separation in the first dimension using the IPGphor Isoelectric Focusing System (Amersham Biosciences). Protein separation in the second dimension was achieved by SDS-12% polyacrylamide gel electrophoresis (PAGE).
Isolation of cytosolic cytochrome c
Cytosolic protein fractions were prepared as previously described.34 Cytochrome c was identified in the cytosolic fractions by Western blotting.
Apoptosis analysis
Three assays were used for analysis of apoptotic cells. These assays produced comparable results when we used them to test identical samples of DP16.1/p53ts cells.
TUNEL assay.
Cells were fixed in 4% formaldehyde followed by 70% ethanol. Then, 3 × 105 cells were resuspended in a 50-μL labeling reaction (25 mM Tris-HCl [pH 6.6], 200 mM potassium cacodylate, 2.5 mM CoCl2, 20 μM biotin-16-deoxyuridine triphosphate [dUTP], 10 μM deoxythymidine triphosphate [dTTP], 1.25 mg/mL BSA, and 12.5 U terminal transferase) and incubated at 37°C for 45 minutes. Cells were washed twice in PBS and incubated with FITC-conjugated avidin in 200 μL 4 × standard sodium citrate (SSC) buffer (1 × SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 5% skim milk and 0.1% Tween 20 for 30 minutes at room temperature. Apoptotic cells stained by FITC fluorescence were analyzed by flow cytometry.
Annexin V staining.
Staining of apoptotic cells with phycoerythrin (PE)–conjugated annexin V was performed according to the supplier (BD Biosciences Pharmingen). Apoptotic cells were analyzed by flow cytometry.
Cell count based on morphology.
Cells were suspended in PBS containing trypan blue dye and counted under a light microscope. Cells stained blue were excluded from analysis. The percentage of apoptotic cells was based on the number of apoptotic cells in a total of 200 cells. Apoptotic cells were identified on the basis of their typical morphology (condensed nuclei and cell shrinkage).
Measurement of mitochondrial membrane potential, reactive oxygen species, glutathione, and free calcium
An EPICS Elite cell sorter (Beckman Coulter), equipped with 3 lasers emitting at 325 nm, 488 nm, and 633 nm, was used to analyze reactive oxygen species (ROS), glutathione content, intracellular free calcium, and mitochondrial membrane potential. All fluorescent dyes were purchased from Molecular Probes (Eugene, OR) and the staining methods were described in detail elsewhere.35 Briefly, 5 μM carboxy-dichlorofluorescein diacetate (carboxy-DCFDA) was used to detect ROS. Carboxy-DCFDA was excited at 488 nm and fluorescence collected at 525 nm. As a positive control for the generation of ROS, the oxidant tert-butyl hydroperoxide (Sigma-Aldrich) was added to the cells 15 minutes before analysis by flow cytometry. Monobromobimane (40 μM) was used to detect the cellular content of reduced glutathione. Acetoxymethyl ester (3 μM indo-1) was used to detect intracellular calcium. Both monobromobimane and indo-1 were excited at 325 nm; the monobromobimane fluorescence was collected with a 440-nm bandpass filter and the intracellular free calcium was measured as a ratio of calcium-bound (405 nm) and calcium-free (525 nm) emissions of indo-1. DiIC1(5) (40 nM) was used to measure mitochondrial membrane potential. DiIC1(5) was excited at 633 nm and fluorescence collected at 675 nm. PI (5 μg/mL) was added to all samples to identify cells that had lost cytoplasmic membrane integrity.
Electrophoretic mobility shift assay
Assays for the DNA-binding activity of STAT536 and NFκB37 were performed with minor changes. To prepare nuclear extracts, EPO-treated or untreated cells were lysed in buffer A (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.8], 20% glycerol, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM EDTA [ethylenediaminetetraacetic acid], 1 mM DTT, 0.1% NP40, and protease inhibitor cocktail [Roche Applied Science, Indianapolis, IN]) and kept on ice for 5 minutes. Extracts were centrifuged and the nuclear pellet was lysed in 3 volumes of buffer A modified to contain 400 mM NaCl and 1% NP40. A 20-μL binding reaction mixture contained 4 μL nuclear lysate (10 μg protein), 1 μg poly(dI-dC), 5 × 104 cpm 32P-labeled double-stranded probe, 20 mM HEPES (pH 7.8), 100 mM NaCl, 1 mM DTT, 1 mM MgCl2, 0.5 mM EDTA, and 8% glycerol. An end-labeled double-stranded oligonucleotide derived from theβ-casein promoter (5′-AGATTTCTAGGAATTCAAATC-3′) was used as the probe for STAT5 and an unlabeled oligonucleotide from the DUB-1 promoter (5′-TAACAGGAAATAATGACTAAG-3′) was used as a negative control in the competition reaction.36An end-labeled oligonucleotide probe derived from the HIV-1 long terminal repeat (LTR; 5′-CGGAAAGTCCCCAGCGGAAAGTCCCTGAT-3′) was used to measure NFκB DNA-binding activity. Tumor necrosis factor α (TNF-α, gift from C. Richardson, Ontario Cancer Institute, Toronto, ON, Canada) was used to stimulate the DNA-binding activity of NFκB.
Transient expression of DN JAK2 and STAT5
Two DN JAK2 expression constructs were used in this study. pEFBOS-JAK2Δ829 contains a carboxy-terminal truncation of JAK2 and pEFBOS-JAK2-DK contains point mutations in the kinase domain resulting in the loss of kinase activity.11 The STAT5 DN construct in pcDNA3 encodes a truncated form of STAT5 with a stop codon at amino acid position 650, producing a protein of approximately 72 kDa.36 Transient transfection of the DN JAK2 constructs into DP16.1/p53ts cells was performed with the Superfect reagent (Qiagen, Valencia, CA) according to protocols supplied by the manufacturer. After transfection, cells were cultured with or without EPO (1 U/mL) at 32°C overnight and collected for apoptosis analysis by TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) staining. The DN STAT5 construct was cotransfected into DP16.1/p53ts cells with a CD20 expression plasmid using the FuGene reagent (Roche Applied Science). Eighteen to 20 hours after transfection, cells were cultured with or without EPO (1 U/mL) at 32°C overnight. A gate was set to select the CD20+ cells and annexin V staining was used to measure apoptosis.
Measurement of STAT5 phosphorylation by flow cytometry
The DP16.1/p53ts cells (2 × 106), either left untreated or treated with EPO (1 U/mL) for 15 minutes, were fixed in cold 70% methanol on ice for 30 minutes, washed 3 times with cold PBS containing 0.5% BSA (PBS-BSA), resuspended in PBS containing goat serum, and incubated at room temperature for 45 minutes. Cells were spun, resuspended in 200 μL PBS-3% BSA containing phospho-Stat5 (Tyr694) antibodies (New England Biolabs) used at a 1:200 dilution, and incubated at 4°C for 16 hours. Cells were washed twice with cold PBS-BSA containing 0.05% Triton-X 100, once with PBS-BSA, resuspended in 200 μL PBS-3% BSA containing secondary antibody (FITC-conjugated rabbit anti–mouse immunoglobulin G) used at a 1:1000 dilution, and incubated at room temperature for 1 hour in the dark. Cells were washed 3 times in PBS-BSA and analyzed by flow cytometry (FACScan, BD Biosciences).
Results
EPO blocks p53-dependent apoptosis
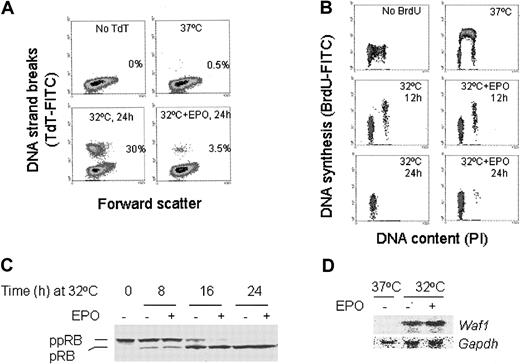
The DP16.1/p53ts cells were transferred to a 32°C incubator and cultured in the presence or absence of EPO. As measured by the TUNEL assay, p53 activation resulted in 30% cell death by 24 hours (Figure 1A). The surviving cells were arrested in the G1 phase of the cell cycle (Figure 1B). Nearly all cells were dead by 72 hours after p53 activation (data not shown). In the presence of EPO, however, the extent of cell death at 24 hours was reduced to 3.5% (Figure 1A) and the cells underwent a prolonged and viable blockade in G1(Figure 1B and data not shown). The p53-mediated G1 arrest was associated with an increase in the hypophosphorylated isoforms of pRB in both EPO-treated and untreated cells (Figure 1C) consistent with p53-dependent induction of the cyclin-dependent kinase inhibitor p21WAF1 (Figure 1D). In addition, EPO did not prevent p53-dependent induction of Bax38 andPidd,39 2 genes implicated in p53-mediated apoptosis (data not shown). These data indicate that EPO does not block the sequence-specific transactivation function of p53 and that EPO-mediated signals interfere with p53-dependent apoptosis but not with p53-dependent G1 arrest.
EPO suppresses p53-dependent apoptosis.
(A) TUNEL assay of DP16.1/p53ts cells grown at 37°C or at 32°C for 24 hours, with or without EPO (1 U/mL). Apoptotic cells were identified by their fluorescence (TdT-FITC) after the fragmented DNA was labeled with biotin-dUTP and avidin-FITC. The numbers shown in each panel represent the percentage of total cells undergoing apoptosis. (B) Pulse labeling with BrdU to detect DNA synthesis. DP16.1/p53ts cells were cultured at 37°C or at 32°C for 12 hours, or 24 hours with or without EPO and then labeled with BrdU for 30 minutes to detect cells undergoing DNA synthesis. Cells were fixed and incorporated BrdU was detected with FITC-conjugated BrdU antibody. BrdU-FITC staining indicates active DNA synthesis and PI staining reveals the DNA content and. hence, the cell cycle position of a cell. (C) DP16.1/p53ts cells were cultured at 32°C for the time periods indicated in the presence or absence of EPO. Cell extracts were prepared and subjected to SDS-PAGE and Western blotting for pRB protein. ppRB indicates hyperphosphorylated RB; pRB, hypophosphorylated RB. (D) Northern blot for Waf-1 mRNA expression. Total RNA was extracted from DP16.1/p53ts cells grown at 37°C or at 32°C for 16 hours with or without EPO. Then 10 μg RNA from each sample was separated on a 1% agarose gel containing formaldehyde and transferred to a nylon membrane. The RNA blot was probed sequentially for Waf-1 andGapdh.
EPO suppresses p53-dependent apoptosis.
(A) TUNEL assay of DP16.1/p53ts cells grown at 37°C or at 32°C for 24 hours, with or without EPO (1 U/mL). Apoptotic cells were identified by their fluorescence (TdT-FITC) after the fragmented DNA was labeled with biotin-dUTP and avidin-FITC. The numbers shown in each panel represent the percentage of total cells undergoing apoptosis. (B) Pulse labeling with BrdU to detect DNA synthesis. DP16.1/p53ts cells were cultured at 37°C or at 32°C for 12 hours, or 24 hours with or without EPO and then labeled with BrdU for 30 minutes to detect cells undergoing DNA synthesis. Cells were fixed and incorporated BrdU was detected with FITC-conjugated BrdU antibody. BrdU-FITC staining indicates active DNA synthesis and PI staining reveals the DNA content and. hence, the cell cycle position of a cell. (C) DP16.1/p53ts cells were cultured at 32°C for the time periods indicated in the presence or absence of EPO. Cell extracts were prepared and subjected to SDS-PAGE and Western blotting for pRB protein. ppRB indicates hyperphosphorylated RB; pRB, hypophosphorylated RB. (D) Northern blot for Waf-1 mRNA expression. Total RNA was extracted from DP16.1/p53ts cells grown at 37°C or at 32°C for 16 hours with or without EPO. Then 10 μg RNA from each sample was separated on a 1% agarose gel containing formaldehyde and transferred to a nylon membrane. The RNA blot was probed sequentially for Waf-1 andGapdh.
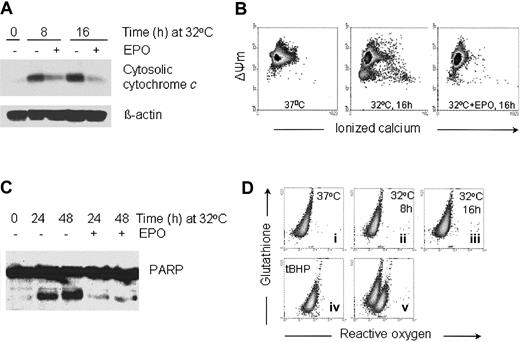
EPO inhibits the release of cytochrome c, the decrease in mitochondrial membrane potential, and the activation of caspases associated with p53-dependent apoptosis
It has been shown that p53-dependent apoptosis proceeds through a pathway that involves Apaf-1 and caspase 9 activation downstream of mitochondrial cytochrome c release.40,41 We wished to determine if p53-dependent apoptosis in DP16.1/p53ts cells involves mitochondrial cytochrome c release and whether EPO promotes survival by inhibiting the release of cytochrome c. DP16.1/p53ts cells were cultured at 32°C for increasing periods of time and cytosolic fractions were prepared for Western blotting. Cytochrome c levels in the cytosol were significantly increased by 8 hours after the temperature shift (Figure2A). In other experiments, cytochromec release was evident as early as 4 hours after the temperature shift (data not shown). The kinetics of cytochromec release were consistent with the kinetics of p53-mediated cell death.29 The release of cytochrome c from the mitochondria was reduced markedly when the cells were cultured in the presence of EPO (Figure 2A).
EPO inhibits the release of cytochrome c, the decrease in mitochondrial membrane potential, and the activation of caspases associated with p53-dependent apoptosis.
(A) Cytosolic protein extracts were prepared from the DP16.1/p53ts cells that were cultured at 32°C for the time periods indicated with or without EPO. Proteins (30 μg) were fractionated by SDS-PAGE, immunoblotted separately with antibodies against cytochromec and β-actin, and visualized by enhanced chemiluminescence. (B) The mitochondrial membrane potential (ΔΨm) and free calcium content of DP16.1/p53ts cells cultured at 32°C in the presence or absence of EPO were measured by flow cytometry. (C) DP16.1/p53ts cells were cultured at 32°C in the presence or absence of EPO for the times indicated. Protein extracts were prepared and analyzed by SDS-PAGE and immunoblotting with anti-PARP antibodies. (D) The generation of ROS and glutathione content of DP16.1/p53ts cells cultured at 37°C or 32°C were measured by flow cytometry (i-iii). ROS production in DP16.1/p53ts cells in response to treatment with Tert-butyl-hydroperoxide is shown in panel iv (tBHP, 70 μM). Panel v represents an overlay of panels ii and iv.
EPO inhibits the release of cytochrome c, the decrease in mitochondrial membrane potential, and the activation of caspases associated with p53-dependent apoptosis.
(A) Cytosolic protein extracts were prepared from the DP16.1/p53ts cells that were cultured at 32°C for the time periods indicated with or without EPO. Proteins (30 μg) were fractionated by SDS-PAGE, immunoblotted separately with antibodies against cytochromec and β-actin, and visualized by enhanced chemiluminescence. (B) The mitochondrial membrane potential (ΔΨm) and free calcium content of DP16.1/p53ts cells cultured at 32°C in the presence or absence of EPO were measured by flow cytometry. (C) DP16.1/p53ts cells were cultured at 32°C in the presence or absence of EPO for the times indicated. Protein extracts were prepared and analyzed by SDS-PAGE and immunoblotting with anti-PARP antibodies. (D) The generation of ROS and glutathione content of DP16.1/p53ts cells cultured at 37°C or 32°C were measured by flow cytometry (i-iii). ROS production in DP16.1/p53ts cells in response to treatment with Tert-butyl-hydroperoxide is shown in panel iv (tBHP, 70 μM). Panel v represents an overlay of panels ii and iv.
Release of cytochrome c into the cytosol is usually preceded or accompanied by a drop in the mitochondrial membrane potential. We observed a drop in the mitochondrial membrane potential when DP16.1/p53ts cells were cultured at 32°C for 16 hours (Figure 2B). Intracellular free calcium also increased in a subpopulation that had suffered loss of mitochondrial membrane potential (Figure 2B). Release of ionized calcium is an indication of disturbance in mitochondrial function. In cells treated with EPO, both the decrease in mitochondrial membrane potential and the increase in free calcium were inhibited (Figure 2B). In addition, we detected cleavage of the caspase 3 substrate, poly(ADP-ribose) polymerase (PARP) during p53-dependent apoptosis and found that PARP cleavage was effectively reduced in response to EPO (Figure 2C). Taken together, these results demonstrate that p53-mediated cell death involves signaling through the mitochondria and that EPO provides protection from p53-dependent apoptosis by acting at an early stage to preserve mitochondrial integrity and prevent caspase activation.
We then looked for an association between p53-dependent apoptosis and the generation of ROS in DP16.1/p53ts cells because p53-dependent apoptosis has been reported to be dependent on ROS production.42-44 DP16.1/p53ts cells were cultured at 32°C and collected at 0, 8 and 16 hours, and the level of ROS and glutathione determined using a flow cytometric assay. We did not detect any increase in ROS or loss of glutathione content after p53 was activated (Figure 2D). DP16.1/p53ts cells treated with the oxidant tert-butyl hydroperoxide served as a positive control and showed the expected production of ROS and loss of glutathione content. In additional experiments, we were unable to detect ROS at earlier time points (2 hours, 4 hours) or later time points (20 hours, 24 hours) after p53 activation (data not shown). These findings indicate that in DP16.1/p53ts cells, p53 activation leads to apoptosis through a pathway that does not involve the generation of ROS.
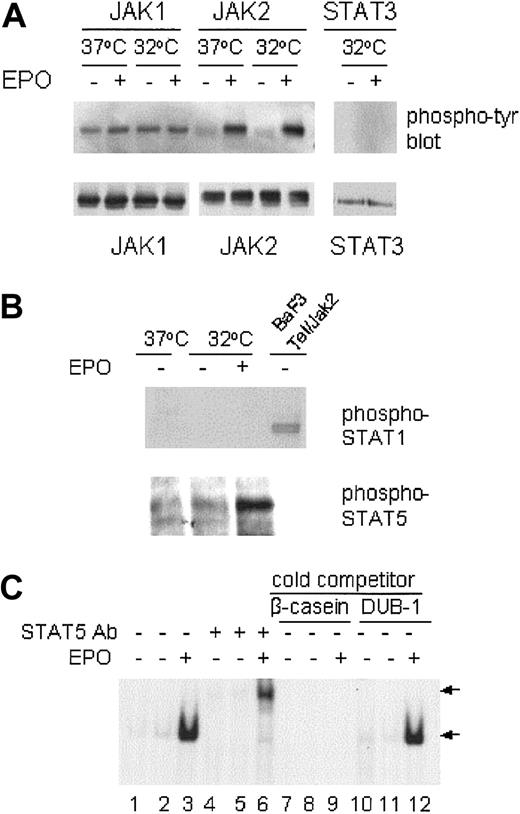
EPO inhibition of p53-dependent apoptosis is associated with JAK2 and STAT5 activation but not JAK1 activation
Binding of the SFFVp env-related gp55 glycoprotein to the EPO-R is associated with constitutive JAK1 activation in SFFVP-infected cells,45 whereas ligand-induced EPO-R homodimerization leads to activation of the cytoplasmic tyrosine kinase JAK2. We wished to determine if the JAK/STAT pathway could be activated in SFFVP-transformed DP16.1/p53ts cells on EPO-R stimulation and whether activation of this pathway was associated with the suppression of p53-dependent apoptosis. DP16.1/p53ts cells were cultured at 37°C or at 32°C for 12 hours and then treated with EPO for 15 minutes just prior to lysis. JAK1 and JAK2 were immunoprecipitated from the cell lysates with anti-JAK1 and anti-JAK2 antibodies, respectively. The immunoprecipitates were analyzed by Western blotting with antiphosphotyrosine antibody PY20 or with anti-JAK1 or anti-JAK2 antibody. As shown in Figure3A, only JAK1 was constitutively tyrosine phosphorylated and EPO treatment did not increase JAK1 phosphorylation. There was no change in JAK1 phosphorylation whether or not p53 was activated at 32°C. In contrast, there was very little tyrosine phosphorylation of JAK2 in DP16.1/p53ts cells cultured at 37°C or 32°C. Within 15 minutes of EPO addition to the culture medium, tyrosine phosphorylation of JAK2 was markedly increased.
JAK/STAT activation in response to EPO.
DP16.1/p53ts cells were cultured at 37°C or at 32°C for 12 hours, then treated with EPO for 15 minutes and lysed immediately for immunoprecipitation and Western blotting. (A) JAK1, JAK2, and STAT3 proteins were immunoprecipitated with specific antibodies and analyzed by Western blotting with antiphosphotyrosine antibodies. The same blots were stripped and reprobed with JAK1, JAK2, or STAT3 antibodies. (B) DP16.1/p53ts cells cultured as above were lysed and subjected to SDS-PAGE and Western blotting with phospho-specific antibodies against STAT1 or STAT5 (Tyr694). An extract prepared from BAF3 cells expressing a Tel-Jak2 fusion protein in which STAT1 is phosphorylated served as a positive control for the phospho-STAT1 antibody. (C) EMSA for STAT5 DNA-binding activity. DP16.1/p53ts cells were cultured at 37°C (lanes 1, 4, 7, and 10) or 32°C for 12 hours (lanes 2, 3, 5, 6, 8, 9, 11, and 12). Nuclear extracts were prepared and incubated with a radiolabeled oligonucleotide probe derived from the β-caseinpromoter. An antibody against STAT5 was included in the binding reaction in lanes 4 to 6. For competition analysis, a 50-fold molar excess of unlabeled β-casein oligonucleotide (lanes 7-9) or an unrelated DUB-1 promoter oligonucleotide (lanes 10-12) was added to the binding reaction. The DNA-binding complexes are indicated by the bottom arrow and the supershifted complexes are indicated by the top arrow.
JAK/STAT activation in response to EPO.
DP16.1/p53ts cells were cultured at 37°C or at 32°C for 12 hours, then treated with EPO for 15 minutes and lysed immediately for immunoprecipitation and Western blotting. (A) JAK1, JAK2, and STAT3 proteins were immunoprecipitated with specific antibodies and analyzed by Western blotting with antiphosphotyrosine antibodies. The same blots were stripped and reprobed with JAK1, JAK2, or STAT3 antibodies. (B) DP16.1/p53ts cells cultured as above were lysed and subjected to SDS-PAGE and Western blotting with phospho-specific antibodies against STAT1 or STAT5 (Tyr694). An extract prepared from BAF3 cells expressing a Tel-Jak2 fusion protein in which STAT1 is phosphorylated served as a positive control for the phospho-STAT1 antibody. (C) EMSA for STAT5 DNA-binding activity. DP16.1/p53ts cells were cultured at 37°C (lanes 1, 4, 7, and 10) or 32°C for 12 hours (lanes 2, 3, 5, 6, 8, 9, 11, and 12). Nuclear extracts were prepared and incubated with a radiolabeled oligonucleotide probe derived from the β-caseinpromoter. An antibody against STAT5 was included in the binding reaction in lanes 4 to 6. For competition analysis, a 50-fold molar excess of unlabeled β-casein oligonucleotide (lanes 7-9) or an unrelated DUB-1 promoter oligonucleotide (lanes 10-12) was added to the binding reaction. The DNA-binding complexes are indicated by the bottom arrow and the supershifted complexes are indicated by the top arrow.
We examined tyrosine phosphorylation of the JAK2 substrates STAT1, STAT3, and STAT5 in DP16.1/p53ts cells in response to EPO stimulation. STAT3 and STAT5 protein are expressed in these cells but we could not detect STAT1 protein by Western blotting. Constitutive activation of these STATs was not observed. Within 15 minutes of EPO stimulation, tyrosine phosphorylation of STAT5, but not of STAT1 or STAT3, was readily detected (Figure 3A-B). In addition, EPO stimulation rapidly induced STAT5 DNA-binding activity as detected by its interaction with a DNA sequence derived from theβ-casein promoter (Figure 3C). Inclusion of a STAT5-specific antibody in the binding reaction resulted in a supershifted STAT5/DNA complex and served to confirm the presence of STAT5 in the complex. Formation of the STAT5/DNA complex was prevented in the presence of excess unlabeledβ-casein promoter DNA but not by an excess of an unrelated oligonucleotide derived from the DUB-1promoter (Figure 3C). These results indicate that JAK1 is constitutively activated in the Friend erythroleukemia cell line DP16.1/p53ts and that EPO stimulation leads to the rapid activation of the JAK2-STAT5 signaling pathway.
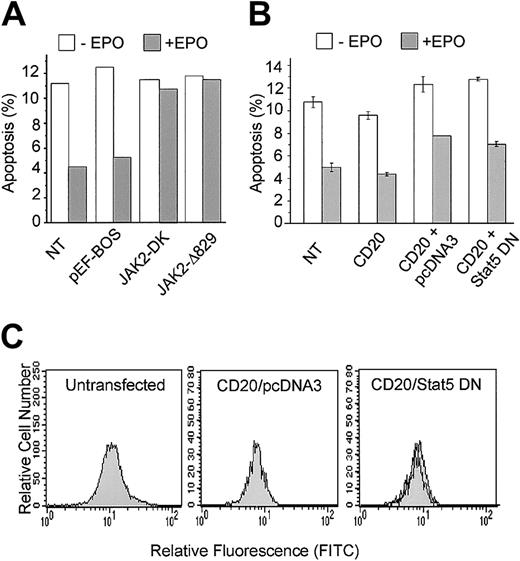
EPO-mediated inhibition of p53-dependent apoptosis requires JAK2
To assess the importance of JAK2 in EPO suppression of p53-mediated apoptosis, we used DN JAK2 mutants to disrupt endogenous JAK2 function. JAK2Δ829 contains a carboxy-terminal truncation and JAK2-DK contains point mutations in the kinase domain resulting in the loss of kinase activity.11 Both mutants are effective in preventing EPO-dependent cell proliferation and survival.11 DP16.1/p53ts cells were transfected with the DN JAK2 mutants and incubated at 32°C to activate p53 in the presence or absence of EPO. Cells were collected 12 hours after p53 activation and the proportion of cells undergoing apoptosis was measured by the TUNEL assay as described in “Materials and methods.” The results, presented in Figure 4A, show that transient expression of both DN JAK2 mutants, but not transfection with the empty vector, effectively blocked the ability of EPO to promote survival of cells with activated p53. These data indicate that JAK2 function is required for EPO inhibition of p53-dependent apoptosis.
DN-JAK2 but not DN-STAT5 inhibits EPO-mediated survival.
(A) DP16.1/p53ts cells were transfected with expression plasmids containing DN-JAK2: JAK2-DK encodes JAK2 with mutations in the kinase domain and JAK2Δ829 encodes JAK2 with a truncated kinase domain; pEF-BOS is the empty vector. After 12 hours at 32°C in the presence or absence of EPO, cultures were fixed and the proportion of cells undergoing apoptosis was measured by the TUNEL assay. The data presented are representative of 2 independent experiments. NT indicates cells that were not transfected. (B) DP16.1/p53ts cells were cotransfected with vectors expressing CD20 together with DN-STAT5 or empty vector (pcDNA3). Cells were cultured as described in the legend to Panel A, fixed, and stained for CD20 expression and for apoptosis with PE-conjugated annexin V, and analyzed by flow cytometry. The percentage of CD20+ cells undergoing apoptosis is presented as the means ± SEM (n = 3). (C) Untransfected DP16.1 cells (left panel), DP16.1 cells transfected with CD20 and empty pcDNA3 vector (middle panel), or DP16.1 cells transfected with CD20 and DN STAT5 (right panel) were treated with EPO for 15 minutes, fixed, and stained with phospho-specific antibodies against STAT5 (Tyr694). Cells expressing phosphorylated STAT5 were detected by flow cytometry using an anti–rabbit IgG conjugated to FITC. The histograms show the relative fluorescence for FITC of cells that were either left untreated (filled histogram) or treated with EPO (empty histogram) 24 hours after transfection.
DN-JAK2 but not DN-STAT5 inhibits EPO-mediated survival.
(A) DP16.1/p53ts cells were transfected with expression plasmids containing DN-JAK2: JAK2-DK encodes JAK2 with mutations in the kinase domain and JAK2Δ829 encodes JAK2 with a truncated kinase domain; pEF-BOS is the empty vector. After 12 hours at 32°C in the presence or absence of EPO, cultures were fixed and the proportion of cells undergoing apoptosis was measured by the TUNEL assay. The data presented are representative of 2 independent experiments. NT indicates cells that were not transfected. (B) DP16.1/p53ts cells were cotransfected with vectors expressing CD20 together with DN-STAT5 or empty vector (pcDNA3). Cells were cultured as described in the legend to Panel A, fixed, and stained for CD20 expression and for apoptosis with PE-conjugated annexin V, and analyzed by flow cytometry. The percentage of CD20+ cells undergoing apoptosis is presented as the means ± SEM (n = 3). (C) Untransfected DP16.1 cells (left panel), DP16.1 cells transfected with CD20 and empty pcDNA3 vector (middle panel), or DP16.1 cells transfected with CD20 and DN STAT5 (right panel) were treated with EPO for 15 minutes, fixed, and stained with phospho-specific antibodies against STAT5 (Tyr694). Cells expressing phosphorylated STAT5 were detected by flow cytometry using an anti–rabbit IgG conjugated to FITC. The histograms show the relative fluorescence for FITC of cells that were either left untreated (filled histogram) or treated with EPO (empty histogram) 24 hours after transfection.
EPO-mediated inhibition of p53-dependent apoptosis is not dependent on STAT5
To assess the importance of STAT5 in EPO suppression of p53-mediated apoptosis, a DN form of STAT536 truncated at amino acid 650 was transiently expressed in DP16.1/p53ts cells. This mutant is defective in tyrosine phosphorylation, heterodimerization, DNA binding, and transcriptional activation. It acts as a DN form likely by blocking wild-type STAT binding to the intracytoplasmic domain of the EPO-R.36 46 In a preliminary series of experiments, we found that expression of DN STAT5 did not diminish the ability of EPO to promote survival of DP16.1/p53ts cells cultured at 32°C to activate p53. To confirm this finding, a vector encoding DN STAT5 was cotransfected with an expression vector encoding the B-cell surface marker CD20. Transfected cells were identified by CD20 staining and the proportion of CD20+ cells undergoing p53-dependent apoptosis at 32°C in the presence or absence of EPO was determined by annexin V staining and flow cytometry. EPO suppressed apoptosis to the same extent in CD20+ cells transfected with DN STAT5 as in CD20+ cells transfected with empty vector (Figure 4B).
To ensure that the DN STAT5 construct was effective at inhibiting the activation of endogenous STAT5 in response to EPO, we measured the level of phosphorylated STAT5 in cells that were transfected with CD20 and DN STAT5. Cells were fixed and stained with a phospho-specific antibody against STAT5 (Tyr694), and the level of staining was measured using a flow cytometry-based assay. The left panel in Figure 4C demonstrates that EPO stimulation of untransfected DP16.1 cells resulted in STAT5 phosphorylation that could be detected using this assay. Furthermore, STAT5 was phosphorylated in response to EPO in CD20+ cells transfected with CD20 and empty vector (Figure4C, middle panel), but not in CD20+ cells transfected with CD20 and the DN STAT 5 construct (Figure 4C, right panel). Together, these data indicate that EPO-mediated survival of DP16.1/p53ts cells at 32°C requires JAK2 but is independent of STAT5.
The PI3′K/PKB-signaling pathway limits p53-dependent apoptosis but is not required for EPO-mediated protection
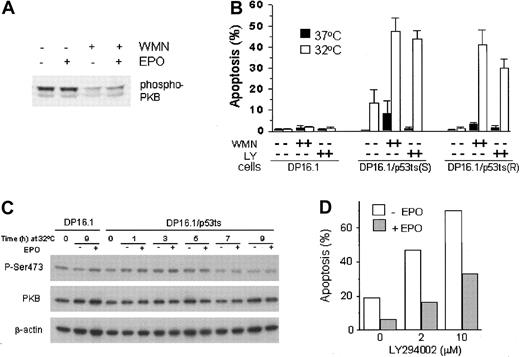
PI3′K is one of several molecules that is recruited to the activated EPO-R either directly47 or indirectly through Gab148 or IRS-2.49 To determine if the PI3′K pathway is constitutively active in gp55-expressing SFFVP-transformed erythroleukemia cells, or whether this pathway can be induced following EPO stimulation, we examined the phosphorylation state of Akt/PKB, a key signaling effector of the PI3′K pathway essential for the survival of at least some cell types.50 51 DP16.1/p53ts cells were treated with EPO in the presence or absence of wortmannin (100 nM), a PI3′K inhibitor, and cell extracts were prepared for analysis by Western blotting using a phospho-PKB antibody that specifically recognizes PKB with phosphoserine at position 473. Figure 5A shows that PKB was constitutively phosphorylated on Ser473 and that addition of EPO to the culture medium did not cause a further increase in PKB phosphorylation. Wortmannin treatment reduced PKB phosphorylation whether or not EPO was present. This indicates that PI3′K activity is an upstream activator of PKB in DP16.1 cells.
The involvement of PI3′K/PKB signaling in p53-dependent apoptosis and in EPO-mediated cell survival.
(A) Constitutive activation of PKB in DP16.1/p53ts cells. DP16.1/p53ts cells were left untreated, treated with EPO alone (15 minutes), wortmannin (WMN, 100 nM for 60 minutes) alone, or with both (WMN for 60 minutes followed by EPO for 15 minutes). Cell extracts were prepared and analyzed by SDS-PAGE and immunoblotting with an antibody specific for phosphorylated PKB (Ser473). (B) Activated PI3′K limits p53-dependent apoptosis. Cells were cultured at 37°C or 32°C for 12 hours in the presence or absence of 100 nM wortmannin (WMN) or 5 μM LY294002 (LY). The percentage of cells that showed evidence of apoptosis was determined on the basis of morphology as described in “Materials and methods” and is presented as the mean ± SEM (n = 3). DP16.1, parental p53− cells; DP16.1/p53ts(S), sensitive subclone that undergoes p53-dependent apoptosis at 32°C; DP16.1/p53ts(R), resistant subclone that undergoes p53-dependent growth arrest but not apoptosis when cultured at 32°C. (C) EPO does not prevent reduction in the level of phosphorylated (Ser473) PKB. DP16.1/p53ts cells or parental DP16.1 cells were harvested at 0 to 9 hours (as indicated) after shifting to 32°C. Then, 50 μg whole cell lysate (in 2 × SDS-loading buffer) was applied to a polyacrylamide gel, separated by electrophoresis, and blotted onto a PDVF membrane. The membrane was immunoblotted sequentially with antiphosphoserine 473 PKB, total PKB (Cell Signaling), and β-actin (Sigma) antibodies. (D) EPO-mediated cell survival in the presence of LY294002. DP16.1/p53ts cells were cultured at 32°C for 12 hours. EPO and LY294002 were added to the cultures at the time of the temperature shift. The percentage of cells undergoing apoptosis was measured by the TUNEL assay.
The involvement of PI3′K/PKB signaling in p53-dependent apoptosis and in EPO-mediated cell survival.
(A) Constitutive activation of PKB in DP16.1/p53ts cells. DP16.1/p53ts cells were left untreated, treated with EPO alone (15 minutes), wortmannin (WMN, 100 nM for 60 minutes) alone, or with both (WMN for 60 minutes followed by EPO for 15 minutes). Cell extracts were prepared and analyzed by SDS-PAGE and immunoblotting with an antibody specific for phosphorylated PKB (Ser473). (B) Activated PI3′K limits p53-dependent apoptosis. Cells were cultured at 37°C or 32°C for 12 hours in the presence or absence of 100 nM wortmannin (WMN) or 5 μM LY294002 (LY). The percentage of cells that showed evidence of apoptosis was determined on the basis of morphology as described in “Materials and methods” and is presented as the mean ± SEM (n = 3). DP16.1, parental p53− cells; DP16.1/p53ts(S), sensitive subclone that undergoes p53-dependent apoptosis at 32°C; DP16.1/p53ts(R), resistant subclone that undergoes p53-dependent growth arrest but not apoptosis when cultured at 32°C. (C) EPO does not prevent reduction in the level of phosphorylated (Ser473) PKB. DP16.1/p53ts cells or parental DP16.1 cells were harvested at 0 to 9 hours (as indicated) after shifting to 32°C. Then, 50 μg whole cell lysate (in 2 × SDS-loading buffer) was applied to a polyacrylamide gel, separated by electrophoresis, and blotted onto a PDVF membrane. The membrane was immunoblotted sequentially with antiphosphoserine 473 PKB, total PKB (Cell Signaling), and β-actin (Sigma) antibodies. (D) EPO-mediated cell survival in the presence of LY294002. DP16.1/p53ts cells were cultured at 32°C for 12 hours. EPO and LY294002 were added to the cultures at the time of the temperature shift. The percentage of cells undergoing apoptosis was measured by the TUNEL assay.
To assess the importance of constitutive PI3′K/PKB signaling for the survival of SFFVP-transformed cells under normal growth conditions and under conditions in which p53 is activated, we inhibited this pathway with the PI3′K inhibitors wortmannin (100 nM) and LY294002 (5 μM). DP16.1 and DP16.1/p53ts cells were cultured at 37°C or 32°C for 12 hours in the presence of absence of the inhibitors. Apoptotic cells were identified on the basis of their typical morphology and enumerated under the microscope. This method for scoring apoptosis was found to be comparable with the TUNEL assay. Wortmannin and LY294002 had little or no effect on the viability of the parental DP16.1 cells, cultured at either temperature, or on DP16.1/p53ts cells cultured at 37°C (Figure 5B). In the presence of activated p53 at 32°C, however, the extent of p53-dependent apoptosis was markedly increased on PI3′K inhibition. We confirmed this observation using a subclone of DP16.1/p53ts cells (R) that was selected on the basis of its inability to undergo p53-dependent apoptosis at 32°C. At 37°C, PI3′K inhibition had no effect on the apoptotic index of these cells. At 32°C, however, PI3′K inhibition resulted in significant cell death (Figure 5B). These results indicate that an intrinsic survival pathway involving PI3′K and PKB is constitutively activated in DP16.1 cells and that this pathway effectively limits p53-dependent apoptosis.
The PTEN tumor suppressor gene (phosphatase and tensin homolog) was recently shown to be regulated at the transcriptional level by p53.52PTENencodes a lipid phosphatase that removes the 3′-phosphate on phosphatidylinositol-3,4,5-triphosphate (PIP3). PI3′K-dependent synthesis of PIP3 is required for the phosphorylation and activation of PKB by PDK1. By decreasing the level of PIP3, PTEN serves as a negative regulator of the PI3′K/PKB pathway. In DP16.1/p53ts cells, activation-specific phosphorylation of PKB was reduced at time points coincident with maximal p53-dependent PTEN induction.52 This observation raised the possibility that EPO might promote survival by maintaining PKB in its phosphorylated and activated state. The blot shown in Figure 5C demonstrates that PKB phosphorylation was reduced at 7 hours following p53 activation at 32°C and that this reduction was unaffected by EPO. Hence, EPO does not appear to act through PKB.
To confirm that the protection afforded by EPO is not mediated through the PI3′K/PKB pathway, we treated DP16.1/p53ts cells with EPO at 32°C in the presence of increasing concentrations of the LY294002 inhibitor (Figure 5D). As expected, the extent of p53-dependent apoptosis increased on addition of LY294002. Nevertheless, EPO was still able to reduce apoptosis and promote cell survival. EPO led to a 65% reduction of p53-mediated cell death at 0 μM and 2 μM LY294002, and a 55% reduction of apoptosis in the presence of 10 μM LY294002 (Figure 5D). Hence, EPO retains the ability to protect DP16.1/p53ts cells from p53-dependent cell death in the presence of LY294002.
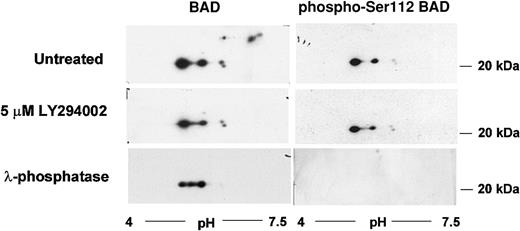
The proapoptotic protein BAD is phosphorylated on multiple serine residues. The PKB-mediated phosphorylation on Ser136 results in its inactivation. We were interested, therefore, in determining whether the intrinsic PI3′K/PKB-dependent survival pathway acts through BAD. We performed an analysis of BAD protein isoforms by 2-dimensional gel electrophoresis and Western blotting. At least 7 BAD protein isoforms were detected in proliferating DP16.1/p53ts cells (Figure 6). Three of these isoforms were lost when cells were treated with the PI3′K inhibitor LY294002 or with λ-phosphatase indicating that that they could represent PKB-dependent BAD phospho-forms (Figure 6). Commercially available Ser136 phospho-specific BAD antibodies did not detect any form of BAD in DP16.1 samples nor in other samples that served as positive controls. Hence, the identification of these phospho-forms remains tentative. We were able to show, however, that a Ser112 phospho-specific BAD antibody recognized the remaining 4 BAD protein isoforms and not the 3 LY294002-sensitive isoforms (Figure 6). Ser112 is believed to be targeted for phosphorylation by a MEK-dependent pathway. These data are consistent with the possibility that BAD is phosphorylated and inactivated by the intrinsic survival pathway.
BAD protein phosphorylation by the PI3′K/PKB pathway.
DP16.1/p53ts cells were left untreated or treated with LY294002 (5 μM) for 60 minutes. Cell extracts were prepared and analyzed by 2-dimensional gel electrophoresis followed by Western blot analysis as described in “Materials and methods.” A portion of the untreated DP16.1/p53ts extract was incubated with λ-phosphatase (20 U/μL) prior to isoelectric focusing in the first dimension and SDS-PAGE in the second dimension. BAD proteins were visualized by immunoblotting with antibodies against BAD or with phospho-specific antibodies recognizing Ser112-phosphorylated BAD.
BAD protein phosphorylation by the PI3′K/PKB pathway.
DP16.1/p53ts cells were left untreated or treated with LY294002 (5 μM) for 60 minutes. Cell extracts were prepared and analyzed by 2-dimensional gel electrophoresis followed by Western blot analysis as described in “Materials and methods.” A portion of the untreated DP16.1/p53ts extract was incubated with λ-phosphatase (20 U/μL) prior to isoelectric focusing in the first dimension and SDS-PAGE in the second dimension. BAD proteins were visualized by immunoblotting with antibodies against BAD or with phospho-specific antibodies recognizing Ser112-phosphorylated BAD.
EPO stimulation leads to increased expression of Bcl-XL
Overexpression of Bcl-2 and its family member Bcl-XLhave been shown to overcome p53-induced apoptosis.53-55 We were unable to detect Bcl-2 expression in DP16.1/p53ts cells. To determine if endogenous Bcl-XL expression was increased in DP16.1/p53ts cells in response to EPO stimulation, cell extracts were prepared at 8, 16, and 24 hours after p53 was activated at 32°C and subjected to Western blotting. We found that Bcl-XL protein expression was not markedly influenced by p53 activation at 32°C. A gradual accumulation of Bcl-XL protein was observed after addition of EPO reaching approximately 5-fold after 24 hours (Figure7A). This increase in Bcl-XLprotein was associated with only a 1.5-fold increase inBcl-XL mRNA (data not shown). Hence, in DP16.1/p53ts cells, Bcl-XL expression appears to be regulated primarily through a posttranscriptional mechanism in response to EPO. Bax protein and RNA expression increased approximately 2-fold in response to p53 activation and these levels were not influenced by EPO (Figure 7A and data not shown).
Bcl-XL and Bax protein expression in DP16.1/p53ts cells.
(A) DP16.1/p53ts cells were cultured at 32°C in the presence or absence of EPO for the times indicated. Protein extracts were fractionated by SDS-PAGE and immunoblotted separately with antibodies against Bcl-XL and Bax. Proteins were visualized by enhanced chemiluminescence. (B) DP16.1/p53ts cells were cultured at 32°C in the presence or absence of EPO for 24 hours. Cell extracts were prepared and analyzed by 2-dimensional gel electrophoresis followed by Western blot analysis using antibodies against Bcl-XL. A portion of the extract prepared from the EPO-stimulated cells was incubated with λ-phosphatase (20 U/μL) prior to analysis. The arrow in the top panel points to the more slowly migrating isoform of Bcl-XL that is lost on EPO stimulation.
Bcl-XL and Bax protein expression in DP16.1/p53ts cells.
(A) DP16.1/p53ts cells were cultured at 32°C in the presence or absence of EPO for the times indicated. Protein extracts were fractionated by SDS-PAGE and immunoblotted separately with antibodies against Bcl-XL and Bax. Proteins were visualized by enhanced chemiluminescence. (B) DP16.1/p53ts cells were cultured at 32°C in the presence or absence of EPO for 24 hours. Cell extracts were prepared and analyzed by 2-dimensional gel electrophoresis followed by Western blot analysis using antibodies against Bcl-XL. A portion of the extract prepared from the EPO-stimulated cells was incubated with λ-phosphatase (20 U/μL) prior to analysis. The arrow in the top panel points to the more slowly migrating isoform of Bcl-XL that is lost on EPO stimulation.
To investigate if EPO might control the expression or activation of Bcl-XL through posttranslational changes, we performed an analysis of Bcl-XL protein isoforms by 2-dimensional gel electrophoresis and Western blotting (Figure 7B). Exposure of the cells to EPO resulted in loss of a more slowly migrating form of Bcl-XL (in the second SDS-PAGE dimension) and the appearance of 3 novel, phosphatase-sensitive isoforms (in the first isoelectric-focusing [IEF] dimension). Together, these findings are consistent with the view that Bcl-XLexpression in DP16.1 cells is posttranscriptionally regulated in response to EPO by both phosphorylation-dependent and -independent mechanisms. Moreover, they raise the possibility that Bcl-XL may be an important mediator of EPO-dependent cell rescue. The biochemical basis and functional consequences of these Bcl-XL modifications are being investigated.
NFκB is not required for EPO-induced survival signaling
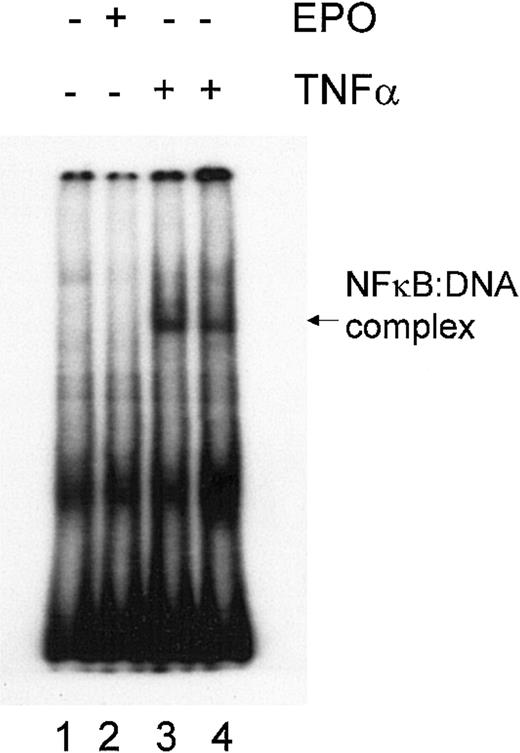
The transcription factor NFκB is an important component of cell survival signaling pathways in many cell types and can be activated by various cytokines. In neurons, NFκB activation appears to be involved in EPO-mediated signaling and in EPO-mediated neuroprotection.56 To evaluate the potential role of NFκB in mediating protection by EPO, we performed an electrophoretic mobility shift assay (EMSA) to measure the DNA-binding activity of NFκB in DP16.1/p53ts cells grown with or without EPO. Nuclear extracts prepared from cells treated with TNF-α for 15 and 30 minutes served as positive controls (Figure 8). No DNA-binding activity of NFκB was detected before or after EPO stimulation of the cells. These results indicate that NFκB is not constitutively activated in DP16.1/p53ts cells; moreover, NFκB is not activated after EPO stimulation. Thus, it is unlikely that NFκB is involved in the inhibition of p53-dependent apoptosis.
Lack of NFκB activation in response to EPO.
DP16.1/p53ts cells were left untreated (lane 1), were treated with EPO for 15 minutes (lane 2), or were treated with TNF-α (20 ng/mL) for 15 minutes (lane 3) or 30 minutes (lane 4). NFκB activation was determined using a gel mobility shift assay.
Lack of NFκB activation in response to EPO.
DP16.1/p53ts cells were left untreated (lane 1), were treated with EPO for 15 minutes (lane 2), or were treated with TNF-α (20 ng/mL) for 15 minutes (lane 3) or 30 minutes (lane 4). NFκB activation was determined using a gel mobility shift assay.
Discussion
The evasion of apoptosis, through the activation of survival pathways or through the inactivation of proapoptotic pathways, plays an important role in the development of cancer, and is now widely regarded as a hallmark of malignancy. A number of cytokines have been shown to regulate cell growth by promoting survival and in this study we have investigated the mechanism through which EPO prevents p53-dependent apoptosis. The activation of p53 in DP16.1/p53ts cells at 32°C results in cell cycle blockade in G1 and in apoptosis. EPO prevented p53-dependent apoptosis but had no effect on p53-dependent G1 arrest. Importantly, EPO did not interfere with the transactivation function of p53. This indicates that EPO is not targeting p53 directly but rather that it is activating a survival pathway that counteracts apoptosis initiated by p53. The p53-dependent apoptosis was accompanied by disruption of mitochondrial membrane integrity reflected by a decrease in mitochondrial membrane potential, an increase in intracellular free calcium, and the release of mitochondrial cytochrome c into the cytosol. EPO prevented all of these changes including the subsequent activation of caspases. Hence, EPO acts at an early stage to preserve mitochondrial integrity and to protect cells from p53-dependent apoptosis. This experimental model is intriguing because the EPO-independent SFFVP-transformed cells used in this study retain the ability to respond to EPO. The activation of EPO-R molecules by EPO leading to cell survival is clearly different from the mitogenic activation of these same receptors by the SFFVP-encoded gp55.
We have shown that JAK2 is phosphorylated in response to EPO and that JAK2 activation is necessary for EPO-mediated rescue of p53-induced apoptosis. This is consistent with the fundamental role of JAK2 in EPO signaling. To begin to identify the components of the JAK2-dependent signaling pathway important for survival, we examined STAT family members. STAT5, but not STAT1 or STAT3, was phosphorylated in response to EPO treatment. STAT5 activation was confirmed by its sequence-specific DNA-binding activity in the nuclear extract of DP16.1/p53ts cells treated with EPO. Disruption of endogenous STAT5 function by transient expression of DN STAT5 protein, however, did not interfere with the ability of EPO to promote survival. Hence, STAT5 appears to be dispensable for EPO-mediated survival. Using an entirely different experimental model based on cell lines that express various truncated forms of the EPO-R, Quelle et al12 reported that the ability of EPO to block apoptosis induced by γ irradiation was similarly dependent on JAK2 and independent of STAT5 activation. Our results provide a more direct assessment of JAK2 and STAT5 involvement in EPO-mediated survival using an experimental model where apoptosis is strictly dependent on p53.
We observed an increase in Bcl-XL protein expression and posttranslational modification of Bcl-XL protein in response to EPO. These findings raise the possibility that EPO-dependent signaling pathways may mediate survival through activation of Bcl-XL. The increase in protein expression was not accompanied by a similar increase inBcl-XL mRNA expression. Although there is general agreement that EPO regulates Bcl-XL expression in erythroid cells, there is uncertainty about the underlying mechanism. Our results clearly indicate that Bcl-XLexpression is regulated posttranscriptionally in DP16.1/p53ts cells; however, they do not exclude the possibility of transcriptional regulation. The importance of elevated endogenous Bcl-XLexpression in rescuing DP16.1/p53ts cells from p53-dependent apoptosis remains to be established.
In contrast to the induced activation of JAK2 and STAT5 by EPO, we found that JAK1 and PI3′K were constitutively activated in DP16.1/p53ts cells. JAK1 deficiency in mice leads to severely reduced numbers of thymocytes, pre-B cells, and mature T and B lymphocytes but does not lead to alterations in the development of other hematopoietic lineages; erythropoiesis is normal in JAK1-deficient mice.57 Despite the fact that JAK1 is not required for normal erythroid development and maturation, constitutive activation of JAK1 has been reported in certain transformed cells and can result in cytokine-independent mitogenic signals.58,59 Constitutive activation of JAK1 in Friend virus–transformed erythroleukemia cells was reported previously.45 It is not clear how JAK1 becomes activated in these transformed erythroid cells. One possibility is that the SFFVP-encoded gp55 protein binds to the EPO-R and induces a conformational change in the receptor that is different from the dimerization induced by EPO/EPO-R interaction. In this regard, it is pertinent to note that the Friend virus susceptibility 2(Fv2) locus encodes a truncated form of the Stk receptor tyrosine kinase (Sf-stk) and it has been suggested that Sf-stk is required for gp55-mediated activation of the EPO-R.60,61gp55 can bind to Sf-stk resulting in constitutive tyrosine phosphorylation and activation of Sf-stk.62 Hence, the recruitment of accessory signaling molecules by the activated EPO-R may be different in the presence of bound Sf-stk and gp55. A second possibility to explain the constitutive activation of JAK1 is that DP16.1 cells may have undergone secondary genetic changes after viral infection either in vivo during the natural course of virus-induced erythroleukemia or in culture during establishment of the cell line.
In erythroid cells, PI3′K is activated in response to EPO stimulation. Here we report that the PI3′K/PKB pathway is constitutively activated in SFFVP-transformed DP16.1/p53ts cells. Constitutive activation of PI3′K has been reported in other EPO-independent erythroid cell lines.63,64 Our results demonstrate that PI3′K and PKB represent components of an intrinsic survival pathway that effectively limits p53-dependent apoptosis in DP16.1/p53ts cells. Inhibition of the PI3′K pathway with LY294002 or wortmannin enhanced p53-dependent cell death. These inhibitors had no detectable effect, at least in the short term, when added to cultures of p53− DP16.1 cells or DP16.1/p53ts cells maintained at 37°C, suggesting that these cells are not dependent on the activated PI3′K pathway for their survival in the absence of extracellular stimuli or p53 activation. Our results are consistent with those of Sabbatini and McCormick65 who showed that ectopic expression of constitutively active PI3′K or PKB delayed the onset of p53-mediated apoptosis in baby rat kidney cells transformed by adenovirus E1A and p53ts. PKB mediates survival through phosphorylation of numerous cellular proteins including BAD. Using 2-dimensional gel electrophoresis, we identified at least 7 BAD protein isoforms in DP16.1/p53ts cells. Three of these isoforms were sensitive to phosphatase treatment and to the PI3′K inhibitor LY294002. These data indicate that the intrinsic PI3′K/PKB pathway that is constitutively active in DP16.1 cells results in BAD phosphorylation.
It is possible that p53 activation initiates an apoptotic program that simply overwhelms the intrinsic survival pathway and tips the balance toward cell death. Alternatively, p53-dependent apoptosis may require active suppression of the intrinsic survival pathway. The recent demonstration that p53 can activate transcription ofPTEN supports the latter possibility.52We demonstrate that pharmacologic suppression of the PI3′K/PKB pathway is not sufficient to initiate cell death in the absence of p53 activation in DP16.1/p53ts cells. Moreover, we have shown previously that the induction of the p53-responsive gene, Pidd, is required for apoptosis in these cells.39 Together, these results highlight the complexity of p53-mediated, transcriptionally dependent apoptosis and suggest that it involves not only the activation of proapoptotic gene(s) but also the attenuation of intrinsic PI3′K-dependent survival signals.
The ability of EPO to inhibit p53-dependent apoptosis was not abrogated by pharmacologic inhibition of PI3′K, suggesting that activation of this pathway is not the primary mechanism by which EPO rescues cells from p53-dependent death. The simplest model to account for these observations is the existence of 2 distinct survival pathways: an intrinsic constitutively active survival pathway dependent on PI3′K/PKB and an extrinsic EPO-inducible pathway dependent on JAK2. The demonstration that STAT5 and PI3′K are dispensable for EPO-mediated survival of DP16.1/p53ts cell following p53 activation indicates that other components in the JAK2 signaling pathway are important for survival.
The authors wish to thank Weili Ma for excellent technical assistance.
Prepublished online as Blood First Edition Paper, July 18, 2002; DOI 10.1182/blood-2002-02-0504.
Supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society (S.B.) and by the Canadian Institutes of Health Research (S.B. and D.L.B.). D.L.B. is a research scientist of the National Cancer Institute of Canada and L.B. is supported by a postgraduate scholarship from the Natural Sciences and Engineering Research Council of Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Samuel Benchimol, Ontario Cancer Institute, 610 University Ave, Toronto, ON M5G 2M9, Canada; e-mail:benchimo@uhnres.utoronto.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal