Treatment for patients with stage IV indolent lymphoma ranges from watchful waiting to intensive chemotherapy and stem cell transplantation. In this trial we compared 2 induction regimens followed by 1 year of interferon maintenance therapy. Fludarabine, mitoxantrone (Novantrone), and dexamethasone (FND) were compared with an alternating triple therapy (ATT) regimen (CHOD-Bleo, ESHAP, and NOPP). Maintenance interferon/dexamethasone was given for 1 year in both treatment arms. Endpoints were comparisons of remission rates, survival, failure-free survival (FFS), molecular response rates, and toxicities. One hundred forty-two patients with previously untreated stage IV indolent lymphoma were evaluable (73 on FND; 69 on ATT). The overall response rates were 97% for FND and 97% for ATT (P = .9). The median follow-up is 5.9 years. The 5-year survival rates were 84% with FND and 82% with ATT (P = .9); the 5-year FFS rates were 41% with FND and 50% with ATT (P = .02). In a multivariate analysis, factors predicting for longer FFS were β2-microglobulin less than 3 mg/L (P = .01) and ATT treatment (P = .03). ATT was associated with a substantially higher rate of grade 3-4 toxicities than FND. In conclusion, both regimens were associated with high rates of response and survival. ATT was associated with substantially longer FFS, but it was more toxic than FND.
Introduction
The management of patients with advanced stage indolent lymphomas includes many options, ranging from watchful waiting to intensive chemotherapy. Controversy about treatment alternatives derives in part from the observation that these are incurable patients when treated with conventional regimens. An apparent therapeutic impasse for indolent lymphoma patients using alkylating agent–based regimens is reflected in the observation that no headway has been made in the outcome for patients with advanced stage indolent lymphoma over the past 30 years.1
In the management of relapsed lymphoma, it is common to use agents that are non–cross resistant with standard front-line chemotherapy such as the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen. In 1985, we first investigated the combination of cisplatin, cytosine arabinoside, and dexamethasone (DHAP) in 90 patients with relapsing or refractory lymphoma. This regimen was effective, with an overall response rate of 60%, including complete remission (CR) in 34% and partial remission (PR) in 26%.2 A later variant of DHAP, with the inclusion of etoposide (ESHAP), was also effective in the salvage setting; for patients with relapsed indolent lymphoma, ESHAP induced CR in 37% and PR in 27%.3 In 1988, we elected to incorporate ESHAP with CHOP in the front-line management of patients with stage IV indolent lymphoma. We also incorporated a third regimen, NOPP (mitoxantrone, vincristine, prednisone, and procarbazine), to include the active new agent, mitoxantrone,4 and to build on the observation by the Eastern Cooperative Oncology Group (ECOG) that a procarbazine-containing regimen could induce durable remissions.5
Our prior experience with this alternating triple therapy (ATT) in previously untreated stage IV indolent lymphomas was encouraging. In 138 patients, 65% achieved CR and 30% PR, resulting in 93% 4-year survival and 57% failure-free survival (FFS).6 For a subset of patients on that trial whose peripheral blood was monitored by polymerase chain reaction (PCR) for bcl-2, 68% reverted to negative; hitherto, such “molecular remission” appeared to be attainable only with stem cell transplantation approaches.7 8
In parallel with that ATT experience for previously untreated patients, in 1990 we started a phase 1 study of fludarabine, mitoxantrone (Novantrone), and dexamethasone (FND) in 21 patients with recurrent indolent lymphoma. The overall response rate was 71% and the CR rate 43%.9 A phase 2 study of FND in 51 patients with relapsed indolent lymphoma produced an overall response rate of 94% (47% CR and 47% PR).10
Following these experiences, in 1992 we initiated a randomized trial for patients with stage IV indolent lymphoma, in which we compared FND with ATT. Responding patients in both treatment arms received maintenance interferon/dexamethasone, based on our prior favorable experience with maintenance interferon.11 The current report summarizes the results of that trial, with 5.9- year follow-up.
Patients and methods
Patients
From December 1992 to November 1997, 159 patients with previously untreated stage IV indolent lymphoma were enrolled on this randomized study. A signed informed consent was obtained before treatment, according to institutional policy, explaining the investigational nature of the trial. Eligibility criteria included previously untreated stage IV indolent lymphoma, age younger than 76 years, cardiac ejection fraction equal to 55% or more, negative HIV serology, and absence of prior malignancy. Adequate marrow, hepatic, and renal functions were required, unless these abnormalities were due to lymphoma. Approval was obtained from the institutional review board for this study; informed consent was provided according to the Declaration of Helsinki.
The staging evaluation included a complete physical examination, bilateral iliac crest bone marrow aspirates and biopsies, chest radiograph, computerized tomography (CT) of the abdomen and pelvis, and lymphangiography as clinically indicated. The Ann Arbor stage was determined after review of all clinical, laboratory, pathologic, and radiographic data in a weekly multidisciplinary conference with participating oncologists, radiotherapists, hematopathologists, and radiologists. The histologic criteria of the Working Formulation12 were used for classification of these malignancies at the time the study was active; these classifications included diffuse small lymphocytic (SL), follicular small cleaved (FSC), and follicular mixed (FM) lymphoma. Patients with follicular large cell lymphoma (2 in the FND and 3 in the ATT arm, including one case with follicular and diffuse large cell lymphoma in both treatment arms) were also registered on this study. Some patients with variant SL histologies were also registered, including mantle cell lymphoma (n = 2; 1 patient in each treatment arm), and lymphoma of mucosa-associated lymphoid tissue (MALT; n = 3; FND = 2 and ATT = 1).13 The equivalent current World Health Organization (WHO) categories for the indolent lymphoma types that were included in this trial are as follows: FSC, follicular grade 1; FM, follicular grade 2; follicular large cell lymphoma, follicular grade 3; SL, small lymphocytic lymphoma.14 The SL variants (mantle cell lymphoma, MALT) were not described in the Working Formulation but are now well-defined entities.14
Treatment plan
Patients were randomly assigned to receive ATT or FND. The ATT program is shown in Table 1. FND consisted of fludarabine 25 mg/m2 days 1 to 3, mitoxantrone 10 mg/m2 on day 1, and dexamethasone 20 mg orally days 1 to 5, repeated at 28-day intervals, for a total of 8 cycles.Pneumocystis carinii pneumonia (PCP) prophylaxis with trimethoprim 160 mg and sulfamethoxazole 800 mg orally was taken twice weekly by patients who received FND. If PCP or any other opportunistic infection occurred, subsequent chemotherapy cycles were given without dexamethasone.
Alternating triple therapy (ATT) regimen
| Drugs . | Dose (mg/m2) . | Days . | Route . | Treatment interval (d) . |
|---|---|---|---|---|
| CHOD-Bleo15 | 21 | |||
| Cyclophosphamide | 750 | 1 | IV | |
| Doxorubicin | 50 (total) | 1-2 | IV, CI | |
| Vincristine | 1.4 (total) | 1-2 | IV, CI | |
| Bleomycin* | 4 | 1 | IV | |
| Bleomycin* | 10 (total) | 1-2 | IV, CI | |
| Dexamethasone | 40 (daily)‡ | 1-4 | PO | |
| ESHAP | 21 | |||
| Etoposide | 40 (daily) | 1-4 | I.V | |
| Methylprednisolone | 500 (daily)‡ | 1-5 | IV | |
| Cytarabine† | 2000 | 5 | IV | |
| Cisplatin | 100 (total) | 1-4 | IV, CI | |
| NOPP | 28 | |||
| Mitoxantrone | 10 | 1 | IV | |
| Vincristine | 1.4 | 1 | IV | |
| Procarbazine | 100 (daily) | 1-14 | PO | |
| Prednisone | 100 (daily)‡ | 1-5 | PO |
| Drugs . | Dose (mg/m2) . | Days . | Route . | Treatment interval (d) . |
|---|---|---|---|---|
| CHOD-Bleo15 | 21 | |||
| Cyclophosphamide | 750 | 1 | IV | |
| Doxorubicin | 50 (total) | 1-2 | IV, CI | |
| Vincristine | 1.4 (total) | 1-2 | IV, CI | |
| Bleomycin* | 4 | 1 | IV | |
| Bleomycin* | 10 (total) | 1-2 | IV, CI | |
| Dexamethasone | 40 (daily)‡ | 1-4 | PO | |
| ESHAP | 21 | |||
| Etoposide | 40 (daily) | 1-4 | I.V | |
| Methylprednisolone | 500 (daily)‡ | 1-5 | IV | |
| Cytarabine† | 2000 | 5 | IV | |
| Cisplatin | 100 (total) | 1-4 | IV, CI | |
| NOPP | 28 | |||
| Mitoxantrone | 10 | 1 | IV | |
| Vincristine | 1.4 | 1 | IV | |
| Procarbazine | 100 (daily) | 1-14 | PO | |
| Prednisone | 100 (daily)‡ | 1-5 | PO |
IV, intravenously; CI, continuous infusion; PO, oral.
Bleomycin 4 mg/m2 on day 1, followed by 10 mg IV, CI over 48 hours on days 1 and 2.
Cytarabine 2 g/m2 by 2-hour infusion on day 5 after platinum.
Steroid doses were given at the stated fixed dose, not mg/m2.
ATT chemotherapy continued for a total of 12 cycles, 4 of each regimen.
Patients achieving CR or PR received 3 MU/m2interferon (IFN) alfa 2b subcutaneously daily on days 2 to 14 and dexamethasone 40 mg orally on days 1 to 3 of each maintenance cycle, with repeat cycles monthly for 1 year.
Patient monitoring during therapy
Restaging evaluations, including bone marrow biopsy and CT scan of the abdomen or follow-up of the lymphangiogram, were done at least every 3 months during the first year, and at least every 3 to 6 months during and after maintenance.
Monitoring of bcl-2 rearrangement by PCR was performed on peripheral blood nucleated cells, as previously described.16 Briefly, oligonucleotide primers flanking the crossover sites in most cases of t(14;18) were used to amplify hybrid DNA sequences and to detect subclinical residual disease. PCR tests were done at diagnosis and approximately every 3 months in patients carrying this rearrangement.
Endpoints and statistical methods
Complete remission was defined as complete disappearance of all detectable clinical and radiographic evidence of disease and disappearance of all disease-related symptoms, and normalization of biochemical abnormalities definitely assignable to lymphoma. Unconfirmed CR (CRu) included cases with minimal stable radiographic changes or with persistent lymphoid aggregates in the bone marrow without atypia.17 Partial remission (PR) was defined as a reduction by 50% or more of the sum of the products of the greatest diameters of bidimensionally measurable disease.18 Any other response was considered a failure. The response and endpoint assessments conformed to the published International Workshop response criteria.18 Survival was measured from the time of entry onto the trial until death from any cause, or last follow-up. FFS was defined as the time from entry onto the trial until progression, relapse, or toxic death.19 Deaths from unrelated causes (n = 1 in this study) are censored in the FFS analysis by using this methodology.
Molecular response during the first year was defined as the achievement of PCR-negativity at any point during this period of treatment.20 To avoid selection bias issues, a 12-month landmark method was used for the correlation of FFS and overall survival with molecular response status.20,21 By this method of evaluating outcome by treatment response, patients who fail early do not prejudicially influence the analysis of a postdiagnosis (treatment response) endpoint.20,21 The chi-square test was used to investigate the independence between 2 categorical variables. Survival curves were estimated by using the Kaplan-Meier method.22 The 2-sided log-rank test was used to test the association between variables and survival or FFS. Multivariate analysis was performed by using the Cox proportional hazards regression model to determine which variables affected the duration of FFS and the association of treatment with FFS after adjusting for the role of other factors.23P values were derived from 2-sided tests, and P≤.05 was considered to be statistically significant. Statistical analyses were carried out using SAS 8.0 and Splus 2000.
Results
Demographics
Of 159 patients, 142 were evaluable. Seventeen patients were excluded for the following reasons: (a) for ATT: withdrawal of consent, 8; refusal of follow-up evaluation, 2; and (b) for FND: incorrect histology, 3; withdrawal of consent, 1; lost to follow-up evaluation, 1; ineligibility (age, 76 years), 1; financial issues, 1.
The 2 treatment groups were similar with respect to established prognostic factors (Table 2). The median age of the patients treated with FND was 50 years (range, 26-71 years) and for ATT it was 52 (range, 17-70 years). Forty-two men and 31 women were treated with FND, and 32 men and 37 women received ATT.
Patients' characteristics
| . | FND N = 73 (%) . | ATT N = 69 (%) . |
|---|---|---|
| Histology | ||
| Follicular | 54 (74) | 58 (84) |
| SL and variants | 19 (26) | 11 (16) |
| IPI | ||
| 1 | 50 (68) | 44 (64) |
| 2 | 19 (26) | 22 (32) |
| > 2 | 4 (5) | 3 (4) |
| Age > 60 y | 20 (27) | 20 (29) |
| PS > 1 | 2 (3) | 3 (4) |
| High LDH | 12 (16) | 7 (10) |
| > 1 extranodal sites | 22 (30) | 14 (20) |
| B symptoms | 10 (14) | 5 (7) |
| High β2-microglobulin | 20 (27) | 11 (16) |
| Bulky | 36 (49) | 39 (57) |
| . | FND N = 73 (%) . | ATT N = 69 (%) . |
|---|---|---|
| Histology | ||
| Follicular | 54 (74) | 58 (84) |
| SL and variants | 19 (26) | 11 (16) |
| IPI | ||
| 1 | 50 (68) | 44 (64) |
| 2 | 19 (26) | 22 (32) |
| > 2 | 4 (5) | 3 (4) |
| Age > 60 y | 20 (27) | 20 (29) |
| PS > 1 | 2 (3) | 3 (4) |
| High LDH | 12 (16) | 7 (10) |
| > 1 extranodal sites | 22 (30) | 14 (20) |
| B symptoms | 10 (14) | 5 (7) |
| High β2-microglobulin | 20 (27) | 11 (16) |
| Bulky | 36 (49) | 39 (57) |
PS indicates performance status; LDH, lactate dehydrogenase.
Eighty percent of the patients treated with FND and 75% of the patients treated with ATT completed more than 75% of the assigned treatment courses. Of the 142 patients, 121 (85%) patients started maintenance therapy with IFN/dexamethasone. Among the 121 patients, 85 (70%) completed 1 year of interferon/dexamethasone. The reasons for discontinuation of maintenance therapy were IFN intolerance (mainly, flulike syndrome and fatigue), 23; heart failure, 1; chest pain, 1; hypothyroidism, 1; hyperlipidemia, 1; secondary myelodysplastic syndrome, 1; progressive disease, 3; pregnancy, 1; financial, 1; and unknown, 3.
Response to therapy
Overall, 79% achieved complete remission with FND and 87% with ATT (P = .23). An additional 18% achieved PR with FND, and 10% achieved PR with ATT. The overall response rate (CR + PR) was 97% (Table 3).
Response
| . | Both treatment arms . | FND . | ATT . | P . |
|---|---|---|---|---|
| Evaluable | 142 | 73 | 69 | |
| CR (%) | 90 (63) | 49 (67) | 41 (59) | .233-150 |
| CRu (%) | 28 (20) | 9 (12) | 19 (28) | |
| PR (%) | 20 (14) | 13 (18) | 7 (10) | |
| SD (%) | 1 (1) | 0 (0) | 1 (1.5) | |
| PD (%) | 3 (2) | 2 (3) | 1 (1.5) | |
| CR + CRu + PR (%) | 138 (97) | 71 (97) | 67 (97) |
| . | Both treatment arms . | FND . | ATT . | P . |
|---|---|---|---|---|
| Evaluable | 142 | 73 | 69 | |
| CR (%) | 90 (63) | 49 (67) | 41 (59) | .233-150 |
| CRu (%) | 28 (20) | 9 (12) | 19 (28) | |
| PR (%) | 20 (14) | 13 (18) | 7 (10) | |
| SD (%) | 1 (1) | 0 (0) | 1 (1.5) | |
| PD (%) | 3 (2) | 2 (3) | 1 (1.5) | |
| CR + CRu + PR (%) | 138 (97) | 71 (97) | 67 (97) |
P compares CR + CRu rates between FND and ATT treatment arms.
Complete response (CR plus CRu) occurred more frequently in patients 60 years or younger, with 1 or fewer extranodal sites, low (< 3 mg/L) β2-microglobulin levels, and at least one negative PCR test during treatment (molecular responders). Other pretreatment characteristics did not correlate with CR rates, including international prognostic index (IPI) score,25 treatment arm, or bcl-2 rearrangement (Table 4).
Response and survival by treatment characteristics
| . | CR + CRu (%) . | P . | 5-y FFS rate, % . | P . | 5-y survival rate, % . | P . |
|---|---|---|---|---|---|---|
| Age | ||||||
| < 60 y | 90/102 (88) | .009 | 52 | .02 | 86 | .04 |
| ≥ 60 y | 28/40 (70) | 29 | 74 | |||
| No. extranodal sites | ||||||
| 0-1 | 80/91 (88) | .04 | 46 | .4 | 87 | .2 |
| > 1 | 38/51 (75) | 43 | 76 | |||
| LDH | ||||||
| ≤ 618 IU/L | 103/123 (84) | .6 | 46 | .3 | 86 | .08 |
| > 618 IU/L | 15/19 (79) | 42 | 68 | |||
| β2-Microglobulin | ||||||
| Low | 98/111 (88) | .002 | 53 | .0002 | 86 | .1 |
| High (≥ 3 mg/L) | 20/31 (65) | 14 | 74 | |||
| Histology | ||||||
| Follicular | 95/112 (85) | .3 | 46 | .5 | 82 | .5 |
| SL variants | 23/30 (77) | 43 | 86 | |||
| IPI | ||||||
| 1 | 82/94 (87) | .07 | 52 | .03 | 88 | .03 |
| ≥ 2 | 36/48 (75) | 32 | 74 | |||
| Treatment arm | ||||||
| FND | 58/73 (79) | .2 | 41 | .02 | 84 | .9 |
| ATT | 60/69 (87) | 50 | 82 | |||
| Bcl-2 rearrangement4-150 | ||||||
| Bcl-2 rearranged | 64/72 (89) | .06 | 51 | .04 | 88 | .04 |
| Germ line | 23/31 (74) | 34 | 71 | |||
| Molecular response4-151 | ||||||
| Molecular response | 47/50 (94) | .01 | 64 | .1 | 95 | .1 |
| No molecular response | 13/18 (72) | 28 | 76 |
| . | CR + CRu (%) . | P . | 5-y FFS rate, % . | P . | 5-y survival rate, % . | P . |
|---|---|---|---|---|---|---|
| Age | ||||||
| < 60 y | 90/102 (88) | .009 | 52 | .02 | 86 | .04 |
| ≥ 60 y | 28/40 (70) | 29 | 74 | |||
| No. extranodal sites | ||||||
| 0-1 | 80/91 (88) | .04 | 46 | .4 | 87 | .2 |
| > 1 | 38/51 (75) | 43 | 76 | |||
| LDH | ||||||
| ≤ 618 IU/L | 103/123 (84) | .6 | 46 | .3 | 86 | .08 |
| > 618 IU/L | 15/19 (79) | 42 | 68 | |||
| β2-Microglobulin | ||||||
| Low | 98/111 (88) | .002 | 53 | .0002 | 86 | .1 |
| High (≥ 3 mg/L) | 20/31 (65) | 14 | 74 | |||
| Histology | ||||||
| Follicular | 95/112 (85) | .3 | 46 | .5 | 82 | .5 |
| SL variants | 23/30 (77) | 43 | 86 | |||
| IPI | ||||||
| 1 | 82/94 (87) | .07 | 52 | .03 | 88 | .03 |
| ≥ 2 | 36/48 (75) | 32 | 74 | |||
| Treatment arm | ||||||
| FND | 58/73 (79) | .2 | 41 | .02 | 84 | .9 |
| ATT | 60/69 (87) | 50 | 82 | |||
| Bcl-2 rearrangement4-150 | ||||||
| Bcl-2 rearranged | 64/72 (89) | .06 | 51 | .04 | 88 | .04 |
| Germ line | 23/31 (74) | 34 | 71 | |||
| Molecular response4-151 | ||||||
| Molecular response | 47/50 (94) | .01 | 64 | .1 | 95 | .1 |
| No molecular response | 13/18 (72) | 28 | 76 |
Bulky disease was defined as any mass with a maximum diameter ≥ 5 cm.24 “Germ line” denotes patients for whom neither major breakpoint region (MBR) nor minor cluster region (mcr) rearrangements were detected with PCR in peripheral blood or bone marrow samples pretreatment.
Univariate analysis was used for data; for multivariate analysis of FFS, see text. LDH, lactate dehydrogenase.
For follicular lymphoma patients, excluding 9 for whom bcl-2 status was unknown.
For FFS and overall survival, 12-month landmark analysis was used (see “Patients and methods”).
CHOD-Bleo/ESHAP/NOPP chemotherapy
The CR rate with ATT was 87%. According to histologic subtype, the CR rate was FSC, 88% (30 of 34 patients); FM, 86% (18 of 21); FLCL, 100% (3 of 3); and SL variants, 82% (9 of 11). ATT induced PR in 4 patients with FSC, in 2 with FM, and in 1 patient with SL.
Fludarabine/novantrone/dexamethasone
The CR rate with FND was 79%. The CR rate by cell type was FSC, 87% (28 of 32 patients); FM, 80% (16 of 20); FLCL, 0% (0 of 2); and SL variants, 74% (14 of 19). Three patients with FSC achieved PR, 4 with FM, 1 with FLCL, and 5 with SL variants.
Overall survival
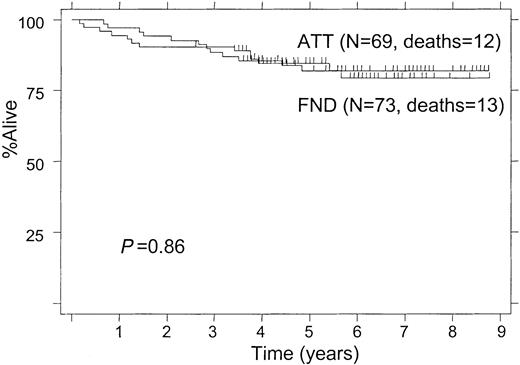
The median follow-up of surviving patients was 5.9 years. The 5-year survival rate was 83%; the median survival has not been reached. There was no survival difference between the 2 treatment arms (82% with ATT and 84% with FND at 5 years, P = .86; Figure 1). When the analysis was limited to follicular lymphomas only, the 5-year survival was 82% with ATT compared with 83% for those receiving FND.
Twenty-five patients have died, 13 following FND and 12 following ATT. On the FND treatment arm, 11 died from progressive disease, 1 from sepsis, and 1 patient died in CR from subsequent small cell lung carcinoma; this was the only patient who was censored in the FFS analysis. On the ATT treatment arm, 8 patients have died from progressive lymphoma, 2 from toxic deaths, 1 from secondary acute myelogenous leukemia (failure in the FFS analysis), and 1 from subsequent hepatoma (failure in the FFS analysis because the lymphoma was not in remission).
In univariate analysis, pretreatment factors that correlated with shorter survival were age 60 years or older, IPI score of 2 or greater, and absence of bcl-2 rearrangement; other clinical features were not substantial (Table 4).
Failure-free survival
Treatment failure occurred in 82 patients; most of these patients received salvage therapy with several regimens, including 1 patient with high-dose therapy and autologous stem cell transplantation (ATT arm), and 10 patients with allogeneic stem cell transplantation (6 in the FND and 4 in the ATT arm).
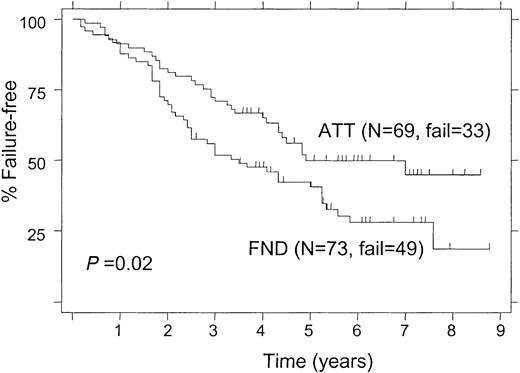
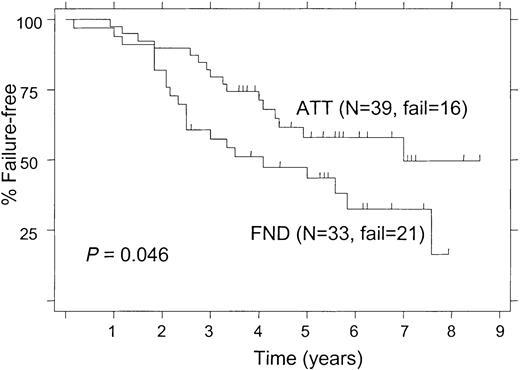
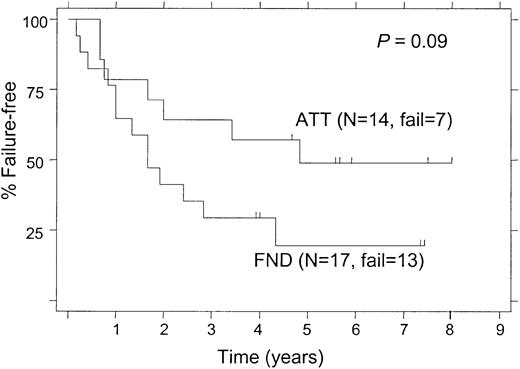
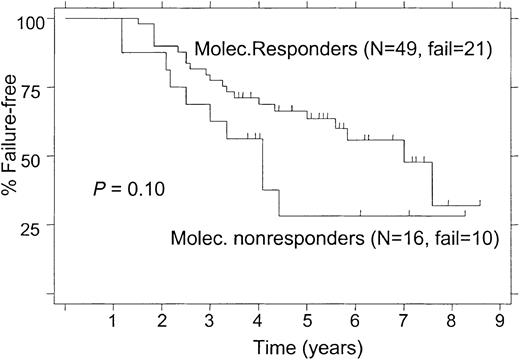
The 5-year FFS was 50% for patients treated with ATT and 41% for those receiving FND (P = .02; Figure2). When the analysis was limited to patients with follicular lymphoma alone, the 5-year FFS rates were still significantly different (52% and 39% for ATT and FND, respectively; P = .027). Among patients with follicular lymphoma who had a bcl-2 rearrangement, the 5-year FFS was significantly longer in patients treated with ATT compared with those treated with FND (58% and 43%, respectively; P = .046; Figure 3). Despite the small number of follicular lymphoma patients without bcl-2 rearrangement (“germ line” patients), there was a nonsignificant trend for longer FFS in patients who received ATT, with a 5-year FFS of 49% compared with 20% for those treated with FND (Figure 4;P = .09).
Failure-free survival of patients with a bcl-2 rearrangement according to the treatment arm.
Failure-free survival of patients with a bcl-2 rearrangement according to the treatment arm.
Failure-free survival of “germ line” follicular lymphoma patients, (ie, no detectable bcl-2 rearrangement), according to the treatment arm.
Failure-free survival of “germ line” follicular lymphoma patients, (ie, no detectable bcl-2 rearrangement), according to the treatment arm.
In univariate analysis, factors predicting for shorter FFS were age 60 years or older, elevated (≥ 3 mg/L) β2-microglobulin, IPI score of 2 or greater, FND therapy, and absence of bcl-2 rearrangement. Other features were not substantial (Table 4).
By multivariate analysis in all 142 patients, factors that independently correlated with prolonged FFS were low β2-microglobulin (P = .01) and ATT therapy (P = .03). Other factors did not reach statistical significance in multivariate analysis, including age younger than 60 years (P = .09). When the multivariate analysis was limited to 103 patients with PCR tests for bcl-2, factors predicting for longer FFS were ATT treatment (P = .002), age younger than 60 years (P = .01), and low β2-microglobulin (P = .02). Detection of bcl-2 rearrangement was not statistically significant in the multivariate analysis for FFS (P = .31).
Molecular monitoring
Response.
Pretreatment bcl-2 gene rearrangement status in the peripheral blood was available in 103 of the 112 follicular lymphoma patients (50 in the FND arm and 53 in the ATT arm). Thirty-three patients (66%) in the FND arm and 39 patients (74%) in the ATT arm had bcl-2 rearrangement in peripheral blood (P = .40).
Thirty-two patients had follow-up PCR tests for bcl-2 in the FND arm and 36 patients in the ATT arm. PCR negativity for bcl-2 was achieved at least once in 26 (81%) of the 32 patients in the FND arm and 24 (67%) of the 36 patients in the ATT arm (P = .17). Among the 26 molecular responders in the FND arm, 21 patients were in clinical CR, 3 in CRu, and 2 in PR. Among the 24 molecular responders in the ATT arm, 17 patients were in clinical CR, 6 in CRu, and 1 in PR.
If PCR negativity were defined more stringently, requiring at least 2 sequential negative PCR tests during the first year, then 18 patients (56%) and 17 patients (47%) achieved molecular response with FND and ATT, respectively (P = .46).
Failure-free survival.
Among 68 patients with follow-up PCR tests for bcl-2, 3 patients had treatment failure during the first year, 2 patients on the ATT arm and 1 on the FND arm. By using the 12-month landmark analysis (see “Patients and methods”), these 3 patients were excluded from the analyses of FFS and survival in relation to the molecular response status (Figure 5 and6). FFS results based on the molecular response status during the first year of treatment are shown in Figure 5.
Failure-free survival according to molecular response status within the first year, 12-month landmark analysis.
(Molec. Responders, PCR-negative status after treatment; Molec. Nonresponders, PCR-positive status after treatment.)
Failure-free survival according to molecular response status within the first year, 12-month landmark analysis.
(Molec. Responders, PCR-negative status after treatment; Molec. Nonresponders, PCR-positive status after treatment.)
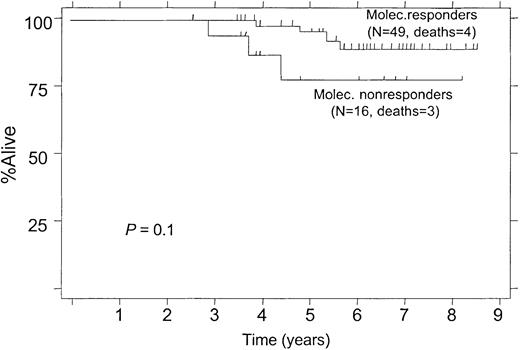
Overall survival according to molecular response status within the first year, 12-month landmark analysis.
(Molec. Responders, PCR-negative status after treatment; Molec. Nonresponders, PCR-positive status after treatment.)
Overall survival according to molecular response status within the first year, 12-month landmark analysis.
(Molec. Responders, PCR-negative status after treatment; Molec. Nonresponders, PCR-positive status after treatment.)
Survival.
At 5 years, 95% of molecular responders were alive compared with 76% of molecular nonresponders (Figure 6; P = .1). The median survival has not been reached in either group.
Toxicity
The toxicity from these chemotherapy regimens was mainly hematologic (Table 5). ATT caused grade 3-4 neutropenia and thrombocytopenia in a substantially higher number of patients than FND. More patients also developed infections on the ATT arm compared with the FND arm. There were 2 cases ofPneumonocystis carinii pneumonia on the FND arm and 2 cases of disseminated fungal infection on the ATT arm. There was one toxic death on the FND arm compared with 2 on the ATT arm. One patient who received ATT died at 2.9 years from secondary acute myelogenous leukemia. Nonhematologic toxicity was less severe on the FND arm, including less grade 3-4 nausea and vomiting, fatigue, electrolyte abnormalities (potassium and magnesium), motor and sensory toxicity, thrombosis, and stomatitis.
Toxicity
| . | FND (N = 73) . | ATT (N = 69) . | . |
|---|---|---|---|
| Grade 3-4 toxicities . | N (%) . | N (%) . | P . |
| Neutropenia | 56 (81) | 65 (94) | .003 |
| Thrombocytopenia | 9 (12) | 54 (78) | < .0001 |
| Infections | 9 (12) | 19 (27) | .022 |
| Hepatotoxicity | 3 (4) | 1 (1) | .33 |
| Nephrotoxicity | 2 (3) | 1 (1) | .59 |
| Cardiotoxicity | 1 (1) | 1 (1) | .97 |
| Nausea/vomiting | 3 (4) | 20 (29) | < .0001 |
| Other | 11 (15) | 24 (35) | .006 |
| . | FND (N = 73) . | ATT (N = 69) . | . |
|---|---|---|---|
| Grade 3-4 toxicities . | N (%) . | N (%) . | P . |
| Neutropenia | 56 (81) | 65 (94) | .003 |
| Thrombocytopenia | 9 (12) | 54 (78) | < .0001 |
| Infections | 9 (12) | 19 (27) | .022 |
| Hepatotoxicity | 3 (4) | 1 (1) | .33 |
| Nephrotoxicity | 2 (3) | 1 (1) | .59 |
| Cardiotoxicity | 1 (1) | 1 (1) | .97 |
| Nausea/vomiting | 3 (4) | 20 (29) | < .0001 |
| Other | 11 (15) | 24 (35) | .006 |
Discussion
The optimal therapy for patients with advanced stage indolent lymphomas has not been defined. Numerous treatment strategies can induce response, but patients inevitably relapse, with a median duration of response of only 2 to 3 years. With standard alkylating agent-based therapy, little if any therapeutic progress has been made in the past 30 years.1 In this randomized trial for patients with stage IV indolent lymphoma, 2 innovative chemotherapeutic regimens, FND and ATT, were compared. Maintenance IFN was administered to all responding patients.
The rationale for comparing 2 nonstandard regimens in this investigational program was our favorable prior experience with both regimens.6 10 Comparably high rates of CR, molecular response, and survival were observed in this trial with both FND and ATT. ATT resulted in substantially longer FFS than FND (50% versus 41% at 5 years), but even the FFS attained with FND (median, 3.5 years) compares favorably with the typical 2- to 3-year median FFS that is achieved with many regimens. In a multivariate analysis, factors that correlated with prolonged FFS were ATT therapy and a low β2-microglobulin. This analysis confirms the previously reported prognostic utility of serum β2-microglobulin.
The superior FFS results attainable with ATT compared with FND have to be placed in perspective with the complexity and toxicity of this regimen. ATT in particular may be difficult to administer in community practice, where the simplicity of single-agent chlorambucil or a standard combination such as COP (cyclophosphamide, vincristine, prednisone) or CHOP still has considerable appeal.
Others' experience with nucleoside analog therapy has, like ours, been promising. Single-agent fludarabine is effective, inducing remission in 65% (CR, 37%) of 42 previously untreated patients,26although it may be inferior to a combination chemotherapy regimen, such as CHVP (cyclophosphamide, doxorubicin, teniposide, prednisone).27 Many investigators have used fludarabine in combination regimens, most often with mitoxantrone (with or without steroids) or cyclophosphamide. With FND and variants, response rates of 69% to 84% have been reported, mostly in the setting of relapse, but also in the front-line setting.28-32 Fludarabine and cyclophosphamide (FC) combinations have been highly effective in both indolent lymphoma33 and chronic lymphocytic leukemia (CLL).34 Consistent with our experience with FND, most others have also reported good tolerance with fludarabine combination regimens.33-36 Some caution is warranted: In an ECOG trial that was designed to build on their favorable phase 1 experience with FC,33 the FC arm was closed early because of excess toxicity.37 Others have used different doses and schedules of FC, with substantially less toxicity than the ECOG experience, including Flinn et al35 who incorporated filgrastim with FC and reported tolerable hematologic and infectious toxicity. Combination regimens such as FND or FC have not been compared in randomized trials with single-agent fludarabine.
The bcl-2 gene is rearranged in 80% to 90% of patients with follicular lymphomas. Eradication of detectable bcl-2 rearranged cells, so called “molecular remission,” correlates with prolonged FFS following either stem cell transplantation8 or some nonmyeloablative chemotherapy regimens.6,20,38-40 In the current trial, both FND and ATT induced molecular remission, but only nonsignificant trends (Figure 5 and 6) for better outcome for molecular responders were noted. Ongoing clinical trials are testing the importance of this surrogate marker. Standardization and consensus about “molecular response” criteria would be useful, as has been done for clinical response criteria.18
We have previously reported that bcl-2 germ line cases have a worse outcome than the bcl-2 rearranged cases.41 Other investigators have found no prognostic significance of bcl-2 rearrangement in relation to the CR rate, survival, or time to progression in a nonhomogeneously treated group of follicular lymphoma patients.42 In the current trial, bcl-2 germ line patients fared worse than patients with detectable bcl-2 gene rearrangement, in keeping with our prior observation.
Until curative treatment strategies are developed, no single trial, not even a randomized one, can resolve all controversies about the therapy of advanced stage indolent lymphoma. The design of our trial does not address whether either ATT or FND are better than standard treatment, such as COP or CHOP. Both FND and ATT induced high rates of CR and molecular response, and patients had long survival with both regimens. The ATT regimen was superior to FND in terms of FFS in this group of patients with stage IV indolent lymphoma, but ATT was considerably more complex and arduous. Longer follow-up will be needed to assess the long-term survival patterns of these patients.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2001-12-0269.
Supported in part by National Cancer Institute Core Grant CA16672 awarded to the University of Texas M. D. Anderson Cancer Center and by a clinical grant from Integrated Therapeutics Group, Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter McLaughlin, Department of Lymphoma-Myeloma, The University of Texas M. D. Anderson Cancer Center, 1400 Holcombe Blvd, Box 429, Houston, TX 77030; e-mail:pmclaugh@mail.mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal