Myelotoxic treatments for oncologic diseases are often complicated by neutropenia, which renders patients susceptible to potentially lethal infections. In these studies of murine hematopoietic stem cell transplantation (HSCT), cotransplantation of lineage-restricted progenitors known as common myeloid progenitors (CMP) and granulocyte-monocyte progenitors (GMP) protects against death following otherwise lethal challenge with either of 2 pathogens associated with neutropenia: Aspergillus fumigatus and Pseudomonas aeruginosa. Cotransplantation of CMP/GMP resulted in a significant and rapid increase in the absolute number of myeloid cells in the spleen, most of which were derived from the donor CMP/GMP. Despite persistent peripheral neutropenia, improved survival correlated with the measurable appearance of progenitor-derived myeloid cells in the spleen. A marked reduction or elimination of tissue pathogen load was confirmed by culture and correlated with survival. Localization of infection by P aeruginosa and extent of disease was also assessed by in vivo bioluminescent imaging using a strain ofP aeruginosa engineered to constitutively express a bacterial luciferase. Imaging confirmed that transplantation with a graft containing hematopoietic stem cells and CMP/GMP reduced the bacterial load as early as 18 hours after infection. These results demonstrate that enhanced reconstitution of a tissue myeloid pool offers protection against lethal challenge with serious fungal and bacterial pathogens.
Introduction
Numerous disease states—naturally occurring or arising as a result of therapy—result in neutropenia that is associated with an increased susceptibility to a variety of bacterial and fungal pathogens.1-4 Two pathogens with the highest mortality rate are the Gram-negative bacteria Pseudomonas aeruginosa and the fungus Aspergillus fumigatus.1,2,5-8 Once infection is established, death may result despite the resolution of neutropenia. This raises the question of whether blood levels of neutrophils or tissue levels of myelomonocytic cells are more proximally responsible for innate immunity to these infections. Administration of granulocyte colony-stimulating factor (G-CSF) or granulocyte-monocyte colony-stimulating factor (GM-CSF) following myeloablation and hematopoietic cell transplantation (HCT) has shortened the neutropenic period. However, administration of these growth factors has not definitively been shown to decrease the incidence of infection, rates of hospitalization, or mortality. Although Gram-positive bacteria are more frequently cultured from the blood of febrile neutropenic patients, Gram-negative rods such as P aeruginosa still carry the highest mortality rate, and sepsis due to P aeruginosais uniquely associated with neutropenia.5 9-14
The rising incidence of invasive aspergillosis in immunocompromised hosts, especially following allogeneic HCT, has been well chronicled during the past 2 decades. Among recipients of allogeneic HCT, 5% to 15% will develop invasive aspergillosis, with a mortality rate of 90% to 100% despite aggressive antifungal therapy.1,2Although it is not the only important risk factor, neutropenia remains the single greatest risk factor for the development of invasive aspergillosis in all populations. It is believed that shortening the duration of neutropenia should reduce the incidence of disease.2 It has been well established in vitro and indirectly shown in animal models that neutrophils are likely the primary effectors against the invasive hyphae of Aspergillusspp.8,15 However, infusions of corticosteroid-mobilized peripheral neutrophils have not resulted in the durable reduction of neutropenia, prevention of aspergillosis, or a definitive enhancement in the treatment of aspergillosis. This failure may be due to the extremely short intravascular residence time of transfused myelomonocytic cells.16-20 Although it is clear that transient increases in peripheral neutrophil counts are not sufficient to protect against opportunistic infections, the question still remains whether blood or tissue levels of myelomonocytic cells determine susceptibility to these fungal pathogens. To develop a model of HCT-related aspergillosis, we transplanted highly purified hematopoietic stem cells (HSCs) into lethally irradiated histocompatible hosts and infected them with lethal doses of aspergillus conidia in the early posttransplantation period. Using this model we were able to study the functional reconstitution of innate immunity resulting from the cotransplantation of different cell subpopulations along with the purified HSCs.
Animal models of invasive aspergillosis have thus far relied on chemotherapy-induced neutropenia or corticosteroid administration to render the animals susceptible to infection.15 21 The lethal dose of aspergillus conidia depends on the route of delivery. Frequently cited lethal doses for aspergillus inoculated intravenously are between 1 × 106and 1 × 108 conidia. In our model, following myeloablative irradiation and HSC rescue of the host animals, the lethal dose of intravenously administered conidia is 100 conidia, 5 logs lower than in previously presented models. More importantly, the response to aspergillus may be studied in the absence of exogenously administered corticosteroids.
We have recently identified and characterized several lineage-restricted hematopoietic progenitors, including the common lymphocyte progenitor (CLP),22 the common myeloid progenitor (CMP) and, downstream of CMP, the granulocyte-monocyte progenitor (GMP) and the megakaryocyte-erythroid progenitor (MEP).23 In pilot experiments leading to this study, cotransplantation of HSC+CMP/GMP, but not HSCs alone, resulted in a detectable increase in the number of splenic myelomonocytic cells by 6 days following myeloablative irradiation and hematopoietic transplantation (D+6). In contrast, there was no significant shortening of the duration of peripheral blood neutropenia in either group. Therefore, we determined if enhanced tissue levels of hematopoietic subpopulations in mice that underwent cotransplantation with HSCs and CMP/GMP would protect against lethal challenges of either invasive aspergillosis or virulent P aeruginosa.
In both models of infections, cotransplantation of CMP/GMP resulted in improved survival and reduced pathogen loads. These results clearly implicate tissue rather than blood levels of myelomonocytic cells in the protection against these opportunistic pathogens.
Materials and methods
Mice
The congenic strains of C57BL/Ka-Thy1.1 (CD45.2) and C57BL/Ka-Thy1.1 (CD45.1) mice were used for donor cell populations, and their F1 generation was used as hosts. This distinction allows the F1 progeny to be used as simultaneous hosts to both CD45 congenic strains, thus permitting differentiation between host and 2 independent donor cell populations. All mice were bred and maintained at the Research Animal Facility at Stanford University School of Medicine. Donor mice were used at 6 to 8 weeks and recipients at 12 to 16 weeks.
Irradiation
Recipient mice were irradiated with a 200 kV x-ray machine (Philips RT250, Shelton, CT). A total dose of 950 cGy (9.5 Gy) was administered in 2 fractions 3 hours apart. All mice were given acid water before irradiation and antibiotic water (1.1 g/L neomycin sulfate and 106 U/L polymyxin B sulfate) after irradiation to reduce the chance of infection from other opportunistic pathogens.
Isolation of hematopoietic stem cells
Whole bone marrow cells were depleted of mature erythrocytes via ammonium chloride lysis and enriched for c-kit+ cells by staining with biotinylated rat antimouse c-kit (3C11), followed by streptavidin immunomagnetic beads (Miltenyi Biotec, Auburn, CA). The enriched cells were then stained with phycoerythrin (PE)–conjugated lineage markers: CD3 (145-2C11; Pharmingen, San Diego, CA), CD4 (Gk1.5), CD5 (53-7.8), CD8 (53-6.7), B220 (6B2), Ter119, Mac-1 (M1/70), and Gr-1 (RB6-8C5). They were also stained with the following stem cells markers: fluorescein isothiocyanate (FITC)–conjugated anti-Thy1.1 (19XE5), Texas Red–conjugated anti–Sca-1 (E13-161), and allophycocyanin (APC)–conjugated anti–c-kit (2B8).24 25To ensure purity, hematopoietic stem cells were double-sorted using a modified fluorescence-activated cell sorter (FACS Vantage, BD Biosciences, San Jose, CA) equipped with a 488-nm argon and a 599-nm dye laser (made available through the FACS shared user group at Stanford University). Dead cells were excluded from sort gates by propidium iodide staining.
Isolation of CMP/GMP populations
Whole bone marrow cells were depleted of mature erythrocytes by ammonium chloride lysis and stained with biotinylated rat antibodies against the lineage (Lin) markers, IL-7Rα–chain (A7R34), CD19 (1D3; Pharmingen), immunoglobulin M (IgM) (R6-60.2; Pharmingen), Thy 1.1 (19XE5), and unconjugated rat antibodies against Ter119, Gr-1 (RB6-8C5), and cyanine (Cy)–Chrome–conjugated rat antimouse CD45R/B220 (RA3-6B2; Pharmingen). The Lin+ cells were partially removed following incubation with sheep antirat Dynabeads (Dynal, Oslo, Norway) and magnetic depletion. To visualize the remaining Lin+ population, the cells were stained with CD45R/B220 Cy-Chrome (RA3-6B2; Pharmingen) and streptavidin-Cy5-PE (RED670; Gibco, Gaithersburg, MD). To visualize the CMP/GMP markers, the cells were stained with APC-conjugated anti–c-kit (2B8), Texas-Red–conjugated anti–Sca-1 (E13-161), FITC-conjugated anti-CD34 (RAM 34; Pharmingen), and PE-conjugated anti-CD16/32 (2.4G2). The progenitor populations were sorted using the FACS Vantage described above.
Transplantation of hematopoietic cells
Purified hematopoietic cells were transplanted into the irradiated host mice via retro-orbital injection. Mice were anesthetized using inhaled Metofane (Mallinckrodt Veterinary, Mundelein, IL). A sterile 22-gauge syringe containing the cells in a total of 100 μL sterile media was inserted into the posterolateral aspect of the orbital cavity and injected into the vasculature. Mice were observed until fully recovered from anesthesia.
Preparation and inoculation of aspergillus conidia
A clinical isolate of A fumigatus that had caused fatal sinusitis in a patient following allogeneic bone marrow transplantation was plated onto a fresh Sabouraud dextrose agar (BD Biosciences, Cockeysville, MD) plate and incubated for 48 hours at 37°C. Ten milliliters of sterile saline was poured onto the surface of the fungal lawn, which was then scraped gently with an inoculating loop. Conidia were spun down and resuspended in sterile normal saline. The resultant suspension was examined for hyphal elements, and the concentration of conidia was determined for each experiment by both counts and colony growth. Suspensions were kept at 4°C. All mice challenged with A fumigatus received 100 conidia in a total of 150 μL sterile normal saline injected via the lateral tail vein.
Fungal culture of target organs
Mice were killed on the specified study days. The spleen, liver, kidneys, lungs, and brains from each mouse were weighed. A fraction of each tissue was homogenized in 2 mL Dulbecco media in the absence of serum. Tissue homogenate (0.3 mL) was plated on freshly made Sabouraud dextrose agar plates. Colonies were counted on days (D) 3, 5, 7, and 10 following plating. Fragments of each organ were fixed for histologic examination.
Preparation and administration of bioluminescent P aeruginosa
A modified bioluminescent strain of the bacterium P aeruginosa (XEN5; Xenogen, Alameda, CA) was used in this model of bacterial infection following hematopoietic stem cell transplantation (HSCT). The lethal doses for 50% of infected animals (LD50) for the parental and the engineered strains, observed in mouse sepsis and pneumonia models, were not significantly different, indicating that the genetic manipulation did not alter virulence.26-29 For infection of mice, P aeruginosaXEN5 was grown to midlog phase at 37°C with constant rotation, and the concentration of bacteria was estimated spectrophotometrically at 600 nm (OD600). Bacteria were diluted in phosphate-buffered saline (PBS; Gibco) for inoculation, and the bacterial concentration in the inoculum was confirmed by determining colony-forming units (CFUs). All mice were matched by weight (18-22 g) and were infected on D+7 after transplantation with 300 to 500 CFUs via intraperitoneal inoculation.
Bioluminescent imaging
Mice were anesthetized with Avertin (250 μg/kg, administered intraperitoneally). At the time of imaging, animals were placed in a light-tight chamber, and photons emitted from luciferase within the animal and transmitted through the tissue were collected for 5 minutes using an in vivo imaging system employing a cooled charge couple device (CCD) camera (IVIS, Xenogen). This imaging technology was initially developed in a murine model of Salmonellainfection.30-32 Using applications in LivingImage software (Xenogen), gray-scale reference images were collected under low light, and pseudocolor images representing the intensity of the bioluminescent signal from the animal were generated in complete darkness. The 2 images were then superimposed. Image composites and annotations were added using additional software: Igor (Wavemetrics, Seattle, WA) and Canvas (Deneba, Miami, FL).
Bacterial CFU quantitation
Following imaging, the mice were killed and the organs (liver, spleen, heart, and lungs) were harvested. Tissues were homogenized and serially diluted in PBS (10-fold) and plated onto Luria broth (LB) agar to determine CFUs per gram of tissue. The plates were incubated overnight at 37°C, and colonies were counted the following day.
G-CSF administration
Human recombinant G-CSF (hG-CSF; Amgen, Thousand Oaks, CA) was administered at a dose of 250 μg/kg/d. The experimental mice were weighed after irradiation, and the appropriate dilutions of G-CSF were made with sterile saline. G-CSF was administered subcutaneously beginning on D+1 and following through D+5.
In vivo neutrophil subset depletion
A single intraperitoneal dose, 1 mg, of the antibody against the neutrophil marker, anti–Gr-1 (RB6-8C5), was administered on D+5. To verify depletion on D+7, immunophenotypic analysis was performed on bone marrow and splenic cells from a representative Gr-1–depleted animal of the HSC-only and HSC+CMP/GMP transplantation groups.
Statistical methods
All samples from each experiment were analyzed on the same day to minimize variability. Following consultation with Dr Ruby Wong from the Department of Biostatistics at Stanford University, a 2-tailed t test was performed on the log values of groups to compare changes due to graft composition and/or treatment. The nominal values were used in calculating the means and the SEMs.
Results
Experimental model
The purpose of these experiments was to determine (1) the role of highly purified myelomonocytic progenitors on the development of innate immune resistance to otherwise lethal doses of A fumigatusor P aeruginosa and (2) whether it is the tissue or blood levels of these effectors that correlates best with protection. To do so, we established a model of radioprotection with modest doses of congenic HSCs wherein there is a significant posttransplantation period of tissue and blood myelomonocytopenia, during which as few as 100 conidia of A fumigatus or 300 to 500 CFUs of P aeruginosa are rapidly lethal. In this background the cotransplantation of CMP/GMP with HSCs leads to a rapid development of myelomonocytic cells with a significant burst that persists for several days. Although the burst size is large, the short residence time of monocytes and neutrophils (< 4 hours)19 33 in the blood results in mice with significant tissue levels but no detectable white blood cells (WBCs) in the blood. Peripheral blood counts determined on D+7 (HSC+CMP/GMP, n = 7; HSCs alone, n = 8) and between D+24 and D+35 (HSC+CMP/GMP, n = 11; HSCs alone, n = 21) showed total counts of less than 1 000 cells per microliter and less than 4 000 cells per microliter in all mice at those time points, respectively. Thus, this mouse model can be used to test the experimental questions stated above.
Two separate series of experiments were performed to study whether we could shorten the period of functional neutropenia by cotransplanting CMP/GMP with HSCs. Following transplantation, animals were challenged with lethal doses of 1 of 2 pathogens of clinical importance: the opportunistic fungus A fumigatus or the bacteria P aeruginosa. In each series of experiments, the preparation of the animals was identical. Following lethal irradiation, mice received grafts of either (1) 200 HSCs alone or (2) 200 HSCs with 10 000 CMP and 20 000 GMP.
Survival of mice challenged with A fumigatus
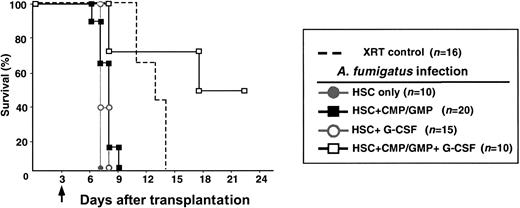
Survival following intravenous challenge with A fumigatuswas dependent on the timing of challenge in relation to lethal irradiation and reconstitution. Mice reconstituted with grafts containing 200 HSCs alone never survived if challenged before D+11. Cotransplantation of CMP (CD16/32loCD34+c-kithiSca-1−lin−) and GMP (CD16/32+CD34+c-kithiSca-1−lin−) with HSCs improved survival if mice were challenged on D+7 (67%), D+9 (100%), or D+11(100%). However, none of these mice survived if challenged on D+3 (Figure 1). A fumigatus was routinely cultured from the homogenized spleens, kidneys, and brains of moribund mice, but no fungus has been isolated from organs of mice surviving more than 1 week after challenge (data not shown).
Survival analysis of mice that underwent transplantation with HSCs and HSC+CMP/GMP against A fumigatus when administered at different times transplantation.
Following total body irradiation (9.5 Gy [950 cGy]), groups of mice were given 200 HSCs only (gray filled circles) or 200 HSCs plus 1 × 104 CMP and 2 × 104 GMP (black filled squares). All groups were infected with Aspergillus fumigatus (100 conidia, intravenously) at a designated time after transplantation (indicated by arrows). Animals not undergoing transplantation that were not infected served as controls for the lethal irradiation dose (broken line). (A) None of the groups survived when infected on day 3 after transplantation (D+3). All mice died within 3 to 5 days after infection. (B) All mice that received only HSCs succumbed to infection when challenged at D+7. However, the survival rate of the mice that underwent cotransplantation with CMP/GMP increased to 67% (P = .002) when similarly challenged. When the time of infection was delayed further to D+9 and D+11 (C,D, respectively), the survival rate of the CMP/GMP group improved to 100%, and mice with HSC-only transplantations showed improved survival rates (22% and 50%, respectively).
Survival analysis of mice that underwent transplantation with HSCs and HSC+CMP/GMP against A fumigatus when administered at different times transplantation.
Following total body irradiation (9.5 Gy [950 cGy]), groups of mice were given 200 HSCs only (gray filled circles) or 200 HSCs plus 1 × 104 CMP and 2 × 104 GMP (black filled squares). All groups were infected with Aspergillus fumigatus (100 conidia, intravenously) at a designated time after transplantation (indicated by arrows). Animals not undergoing transplantation that were not infected served as controls for the lethal irradiation dose (broken line). (A) None of the groups survived when infected on day 3 after transplantation (D+3). All mice died within 3 to 5 days after infection. (B) All mice that received only HSCs succumbed to infection when challenged at D+7. However, the survival rate of the mice that underwent cotransplantation with CMP/GMP increased to 67% (P = .002) when similarly challenged. When the time of infection was delayed further to D+9 and D+11 (C,D, respectively), the survival rate of the CMP/GMP group improved to 100%, and mice with HSC-only transplantations showed improved survival rates (22% and 50%, respectively).
Effect of anti–Gr-1 antibody administration early after transplantation
Administration of anti–Gr-1 antibody has been shown to deplete splenic myeloid lineage cells for up to 5 days.34-36 In these experiments, the administration of 1 mg anti–Gr-1 antibody on D+5 resulted in a profound depletion (> 99%) of bone marrow and splenic neutrophils on the day of challenge with A fumigatus(D+7). However, this procedure did not reduce the B- and T-cell populations. In contrast to the 67% survival observed in mice that underwent cotransplantation with HSCs and CMP/GMP and then were challenged with A fumigatus on D+7 (Figure 1), the posttransplantation administration of anti–Gr-1 antibody abrogated this protection, resulting in a significantly decreased survival (10%; Figure 2).
Survival analysis following total neutrophil depletion.
The depletion of neutrophils abrogated the protective response againstA fumigatus demonstrated by the myeloid progenitors (CMP/GMP). (A) On D+5, anti–Gr-1 (1 mg) was given to mice that underwent transplantation with HSCs (gray filled triangles) or HSC+CMP/GMP (black filled squares). The anti–Gr-1 treatment groups were infected on D+7 along with a group of untreated mice that underwent transplantation with HSCs (gray filled circles). The survival of mice that underwent transplantation with HSCs+CMP/GMP and were then treated with anti–Gr-1 was reduced to equivalence to animals that underwent transplantation with HSCs only as opposed to their nondepleted counterparts, which have demonstrated a survival rate of 67% in this series of experiments. Arrows represent anti–Gr-1 administration and A fumigatus challenge. An HSC-only transplantation group (gray open circles) served as control for hematopoietic reconstitution. (B) FACS analysis of splenocytes from mice that underwent transplantation with HSCs only or HSC+CMP/GMP. To assess the extent of neutrophil depletion, an uninfected representative animal from each group was treated with anti–Gr-1 on D+5 and analyzed on D+7. The values indicate the percentage of neutrophils (Mac-1+Gr-1+) of total live splenocytes.
Survival analysis following total neutrophil depletion.
The depletion of neutrophils abrogated the protective response againstA fumigatus demonstrated by the myeloid progenitors (CMP/GMP). (A) On D+5, anti–Gr-1 (1 mg) was given to mice that underwent transplantation with HSCs (gray filled triangles) or HSC+CMP/GMP (black filled squares). The anti–Gr-1 treatment groups were infected on D+7 along with a group of untreated mice that underwent transplantation with HSCs (gray filled circles). The survival of mice that underwent transplantation with HSCs+CMP/GMP and were then treated with anti–Gr-1 was reduced to equivalence to animals that underwent transplantation with HSCs only as opposed to their nondepleted counterparts, which have demonstrated a survival rate of 67% in this series of experiments. Arrows represent anti–Gr-1 administration and A fumigatus challenge. An HSC-only transplantation group (gray open circles) served as control for hematopoietic reconstitution. (B) FACS analysis of splenocytes from mice that underwent transplantation with HSCs only or HSC+CMP/GMP. To assess the extent of neutrophil depletion, an uninfected representative animal from each group was treated with anti–Gr-1 on D+5 and analyzed on D+7. The values indicate the percentage of neutrophils (Mac-1+Gr-1+) of total live splenocytes.
Effect of G-CSF on susceptibility to A fumigatus
G-CSF has been shown to mobilize cells into the periphery, accelerate the differentiation of progenitor cells into their mature progeny, and possibly qualitatively enhance function of mature neutrophils.37 In an attempt to shorten the period of susceptibility further in mice that underwent transplantation with HSCs or cotransplantation with HSCs and CMP/GMP, 250 μg/kg human G-CSF was administered intravenously on D+1 through D+5. This dose has been previously demonstrated to accelerate neutrophil recovery following neutropenia in the mouse. The administration of G-CSF failed to improve survival of mice that underwent transplantation with HSCs alone. In contrast, 50% of the mice that underwent cotransplantation with CMP/GMP and received this short course of G-CSF survived at least through D+35, even when infected with A fumigatus on D+3. None of the mice challenged on D+3 that received grafts containing HSCs and CMP/GMP survived in the absence of G-CSF (Figure3). Therefore, the administration of G-CSF to the CMP/GMP group further shortens the period of susceptibility to invasive aspergillosis.
G-CSF improves survival against a lethal challenge withA fumigatus.
To assess the effects of G-CSF, groups of mice that underwent transplantation with 200 HSCs only or 200 HSCs plus 1 × 104 CMP and 2 × 104 GMP were given hG-CSF (250 μg/kg) subcutaneously at daily intervals on D+1 through D+5. Without G-CSF, mice that underwent transplantation with HSCs only (gray filled circles) or with HSC+CMP/GMP (black filled squares) did not survive when challenged with A fumigatus on D+3. However, there was a marked improvement in survival in groups that underwent cotransplantation with CMP/GMP and were treated with G-CSF (black open squares), whereas G-CSF treatments in mice with HSCs only did not improve their survival rate (gray open circles;P = .013). Animals not undergoing transplantation that were not infected served as controls for the lethal irradiation dose (broken line). The arrow indicates day of challenge with A fumigatus.
G-CSF improves survival against a lethal challenge withA fumigatus.
To assess the effects of G-CSF, groups of mice that underwent transplantation with 200 HSCs only or 200 HSCs plus 1 × 104 CMP and 2 × 104 GMP were given hG-CSF (250 μg/kg) subcutaneously at daily intervals on D+1 through D+5. Without G-CSF, mice that underwent transplantation with HSCs only (gray filled circles) or with HSC+CMP/GMP (black filled squares) did not survive when challenged with A fumigatus on D+3. However, there was a marked improvement in survival in groups that underwent cotransplantation with CMP/GMP and were treated with G-CSF (black open squares), whereas G-CSF treatments in mice with HSCs only did not improve their survival rate (gray open circles;P = .013). Animals not undergoing transplantation that were not infected served as controls for the lethal irradiation dose (broken line). The arrow indicates day of challenge with A fumigatus.
Effect of CMP/GMP cotransplantation on both donor- and host-derived myeloid cells
The appearance of CMP/GMP-derived cells in the spleen correlated with improved survival. Flow cytometric analyses of splenic subpopulations were performed on D+4 and D+7 to analyze the expression of myelomonocytic lineage markers, Mac-1 and Gr-1. Cells expressing high levels of both Mac-1 and Gr-1 are predominantly neutrophils, whereas the Mac-1+Gr-1− cells are predominantly macrophages.33 Although the paucity of live splenic cells limits analysis of cellular subpopulations on D+4 after transplantation, no difference in the total number of cells or percentages of Mac-1+ cells was detected regardless of graft composition or administration of G-CSF. Analysis on D+7 revealed significant differences in the splenic myeloid populations between the different groups. In the absence of G-CSF, cotransplantation of CMP/GMP with HSCs resulted in increased absolute numbers of splenocytes, including splenic neutrophils, most of which were derived from the donor CMP/GMP (67.4%). Analysis of the CD45+ splenocyte population also confirmed a significant increase in the percentage of this population that was Mac-1+Gr-1+ (28%) when compared with mice that contained grafts containing only HSCs (16%; Table1).
Derivation of Mac-1+Gr-1+splenocytes on D+7 after transplantation
| . | Graft composition without G-CSF . | Graft composition with G-CSF . | ||
|---|---|---|---|---|
| HSCs, n = 10 . | HSC + CMP/GMP, n = 5 . | HSCs, n = 7 . | HSC + CMP/GMP, n = 6 . | |
| Total splenocytes | 111.1 ± 12.9 | 181.5 ± 12.9* | 63.4 ± 7.5 | 300.8 ± 40.0*,† |
| Total CD45+splenocytes | 82.7 ± 14.0 | 105.0 ± 26.2 | 43.1 ± 7.5 | 142.0 ± 20.6*,† |
| Total Mac-1+Gr-1+ | 13.3 (16%) | 29.8 (28%)* | 3.7 (8.6%) | 29.5 (21%)*,† |
| Derivation | ||||
| Donor HSCs | 11.6 ± 4.2 | 4.7 ± 1.2 | 2.5 ± 0.7 | 6.4 ± 1.4† |
| Donor CMP/GMP | — | 20.1 ± 4.9 | — | 14.9 ± 1.8 |
| Host | 1.6 ± 0.3 | 5.0 ± 1.6 | 1.2 ± 0.5 | 8.2 ± 5.5† |
| . | Graft composition without G-CSF . | Graft composition with G-CSF . | ||
|---|---|---|---|---|
| HSCs, n = 10 . | HSC + CMP/GMP, n = 5 . | HSCs, n = 7 . | HSC + CMP/GMP, n = 6 . | |
| Total splenocytes | 111.1 ± 12.9 | 181.5 ± 12.9* | 63.4 ± 7.5 | 300.8 ± 40.0*,† |
| Total CD45+splenocytes | 82.7 ± 14.0 | 105.0 ± 26.2 | 43.1 ± 7.5 | 142.0 ± 20.6*,† |
| Total Mac-1+Gr-1+ | 13.3 (16%) | 29.8 (28%)* | 3.7 (8.6%) | 29.5 (21%)*,† |
| Derivation | ||||
| Donor HSCs | 11.6 ± 4.2 | 4.7 ± 1.2 | 2.5 ± 0.7 | 6.4 ± 1.4† |
| Donor CMP/GMP | — | 20.1 ± 4.9 | — | 14.9 ± 1.8 |
| Host | 1.6 ± 0.3 | 5.0 ± 1.6 | 1.2 ± 0.5 | 8.2 ± 5.5† |
Data are expressed as mean cell counts ± SEM (×104). Values in parentheses indicate the percentage of the CD45+ population that was Mac-1+Gr-1+.
Significantly different from HSCs without G-CSF (P < .05).
Significantly different from HSCs with G-CSF (P < .05).
The administration of G-CSF to mice that had received grafts containing CMP and GMP resulted in a significantly greater number of live splenocytes, CD45+ splenocytes, and Mac-1+Gr-1+ cells when compared with mice that received grafts of HSCs only, with or without G-CSF. However, the absolute number of Mac-1+Gr-1+ in these mice was not significantly different from the HSC+CMP/GMP group that did not receive G-CSF (Table 1). By D+7, less than 1% of all splenocytes were monocytes or macrophages in all mice regardless of graft composition. Analysis of peripheral blood confirmed that all mice were neutropenic with an absolute neutrophil count less than 500/μL through D+35. Thus, blood levels of neutrophils are not responsive to these doses of HSC+CMP/GMP with or without G-CSF. Moreover, blood neutrophil levels do not predict susceptibility to aspergillosis. The observation that mice receiving HSC-only grafts were able to survive challenge if infection was delayed until at least D+11 confirms the finding that peripheral neutropenia does not predict susceptibility to invasive disease. We also noticed a potentially disturbing effect of G-CSF on mice that underwent transplantation with HSCs only. Donor HSC-derived Mac-1+Gr-1+ cells decreased in G-CSF treated mice compared with HSCs alone. This deserves more study to test if this is organ specific and if it somehow could compromise the HSC-restored host.
Survival following lethal challenge with Pseudomonas aeruginosa
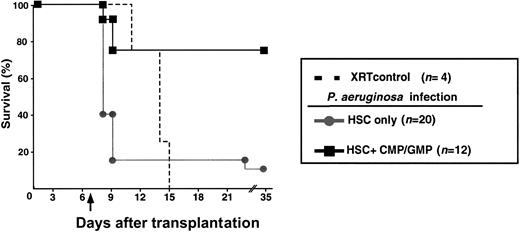
Infection by P aeruginosa has a close association with neutropenia. In most series, P aeruginosa infection is frequently associated with a lethal outcome. Unlike fungal disease, patients are at risk for infection by P aeruginosa even after only brief periods of neutropenia. The XEN5 strain of P aeruginosa constitutively expresses a heterodimeric bacterial luciferase and the enzymes necessary for substrate biosynthesis from the lux operon originally obtained from the bacterium Photorhabdus luminescens. Approximately 300 CFUs of P aeruginosa XEN5 were injected intraperitoneally into mice on D+7 after transplantation. In a series of 3 experiments, 75% of mice that received grafts containing CMP/GMP in addition to HSCs survived. This was in contrast to only 10% of mice that received grafts containing only HSCs (Figure4). All mice except for one in the HSC group that survived for 72 hours after infection remained alive for the remainder of the 35-day observation period.
Survival of mice against lethal infection withPseudomonas aeruginosa.
Groups of mice were challenged with P aeruginosa (D+7, 300 CFUs, intraperitoneally) following total body irradiation and reconstitution with 200 HSCs (gray filled circles) or with 200 HSCs plus 1 × 104 CMP and 2 × 104 GMP (black filled squares; P = .0002). Mice that succumbed to infection became moribund as early as 18 hours after infection. Animals that survived beyond D+10 remained healthy throughout the experimental period. Animals not undergoing transplantation that were not infected served as controls for the lethal irradiation dose (broken line). The arrow indicates day of challenge with P aeruginosa.
Survival of mice against lethal infection withPseudomonas aeruginosa.
Groups of mice were challenged with P aeruginosa (D+7, 300 CFUs, intraperitoneally) following total body irradiation and reconstitution with 200 HSCs (gray filled circles) or with 200 HSCs plus 1 × 104 CMP and 2 × 104 GMP (black filled squares; P = .0002). Mice that succumbed to infection became moribund as early as 18 hours after infection. Animals that survived beyond D+10 remained healthy throughout the experimental period. Animals not undergoing transplantation that were not infected served as controls for the lethal irradiation dose (broken line). The arrow indicates day of challenge with P aeruginosa.
Bioluminescent imaging to determine tissue bacterial distribution and CFU determination
Mice were imaged at 18 hours after infection with P aeruginosa XEN5 using the IVIS system. The bioluminescent images in Figure 5 demonstrate the ability to monitor the protection provided by grafts of HSCs only or HSC+CMP/GMP against P aeruginosa in real time. The bioluminescent signals of 5 moribund infected mice that received grafts of HSCs alone contrasted the lack of detectable signal in contemporaneous images from 5 infected littermates that received a graft containing CMP/GMP (Figure 5). The bioluminescent images of the mice display the relative location and concentration of luminescent bacteria in vivo. After imaging, distribution of P aeruginosa XEN5 in the moribund mice and healthy mice was confirmed by CFU determination in liver, spleen, lung, and heart tissues harvested at 18 hours after infection (Table2). Nine of the 10 mice in the HSC group were moribund compared with 1 of the 5 mice in the CMP/GMP group. The tissue bacterial load correlated with clinically apparent illness and the bioluminescent signals in each mouse. Even at this early time point, there was a 1- to 3-log decrease in the tissue bacterial load of the mice that received CMP/GMP when compared with the mice that received HSCs alone. No bacteria were detected in any mouse that survived and was without evidence of illness 72 hours after infection (data not shown). These results confirm the rapid proliferation and dissemination of P aeruginosa. Animals that were able to clear the pathogen appeared to do so shortly after infection (< 18 hours).
Contemporaneous images representing the bioluminescence of mice 18 hours after intraperitoneal inoculation of 300 CFUs ofP aeruginosa XEN5.
This strain of Pseudomonas constitutively expresses a bacterial luciferase and the enzymes necessary for the substrate biosynthesis from the lux operon. The color scale represents bioluminescence intensity (photon counts) in the images as measured using IVIS. Mice that received grafts containing only HSCs (A) demonstrated a strong bioluminescent signal that contrasts the lack of signal in the infected mice that received a graft containing HSCs and CMP/GMP. Rapid dissemination of P aeruginosa occurred in mice that received HSC-only grafts. In contrast, cotransplantation with CMP/GMP prevented bacterial dissemination and subsequent morbidity as evidenced by the bioluminescent images (B) and CFU determination.
Contemporaneous images representing the bioluminescence of mice 18 hours after intraperitoneal inoculation of 300 CFUs ofP aeruginosa XEN5.
This strain of Pseudomonas constitutively expresses a bacterial luciferase and the enzymes necessary for the substrate biosynthesis from the lux operon. The color scale represents bioluminescence intensity (photon counts) in the images as measured using IVIS. Mice that received grafts containing only HSCs (A) demonstrated a strong bioluminescent signal that contrasts the lack of signal in the infected mice that received a graft containing HSCs and CMP/GMP. Rapid dissemination of P aeruginosa occurred in mice that received HSC-only grafts. In contrast, cotransplantation with CMP/GMP prevented bacterial dissemination and subsequent morbidity as evidenced by the bioluminescent images (B) and CFU determination.
Tissue load of mice infected with P aeruginosa on D+1 after infection
| . | HSCs, n = 10 . | HSC + CMP/GMP, n = 5 . | P . |
|---|---|---|---|
| Liver | 6.4 × 106 ± 3 × 106 | 7.7 × 105 ± 7 × 105 | .086 |
| Spleen | 9.0 × 106 ± 4 × 106 | 7.7 × 104 ± 7 × 104 | .014 |
| Lungs | 1.6 × 107 ± 8 × 106 | 4.2 × 104 ± 2 × 104 | .027 |
| Heart | 1.6 × 106 ± 1 × 106 | 1.3 × 104 ± 1 × 104 | .019 |
| . | HSCs, n = 10 . | HSC + CMP/GMP, n = 5 . | P . |
|---|---|---|---|
| Liver | 6.4 × 106 ± 3 × 106 | 7.7 × 105 ± 7 × 105 | .086 |
| Spleen | 9.0 × 106 ± 4 × 106 | 7.7 × 104 ± 7 × 104 | .014 |
| Lungs | 1.6 × 107 ± 8 × 106 | 4.2 × 104 ± 2 × 104 | .027 |
| Heart | 1.6 × 106 ± 1 × 106 | 1.3 × 104 ± 1 × 104 | .019 |
Mice in each transplantation group were infected with 500 CFUs ofP aeruginosa intraperitoneally on D+7 after transplantation. Eighteen hours after infection, the indicated organs were harvested and cultured to determine bacterial load. The CFUs are calculated as the means ± SEM.
Discussion
In these studies we demonstrated that cotransplantation of highly purified myeloid-restricted progenitors, CMP and GMP, along with highly purified HSCs enhanced reconstitution of a functional myeloid response to a lethal challenge with either the opportunistic fungus A fumigatus or the bacterium P aeruginosa. The improvement in survival was correlated with a significant reduction in or an elimination of organisms. Histologic examination confirmed the elimination of organisms in surviving mice and dissemination of either fungal or bacterial organisms in mice that succumbed to the pathogen challenge.
In these studies, the routes of administration of the pathogens were selected deliberately. The natural reservoirs for P aeruginosa are water and the gastrointestinal tract. This bacterium most commonly enters the body via defects in the integrity of the gastrointestinal tract or via an intravenous catheter, and bacteremia rapidly ensues. Therefore, the distribution of P aeruginosa following intraperitoneal injection approximates that observed in the initial phases of systemic disease following natural infection. In contrast, A fumigatus is a ubiquitous fungus. The most common route of inoculation is via inhalation of the fungal conidia (spores). In the immunocompromised host, sporulation and subsequent formation of hyphal forms may result in an angioinvasive infection. The primary immune effectors against Aspergillusspp depend on the developmental form of the fungus. Pulmonary macrophages are primarily responsible for clearance of inhaled conidia.1 In contrast, neutrophils appear to be necessary for clearance of the newly budding conidia and the branching, invasive hyphal forms.1 Direct inoculation or introduction via an intravenous catheter is rarely seen. Nevertheless, conidia were administered via intravenous injection to ensure challenge with a precise number of conidia, which allowed us to determine the potential benefit of enhanced myeloid reconstitution against invasive hyphae in the absence of potentially confounding influence of alveolar macrophages. Histologic analysis confirmed that, within 72 hours, the conidia disseminated and resulted in invasive hyphal forms throughout the spleen, liver, kidneys, brain, and lungs.
In the natural course of invasive aspergillosis in an immunocompromised host, it is uncommon to observe a profound local neutrophil infiltration even when infection is successfully resolved. Despite this fact, numerous investigators have confirmed the crucial role of the myeloid cell in protecting against invasive aspergillosis. Significant neutrophilic infiltration was not seen in histologic evaluation of tissue from moribund or healthy mice after infection in this study. Similarly, although neutrophilic infiltration may be seen in focal human infection due to P aeruginosa, such infiltration is not typically seen in disseminated or overwhelming infection. The histologic examination of the organs from moribund mice in this study revealed collections of Gram-negative rods without neutrophilic infiltration. Because recovery from infection due to A fumigatusand P aeruginosa can occur in the absence of visible immune response, such histologic evaluation does not correlate with clinical outcome or negate the demonstrated role of the neutrophil.
The systemic distribution of P aeruginosa was noninvasively assessed using in vivo bioluminescent imaging in the transplantation models. This imaging modality can be used to monitor disease progression without the need to kill the animals. Observations made by imaging were confirmed by determining CFUs at selected time points. In the CMP/GMP graft group, it was apparent at 18 hours after infection which mice were likely to survive the infection and which were destined to succumb to infection. The intensity of the bioluminescent signal correlated with clinically apparent illness and survival of the mice. The prediction of one moribund mouse in the CMP/GMP grafted out of 5 (80% survival) correlated well with the previous data obtained by survival counts (75% survival). The sensitivity of the imaging permitted detection of low bacterial loads in the tissues. In this series of experiments, the bacterial tissue burden correlated with the degree and distribution of photons as detected by in vivo imaging. Based on the measurement of bacterial CFUs in this model using mice of the C57BL/Ka strain, 105 CFUs per gram of tissue could be detected by the bioluminescent technique.
An important finding in these studies is that peripheral blood neutropenia alone does not predict risk of death due to either of these pathogens. The cotransplantation of CMP/GMP along with HSCs did not result in detectable progenitor-derived blood neutrophils at any time point, whereas CMP/GMP-derived myeloid cells could be found in the spleen despite the fact that the mice remained neutropenic. As early as D+7 after transplantation, it was determined that the cells accounting for the increase in the absolute number of splenic myeloid cells were derived from the cotransplanted CMP and GMP. Furthermore, antibody depletion of these splenic myeloid cells with anti–Gr-1 antibody resulted in the elimination of the enhanced innate immunity resulting from the cotransplantation of myelomonocytic progenitor cells. While other tissues were not specifically studied, splenic reconstitution likely reflects that which occurs in other hematopoietic tissue in the mouse. These findings suggest that quantitation of live cells in the splenic or peripheral blood compartments does not provide adequate measure of the available Gr-1+ effector cells.
The administration of G-CSF following cotransplantation resulted in the shortening of the period of susceptibility to lethal challenge withA fumigatus. Half of the mice that underwent cotransplantation with CMP/GMP and subsequently administered G-CSF survived challenge on D+3 after transplantation. In contrast, there was a 100% mortality in similarly challenged mice that underwent cotransplantation that had not received G-CSF. Although the shortening of the period of susceptibility following G-CSF administration was not accompanied by an increase in the absolute number of splenic myeloid cells, there was a significant increase in the survival of animals following lethal infection. The increased survival may be due to the enhancement of neutrophil differentiation, function, and survival following administration of G-CSF.38 39 These findings suggest that a relatively small increase in either the number of neutrophils or an enhancement of the function of neutrophils in tissue improved destruction of fungal hyphae.
Despite decades of attempts, transfusion of donor neutrophils mobilized by the administration of corticosteroids has never been shown to provide durable protection against fungal or bacterial infections. This is not surprising because these cells have a very short life span. Similarly, although administration of G-CSF or GM-CSF has been shown to decrease the duration of neutropenia following chemotherapy, there is no proven efficacy of these therapies in the prevention or treatment of infectious complications.20 The efficacy of these therapies may well be limited by the significant depletion of the myeloid pools available for mobilization and differentiation following myelotoxic chemotherapy.40 41 Our data demonstrate that the supplementation of the myeloid progenitor cells by cotransplantation of CMP/GMP with HSCs, with or without the concomitant administration of G-CSF, can prevent the establishment of lethal, invasive fungal disease.
There are potential therapeutic implications of these findings. The subsets of CMP and GMP can be mobilized into the peripheral blood of mice, and we have preliminary evidence that human CMP/GMP can also be mobilized into the peripheral blood following administration of cyclophosphamide and G-CSF. Thus, it may be possible to engineer grafts that contain HSCs and CMP/GMP but are devoid of T cells for allotransplantation and devoid of malignant cells for autotransplantation. Of potentially more widespread application is donor transfusion of these myeloid progenitors following chemotherapeutic regimens or myeloablative preparative regimens for HCT. This would be expected to decrease significantly the risk of infectious complications due to neutropenia. We are currently testing the hypothesis that these transfusions could be fully allogeneic.
We are grateful to Libuse Jerabek and Lucino Hidalgo for their assistance in executing the experiments and Motonari Kondo, Jos Domen, and Judith Shizuru for their invaluable advice.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-05-1552.
Supported by National Institutes of Health grants NIAID 1K11A101197, CA42551, HD37543, 1RO1CA86017, 5T32 DK07762, 2P01CA49605 American Society for Blood and Marrow Transplantation (ASBMT)/Roche New Investigator Award, and by unrestricted gifts from the Mary L. Johnson and Hess Research Funds, and Center for Clinical Immunology at Stanford.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Janice M. Y. Brown, Divisions of Bone Marrow Transplantation and Infectious Diseases, Stanford University Medical Center, H1353, 300 Pasteur Dr, Stanford, CA; e-mail:wesbrown@stanford.edu.

![Fig. 1. Survival analysis of mice that underwent transplantation with HSCs and HSC+CMP/GMP against A fumigatus when administered at different times transplantation. / Following total body irradiation (9.5 Gy [950 cGy]), groups of mice were given 200 HSCs only (gray filled circles) or 200 HSCs plus 1 × 104 CMP and 2 × 104 GMP (black filled squares). All groups were infected with Aspergillus fumigatus (100 conidia, intravenously) at a designated time after transplantation (indicated by arrows). Animals not undergoing transplantation that were not infected served as controls for the lethal irradiation dose (broken line). (A) None of the groups survived when infected on day 3 after transplantation (D+3). All mice died within 3 to 5 days after infection. (B) All mice that received only HSCs succumbed to infection when challenged at D+7. However, the survival rate of the mice that underwent cotransplantation with CMP/GMP increased to 67% (P = .002) when similarly challenged. When the time of infection was delayed further to D+9 and D+11 (C,D, respectively), the survival rate of the CMP/GMP group improved to 100%, and mice with HSC-only transplantations showed improved survival rates (22% and 50%, respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/13/10.1182_blood-2002-05-1552/4/m_h82423539001.jpeg?Expires=1769105504&Signature=dBK4StxpFmOTHKoOxMID4QadrPUqLlmPkxWWUcwE1wqVOk0MZc8pcjcbFgjUd-HC6QXxZqk2Fuv8t25m-PNEJaRKPlRzZnQLcHb-eaU0drRN0Yqqu25X8GX2DF-0BKzxyag0fDXNrogpOjM7BgxWm4MkTBnCtGqOenYo3AlN58b2pWkhEdtEX2bTrpnsGhlsiDyRGYWsA0haWdATJWa7XkT3bI-1fwWT3sFovSPovGGT41bMVJi5zyjs9Dhv7iiD7aOxcJFkBe1nFPojhDlFLYkpdkUaePMTkj4LEOpdHwbpioUs~FwK1jqI0McBj1ru0nUWRVAi-WTvmcP2Z1Ao2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal