Thrombotic thrombocytopenic purpura (TTP) is a devastating thrombotic disorder caused by widespread microvascular thrombi composed of platelets and von Willebrand factor (VWF). The disorder is associated with a deficiency of the VWF-cleaving metalloprotease, ADAMTS-13, with consequent accumulation of ultralarge (UL) VWF multimers in the plasma. ULVWF multimers, unlike plasma forms of VWF, attach spontaneously to platelet GP Ibα, a component of the GP Ib-IX-V complex. We have found that ULVWF multimers secreted from stimulated endothelial cells (ECs) remained anchored to the endothelial surface where platelets and Chinese hamster ovary cells expressing the GP Ib-IX-V complex attached to form long beads-on-a-string structures in the presence of fluid shear stresses in both the venous (2.5 dyne/cm2) and arterial (20 and 50 dyne/cm2) ranges. Although measurement of the activity of the ADAMTS-13 VWF-cleaving metalloprotease in vitro requires prolonged incubation of the enzyme with VWF under nonphysiologic conditions, EC-derived ULVWF strings with attached platelets were cleaved within seconds to minutes in the presence of normal plasma (containing approximately 100% ADAMTS-13 activity) or in the presence of partially purified ADAMTS-13. By contrast, the strings persisted for the entire period of perfusion (10 minutes) in the presence of plasma from patients with TTP containing 0% to 10% ADAMTS-13 activity. These results suggest that cleavage of EC-derived ULVWF multimers by ADAMTS-13 is a rapid physiologic process that occurs on endothelial cell surfaces.
Introduction
von Willebrand factor (VWF) is a large plasma glycoprotein involved in several processes key to normal hemostasis. VWF that becomes affixed to the subendothelial matrix mediates the initial adhesion of platelets at sites of endothelial denudation by binding the platelet GP Ib-IX-V complex. VWF also plays a crucial role in the fluid-phase aggregation of platelets that occurs at sites of very high shear stress, initiating platelet activation through a shear-induced interaction with GP Ib-IX-V and also serving as the glue that holds the platelets together by binding the activated form of integrin αIIbβ3. VWF is also involved in maintenance of the soluble coagulation system, binding coagulation factor VIII and thereby stabilizing it from degradation.
VWF is synthesized in only 2 sites: in megakaryocytes, where it is stored in α-granules that are later partitioned into platelets, and in endothelial cells, where it is either secreted constitutively or stored in granules called Weibel-Palade bodies for secretion on endothelial stimulation. VWF undergoes a number of intracellular processing steps after synthesis of the 2791–amino acid precursor protein.1 In the endoplasmic reticulum, the precursors form disulfide-linked dimers through C-terminal cysteine residues. After transport to the Golgi, the dimers join through N-terminal disulfide bonds to form multimers, in a process requiring the participation of the 741–amino acid propeptide, which is then cleaved by a furinlike enzyme before trafficking of the VWF into storage granules.2,3 It is unknown what limits the growth of multimers, or what determines their ultimate length. After multimerization, a portion of the VWF is stored in the endothelial Weibel-Palade bodies or platelet α-granules, from where they are released on stimulation of the respective cells. Stored and newly secreted VWF is rich in ultralarge (UL) forms, which are hyperreactive in their capacity to bind the GP Ib-IX-V complex.4 On their secretion, ULVWF multimers are processed further, by limited proteolysis by a recently characterized plasma metalloprotease called ADAMTS-13.
ADAMTS-13 reduces the size of large and ultralarge5-7 VWF multimers to smaller forms in vitro by specifically cleaving the Y842/M843 peptide bond in the VWF A2 domain, generating 176-kDa and 140-kDa fragments that are found in the normal circulation.8-10 ADAMTS-13 activity is currently measured in vitro by using static assays that require prolonged incubation (up to 24 hours) under nonphysiologic conditions (low ionic strength buffer containing barium ion and urea or guanidine at a pH of 8-9).10 The inefficiency of this reaction suggests that the in vitro assays lack one or more conditions that allow rapid proteolytic cleavage of ULVWF multimers in vivo. The importance of this proteolytic processing is perhaps best illustrated by the severe consequences of ADAMTS-13 deficiency, which is associated with a severe thrombotic disorder known as thrombotic thrombocytopenic purpura (TTP).
TTP is characterized by microvascular thrombosis, consumptive thrombocytopenia, organ ischemia, and hemolytic anemia.11,12 The microthrombi are composed of platelets and von Willebrand factor (VWF).11 If left untreated, the disorder is rapidly progressive and almost uniformly fatal.
In the current study, we observed that ULVWF multimers secreted from endothelial cells (ECs) are anchored to the endothelial surface as extraordinarily long stringlike structures capable of binding platelets and Chinese hamster ovary (CHO) cells that express surface GP Ibα. These ULVWF multimeric strings were cleaved rapidly in the presence of normal plasma or partially purified ADAMTS-13, but not by plasma from any of the 14 patients with TTP.
Patients, materials, and methods
Platelet and plasma preparations
Under a protocol approved by the Institutional Review Board of the Baylor College of Medicine, blood was drawn from 34 healthy donors who had no history of thrombosis and had not ingested medications in the 2 weeks preceding phlebotomy. All donors signed consent forms before blood was drawn. The donor pool consisted of 19 women and 15 men, ranging in age from 22 to 56 years.
Blood was drawn into acid-citrate dextrose (ACD) anticoagulant (85 mM sodium citrate, 111 mM glucose, and 71 mM citric acid, 10% vol/vol). To isolate platelets, the whole blood was first centrifuged at 150g for 15 minutes at 24°C to obtain platelet-rich plasma (PRP), which was then centrifuged at 900g for 10 minutes to obtain platelets and platelet-poor plasma (PPP). The platelet pellets were washed once with a CGS buffer (13 mM sodium citrate, 30 mM glucose, and 120 mM sodium chloride, pH 7.0) and resuspended in Ca++, Mg++-free Tyrode buffer (138 mM sodium chloride, 5.5 mM glucose, 12 mM sodium bicarbonate, 2.9 mM potassium chloride, and 0.36 mM dibasic sodium phosphate, pH 7.4). PRP was also used in some experiments.
TTP patients
Plasma from 14 patients with TTP was obtained from both citrated and ACD blood (Table 1). Among them, 4 patients carried the diagnosis of familial chronic relapsing TTP of childhood and 10 carried the diagnosis of adult acquired idiopathic TTP. Of those with adult TTP, the presence of ADAMTS-13 inhibitors was documented in 3 patients. The VWF-cleaving activity in these patients' plasmas ranged from 0% to 10% as determined by a standardized static assay that used ULVWF from the supernatant of histamine-stimulated ECs as a substrate.10 13
Information about the TTP patients
| Patient code . | Sex . | Diagnosis . | ADAMTS-13 activity % static assay . | ADAMTS-13 inhibitor . |
|---|---|---|---|---|
| 1 | M | Familial TTP | 0 | − |
| 2 | M | Familial TTP | 0 | − |
| 3 | F | Familial TTP | 0 | − |
| 4 | M | Familial TTP | 0 | − |
| 5 | M | Adult acquired idiopathic TTP | 0 | − |
| 6 | F | Adult acquired idiopathic TTP | 0 | − |
| 7 | F | Adult acquired idiopathic TTP | 0 | + |
| 8 | M | Adult acquired idiopathic TTP | 10 | − |
| 9 | M | Adult acquired idiopathic TTP | 0 | − |
| 10 | F | Adult acquired idiopathic TTP | 0 | − |
| 11 | F | Adult acquired idiopathic TTP | 10 | − |
| 12 | M | Adult acquired idiopathic TTP | 0 | + |
| 13 | M | Adult acquired idiopathic TTP | 10 | + |
| 14 | F | Adult acquired idiopathic TTP | 0 | − |
| Patient code . | Sex . | Diagnosis . | ADAMTS-13 activity % static assay . | ADAMTS-13 inhibitor . |
|---|---|---|---|---|
| 1 | M | Familial TTP | 0 | − |
| 2 | M | Familial TTP | 0 | − |
| 3 | F | Familial TTP | 0 | − |
| 4 | M | Familial TTP | 0 | − |
| 5 | M | Adult acquired idiopathic TTP | 0 | − |
| 6 | F | Adult acquired idiopathic TTP | 0 | − |
| 7 | F | Adult acquired idiopathic TTP | 0 | + |
| 8 | M | Adult acquired idiopathic TTP | 10 | − |
| 9 | M | Adult acquired idiopathic TTP | 0 | − |
| 10 | F | Adult acquired idiopathic TTP | 0 | − |
| 11 | F | Adult acquired idiopathic TTP | 10 | − |
| 12 | M | Adult acquired idiopathic TTP | 0 | + |
| 13 | M | Adult acquired idiopathic TTP | 10 | + |
| 14 | F | Adult acquired idiopathic TTP | 0 | − |
− indicates negative; and +, positive for ADAMTS-13 inhibitors.
Endothelial culture
Under a protocol approved by the Institutional Review Board of the Baylor College of Medicine, endothelial cells were obtained from human umbilical veins (HUVECs) or arteries (HUAECs) as described previously.14 The umbilical cords were first washed with phosphate buffer (140 mM NaCl, 0.4 mM KCl, 1.3 mM NaH2PO4, 1.0 mM Na2HPO4, 0.2% glucose, pH 7.4) and then infused with a collagenase solution (0.02%; Invitrogen Life Technologies, Carlsbad, CA). After a 30-minute incubation at room temperature, the cords were rinsed with 100 mL phosphate buffer. Eluates containing endothelial cells were centrifuged at 250g for 10 minutes. The cell pellets were resuspended in Medium 199 (Invitrogen Life Technologies) containing 20% heat-inactivated fetal calf serum and 0.2 mM L-glutamine. The endothelial cells were then plated on a culture dish coated with 1% gelatin and grown until confluent (3-5 days).
Endothelial cells from human coronary artery (HCAEC) and human microvasculature (HMVEC) were purchased from Cambrex (East Rutherford, NJ) and cultured in endothelial growth medium containing bovine brain extract, human epidermal growth factor, hydrocortisone, GA-1000 (gentamicin and amphotericin B), and fetal bovine serum (2%). Cells were maintained according to the manufacturer's instructions and used within 4 to 5 passages.
We also used a human endothelial cell line (ECV304) defective in VWF synthesis in some of the studies. This cell line is derived from human umbilical vein endothelial cells15 and was shown to be of endothelial origin by the presence of Weibel-Palade bodies and positive staining for the endothelial marker PHM5.
Endothelial cells were activated with 25 μM histamine (Sigma-Aldrich, St Louis, MO) for 10 minutes at room temperature before the perfusion experiments.
Parallel-plate flow chamber
The formation and cleavage of VWF strings was studied under flow in a parallel-plate flow chamber system and observed by phase-contrast video microscopy. The parallel-plate flow chamber is composed of a polycarbonate slab, a silicon gasket, and a glass coverslip.16 The endothelial cells are grown as a monolayer on the coverslip.17 A syringe pump connected to the outlet port draws the platelet suspension through the chamber at defined flow rates to generate specific wall shear stresses. Shear stresses of 2.5, 20, and 50 dyne/cm2 were used in the experiments reported here. The chamber was kept at 37°C with a thermostatic air bath during the experiments.
The assembled parallel-plate flow chamber was mounted onto an inverted-stage microscope (Eclipse TE300; Nikon, Garden City, NY) equipped with a high-speed digital camera (Model Quantix; Photometrics, Tucson, AZ). Acquired images were analyzed offline by using MetaMorph software (Universal Images, West Chester, PA). The strings were quantitated by counting individual strings in 20 continuous view fields (× 400).
ADAMTS-13
ADAMTS-13 was purified to the diethylaminoethyl (DEAE) column step from factor VIII/VWF concentrate by the method described earlier.6 Purified ADAMTS-13 slowly cleaved VWF in overnight incubation in the presence of 1 M urea, yielding only 176-kDa and 140-kDa fragments as detected on reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels.
Antibodies
The anti-VWF monoclonal antibody 6G1, which blocks the binding of GP Ibα, was a kind gift from Michael C. Berndt of the Baker Medical Research Institute, Melbourne, Victoria, Australia. AK2, a GP Ibα monoclonal antibody that blocks VWF binding, was purchased from RDI (Flanders, NJ). Polyclonal anti-VWF was purchased from DAKO (Carpinteria, CA).
Results
CHO cells expressing the GP Ib-IX complex align in stringlike structures on the surface of histamine-stimulated HUVECs
While investigating the role of the GP Ib-IX complex in platelet-endothelial interactions, we observed that when CHOαβIX cells (expressing the GP Ib-IX-V complex) were perfused over histamine-stimulated HUVECs (at 2.5 dyne/cm2 shear stress), adherent cells sometimes aligned in the direction of flow, forming beads-on-a-string structures that moved back and forth in the fluid stream as if connected by an invisible string (Figure1A). There were typically between 5 and 10 cells per string. In contrast, when CHOβIX cells, which lack GP Ibα, were perfused over the activated endothelial cells, they neither adhered nor formed strings (Figure 1B). Further, formation of the stringlike structures required endothelial activation.
Adhesion of CHO cells on stimulated endothelial cells.
CHO cells expressing either the GP Ib-IX-V complex (CHOαβIX cells) or a partial complex lacking GP Ibα (CHOβIX cells) were perfused at a constant shear stress of 2.5 dyne/cm2 over a monolayer of HUVECs. CHOαβIX cells (A), but not CHOβIX cells (B), adhered to endothelial cells in a stringlike fashion. The figure is representative of 5 separate experiments. Original magnifications × 200.
Adhesion of CHO cells on stimulated endothelial cells.
CHO cells expressing either the GP Ib-IX-V complex (CHOαβIX cells) or a partial complex lacking GP Ibα (CHOβIX cells) were perfused at a constant shear stress of 2.5 dyne/cm2 over a monolayer of HUVECs. CHOαβIX cells (A), but not CHOβIX cells (B), adhered to endothelial cells in a stringlike fashion. The figure is representative of 5 separate experiments. Original magnifications × 200.
Platelets also adhere to long strings attached to stimulated endothelial cells
To determine the potential physiologic relevance of the GP Ib-interactive strings, we performed the same experiments with washed platelets. Washed human platelets perfused over a monolayer of histamine-stimulated HUVECs at 2.5 dyne/cm2 shear stress adhered to and formed similar beads-on-a-string structures on the endothelial surface (Figure 2Ai). These structures only formed on the stimulated (Figure 2Ai) but not unstimulated ECs (Figure 2Aii). They moved together back and forth in the fluid stream (Figure 2B). In some cases, the strings were astonishingly long, holding tens or even hundreds of platelets, and having lengths up to several millimeters (Figure 2C). We did not observe the formation of VWF-platelet strings on the endothelial surface that had previously been perfused with normal plasma (data not shown).
Platelet adhesion on stimulated endothelial cells.
Washed normal platelets were perfused at a constant shear stress of 2.5 dyne/cm2 over a monolayer of HUVECs. (A) Long lines of platelets aligned as “beads on a string” above histamine-stimulated (i), but not unstimulated (ii), HUVECs. Original magnification × 400. (B) Platelet strings repeatedly stretch and relax in the fluid stream. (C) The length of strings varied but could be several millimeters long (bar = 100 μm). The figure is representative of 86 separate experiments using HUVECs and platelets from 34 different donors.
Platelet adhesion on stimulated endothelial cells.
Washed normal platelets were perfused at a constant shear stress of 2.5 dyne/cm2 over a monolayer of HUVECs. (A) Long lines of platelets aligned as “beads on a string” above histamine-stimulated (i), but not unstimulated (ii), HUVECs. Original magnification × 400. (B) Platelet strings repeatedly stretch and relax in the fluid stream. (C) The length of strings varied but could be several millimeters long (bar = 100 μm). The figure is representative of 86 separate experiments using HUVECs and platelets from 34 different donors.
Long strings are ULVWF multimers
To determine whether the strings were truly composed of ULVWF, we evaluated platelet attachment to the strings in the presence of monoclonal antibodies that block the GP Ibα–VWF interaction: either 6G1, which binds VWF, or AK2, which binds GP Ibα. Under conditions identical to those used to induce platelet binding to the endothelial strings in the absence of antibody, both antibodies completely inhibited platelet binding (Figure 3A-B), whereas a control immunoglobulin G (IgG) did not (Figure 3C). Experiments with a transformed endothelial cell line (ECV304) that is defective in VWF synthesis provided further evidence that the strings are made up of VWF, as platelets did not form stringlike structures on histamine-stimulated ECV304 (Figure 3D).15 Finally, polystyrene beads coated with a polyclonal anti-VWF antibody also attached to the long strings when perfused over stimulated ECs (Figure3E), confirming that the strings are indeed composed of the EC-derived ULVWF.
The strings were formed by tethering platelets to ultralarge VWF multimers via GP Ibα of the GP Ib-X complex.
The formation of strings with adherent platelets was blocked by monoclonal antibodies against either VWF (6G1; A) or GP Ibα (AK2; B) but not by a control mouse IgG (C). Original magnifications × 400. The platelet strings did not form above the surface of endothelial cells incapable of VWF synthesis (ECV304; D). Polystyrene beads coated with a polyclonal anti-VWF antibody also adhered in strings on activated HUVECs (E). Each figure is representative of 4 to 6 separate experiments.
The strings were formed by tethering platelets to ultralarge VWF multimers via GP Ibα of the GP Ib-X complex.
The formation of strings with adherent platelets was blocked by monoclonal antibodies against either VWF (6G1; A) or GP Ibα (AK2; B) but not by a control mouse IgG (C). Original magnifications × 400. The platelet strings did not form above the surface of endothelial cells incapable of VWF synthesis (ECV304; D). Polystyrene beads coated with a polyclonal anti-VWF antibody also adhered in strings on activated HUVECs (E). Each figure is representative of 4 to 6 separate experiments.
Influence of fluid shear stress and endothelial cell type on the formation of ULVWF multimeric strings
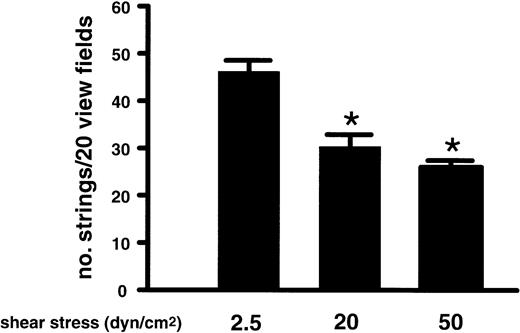
The experiments until now examined string formation at venular shear stresses. It was, therefore, important to determine whether the platelets would attach and also form beads-on-a-string structures on ULVWF at arterial or arteriolar shear stresses, where the GP Ib-VWF interaction is believed to be more important for achieving hemostasis and also where the platelet-VWF thrombi characteristic of TTP are known to form. Washed platelets were perfused over the stimulated endothelial cells under shear stresses of 20 or 50 dyne/cm,2 both of which are often encountered in the arterial circulation.18The VWF strings also formed under these conditions (Figure4), although the numbers of strings formed on the endothelial surface were lower under both 20 and 50 dyne/cm2 of shear stress than at 2.5 dyne/cm,2a shear stress normally found in venules. These results demonstrate that the ULVWF strings form under conditions of both low-shear venous flow and high-shear arterial flow.
Formation of ULVWF strings under various conditions of shear stress.
Washed platelets were perfused over histamine-stimulated HUVECs at flow rates that generated fluid shear stresses of 2.5, 20, or 50 dyne/cm2. Formation of the ULVWF strings with adherent platelets was evaluated at the end of 2 minutes of perfusion and quantified in 20 continuous view fields (× 400). The results are expressed as means ± SEM, * Student t test, n = 4,P < .05.
Formation of ULVWF strings under various conditions of shear stress.
Washed platelets were perfused over histamine-stimulated HUVECs at flow rates that generated fluid shear stresses of 2.5, 20, or 50 dyne/cm2. Formation of the ULVWF strings with adherent platelets was evaluated at the end of 2 minutes of perfusion and quantified in 20 continuous view fields (× 400). The results are expressed as means ± SEM, * Student t test, n = 4,P < .05.
To determine if the formation of the VWF strings is unique to HUVECs, we have also tested cells from arterial endothelium. Primary cultures of human endothelial cells from umbilical artery or from early passages (4-5 passages) of coronary artery endothelium and lung microvascular endothelium were stimulated with 25 μM histamine to induce release of ULVWF. Perfused washed human platelets adhered to attached ULVWF multimers on each of the different endothelial cell types (Figure5). The numbers of ULVWF strings that formed on histamine-stimulated primary cultures of endothelial cells from umbilical veins or arteries were significantly greater than those formed on the coronary or microvascular endothelial cells (Figure 5). It is unknown whether this decrease is a result of the higher passage number of the latter cell types, a well-known determinant of VWF production, or represents an inherent difference in VWF production between the endothelia of different vascular beds.
Formation of ULVWF strings on different types of endothelial cells.
Washed normal platelets were perfused over histamine-stimulated endothelial cells from human umbilical veins (HUVECs) and arteries (HUAECs), as well as from coronary arteries (HCAECs) and lung microvasculature (HMVECs), under a shear stress of 2.5 dyne/cm2. Formation of ULVWF strings with adherent platelets was evaluated after 2 minutes of perfusion and quantified in 20 continuous view fields (× 400). The results are expressed as means ± SEM, * Student t test, n = 7,P < .05.
Formation of ULVWF strings on different types of endothelial cells.
Washed normal platelets were perfused over histamine-stimulated endothelial cells from human umbilical veins (HUVECs) and arteries (HUAECs), as well as from coronary arteries (HCAECs) and lung microvasculature (HMVECs), under a shear stress of 2.5 dyne/cm2. Formation of ULVWF strings with adherent platelets was evaluated after 2 minutes of perfusion and quantified in 20 continuous view fields (× 400). The results are expressed as means ± SEM, * Student t test, n = 7,P < .05.
Long ULVWF strings are cleaved by ADAMTS-13 present in normal plasma
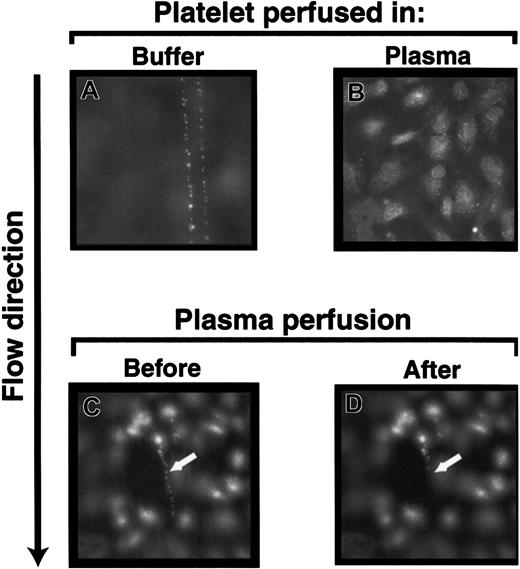
In the previous experiments, the platelets that adhered to the ULVWF strings were in plasma-free buffer. When platelet-rich plasma instead of washed platelets in buffer was perfused over stimulated HUVECs, the ULVWF strings with adherent platelets either never formed or were cleaved instantaneously (Figure6B). In addition, when preformed ULVWF strings with adherent platelets were perfused with platelet-poor plasma from any of the 34 healthy donors, all strings were cleaved within 2 minutes (Figure 6C-D). The strings were usually cleaved in 1 or 2 steps at or near their upstream attachment sites, releasing the ULVWF multimers with adherent platelets. We did not observe the reformation of the platelet-VWF strings on the endothelial surface that had previously been treated with either normal plasma or partially purified ADAMTS-13 (data not shown).
Normal plasma contains VWF-cleaving activity.
The ULVWF strings with adherent platelets formed when washed platelets (A), but not platelet-rich plasma (B), were perfused over histamine-stimulated HUVECs. The ULVWF strings with adherent platelets that formed by perfusing washed platelets (C; arrow) were cleaved when normal platelet-poor plasma was perfused subsequently (D; arrow). The figure is representative of 28 separate experiments. Original magnifications × 400.
Normal plasma contains VWF-cleaving activity.
The ULVWF strings with adherent platelets formed when washed platelets (A), but not platelet-rich plasma (B), were perfused over histamine-stimulated HUVECs. The ULVWF strings with adherent platelets that formed by perfusing washed platelets (C; arrow) were cleaved when normal platelet-poor plasma was perfused subsequently (D; arrow). The figure is representative of 28 separate experiments. Original magnifications × 400.
The rapid cleavage of the ULVWF-platelet strings in the presence of normal plasma suggested that this might be the action of the recently characterized metalloprotease, ADAMTS-13. To examine this possibility more closely, we perfused a buffered solution containing ADAMTS-13 through the chamber containing preformed ULVWF-platelet strings. The strings were cleaved rapidly from the HUVEC surface at a rate similar to that observed with normal plasma (Figure7). The VWF-cleaving activity of the partially purified protease was determined as previously described.8
Cleavage of the ULVWF strings with adherent platelets by normal plasma, TTP plasma, or ADAMTS-13.
Washed normal platelets were perfused over histamine-stimulated HUVECs at 2.5 dyne/cm2 shear stress for 2 minutes to allow formation of ULVWF strings with adherent platelets. Then, either Tyrode buffer, normal plasma, TTP plasma, or partially purified VWF-cleaving metalloprotease was perfused for an additional 2 minutes (for normal plasma and purified VWF-cleaving metalloprotease) or 10 minutes (for buffer or TTP plasma). The number of ULVWF strings with adherent platelets was quantified in 20 continuous view fields (× 400), and the results are expressed as means ± SEM.
Cleavage of the ULVWF strings with adherent platelets by normal plasma, TTP plasma, or ADAMTS-13.
Washed normal platelets were perfused over histamine-stimulated HUVECs at 2.5 dyne/cm2 shear stress for 2 minutes to allow formation of ULVWF strings with adherent platelets. Then, either Tyrode buffer, normal plasma, TTP plasma, or partially purified VWF-cleaving metalloprotease was perfused for an additional 2 minutes (for normal plasma and purified VWF-cleaving metalloprotease) or 10 minutes (for buffer or TTP plasma). The number of ULVWF strings with adherent platelets was quantified in 20 continuous view fields (× 400), and the results are expressed as means ± SEM.
ULVWF strings are not cleaved in the presence of plasma from TTP patients
Because defects in ADAMTS-13 metalloprotease have been proposed as the cause of TTP,12 we tested the capacity of plasma from patients with TTP to cleave the VWF strings. Citrated plasma samples from 14 patients with TTP were evaluated. Each plasma sample contained less than 10% of normal VWF-cleaving metalloprotease activity, as determined by a static assay described previously.19 20Washed normal platelets were suspended in the individual TTP plasma samples and perfused over histamine-stimulated HUVECs. In contrast to the rapid cleavage of ULVWF multimers in the presence of normal plasma (Figure 8B) or partially purified ADAMTS-13 (Figure 7), the ULVWF multimeric strings with adherent platelets were not cleaved in the presence of TTP plasma during the entire 10-minute period of monitoring (Figures 7 and 8A).
Plasma from patients with TTP did not cleave the ULVWF strings with adherent platelets.
The ULVWF strings with adherent platelets remained after 10 minutes of perfusion at 2.5 dyne/cm2 of plasma from patients with TTP (A), whereas the strings were rapidly cleaved when normal plasma was perfused (B). The figure is representative of separate experiments carried out using plasma from 14 patients with TTP or 34 healthy donors. Original magnifications × 400.
Plasma from patients with TTP did not cleave the ULVWF strings with adherent platelets.
The ULVWF strings with adherent platelets remained after 10 minutes of perfusion at 2.5 dyne/cm2 of plasma from patients with TTP (A), whereas the strings were rapidly cleaved when normal plasma was perfused (B). The figure is representative of separate experiments carried out using plasma from 14 patients with TTP or 34 healthy donors. Original magnifications × 400.
When a mixture (1:1 ratio) of the partially purified ADAMTS-13 (or normal plasma) and plasma from 2 patients with adult acquired idiopathic TTP containing anti–ADAMTS-13 antibodies was perfused over the stimulated ECs, the VWF strings formed (Figure9C,E) on the endothelial surface at levels comparable to those perfused with buffer (Figure 9A). In contrast, deficiency in the ADAMTS-13 activity in plasma from 2 patients with familial TTP was corrected by mixing with normal plasma (1:1 volume ratio) or with partially purified ADAMTS-13 (Figure 9D-E).
Effect of TTP plasma on the ULVWF-cleaving activity of ADAMTS-13.
Washed platelets suspended in Tyrode buffer formed the stringlike structures on the stimulated endothelial surface (A), whereas the VWF-platelet strings were cleaved in the presence of purified ADAMTS-13 (B). ADAMTS-13 failed to cleave the VWF strings when it was mixed with plasma from acquired TTP containing anti–ADAMTS-13 antibody (E), but functioned normally when mixed with plasma from congenital TTP with deficient ADAMTS-13 activity (D). Panel E is the summary of multiple sample analyses, and results are expressed as means ± SEM, n = 3.
Effect of TTP plasma on the ULVWF-cleaving activity of ADAMTS-13.
Washed platelets suspended in Tyrode buffer formed the stringlike structures on the stimulated endothelial surface (A), whereas the VWF-platelet strings were cleaved in the presence of purified ADAMTS-13 (B). ADAMTS-13 failed to cleave the VWF strings when it was mixed with plasma from acquired TTP containing anti–ADAMTS-13 antibody (E), but functioned normally when mixed with plasma from congenital TTP with deficient ADAMTS-13 activity (D). Panel E is the summary of multiple sample analyses, and results are expressed as means ± SEM, n = 3.
Discussion
In the current study, we demonstrate that newly released ULVWF forms extremely long stringlike structures on the surface of stimulated endothelium under conditions of flow. These strings support the adhesion of platelets or GP Ib-IX complex–expressing CHO cells and are rapidly cleaved in the presence of normal plasma or partially purified ADAMTS-13 metalloprotease but not in the presence of plasma from patients with the microvascular thrombotic disorder TTP.
ULVWF multimers derived from ECs are hyperreactive and capable of interacting under static conditions with platelet GP Ib-IX-V complexes in the absence of ristocetin or botrocetin. ULVWF multimers are also more reactive with platelet receptors in the presence of high fluid shear stress. It had been postulated that this increased platelet reactivity of the ULVWF multimers resulted from a larger number of GP Ibα binding sites, sites contained within the A1 domain of VWF. However, our recent studies using optical tweezer technology demonstrated that the ULVWF multimers, in fact, form spontaneous high-strength bonds with GP Ibα under conditions in which the plasma forms of VWF fail to bind GP Ibα at all.21 The individual bonds between A1 domains within ULVWF multimers and GP Ibα were 1.5 times stronger than those between A1 domains in plasma VWF and GP Ibα in the presence of modulators. The hyperreactivity of EC-derived ULVWF multimers was also demonstrated in this study—rather than the rolling interaction observed when platelets in flowing blood adhere to plasma VWF,22-24 platelets that attached to ULVWF strings were firmly adherent and were never demonstrated to roll on the strings, even at shear stresses as high as 50 dyne/cm.2
One of the surprising findings of our current study was the extraordinary lengths of the ULVWF multimer strings that formed on the endothelial surface, some reaching several millimeters in length (Figure 2, for example). The length of these ULVWF multimers is much greater than that demonstrated for plasma-derived VWF by rotary shadowing electron microscopy.25 This finding may represent the true length of a single, covalently bound multimer—which in molecular dimensions would rival some of the largest biologic molecules known—or alternatively could result from noncovalent self-association of VWF multimers, either in the Weibel-Palade bodies or after secretion, as has been demonstrated for surface-associated plasma forms of VWF.26 It should be noted that we never observed the step-wise growth of the stringlike structures, as might be expected if additional multimers were being sequentially added from the newly released ULVWF. The difficulty in estimating the molecular masses of the largest multimeric forms of VWF by conventional means such as agarose gel electrophoresis renders estimation of ULVWF size problematic.
Whatever the true size of ULVWF multimers, they typically undergo ADAMTS-13–catalyzed proteolysis on their release from endothelial cells.6 To date, this process has only been studied by using in vitro static assays.8-10 With the use of these techniques, the VWF-cleaving metalloprotease activity in plasma requires prolonged incubation (up to 24 hours) with large or unusually large VWF multimers and nonphysiologic conditions. This finding suggests that conditions absent in the in vitro assay allow much more rapid cleavage in vivo, a conclusion supported by the rapid in vivo clinical response of TTP patients to infusion/exchange using plasma products containing ADAMTS-13.25
A recent report by Andre et al27 described GP Ibα-mediated platelet adhesion in vivo to mouse venular endothelium stimulated with either the calcium ionophore A23187 or histamine. This process was dependent on the release of stored VWF from the endothelial cells, as it did not occur in VWF-deficient mice. Of interest, platelet adhesion to the stimulated endothelium began within 15 seconds of stimulation and peaked after about 1 minute, with a subsequent rapid drop-off in the number of adherent platelets. This phenomenon may represent a process in which the capacity of plasma ADAMTS-13 to cleave newly released ULVWF multimers is overwhelmed, allowing the ULVWF multimers to remain transiently on the endothelial surface where they can support platelet adhesion. In contrast to our findings, Andre et al27 observed some of the platelets translocating on the VWF.
Our studies suggest possible mechanisms for rapid and efficient ULVWF multimer cleavage in vivo. In the absence of VWF-cleaving metalloprotease activity, ULVWF multimers from stimulated ECs formed long stringlike structures under shear stresses similar to those in both the venous and the partially obstructed arterial circulation. The strings were cleaved rapidly (seconds to 2 minutes) in the presence of plasma ADAMTS-13. This accelerated cleavage of ULVWF multimers in our system as compared with previously described static systems may result either from the presence of the endothelial cell surface, which could provide an anchor or cofactors for the metalloprotease, or from the presence of fluid shear stresses that impart tensile stretch to the EC-bound multimers. Even a low level of fluid shear stress may allow the ULVWF multimers to adopt an extended conformation that exposes the cleavage sites or, alternatively, optimizes EC or ULVWF binding of the metalloprotease. In either case, our studies suggest that rapid and efficient cleavage of EC-derived ULVWF multimers by ADAMTS-13 occurs on EC surfaces.
Failure to cleave ULVWF multimers has dangerous clinical consequences in TTP. The ULVWF multimer strings with adherent and aggregated platelets may occlude small blood vessels in situ. ULVWF-platelet clumps may also dislodge under high shear stress and obstruct smaller vessels downstream. Furthermore, platelets could be activated on adhesion and release proinflammatory substances with the potential to activate or damage endothelial cells.
The cleavage of ULVWF on the endothelial surface raises the possibility that ADAMTS-13 may have to anchor to the endothelial surface to be fully functional, although direct evidence for this mechanism is lacking. If anchorage is necessary for activity, defects in anchorage could inhibit ADAMTS-13 activity, with the static enzyme assay likely to report normal or moderately compromised VWF protease activity. In accord with this possibility, a report was recently published about a patient with a familial type of relapsing TTP and normal ADAMTS-13 activity in plasma.28 This patient may have a defect in ADAMTS-13 attachment to EC surfaces in vivo. One possible means by which ADAMTS-13 could attach to EC surfaces is through its thrombospondin-1–like domains interacting with endothelial CD36. This possibility would provide a pathophysiologic mechanism by which antibodies to CD36, a thrombospondin-1 receptor on stimulated ECs, could cause acquired TTP, as has been recently reported.29It is not yet known if toxin- or chemical-induced defects in the attachment of ADAMTS-13 to ECs contributes to the pathophysiology of the hemolytic-uremic syndrome or chemotherapy/transplantation-associated microangiopathy, which are other microvascular thrombotic microangiopathies not accompanied by severe reduction in plasma ADAMTS-13 activity.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2002-05-1401.
Supported by grants HL64796, 1-P50-HL65967, and HL18673 from the National Institutes of Health; a Grant-in-Aid from the American Heart Association-Texas Affiliate; and the Mary R. Gibson Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jing-fei Dong, Thrombosis Research Section, Department of Medicine, BCM286, N1319, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030; e-mail:jfdong@bcm.tmc.edu.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal