Abstract
Up-regulation of folate receptor (FR) type-β in acute myelogenous leukemia (AML) by all-trans retinoic acid (ATRA) and its restricted normal tissue distribution makes it a potential target for therapeutic intervention. The FR-β in peripheral blood granulocytes was unable to bind folate and appeared to have a variant GPI membrane anchor, evident from its insensitivity to phosphatidylinositol-specific phospholipase C but not nitrous acid. Granulocyte FR-β lacked mutations, and neither deglycosylation nor detergent solubilization restored folate binding. The posttranslational modification causing its nonfunctionality was evidently absent in FR-β from AML cells from patient marrow, which bound folate. From flow cytometric analysis of 78 AML bone marrow specimens of different subtypes, 68% expressed FR-β, most of which were also CD34+. In model cell lines that are FR (−) (KG-1a, L1210, and Chinese hamster ovary [CHO]) or FR (+) (KG-1, L1210 JF, and recombinant CHO-FR–β), selective FR-mediated binding and cytotoxicity was obtained using folate-coated liposomes encapsulating fluorescent calcein (f-L-calcein) and doxorubicin (f-L-DOX), respectively, which could be blocked by 1 mM free folic acid. In the FR-β–expressing KG-1 human AML cells, treatment with ATRA further increased this specificity. In mouse ascites leukemia models generated using L1210JF or KG-1 cells, increased median survival times were obtained with f-L-DOX treatment compared to nontargeted L-DOX. In the KG-1 model, ATRA treatment increased the cure rate with f-L-DOX from 10% to 60%. The above combined data from our 2 laboratories further support the feasibility and potential usefulness of selective ATRA-facilitated liposomal drug delivery in FR-β (+) AMLs.
Introduction
Acute myelogenous leukemia (AML) is the most common type of acute leukemia in adults. Standard chemotherapy results in a 70% complete remission rate in AML patients.1 Treatment with drugs such as anthracyclines, however, is associated with severe side effects such as myelosuppression and dose-limiting cardiotoxicity and a high incidence of relapse.2 Relapsed disease is frequently refractory to chemotherapy and exhibits multidrug resistance (MDR).3
A potential means of treating AML is by targeted drug delivery through liposomes. Liposomal delivery of drugs has been shown to extend their systemic circulation time, reduce dose-limiting toxicity, and overcome MDR.4-7 Liposomal anthracyclines have reached clinical use for the treatment of Kaposi sarcoma5 and are in clinical trial for solid tumors and leukemias.8-14 Liposomal delivery has also been shown to increase anthracycline cytotoxicity in tumor cells exhibiting MDR.6,7,15-17 The efficacy of liposomal drugs can potentially be further enhanced by selective targeting to tumor cells through a cell surface molecule that is differentially expressed on tumor cells.18 19
The folate receptor (FR) is a promising target because of its narrow tissue specificity, its overexpression in malignant tissues, and its ability to bind and internalize folic acid conjugates. Of the 3 human folate receptor isoforms,20-22 2 (FR-α and FR-β) are attached to the cell surface through a glycosyl-phosphatidylinositol (GPI) membrane anchor,20,23 whereas the third (FR-γ) is constitutively secreted.24 The expression of FR-α in normal tissues is restricted to certain epithelial cells, where it is inaccessible by the circulation.25,26 FR-α is found to be amplified in many epithelial-lineage human tumors, including approximately 90% of ovarian carcinomas.26-29 A wide variety of experimental therapies,30 including drug-loaded liposomes,31-34 have successfully targeted FR-α selectively in gynecologic and other tumors. The expression of FR-β in normal tissues is restricted to placenta and hematopoietic cells, where it is expressed in the myelomonocytic lineage and is particularly elevated during neutrophil maturation or during monocyte or macrophage activation.29,35,36 FR-β is also known to be expressed in chronic myelogenous leukemia (CML) and in AML.22,37,38FR-β from membrane preparations from CML and AML spleen samples and activated macrophage bound folic acid,22,36 but a protein in CD34+ cells immunoreactive to anti-FR antiserum did not.39 FR-β expression in KG-1 AML cells and in primary AML blasts obtained from the bone marrow of AML patients may be further induced in vitro up to 20-fold by brief (1- to 5-day) treatment with all-trans retinoic acid (ATRA) in a manner independent of the induction of cell differentiation and growth inhibition.40 These findings raise the possibility of selective targeting of drugs to FR-β in AML cells.
Here we present additional data on the extent of FR-β expression in AML cells from a large number of patients and its coexpression with CD34. We also demonstrate that FR-β in normal neutrophils is unable to bind folate because of posttranslational modification, in contrast to AML cells. We further report the in vitro binding and cytotoxicity of folate-coated liposomal DOX (f-L-DOX) in leukemic cells, particularly in combination with ATRA, and the in vivo therapeutic efficacy of this approach in mice engrafted with FR (+) murine leukemia L1210JF or KG-1 human AML cells. The combined studies from our 2 laboratories support the feasibility of delivering liposomal therapeutics to AML cells by combining FR-β targeting and ATRA treatment.
Materials and methods
Cell lines
L1210, CHO-K1, KG-1, and KG-1a cells were purchased from American Type Culture Collection (Rockville, MD). The murine FR-α (+) subline L1210JF, generated by adapting L1210 cells in low folate culture media, was kindly provided by Dr J. Fan (National Institutes of Health, Bethesda, MD). FR-β–transfected Chinese hamster ovary (CHO-FR–β) cells were generated as described previously.41 The L1210, L1210JF, CHO-K1, and CHO-FR–β cells were cultured at 37°C in 5% CO2 in folate-free RPMI 1640 medium, supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 292 μg/mL l-glutamine, and 10% fetal bovine serum (FBS; Gibco-BRL, Grand Island, NY) as the only source of folate. KG-1 and KG-1a cells were cultured in media supplemented with 20% FBS.
Antibodies and reagents
Rabbit antisera to purified FR-β was generated as described previously.21 Phycoerythrin (PE)–anti-CD34 mouse mAb and allophycocyanin (APC)–anti-CD45 mouse mAb were purchased from Becton Dickinson (Bedford, MA). Fluorescein isothiocyanate (FITC)–goat anti-rabbit IgG was purchased from Cappel (Organon Teknika, Durham, NC). ATRA, folic acid, cholesterol (Chol), calcein, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and DOX were purchased from Sigma-Aldrich (St Louis, MO). Phosphatidylinositol-specific phospholipase C (PI-PLC) was purchased from Boehringer Mannheim (Indianapolis, IN). Distearoylphosphatidylcholine (DSPC) and methoxy-polyethyleneglycol (MWt approximately 2000)-distearoylphosphatidylethanolamine (mPEG-DSPE) were purchased from Avanti Polar Lipids (Alablaster, AL). [3H] Folic acid was purchased from Moravek Biochemicals (Brea, CA). BCA protein assay kit was purchased from Pierce Chemical (Rockford, IL). FITC-folate and folate-polyethyleneglycol (MWt approximately 3350)-distearoylphosphatidylethanolamine (folate-PEG-DSPE) were synthesized as described previously.32 42
Flow cytometry analysis
Leukocytes from normal peripheral blood were prepared by centrifugation of whole blood (10 mL) for 10 minutes at 400gand incubating the cells from the buffy coat with 11 mL lysis buffer (155 mM NH4Cl, 10 mM KHCO3, and 1 mM EDTA) at room temperature for 15 minutes. Leukocytes were sedimented by centrifugation at 400g for 10 minutes. Red blood cell debris was removed gently together with the supernatant fluid, and the leukocytes were washed twice with 10 mM sodium phosphate buffer (pH 7.5) and 150 mM NaCl (phosphate-buffered saline [PBS]).
Mononuclear cells from normal bone marrow were separated by Ficoll–Hypaque gradient centrifugation (Pharmacia, Gaithersburg, MD). The cells, recovered from the interphase, were washed with PBS containing 1% normal goat serum and 0.1% sodium azide (buffer B) and finally were resuspended in the same buffer. Leukemic blasts were received frozen from the Pediatric Oncology Group tissue bank (St Jude's Children's Hospital, Memphis, TN). The cells were rapidly thawed under hot running water and were immediately resuspended at 37°C in RPMI 1640 media containing 30% FBS. Viability of the leukemic blasts was greater than 90% based on trypan blue exclusion. From CD45/side scatter data, more than 90% of the cells appeared to be leukemic blasts.
For immunostaining, cells (1 × 106) first were incubated on ice with 20% human normal AB serum (Gel-Freeze, Milwaukee, WI) for 30 minutes. They then were washed once with buffer B, followed by incubation with either anti-FR antibody or normal rabbit immunoglobulin (negative control) in 120 μL buffer B for 60 minutes on ice with intermittent mixing. PE-conjugated anti-CD34 antibody, together with APC-conjugated anti-CD45 antibody, was added, and the incubation was continued for 30 minutes. Cells were washed twice with buffer B. FITC-goat anti-rabbit IgG (1:500 dilution) was added to the cells suspended in 0.5 mL buffer B and was incubated for 30 minutes on ice with intermittent gentle mixing. After 2 washes with buffer B, the cells were mixed with 300 μL 1% paraformaldehyde and stored at 4°C. Cells were examined on a flow cytometer within 24 hours. The threshold to determine a positive signal by flow cytometry was obtained with normal rabbit IgG isotype control.
To measure the binding of FITC-folate to whole cells, the cells were first washed with low pH buffer (10 mM sodium acetate, pH 4, 150 mM NaCl, 7 mM glucose) at 4°C to remove endogenous bound folate, followed by 2 washes with PBS and incubation with the reagent (10 nM) in PBS at 4°C for 30 minutes. Cells were then washed with PBS and analyzed by flow cytometry. In parallel, the cells were preincubated for 10 minutes with folic acid (1 μM) before the addition of FITC-folate.
Cell lysates and Western blots
Cell lysates were prepared using Triton X-100 as described previously and subjected to Western blot analysis by probing with rabbit anti–FR-β antibody, as previously described.40Band intensities were estimated by using NIH Image software.
PI-PLC cleavage
As described previously,43 cells were washed with PBS, sedimented at 1000g at 4°C, and resuspended in a digesting buffer (25 mM Tris-HCl, pH 7.5, 250 mM sucrose, 10 mM glucose, 1% BSA). Cells (106) were then treated with 0.3 U PI-PLC for 1 hour at 37°C and washed twice with cold PBS.
Nitrous acid cleavage
As described previously,43 crude membranes were suspended in 50 mM sodium acetate, pH 3.5, containing 0.165 M freshly dissolved NaNO2 and were incubated for 6 hours at room temperature and washed with PBS.
[3H] Folic acid binding assay
As described previously,43 cells (2 × 106 per mL) were washed successively at 4°C with pH 4 buffer (10 mM sodium acetate, 150 mM NaCl, 7 mM glucose) and PBS to remove endogenous folate. Cells (106) were then incubated with 1.2 pmol [3H] folic acid in 0.5 mL PBS at 37°C for 30 minutes, sedimented at 1000g, chilled to 4°C, and washed with cold PBS. Radioactivity in the pellet was measured by liquid scintillation counting.
RT-PCR and cDNA sequence analysis
Total RNA from cells was isolated by using the guanidinium thiocyanate-phenol-chloroform single-step extraction method (Stratagene). RNA was reverse transcribed and amplified by PCR, as described previously.22 Several combinations of oligonucleotide probes, corresponding to various sequences in the FR-β cDNA, were used for the PCR amplification including (1) GATCTATTGCCTACTTAGAGAGAGGC and ACAACTTTAACTGGGACCACTGC and (2) GTGGTCCCAGTTAAAGTTGTACAGG and GGGTTAGGTGACTAATAGAAGCATGC. PCR products were purified after electrophoresis from a 1% agarose gel using the Geneclean kit (BIO101, La Jolla, CA). Purified PCR products (0.5 mg DNA each) were subjected to DNA sequence analysis using PCR sequencing on a Perkin Elmer GeneAmp PCR System 9600 (Shelton, CT).
Deglycosylation
Detergent extracts of crude cell membranes were treated with N-glycanase (Boehringer-Mannheim) to deglycosylate FR-β, as described previously.44
Liposome preparation
Liposomes composed of DSPC/Chol/folate-PEG-DSPE (60:40:0.1, mol/mol) encapsulating 50 mM calcein were prepared by polycarbonate membrane extrusion, as described previously.32 Calcine and phospholipid concentrations were determined by absorption at 495 nm and a colorimetric assay,45 respectively. Liposomal entrapment efficiency was between 6% and 10%. For FR-targeted liposomes containing DOX (f-L-DOX), a composition of DSPE/Chol/mPEG-DSPE/folate-PEG-DSPE (60:36:4:0.1, mol/mol) was used. DOX was incorporated by remote loading, as described previously.46 DOX concentrations in the liposomal samples were measured by absorption at 480 nm. DOX loading efficiency was greater than 95%. Liposome size distribution was measured by light scattering on a Nicomp 370 submicron particle analyzer (Particle Sizing System, Santa Barbara, CA). Mean liposomal diameters were between 100 and 110 nm for all preparations. Liposome samples were stored at 4°C for up to 2 weeks and showed no significant (less than 1%) leakage of calcein or DOX during this period.
Cellular uptake of f-L-calcein
Approximately 106 cells were incubated in triplicate with f-L-calcein or L-calcein (20 μM calcein) in folate-free RPMI 1640 media (with or without 1 mM folic acid) for 1 hour at 37°C. Cells were then washed 3 times with cold PBS and examined by fluorescence microscopy or flow cytometry. Fluorescence microscopy was carried out on a Zeiss Axioshop Epifluorescence Microscope with an Optronics 3 chip low-light level color CCD camera attachment (Thornwood, NY). Digital images were analyzed using the NIH Image software. Flow cytometry was performed on a Beckman Coulter Elite Flow Cytometer, using at least 105 cells (Miami, FL). Cellular uptake was presented as the relative fluorescence index. For the AML KG-1 and KG-1a cells, cellular uptake was also determined with cells exposed for 5 days to 1 μM ATRA. For KG-1 cells, f-L-calcein uptake was also measured with cells pretreated with PI-PLC.
Determination of the in vitro cytotoxicity of f-L-DOX to leukemia cells
Cells were aliquoted into 24-well plates at a density of approximately 2 × 105 cells per well (in 300 μL). DOX dose range tested was 1 mM to 61 nM. Cells were incubated in quadruplicate with 1:4 serial dilutions of f-L-DOX, L-DOX, or free DOX for 2 hours at 37°C, washed 3 times with cold PBS, and cultured for an additional 72 hours in fresh media. For cell viability determination, 30 μL of 5 mg/mL MTT was added, followed by 2-hour incubation at 37°C. Cells were then sedimented by centrifugation at 1000g for 3 minutes, and the pellet was dissolved in 150 μL isopropanol containing 0.1 M HCl, which was then transferred to the wells of a 96-well plate. Absorption at 570 nm was read on a Dynatech MR-600 microplate reader. Concentrations of DOX required for 50% growth inhibition (IC50) were then obtained from the OD570 versus DOX concentration curves. For KG-1 and KG-1a cells, the MTT assay was also performed on cells that had been cultured for 5 days in media containing 1 μM ATRA. For receptor blocking studies, folic acid (1 mM) was added to media during drug exposure.
Evaluation of the therapeutic efficacy of f-L-DOX in murine leukemia models
Antileukemic activity of f-L-DOX was evaluated in 2 FR (+) murine leukemia ascites tumor models. The first model consists of DBA/2 mice (Charles River, Wilmington, MA) carrying ascites tumors from the murine FR (+) L1210JF cells, an FR (+) subline of murine lymphocytic leukemia cell line L1210. Male mice (18-22 g) were placed on a folate-deficient diet (AIN-90G; Dyets, Bethlehem, PA) on arrival and for at least 1 week before leukemia cell inoculation. Normal rodent chow was not used because it contains approximately 3.19 mg/kg folic acid, which leads to supraphysiological serum folate. Mice (in groups of 8) were injected intraperitoneally with 1 × 106 L1210JF cells on day 0 and then were given 3 intraperitoneal injections (in 50 μL) of various DOX formulations or saline control on days 1, 5, and 9. Animal survival was then monitored daily until day 80. The second model consists of severe combined immunodeficient (SCID) mice (Charles River, Wilmington, MA) carrying ascites tumor from KG-1 cells. Female CB.17 SCID (scid/scid, 18-22 g) mice were inoculated intraperitoneally with 106 cells on day 0. Without treatment, visible ascites fluid developed at approximately day 30. Peritoneal exudate cells were collected by peritoneal lavage with 5 mL Hanks balanced salt solution, pelleted, and resuspended in RPMI 1640 media. These cells exhibited a morphology similar to that of KG-1 cells maintained in vitro. Half the mice received daily intraperitoneal injections of 10 mg/kg ATRA from days 1 through 5. Mice (in groups of 8) then received intraperitoneal injections (in 50 μL) of the DOX formulations or saline control on days 1, 5, and 9. Animal survival was then monitored daily until day 100. Comparisons of animal survival data were carried out by a log-rank test.
Results
Ligand binding by FR-β in granulocytes and AML cells
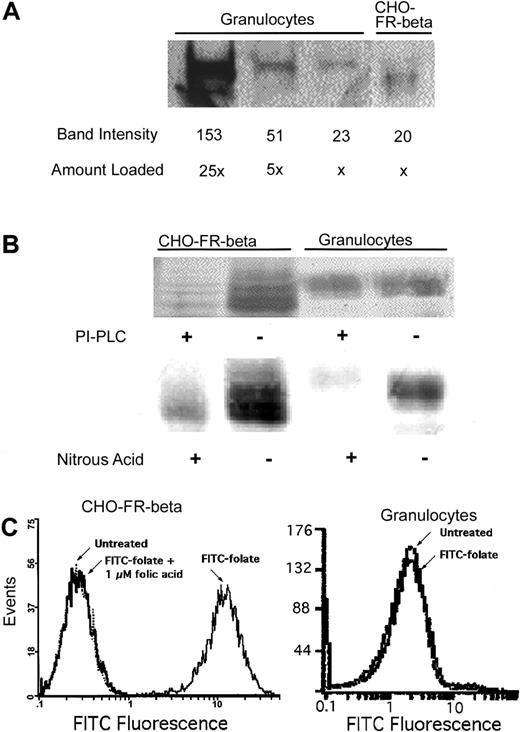
Because neutrophils express the highest level of FR-β in normal hematopoiesis, it was of interest to examine its ligand-binding property in those cells. Western blots of membrane preparations from normal peripheral blood granulocytes probed with antibody to FR-β gave a diffuse band with an apparent molecular weight slightly higher than that of FR-β from recombinant CHO–FR-β cells (Figure1A). From a semiquantitative analysis of the Western blot in Figure 1, the level of FR-β expressed in granulocytes is comparable to that of the recombinant CHO–FR-β cells. In contrast to FR-β in CHO–FR-β cells, the receptor in the granulocytes could not be released from the cell surface with PI-PLC, a diagnostic test for the GPI membrane anchor (Figure 1B). Several tissues are known to confer modification on the inositol ring of the GPI anchor, causing resistance to PI-PLC.43 In such instances, the anchor may be cleaved by nitrous acid.43When membranes prepared from granulocytes were treated with nitrous acid, FR-β was released, suggesting the presence of a modified GPI anchor for FR-β in these cells (Figure 1B). FR expressed in granulocytes, however, was unable to bind FITC-folate, unlike that in CHO–FR-β cells (Figure 1C).
Expression and properties of FR-β in peripheral blood granulocytes and in recombinant CHO–FR-β cells.
(A) FR-β expression detected in cell lysate by Western blot using anti–FR-β antibody. Band intensities were estimated using NIH Image software. (B) Differential sensitivity of FR-β GPI anchors to PI-PLC and nitrous acid; whole cells were treated with PI-PLC, and the cell lysates were analyzed by Western blot using anti–FR-β antibody. Alternatively, cell membranes were treated with nitrous acid and similarly analyzed. (C) Cellular binding of FITC-folate determined by flow cytometry. 10 nM FITC-folate was used with or without preincubation with 1 μM folic acid, as described in “Materials and methods.”
Expression and properties of FR-β in peripheral blood granulocytes and in recombinant CHO–FR-β cells.
(A) FR-β expression detected in cell lysate by Western blot using anti–FR-β antibody. Band intensities were estimated using NIH Image software. (B) Differential sensitivity of FR-β GPI anchors to PI-PLC and nitrous acid; whole cells were treated with PI-PLC, and the cell lysates were analyzed by Western blot using anti–FR-β antibody. Alternatively, cell membranes were treated with nitrous acid and similarly analyzed. (C) Cellular binding of FITC-folate determined by flow cytometry. 10 nM FITC-folate was used with or without preincubation with 1 μM folic acid, as described in “Materials and methods.”
To test for possible primary sequence variation in the FR expressed in neutrophils, total mRNA from these cells was reverse transcribed, and the cDNA was amplified by PCR using combinations of primers corresponding to the sequence of FR-β (see “Materials and methods”). No sequence divergence was observed from the known cDNA sequence of FR-β (results not shown), suggesting that the inability of FR-β expressed in neutrophils to bind ligand is likely because of cell type–specific posttranslational modification. Both the nature of N-glycosylation and modifications of the GPI anchor can alter the ligand-binding properties of proteins, but it has been shown that FR-β may be completely deglycosylated without loss of its ability to bind folic acid.21 It has also been shown that modification of the GPI anchor in murine FR-α in a specific cell type altered its affinity for folate and that this effect was reversed on membrane solubilization with nonionic detergent.43 Because neither deglycosylation nor detergent solubilization (see “Materials and methods”) could restore the ligand-binding property of the receptor (results not shown), the nature of the modification in FR-β in neutrophils that disrupts its ligand-binding property remains unknown. In contrast to peripheral blood granulocytes, FR-β (+) AML cells bound [3H] folic acid to the extent (0.72 ± 0.13 pmol per 106 cells) predicted by the relative level of FR-β expression from flow cytometry using anti–FR-β antibody. Furthermore, the binding of [3H] folic acid by the FR-β (+) AML cells could be blocked by preincubation with 10 nM folic acid, indicating that the binding was FR specific.
Frequency of FR-β expression in AML
Using a limited number of samples, FR-β has been shown to be differentially expressed in myeloid leukemia.35 FR-β expression in AML can be further induced by retinoids.40In this study, we analyzed 78 AML samples for FR-β expression. Table1 is a summary of FR-β expression in AML cells obtained from patient marrow examined by flow cytometry. CD34 expression in the leukemia samples is also noted (Table 1). The data show that most (68%) of the AML samples tested were positive for FR-β. Furthermore, in most (38 of 53) of the FR-β (+) AML bone marrow samples, 100% of the cells expressed FR-β. In addition, 66% of the FR-β (+) samples were also CD34+ (Table1).
Expression of FR-β and CD34 in AML patient bone marrow aspirates determined by flow cytometry
| FAB class (total) . | FR-β+ . | FR-β+/CD34+ . |
|---|---|---|
| M0 (1) | 0 | 0 |
| M1 (9) | 3 | 2 |
| M2 (10) | 4 | 4 |
| M3 (4) | 3 | 0 |
| M4 (13) | 11 | 9 |
| M5 (11) | 11 | 6 |
| M6 (2) | 2 | ND |
| ND (28) | 19 | 14 |
| FAB class (total) . | FR-β+ . | FR-β+/CD34+ . |
|---|---|---|
| M0 (1) | 0 | 0 |
| M1 (9) | 3 | 2 |
| M2 (10) | 4 | 4 |
| M3 (4) | 3 | 0 |
| M4 (13) | 11 | 9 |
| M5 (11) | 11 | 6 |
| M6 (2) | 2 | ND |
| ND (28) | 19 | 14 |
Total number of AML samples tested: 78.
Number of samples in which 100% of the cells were FR-β+: 38.
Number of samples in which 80%-90% of cells were FR-β+: 4.
Number of samples in which 30%-60% of cells were FR-β+: 8.
Total number of FR-β+ AML samples: 53.
Percentage FR-β+ samples: 68.
Percentage positivity represents the fraction of cells in each sample that gave a positive peak-shift with anti–FR-β antibody relative to the threshold for each sample defined by the peak position of the antibody isotype control.
ND indicates not determined.
Uptake of f-L-calcein by FR-β–expressing cells
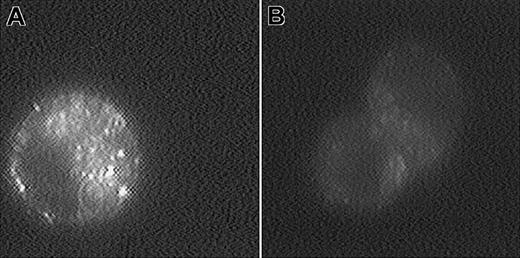
The cellular uptake of f-L-calcein was determined in FR (+) human KG-1 AML, murine L1210JF ALL (expressing murine FR-α), and CHO–FR-β cells and in FR (−) KG-1a, L1210, and CHO-K1 cells. L-calcein was used as a nontargeted control. Uptake of the liposomes in KG-1 cells was also examined by fluorescence microscopy (Figure2). Overall fluorescence was much greater in cells treated with f-L-calcein than in those treated with L-calcein. Intracellular distribution of the fluorescence displayed a punctate pattern in the former (Figure 2). This observation suggests internalization of f-L-calcein through FR-β–mediated endocytosis and sequestration of the liposomal calcein in intracellular endosomal compartments, a pattern similar to the one reported for FR-α–expressing KB human oral cancer cells treated with f-L-calcein,31 suggesting a similar intracellular trafficking pattern for the 2 receptor subtypes.
Fluorescence micrographs of KG-1 cells treated with calcein-containing liposomes.
KG-1 cells were incubated with f-L-calcein (A) or L-calcein (B) for 1 hour at 37°C and were washed 3 times with cold PBS. Images of the live cells were collected with a digital imaging system, as described in “Materials and methods.” Magnification, × 2000.
Fluorescence micrographs of KG-1 cells treated with calcein-containing liposomes.
KG-1 cells were incubated with f-L-calcein (A) or L-calcein (B) for 1 hour at 37°C and were washed 3 times with cold PBS. Images of the live cells were collected with a digital imaging system, as described in “Materials and methods.” Magnification, × 2000.
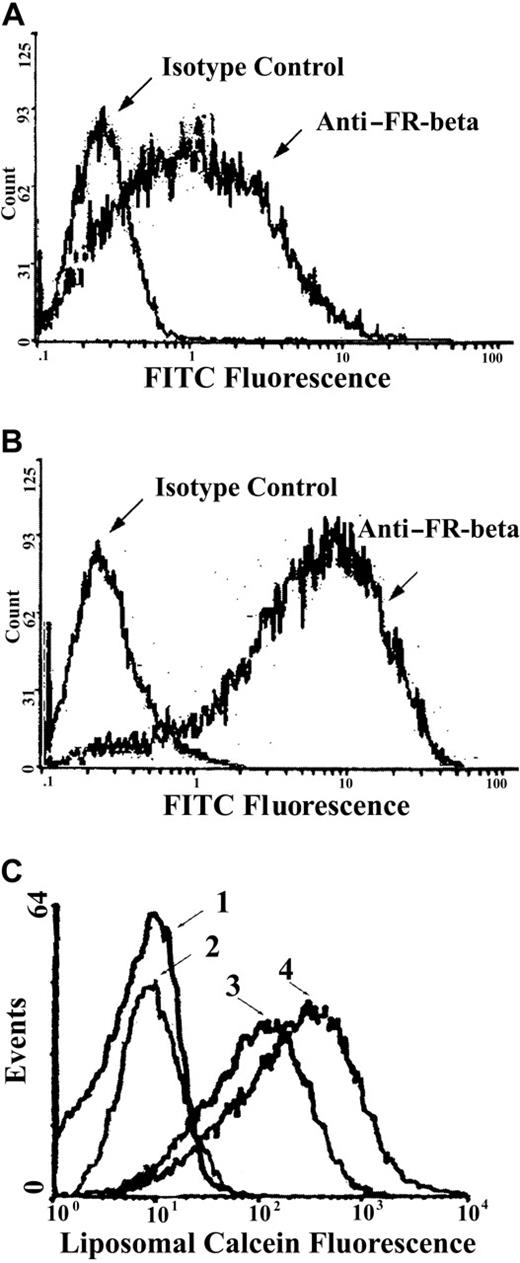
KG-1 cells have previously been reported to up-regulate FR-β in response to ATRA.40 We analyzed by flow cytometry the effect of ATRA treatment of KG-1 cells on FR-β expression and f-L-calcein uptake. As expected, culturing KG-1 cells in the presence of ATRA resulted in an increase in FR-β expression (Figure3A, B). In addition, these cells also showed FR-selective uptake of f-L-calcein but not of L-calcein, with ATRA-treated cells showing further increased uptake of f-L-calcein (Figure 3C). Uptake of f-L-calcein and L-calcein in various FR (+) and FR (−) cell lines was quantified by flow cytometry (Table2). Cellular uptake of f-L-calcein was considerably greater than that of L-calcein among the FR (+) cell lines, but not among the FR (−) cell lines. F-L-calcein uptake was further increased approximately 5-fold in KG-1 cells cultured for 5 days in media containing 1 μM ATRA (Figure 3C; Table 2), presumably because of FR-β induction. In contrast, the FR (−) KG-1a subline did not show FR-β expression or preferential uptake of f-L-calcein, with or without pretreatment with ATRA (Table 2). Cellular uptake of f-L-calcein in FR (+) cells was blocked by 1 mM free folic acid (Table2), indicating that uptake was primarily due to FR. A lower concentration of folic acid (1 μM) did not block folate-L-DOX uptake in FR (+) cells. These data indicate that FR-β is capable of high-affinity binding and internalization of f-L-calcein in a fashion similar to that for FR-α and that up-regulation of FR-β with ATRA could further increase the cellular uptake of f-L-calcein. The data (Table 2) also showed that f-L-calcein uptake in KG-1 cells was blocked by pretreatment with PI-PLC, which releases cell surface FR-β by cleaving its GPI membrane anchor, further confirming the role of the FR.
Effect of ATRA on FR-β expression and f-L-calcein uptake in KG-1 cells.
Cellular FR-β expression was determined by flow cytometry using rabbit anti–FR-β (with normal rabbit IgG as an isotype control) as the primary antibody and FITC-goat anti-rabbit IgG as the secondary antibody. Liposomal uptake was determined by fluorescence of the encapsulated calcein. (A) FR-β expression in untreated KG-1 cells. (B) FR-β expression in KG-1 cells after a 5-day exposure to ATRA (1 μM). (C) Uptake of f-L-calcein and L-calcein by KG-1 cells and the effect of ATRA. Cells were treated with L-calcein without ATRA pretreatment (1), L-calcein with ATRA pretreatment(2), f-L-calcein without ATRA pretreatment (3), or f-L-calcein with ATRA pretreatment (4).
Effect of ATRA on FR-β expression and f-L-calcein uptake in KG-1 cells.
Cellular FR-β expression was determined by flow cytometry using rabbit anti–FR-β (with normal rabbit IgG as an isotype control) as the primary antibody and FITC-goat anti-rabbit IgG as the secondary antibody. Liposomal uptake was determined by fluorescence of the encapsulated calcein. (A) FR-β expression in untreated KG-1 cells. (B) FR-β expression in KG-1 cells after a 5-day exposure to ATRA (1 μM). (C) Uptake of f-L-calcein and L-calcein by KG-1 cells and the effect of ATRA. Cells were treated with L-calcein without ATRA pretreatment (1), L-calcein with ATRA pretreatment(2), f-L-calcein without ATRA pretreatment (3), or f-L-calcein with ATRA pretreatment (4).
Cellular uptake of f-L-calcein by various cell lines determined by flow cytometry
| Cell line . | FR (+) . | FR (−) . | ||||||
|---|---|---|---|---|---|---|---|---|
| KG-1 . | L1210JF . | CHO-FR-β . | KG-1a . | L1210 . | CHO-K1 . | |||
| Pre-exposure to ATRA . | − . | + . | − . | − . | − . | + . | − . | − . |
| L-calcein | 9.3 ± 2.1 | 9.1 ± 1.3 | 5.7 ± 0.6 | 6.1 ± 1.3 | 5.4 ± 1.3 | 5.1 ± 0.3 | 4.7 ± 0.3 | 5.1 ± 2.3 |
| f-L-calcein | 61 ± 17 | 320 ± 78 | 78 ± 9 | 91 ± 12 | 6.1 ± 0.7 | 6.7 ± 0.9 | 7.1 ± 1.2 | 5.7 ± 1.5 |
| f-L-calcein + 1 mM folate | 11 ± 1.4 | 27 ± 2 | 8.3 ± 1.7 | 7.3 ± 0.2 | 5.1 ± 0.5 | 7.3 ± 1.4 | 6.4 ± 1.5 | 4.9 ± 0.7 |
| f-L-calcein + 1 μM folate | 63 ± 11 | ND | 81 ± 13 | ND | ND | ND | ND | ND |
| f-L-calcein + pretreated w/PI-PLC | 13 ± 4 | ND | ND | ND | ND | ND | ND | ND |
| Cell line . | FR (+) . | FR (−) . | ||||||
|---|---|---|---|---|---|---|---|---|
| KG-1 . | L1210JF . | CHO-FR-β . | KG-1a . | L1210 . | CHO-K1 . | |||
| Pre-exposure to ATRA . | − . | + . | − . | − . | − . | + . | − . | − . |
| L-calcein | 9.3 ± 2.1 | 9.1 ± 1.3 | 5.7 ± 0.6 | 6.1 ± 1.3 | 5.4 ± 1.3 | 5.1 ± 0.3 | 4.7 ± 0.3 | 5.1 ± 2.3 |
| f-L-calcein | 61 ± 17 | 320 ± 78 | 78 ± 9 | 91 ± 12 | 6.1 ± 0.7 | 6.7 ± 0.9 | 7.1 ± 1.2 | 5.7 ± 1.5 |
| f-L-calcein + 1 mM folate | 11 ± 1.4 | 27 ± 2 | 8.3 ± 1.7 | 7.3 ± 0.2 | 5.1 ± 0.5 | 7.3 ± 1.4 | 6.4 ± 1.5 | 4.9 ± 0.7 |
| f-L-calcein + 1 μM folate | 63 ± 11 | ND | 81 ± 13 | ND | ND | ND | ND | ND |
| f-L-calcein + pretreated w/PI-PLC | 13 ± 4 | ND | ND | ND | ND | ND | ND | ND |
Values shown represent the means of 3 measurements ± 1 SD.
ND indicates not determined.
In vitro cytotoxicity of f-L-DOX to cultured leukemia cells
FR-targeted liposomal doxorubicin (f-L-DOX) was evaluated for in vitro cytotoxicity in FR (+) and FR (−) leukemia cells by MTT assay. IC50 values (Tables3-4) show that F-L-DOX is 25 times more cytotoxic than L-DOX to the FR-β (+) KG-1 cells in the absence of ATRA and 63 times more cytotoxic with ATRA pretreatment (Tables 3-4). In contrast, no therapeutic advantage or an ATRA-induction effect on cytotoxicity is observed with f-L-DOX in the KG-1a cell line, which is FR-β (−) (Tables 3-4). Superior cytotoxicity of f-L-DOX over L-DOX was also observed in the FR (+) L1210JF cell line, but not in the FR (−) L1210 cells (Tables 3-4). Data in Tables 3 and 4 further show that free folic (1 mM) increased the IC50 of folate-L-DOX in KG-1 cells (ATRA treated and untreated) and L1210JF cells by approximately one order of magnitude. These are in agreement with the observed inhibition of binding of folate-L-DOX to the same cells (Table 2).
Cytotoxicity of f-L-DOX to cultured leukemia cells: IC50 values (μM) for cells incubated in folate-free media
| Leukemia cell line . | KG-1 . | KG-1a . | L1210JF . | L1210 . | ||
|---|---|---|---|---|---|---|
| FR expression status . | FR-β (+) . | FR (−) . | FR-α (+) . | FR (−) . | ||
| Pre-exposure to ATRA . | − . | + . | − . | + . | − . | − . |
| Free DOX | 0.65 | 0.63 | 0.61 | 0.59 | 0.86 | 0.91 |
| f-L-DOX | 0.97 | 0.43 | 19 | 17 | 1.3 | 27 |
| Nontargeted L-DOX | 24 | 27 | 23 | 22 | 34 | 29 |
| Leukemia cell line . | KG-1 . | KG-1a . | L1210JF . | L1210 . | ||
|---|---|---|---|---|---|---|
| FR expression status . | FR-β (+) . | FR (−) . | FR-α (+) . | FR (−) . | ||
| Pre-exposure to ATRA . | − . | + . | − . | + . | − . | − . |
| Free DOX | 0.65 | 0.63 | 0.61 | 0.59 | 0.86 | 0.91 |
| f-L-DOX | 0.97 | 0.43 | 19 | 17 | 1.3 | 27 |
| Nontargeted L-DOX | 24 | 27 | 23 | 22 | 34 | 29 |
Assays were repeated twice. Within each assay, cells were treated in quadruplicate. The error within each group was less than 20%.
Cytotoxicity of f-L-DOX to cultured leukemia cells: IC50 (μM) values for cells incubated in media containing 1 mM folic acid
| Leukemia cell line . | KG-1 . | KG-1a . | L1210JF . | L1210 . | ||
|---|---|---|---|---|---|---|
| FR expression status . | FR-β (+) . | FR (−) . | FR-α (+) . | FR (−) . | ||
| Pre-exposure to ATRA . | − . | + . | − . | + . | − . | − . |
| Free DOX | 0.67 | 0.68 | 0.63 | 0.62 | 0.87 | 0.93 |
| f-L-DOX | 11 | 5.4 | 21 | 19 | 12 | 29 |
| Nontargeted L-DOX | 27 | 29 | 23 | 20 | 35 | 31 |
| Leukemia cell line . | KG-1 . | KG-1a . | L1210JF . | L1210 . | ||
|---|---|---|---|---|---|---|
| FR expression status . | FR-β (+) . | FR (−) . | FR-α (+) . | FR (−) . | ||
| Pre-exposure to ATRA . | − . | + . | − . | + . | − . | − . |
| Free DOX | 0.67 | 0.68 | 0.63 | 0.62 | 0.87 | 0.93 |
| f-L-DOX | 11 | 5.4 | 21 | 19 | 12 | 29 |
| Nontargeted L-DOX | 27 | 29 | 23 | 20 | 35 | 31 |
Assays were repeated twice. Within each assay, cells were treated in quadruplicate. The error within each group was less than 20%.
Therapeutic efficacy of f-L-DOX in murine leukemia models
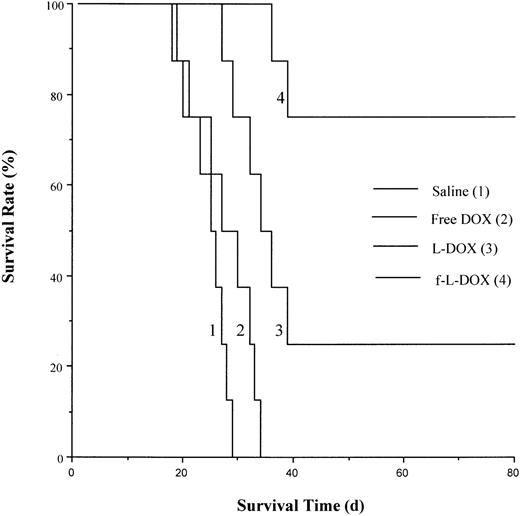
The antileukemic therapeutic efficacy of f-L-DOX was evaluated in 2 FR (+) murine leukemia ascites models. The first model consists of DBA/2 mice with ascites tumors from the FR (+) L1210JF murine leukemia cells. Animals were treated with one of the following by intraperitoneal injection: saline (0.9% NaCl, USP), free DOX (3 mg/kg), L-DOX (5 mg/kg DOX), or f-L-DOX (5 mg/kg DOX). The free DOX dose (3 mg/kg) was chosen to correspond to a value below (at approximately 10% of) its previously reported LD50value47 of approximately 26 mg/kg. The L-DOX or f-L-DOX dose (5 mg/kg) used was equitoxic to 3 mg/kg free DOX, based on previous findings showing that the LD50 of L-DOX was between 1.5 and 2 times higher than that of free DOX in mice.47 48 Median survival time for the 4 groups of mice were 25.5, 28.5, 35, and more than 80 days, respectively (Figure4). The data clearly indicated that L-DOX was more effective than free DOX in prolonging animal survival (P = .0159; log-rank test), presumably because of the prolonged systemic circulation time of L-DOX compared to free DOX. Meanwhile, f-L-DOX was even more efficacious than L-DOX (P = .0259; log-rank test), extending the long-term survival rate to 75%, presumably because of more effective DOX delivery through FR-mediated tumor cell targeting.
Effect of f-L-DOX treatment on the survival of DBA/2 mice carrying L1210JF ascites tumor.
DBA/2 mice inoculated intraperitoneally with L1210JF cells were treated with saline, free DOX, L-DOX, or f-L-DOX, as described in “Materials and methods.” Animal survival was recorded starting from the day of tumor cell inoculation.
Effect of f-L-DOX treatment on the survival of DBA/2 mice carrying L1210JF ascites tumor.
DBA/2 mice inoculated intraperitoneally with L1210JF cells were treated with saline, free DOX, L-DOX, or f-L-DOX, as described in “Materials and methods.” Animal survival was recorded starting from the day of tumor cell inoculation.
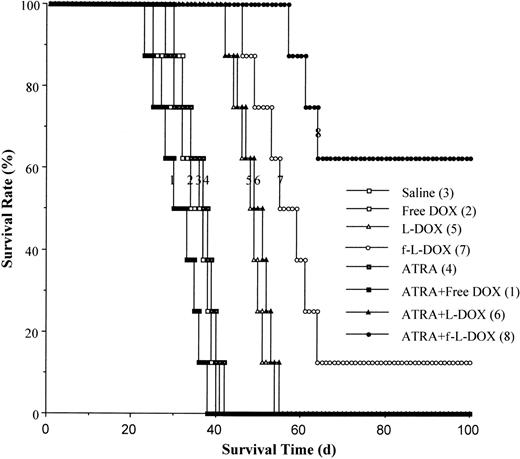
We further evaluated the therapeutic efficacy of f-L-DOX in an ascites tumor xenograft model consisting of CB.17 SCID mice inoculated intraperitoneally with the human KG-1 AML cells. Half the animals received daily intraperitoneal injections of 10 mg/kg ATRA on days 1 through 5. The following formulations were administered by intraperitoneal injection on days 1, 5, and 9: (1) saline; (2) free DOX (3 mg/kg); (3) L-DOX (5 mg/kg); (4) f-L-DOX (5 mg/kg); (5) saline + ATRA; (6) free DOX + ATRA; (7) L-DOX + ATRA; and (8) f-L-DOX + ATRA. Data on animal survival (Figure5) indicate that L-DOX is more effective than free DOX (P = .0001; log-rank test) but does not yield long-term survival (Figure 5). F-L-DOX is more effective than L-DOX (P = .0061; log-rank test), resulting in long-term survival. ATRA cotreatment further improves the long-term survival rate of the f-L-DOX–treated animals from 12.5% to 60% (P = .0190; log-rank test). The pronounced improvement in therapeutic efficacy with ATRA is most likely the result of up-regulation of FR-β expression on KG-1 cells in vivo because ATRA does not induce differentiation or growth inhibition of KG-1 cells in vitro40 and ATRA treatment in the absence of f-L-DOX or the L-DOX control does not enhance survival (Figure 5). These results validate the scheme of enhancing the efficacy of FR-β–targeted therapy through receptor up-regulation using ATRA.
Effect of f-L-DOX treatment on the survival of SCID mice carrying KG-1 ascites tumor.
SCID mice inoculated intraperitoneally with KG-1 cells were treated with saline, free DOX, L-DOX, or f-L-DOX, with or without coinjection of ATRA, as described in “Materials and methods.” Animal survival was recorded starting from the day of tumor cell inoculation.
Effect of f-L-DOX treatment on the survival of SCID mice carrying KG-1 ascites tumor.
SCID mice inoculated intraperitoneally with KG-1 cells were treated with saline, free DOX, L-DOX, or f-L-DOX, with or without coinjection of ATRA, as described in “Materials and methods.” Animal survival was recorded starting from the day of tumor cell inoculation.
Discussion
There are a number of instances in which cell surface antigens have been effectively targeted in the treatment of leukemia. For example, Gemtuzumab ozogamicin (CMA-676), which consists of a cytotoxic drug, calicheamicin, linked to a human monoclonal antibody specific for the myelocyte marker CD33, has recently been approved by the Food and Drug Administration for clinical use.49Rituximab, an antibody against the B-cell–specific antigen CD20, is widely used in the clinic to treat low-grade B-cell lymphoma.50 There has also been progress in the preclinical and clinical development of immunotoxins and of cytokine fusion toxins for the treatment of leukemia.51 In a recent clinical trial, effective therapy of hairy cell leukemia has been reported using a recombinant immunotoxin containing an anti-CD22 variable domain.52 Although a large number of preclinical and clinical studies exploring the folate receptor as a potential therapeutic target have focused primarily on FR type-α in several solid tumors, recent reports35,39 40 and the present study highlight special advantages in the therapeutic targeting of FR-β. They are (1) the expression of FR-β in a large number of (CD34+) AMLs, (2) the inability of FR-β in normal hematopoietic cells to bind folate, (3) specific up-regulation of FR-β in AML cells by brief treatment with ATRA, and (4) greater accessibility of AML cells to systemic circulation than cells in solid tumors.
It has been reported that CD34+ bone marrow cells that express relatively low levels of a protein immunoreactive with anti-FR antiserum did not show detectable binding of folic acid.39However, activated synovial macrophages in rheumatoid arthritis36 and membranes from CML and AML spleens22,37 38 have the ability to bind the ligand. The results of this study clearly demonstrate the inability of FR-β expressed on the surfaces of mature granulocytes to bind folate. In contrast, FR-β expressed in leukemic blasts from bone marrow aspirates of AML patients and the receptor in KG-1 AML cells was able to bind folate. This curious pattern of association of functional FR-β with certain cell types is clearly not caused by mutations in the receptor and is presumably the result of cell type–specific posttranslational modification. Such a differential modification may be reflected in the slightly higher apparent molecular weight of the receptor seen in neutrophils. Cell surface protein in neutrophils immunoreactive with anti–FR-β antibody did, however, display the GPI membrane anchor typical of FR-β. Even though the receptor in neutrophils displayed a variant GPI anchor structure, detergent solubilization experiments could not implicate such a modification as the basis for the inability of neutrophils to bind folate. Deglycosylation experiments also excluded a role for the nature of N-glycosylation in neutrophils in causing the inability of FR-β to bind folate. Therefore, the nature of the functionally relevant cell type–specific posttranslational modification in FR-β remains unclear at this time; however, the foregoing studies underscore the possibility of obtaining selective killing of AML cells by targeting them with folate conjugates.
AML is characterized by the accumulation of poorly differentiated blast cells with limited proliferative capacity. Maintenance of this disease, therefore, appears to be dependent on a smaller population of leukemic stem cells that have extensive proliferative capacity. Divergent theories have been put forth to explain the nature of these proliferative cells and the process of leukemogenesis.53,54 Establishing unifying principles in AML leukemogenesis may be further complicated because of the phenotypic heterogeneity in AMLs. Therefore, it may not be possible to predict whether targeting a particular cell surface protein in AML cells will effectively wipe out the leukemic stem cells. It is encouraging, as the results of this study show, that FR-β is expressed in a wide variety of AMLs. In most of those AMLs, virtually all the leukemic cells are FR-β (+). Moreover, there is a high frequency of coexpression of FR-β and CD34 in these leukemias. Thus, there is a high likelihood that the proliferating leukemic cells also frequently express FR-β. Furthermore, Wang et al40 have reported that the mechanism of ATRA induction of FR-β predicts that AML cells in general should respond to ATRA by up-regulating FR-β. This prediction is based on the fact that the ATRA effect is mediated by all 3 types of nuclear receptors for ATRA (RARα, RARβ, and RARγ) and that, in model AML cells, the ATRA response bypasses the pathway of retinoid-induced differentiation, which is known to be mediated by RARα. In fact, ATRA treatment of primary leukemic cells obtained from patients with FAB M2 and FAB M4 AMLs (which are known to be refractory to ATRA differentiation therapy) resulted in the induction of FR-β.40 Recent studies from the Ratnam laboratory (to be published) show that the FR-β gene is a direct target of the nuclear receptors. Therefore, AML cells expressing any of the 3 nuclear receptors for ATRA may be expected to allow the up-regulation of FR-β by ATRA.
Data on in vitro cellular uptake of f-L-calcein and cytotoxicity of f-L-DOX in cultured FR-β–expressing KG-1 cells are similar to previous findings with FR-targeted liposomes in FR-α–expressing KB oral cancer and HeLa cervical cancer cells.31,32 The cellular uptake showed a strong dependence on available FR on the cell surface and can be increased through ATRA treatment in KG-1 cells. These findings support the notion that, unlike its counterpart in normal hematopoietic cells and mature neutrophils, FR-β expressed on KG-1 AML cells is functional and is capable of mediating internalization of folate-derivatized drug carriers. The high apparent affinity of f-L-calcein to KG-1 cells, as indicated by the inability of 1 μM folic acid to block cellular uptake, was likely a result of multivalent interactions between the liposomes and the cellular FRs. This result also implies that the circulating folate in serum is not high enough to cause significant inhibition of the binding of folate-coated liposomes to the target cells. The median serum folate level in humans has shown an increase since the FDA recommended dietary folate supplementation in the late 1990s from 12.6 μg/L (28.6 nM) to 18.7 μg/L (42.4 nM),55 mostly in the form of 6S-5-methyltetrahydrofolate. Not only is the concentration of circulating folate thus considerably lower than the level required to produce significant competition, but the affinity of the circulating form of folate (6S-5-methyltetrahydrofolate) for the β isoform of FR is approximately 35-fold lower than that of folic acid.56Therefore, serum folate is not likely to have a significant inhibitory effect on the binding of folate-coated liposome to tumor cells expressing FR-β.
F-L-DOX cytotoxicity in cultured leukemic cells was superior to that of L-DOX, showed a strong dependence on FR expression, and was increased in KG-1 cells with ATRA-induction of FR-β. These results are consistent with published reports on the cytotoxicity of f-L-DOX against FR-α–expressing KB and HeLa cells.31,32 Free DOX is known to enter cells efficiently through passive diffusion, resulting in a much lower IC50 value than the nontargeted L-DOX in vitro. This was expected because liposomal entrapment would make L-DOX unavailable for uptake during cellular exposure (2 hours). However, even the nontargeted L-DOX has been shown to be more therapeutically efficacious and to cause fewer side effects than free DOX when evaluated for tumor treatment in vivo.5 In the present study, f-L-DOX showed an IC50 value similar to free DOX even under in vitro conditions. This indicates a highly efficient drug delivery mechanism mediated by FR.
Data from in vivo survival analyses using 2 murine leukemia models show a significant therapeutic advantage of f-L-DOX over L-DOX and free DOX. In SCID mice carrying KG-1 ascites tumor, the therapeutic efficacy of f-L-DOX is further enhanced by administering ATRA, showing the potential advantage of combining FR-β up-regulation and FR-β targeting in leukemia treatment in vivo. In the in vivo KG-1 ascites tumor model, enhancement of median survival and cure rates of f-L-DOX by ATRA appears to be more dramatic than the relatively modest (approximately 2.5-fold) decrease in the IC50 value for the in vitro cytotoxicity of f-L-DOX to KG-1 cells produced by ATRA. This observation may be explained by the possibility that the ATRA-enhanced cytotoxicity of f-L-DOX is relatively greater in the tumorigenic subpopulation of the phenotypically heterogenous KG-1 cells. ATRA induction of FR-β should be particularly beneficial in therapeutic targeting of FR-β in AML because it occurs in AML subtypes (all AMLs except APL) that are refractory to ATRA-differentiation therapy. Previous mechanistic studies of FR-β modulation by ATRA and other retinoids have shown that all 3 types of nuclear receptors for ATRA can mediate FR-β induction.40 Based on this finding, it may be predicted that, even in APL patients unresponsive to the standard ATRA therapy because of blocks in the differentiation pathway, ATRA should elevate FR-β expression. Therefore, FR-β–targeted therapy may be an effective means of eliminating minimal residual disease in APL patients after ATRA-differentiation therapy.
Lee et al57 recently reported that indium-111–labeled FR-targeted and nontargeted control liposomes injected intravenously showed indistinguishable patterns of biodistribution in mice. Recent studies by the same group have shown similar results with FR-targeted and nontargeted liposomes loaded with boron.58 These data suggested that the targeted liposomes were likely to have similar bioavailability in the bone marrow compared with nontargeted liposomes, which are known to be highly bioavailable in the bone marrow.
Current chemotherapy for AML has replaced DOX with the anthracycline drugs idarubicin and daunorubicin.59 Even though DOX is a potent cytotoxic, in its free form, the drug is more toxic than idarubicin and daunorubicin. Delivery of DOX in liposomal formulations alleviates the toxicity concerns while retaining its potency in killing malignant cells with the added advantage of bypassing multidrug resistance. The principles initially elucidated using DOX in this study may be expected to be applicable to other cytotoxic drugs that are effective against leukemic cells.
Because ATRA differentiation therapy is a routine treatment modality for the APL subtype of AML60 and liposomal DOX delivery is already used for the treatment of solid tumors, combining ATRA treatment with FR-targeted liposomal DOX should not meet with significant toxicity concerns in future clinical trials. This combined report from our 2 laboratories offers proof of principle of this concept and warrants further studies using additional murine leukemia models and extension to clinical settings.
We thank Dr Frederick G. Behm for supplying the bone marrow samples from the POG Tissue Bank and Thomas Sawyer in the Pathology Department at the Medical College of Ohio for his assistance in the flow cytometry studies.
Supported by American Cancer Society grant RPG-99-097-01-MGO, Leukemia and Lymphoma Society grant 6113-02 (R.J.L.), and National Institutes of Health R01 grants CA80183 and CA70873 (M.R.).
X.Q.P., X.Z., M.R., and R.J. L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert J. Lee, Division of Pharmaceutics, The Ohio State University, 500 W 12th Ave, Columbus, OH 43210; e-mail:lee.1339@osu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal