Immunosuppressive therapy leads to meaningful hematologic improvement in most patients with aplastic anemia (AA). Failure to respond and a later relapse could be due to deficient numbers of hematopoietic stem cells, inadequate treatment of the immune process, or a nonimmunologic etiology. Interferon-γ (IFN-γ) has been implicated in the pathophysiology of hematopoietic failure in AA. On the basis of previous findings showing overexpression of IFN-γ in bone marrow (BM) and peripheral blood (PB) in this disease, we hypothesized that quantitation of IFN-γ might be applied to predict and monitor responses to immunosuppressive therapy. We measured expression of IFN-γ in lymphocytes obtained from 123 AA patients, using intracellular 2-color fluorescent staining and flow cytometry. Of 70 patients with severe AA, 36 (51%) demonstrated increased IFN-γ in circulating T cells. IFN-γ was detected in only 4 of 53 patients who had recovered from AA. IFN-γ was not found in PB lymphocytes of patients with other hematologic diseases and heavy transfusion burdens or in healthy volunteers. Among 62 AA patients who were assessed before first treatment with immunosuppressive drugs, 27 of 28 (96%) with circulating IFN-γ–containing T cells subsequently responded to therapy; in contrast, only 11 of 34 (32%) patients whose PB lacked IFN-γ lymphocytes improved to transfusion independence. IFN-γ–containing lymphocytes declined following treatment in all cases. Of 17 patients assessed during relapse, IFN-γ was present in T cells prior to the blood count decline in 13, and 12 responded to reinstitution of immunosuppressive drugs. Of 30 BMs tested prior to first treatment, 20, all in responding patients, were positive for IFN-γ, whereas the negative tests were obtained in 10 nonresponding patients. IFN-γ is increased in the PB lymphocytes of many patients with AA, and these cells decline with therapy. The presence of intracellular IFN-γ may predict response to immunosuppressive treatment and also the onset of relapse.
Introduction
Substantial clinical and experimental evidence suggests that the immune system plays an important role in the pathophysiology of acquired aplastic anemia (AA). Various immunosuppressive drugs, including antithymocyte globulin (ATG), cyclosporine (CSA), and high doses of cyclophosphamide or corticosteroids, have produced hematologic improvement in the majority of patients with life-threatening cytopenias1; responses are usually equivalent to independence from the need for transfusion and an increase in neutrophil counts to levels adequate to prevent infection. For example, combined treatment with ATG and CSA produced durable responses in the majority of patients who were treated at the National Institutes of Health Clinical Center (Bethesda, MD)2 and is currently the treatment of choice in older patients and in children without HLA-matched sibling donors. Laboratory data accumulated over the last 2 decades have implicated an underlying immune-mediated destruction of blood-forming cells as pathophysiologic, manifested in vitro by coculture inhibition of hematopoietic colony formation by the patient's lymphocytes, an activated state of circulating cytotoxic lymphocytes, and increased production of cytokines typical of the T-helper 1 (TH1) response, especially interferon-γ (IFN-γ), tumor necrosis factor α, and interleukin-2 (IL-2) (reviewed in Young and Maciejewski3). Acting locally in the bone marrow (BM), activated T cells probably induce Fas-mediated cell death of hematopoietic progenitor and stem cells, ultimately resulting in a marked decrease in the number of these cells leading to severe pancytopenia.
However, not all patients respond to immunosuppressive therapy. In some cases, the quantitative stem cell deficit may be too severe to allow recovery (although assays have not shown a difference between responsive and refractory patients in the number of primitive hematopoietic cells).4,5 Alternatively, the immune system may be refractory to current drug regimens, a situation that can occur in other immunologically mediated disease. Unresponsive patients might also suffer from a nonimmunologic form of BM failure. Unfortunately, none of the research assays of immune system activation or of hematopoietic inhibition have proven useful as clinical tests.6,7 Of course, prediction of responsiveness to treatment based on pathophysiology would be practically useful. Patients, especially children, unlikely to respond to immunosuppressive therapy could be directed early to transplantation methodologies based on alternative donor sources or to experimental protocols8; conversely, patients with a high probability of response might elect to postpone conventional marrow transplantation, which carries significant morbidity and mortality, especially in older age groups, and to be treated first with immunosuppression.9 In addition, there is a high rate of relapse after immunosuppressive therapy,10 11 and evidence of immune system activity could be used to adjust or prolong current drug therapy or to trigger reinstitution of effective treatment before the recurrence of life-threatening cytopenias.
In AA, IFN-γ is a marker of immune system activity and may itself be a mediator of BM cell destruction. Assays of this and other cytokines have usually depended on cumbersome in vitro cell culture, including stimulation of cells with mitogens; insensitive determinations of plasma levels; or measurement of messenger RNA (mRNA) in extracts of cells.12 13 We designed the current study to detect IFN-γ in lymphocytes from patients with AA using new methodologies that measure its intracellular content.
Patients, materials and methods
Patients
We analyzed BM and PB samples obtained from patients with other hematologic disorders, as well as from healthy controls. Informed consent was obtained according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (Bethesda, MD). The diagnosis of aplastic anemia was established by BM biopsy and PB counts according to the criteria of the International Aplastic Anemia and Agranulocytosis Study,14 and severity was classified by the degree of blood count depression (2 of the following 3 criteria: absolute neutrophil count [ANC] below 0.5 × 109/L [500/μL], platelet count below 20 000 × 109/L [20 000/μL], absolute reticulocyte count [ARC] below 60 000 × 109/L [60 000/μL]).15 We studied 123 patients, of whom 70 had severe disease at the time of testing, either at diagnosis or after failing treatment (62 were naive to treatment, whereas 8 had been previously treated but had experienced a relapse). A further 53 patients had recovered to the point that blood counts no longer satisfied severity criteria and they were no longer dependent on transfusion of blood products2 (6 of these patients were also observed during relapse). Serial samples were obtained in some patients at time points prior to therapy, after treatment, and with relapse. We also assayed 30 healthy controls and 17 patients with congenital hemoglobinopathies who had received large numbers of red blood cell transfusions.
Cell separation and culture
BM was obtained by aspiration from the posterior iliac crest into syringes containing media supplemented 1:10 with heparin (O'Neill and Feldman, St Louis, MO). Mononuclear cells were isolated by density gradient centrifugation with the use of lymphocyte separation medium (Organon, Durham, NC). When appropriate, natural lymphocyte-derived IL-2 (Boehringer Mannheim, Indianapolis, IN), phytohemagglutinin (PHA) (Boehringer Mannheim), anti-CD3 monoclonal antibody (mAb) (Pharmingen, San Jose, CA), phorbol 12-myristate 13 acetate (PMA), and calcium ionophore were used for stimulation at concentrations of 10 U/mL and 0.1 μg/mL.
Flow cytometry
Intracellular staining for IFN-γ and IL-4 expression was performed by means of the Pharmingen Intracellular Staining Kit.16 Double-color surface staining was first performed with phycoerythrin (PE)-conjugated anti-CD4 and anti-CD8 mAbs, and then cells were permeabilized by means of a saponin-based method (Pharmingen) and stained with fluorescein isothiocyanate (FITC)–anti–IFN-γ or IL-4 mAbs (Pharmingen and BioSource, Camarillo, CA). Specificity of the antibody was confirmed by showing elimination of staining with a blocking antibody. Then, 10 μg purified unconjugated antibody was added to the fixed/permeabilized cells, which were then incubated for 20 minutes prior to addition of the conjugated antibody. Samples were analyzed by means of the Coulter (Hialeah, FL) EPICS V flow cytometer. Lymphocytes were initially gated by forward scatter/side scatter; secondary gates were set on the basis of staining with isotypic control mAbs so that fewer than 1% of cells stained positive.
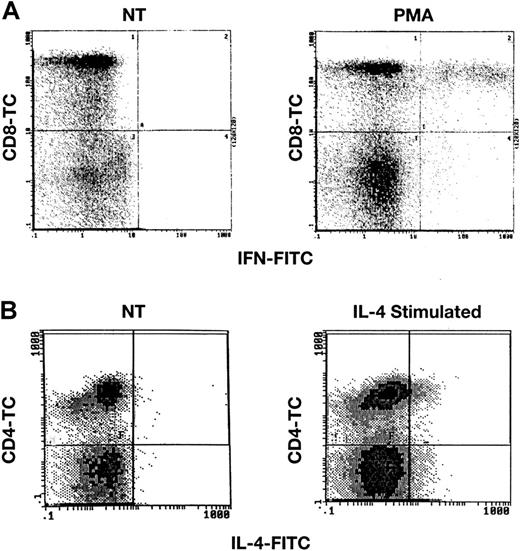
Multiple control experiments were performed to validate the applicability of intracellular staining for cytokines, with the use of previously reported methods.16 First, we stained normal and unstimulated lymphocytes, as well as lymphocytes that had been cultured for varying periods of time, with PMA at 50 ng/mL and calcium ionomycin at 250 ng/mL or with PHA. Unstimulated samples of lymphocytes from 36 healthy volunteers demonstrated no staining for either IFN-γ or IL-4, when compared with isotypic controls (cells stained with a PE-conjugated immunoglobulin-G isotype mAb). Normal lymphocytes that had been exposed to PMA/calcium ionomycin for 6 hours or to PHA for 2 days showed evidence of accumulation of both intracellular IFN-γ and IL-4 (Figure1). Results of flow cytometric experiments were expressed as a ratio of the mean channel fluorescence (MCF) sample/MCF isotype on an arbitrary scale. For example, PHA stimulation of cultured cells resulted in a sample MCF of 1.6 (n = 5), whereas isotype controls registered MCF at 0.10; the ratio was 16. Lymphocytes cultured in the presence of IFN-γ or IL-10, which favors development of the TH1 phenotype, stained primarily for IFN-γ, whereas lymphocytes cocultivated with IL-4 or IL-12, stimuli that favor the development of TH2 cells, stained primarily for IL-4 (data not shown); in these and other experiments, commercial enzyme-linked immunosorbent assays of culture supernatants also confirmed either predominantly IFN-γ or predominantly IL-4 production after specific treatment. When FITC-conjugated IFN-γ was incubated with IFN-γ prior to addition of cells, staining was decreased in a dose-dependent fashion. Similarly, preincubation of cells with unlabeled IFN-γ mAb eliminated staining of cells, while an irrelevant mAb did not (data not shown).
Detection of IFN-γ and IL-4 after stimulation of normal lymphocytes.
PB lymphocytes from healthy donors were stimulated with either PMA and calcium ionomycin or with IL-4 and were then surface stained with CD4-TC or CD8-TC mAbs, and finally intracellularly stained with IFN-γ mAb (panel A) or IL-4 mAb (panel B) as described in “Patients, materials, and methods.” Scattergrams demonstrate staining in stimulated lymphocytes, but no staining in unstimulated cells (not treated; NT).
Detection of IFN-γ and IL-4 after stimulation of normal lymphocytes.
PB lymphocytes from healthy donors were stimulated with either PMA and calcium ionomycin or with IL-4 and were then surface stained with CD4-TC or CD8-TC mAbs, and finally intracellularly stained with IFN-γ mAb (panel A) or IL-4 mAb (panel B) as described in “Patients, materials, and methods.” Scattergrams demonstrate staining in stimulated lymphocytes, but no staining in unstimulated cells (not treated; NT).
We also assessed the reproducibility of intracellular cytokine staining in clinical samples. Normal lymphocytes from 5 donors were divided and cultured with or without IL-2 and PHA. After 48 hours of culture, each sample was stained in duplicate. A high degree of reproducibility was found, as determined either (1) by cells graded as staining brightly or dimly or (2) based on MCF (Pearsonr = 0.97). Duplicate tests performed on blood samples from 46 patients assayed during periods of comparable disease activity (ANC ± 10%; platelet count ± 10%; ARC ± 20%) also showed good reproducibility (Pearson r = 0.89). Finally, mAbs with specificity for IFN-γ from multiple sources (BioSource and Pharmingen) were assayed and showed similar results (n = 27; Pearson r = 0.97).
Statistics
Correlations between tests were measured with the use of the Pearson coefficient of correlation or Fisher exact test where appropriate. The Fisher exact test was used to determine statistical significance.
Results
Intracellular staining for IFN-γ in lymphocytes from patients with hematologic disease and healthy individuals
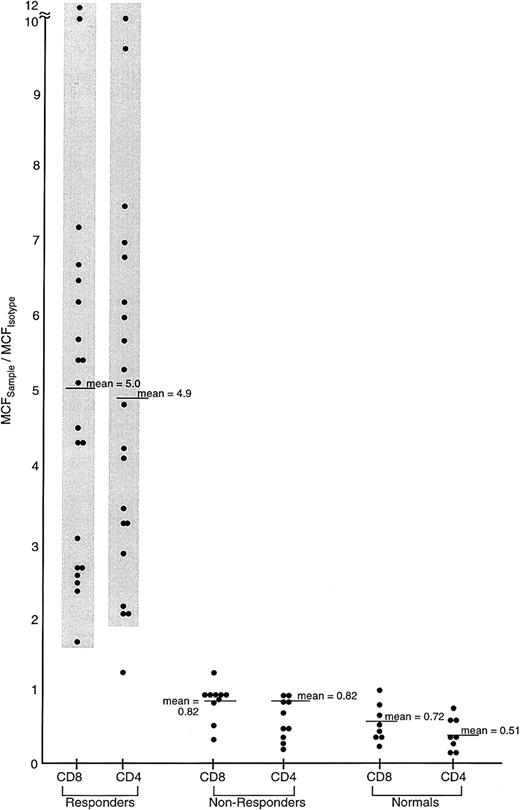
We used intracellular staining to study expression of 2 cytokines, IFN-γ and IL-4, in blood samples from patients with BM failure and from controls. IFN-γ has been directly implicated in hematopoietic cell destruction and is a marker of the TH1/cytotoxic T cell 1(Tc1) immune response; IL-4 was chosen to measure TH2 immune response. Increased numbers of cells staining for IFN-γ were detected in patients with severe AA (Figure 2); both CD4 and CD8 T cells contained IFN-γ (Figure 2). In contrast, IL-4 staining was not increased in most patients (data not shown). Of 62 patients with severe AA, cells, 31 patients scored positive for IFN-γ. In contrast, 17 hematologic controls (patients receiving a large number of transfusions for congenital anemias) and 30 healthy individuals did not show the presence of IFN-γ in PB lymphocytes. Increased expression of IFN-γ also was apparently not related to prior transfusions in severe AA, as some patients with a minimal number of transfusions demonstrated increased cytokine expression (data not shown).
Detection of IFN-γ by intracellular cytokine staining in patients with AA.
Lymphocytes from a patient with severe AA and a healthy control were surface stained with CD4-TC, and CD8-PE and intracellularly stained with mAb IFN-γ–FITC.
Detection of IFN-γ by intracellular cytokine staining in patients with AA.
Lymphocytes from a patient with severe AA and a healthy control were surface stained with CD4-TC, and CD8-PE and intracellularly stained with mAb IFN-γ–FITC.
Intracellular IFN-γ and response to treatment
Sixty-two treatment-naive patients were tested for intracellular IFN-γ before and after entering research protocols for immunosuppressive drug treatment (55 patients were treated with ATG and CSA, 7 with high-dose cyclophosphamide [CTX] and cyclosporin (CSA) (Tables 1 and2). In addition, we tested 20 patients prior to retreatment with immunosuppression for relapse or failure to respond to a first course of ATG. Of the 27 treatment-naive patients whose blood cells contained IFN-γ at or near the time of clinical presentation, all but 1 subsequently responded to treatment with improvement in blood counts, so that they no longer required transfusions and were not susceptible to bacterial infection (Tables 1and 3; Figure3). In contrast, only 11 of 34 patients whose blood cells lacked IFN-γ responded to these therapies (Fisher 2-tailed test, P < .0001) (Tables 2 and 3). After treatment, the percentage of IFN-γ–containing cells fell, usually to control levels (Figure 4). In addition, responding patients also often showed increased IL-4 in CD4 cells after therapy, suggesting a shift in the balance between TH1 and TH2. Cells from responding patients did not stain for IFN-γ after all immunosuppressive medication was discontinued.
Treatment-naive patients who responded to immunotherapy
| Patient . | Disease duration prior to treatment . | Transfusions prior to treatment, no. . | Treatment . | Interval between treatment and response, mo . | Quality of response . | Relapse1-160 . | MCF ratio . |
|---|---|---|---|---|---|---|---|
| 1 | 2 mo | 0 | ATG/CSA | 3 | PR | No | 1.4 |
| 2 | 4 mo | 12 | ATG/CSA | 3 | PR | No | 2.1 |
| 3 | 1 mo | 5 | ATG/CSA | 3 | PR | Yes | 3.4 |
| 4 | 2 mo | 4 | ATG/CSA | 3 | PR | No | 1.2* |
| 5 | 1 mo | 3 | ATG/CSA | 3 | PR | Yes | 1.2* |
| 6 | 2 mo | 4 | ATG/CSA | 3 | PR | Yes | 3.4 |
| 7 | 2 mo | 4 | ATG/CSA | 3 | PR | Yes | 1.4 |
| 8 | 15 d | 2 | ATG/CSA | 3 | PR | No | 7.4 |
| 9 | 2 mo | 7 | ATG/CSA | 3 | PR | Yes | 6.7 |
| 10 | 1 mo | 2 | ATG/CSA | 3 | PR | No | 2.2 |
| 11 | 3 mo | 2 | CTX/CSA | 3 | PR | No | 3.0 |
| 12 | 2 mo | 2 | ATG/CSA | 3 | PR | No | 2.3 |
| 13 | 1 mo | 4 | ATG/CSA | 3 | PR | Yes | 2.4 |
| 14 | 1 mo | 0 | ATG/CSA | 3 | PR | Yes | 1.5 |
| 15 | 2 mo | 4 | CTX/CSA | 3 | CR | Yes | 3.5 |
| 16 | 1 mo | 6 | ATG/CSA | 6 | PR | Yes | 3.0 |
| 17 | 2 mo | 4 | ATG/CSA | 3 | CR | No | 5.2 |
| 18 | 2 mo | 6 | ATG/CSA | 3 | CR | No | 0.4* |
| 19 | 8 mo | 2 | CTX/CSA | 6 | PR | Yes | 1.4 |
| 20 | 4 mo | 2 | ATG/CSA | 3 | PR | Yes | 1.1* |
| 21 | 1 mo | 1 | ATG/CSA | 3 | PR | No | 4.7 |
| 22 | 1 mo | 0 | ATG/CSA | 3 | PR | No | 2.1 |
| 23 | 4 mo | 10 | ATG/CSA | 3 | PR | No | 2.0 |
| 24 | 1 y | 16 | ATG/CSA | 3 | PR | No | 1.9 |
| 25 | 1 mo | 6 | CTX/CSA | 3 | PR | No | 0.4* |
| 26 | 1 mo | 3 | ATG/CSA | 3 | PR | No | 6.5 |
| 27 | 3 mo | 4 | ATG/CSA | 3 | PR | No | 1.2* |
| 28 | 15 d | 2 | ATG/CSA | 6 | PR | No | 4.2 |
| 29 | 1 mo | 3 | ATG/CSA | 6 | PR | No | 7.2 |
| 30* | 2 mo | 6 | ATG/CSA | 3 | PR | No | 1.1* |
| 31* | 1 y | 12 | ATG/CSA | 3 | PR | No | 1.1* |
| 32 | 15 d | 0 | ATG/CSA | 6 | PR | No | 5.3 |
| 33 | 9 d | 3 | ATG/CSA | 3 | PR | No | 2.4 |
| 34 | 1 mo | 2 | ATG/CSA | 3 | PR | No | 1.0* |
| 35 | 15 d | 0 | ATG/CSA | 3 | CR | No | 2.0 |
| 36 | 2 mo | 2 | ATG/CSA | 3 | PR | Yes | 1.2* |
| 37 | 6 mo | 20 | ATG/CSA | 6 | PR | No | 1.2* |
| 38 | 2 mo | 2 | ATG/CSA | 3 | PR | No | 4.7 |
| Patient . | Disease duration prior to treatment . | Transfusions prior to treatment, no. . | Treatment . | Interval between treatment and response, mo . | Quality of response . | Relapse1-160 . | MCF ratio . |
|---|---|---|---|---|---|---|---|
| 1 | 2 mo | 0 | ATG/CSA | 3 | PR | No | 1.4 |
| 2 | 4 mo | 12 | ATG/CSA | 3 | PR | No | 2.1 |
| 3 | 1 mo | 5 | ATG/CSA | 3 | PR | Yes | 3.4 |
| 4 | 2 mo | 4 | ATG/CSA | 3 | PR | No | 1.2* |
| 5 | 1 mo | 3 | ATG/CSA | 3 | PR | Yes | 1.2* |
| 6 | 2 mo | 4 | ATG/CSA | 3 | PR | Yes | 3.4 |
| 7 | 2 mo | 4 | ATG/CSA | 3 | PR | Yes | 1.4 |
| 8 | 15 d | 2 | ATG/CSA | 3 | PR | No | 7.4 |
| 9 | 2 mo | 7 | ATG/CSA | 3 | PR | Yes | 6.7 |
| 10 | 1 mo | 2 | ATG/CSA | 3 | PR | No | 2.2 |
| 11 | 3 mo | 2 | CTX/CSA | 3 | PR | No | 3.0 |
| 12 | 2 mo | 2 | ATG/CSA | 3 | PR | No | 2.3 |
| 13 | 1 mo | 4 | ATG/CSA | 3 | PR | Yes | 2.4 |
| 14 | 1 mo | 0 | ATG/CSA | 3 | PR | Yes | 1.5 |
| 15 | 2 mo | 4 | CTX/CSA | 3 | CR | Yes | 3.5 |
| 16 | 1 mo | 6 | ATG/CSA | 6 | PR | Yes | 3.0 |
| 17 | 2 mo | 4 | ATG/CSA | 3 | CR | No | 5.2 |
| 18 | 2 mo | 6 | ATG/CSA | 3 | CR | No | 0.4* |
| 19 | 8 mo | 2 | CTX/CSA | 6 | PR | Yes | 1.4 |
| 20 | 4 mo | 2 | ATG/CSA | 3 | PR | Yes | 1.1* |
| 21 | 1 mo | 1 | ATG/CSA | 3 | PR | No | 4.7 |
| 22 | 1 mo | 0 | ATG/CSA | 3 | PR | No | 2.1 |
| 23 | 4 mo | 10 | ATG/CSA | 3 | PR | No | 2.0 |
| 24 | 1 y | 16 | ATG/CSA | 3 | PR | No | 1.9 |
| 25 | 1 mo | 6 | CTX/CSA | 3 | PR | No | 0.4* |
| 26 | 1 mo | 3 | ATG/CSA | 3 | PR | No | 6.5 |
| 27 | 3 mo | 4 | ATG/CSA | 3 | PR | No | 1.2* |
| 28 | 15 d | 2 | ATG/CSA | 6 | PR | No | 4.2 |
| 29 | 1 mo | 3 | ATG/CSA | 6 | PR | No | 7.2 |
| 30* | 2 mo | 6 | ATG/CSA | 3 | PR | No | 1.1* |
| 31* | 1 y | 12 | ATG/CSA | 3 | PR | No | 1.1* |
| 32 | 15 d | 0 | ATG/CSA | 6 | PR | No | 5.3 |
| 33 | 9 d | 3 | ATG/CSA | 3 | PR | No | 2.4 |
| 34 | 1 mo | 2 | ATG/CSA | 3 | PR | No | 1.0* |
| 35 | 15 d | 0 | ATG/CSA | 3 | CR | No | 2.0 |
| 36 | 2 mo | 2 | ATG/CSA | 3 | PR | Yes | 1.2* |
| 37 | 6 mo | 20 | ATG/CSA | 6 | PR | No | 1.2* |
| 38 | 2 mo | 2 | ATG/CSA | 3 | PR | No | 4.7 |
PR indicates partial response; CR, complete response.
Cells considered negative for IFN-γ.
Relapse within 2 years of initial response.
Treatment-naive patients who did not respond to immunotherapy
| Patient . | Disease duration prior to treatment . | Transfusions prior to treatment, no. . | Treatment type . | MCF ratio . |
|---|---|---|---|---|
| 1 | 3.5 mo | 4 | ATG/CSA | 1 |
| 2 | 5 mo | 19 | ATG/CSA | 0.58 |
| 3 | 3 mo | 4 | ATG/CSA | 1 |
| 4 | 10 y | 2 | CTX | 0.9 |
| 5 | 3 mo | 3 | ATG/CSA | 1 |
| 6 | 8 y | 4 | ATG/CSA | 0.52 |
| 7 | 4 mo | 20 | ATG/CSA | 0.36 |
| 8 | 8 y | 3 | CTX | 0.54 |
| 9 | 2.5 mo | 4 | CTX | 0.28 |
| 10 | 4 y | 11 | ATG/CSA | 0.58 |
| 11 | 3 mo | 2 | ATG/CSA | 0.39 |
| 12 | 3 mo | 9 | ATG/CSA | 1 |
| 13 | 3 mo | 3 | ATG/CSA | 1 |
| 14 | 3 wk | 3 | ATG/CSA | 1 |
| 15 | 1 mo | 2 | ATG/CSA | 0.7 |
| 16 | 2 wk | 4 | ATG/CSA | 0.8 |
| 17 | 3 mo | 5 | ATG/CSA | 1.2 |
| 18 | 2 mo | 4 | ATG/CSA | 1 |
| 19 | 1 mo | 6 | ATG/CSA | 1.1 |
| 20 | 2 mo | 1 | ATG/CSA | 0.66 |
| 21 | 2 wk | 8 | ATG/CSA | 0.89 |
| 22 | 2 wk | 3 | ATG/CSA | 0.7 |
| 23 | 3 wk | 4 | ATG/CSA | 2.8* |
| 24 | 2 wk | 5 | ATG/CSA | 1.0 |
| Patient . | Disease duration prior to treatment . | Transfusions prior to treatment, no. . | Treatment type . | MCF ratio . |
|---|---|---|---|---|
| 1 | 3.5 mo | 4 | ATG/CSA | 1 |
| 2 | 5 mo | 19 | ATG/CSA | 0.58 |
| 3 | 3 mo | 4 | ATG/CSA | 1 |
| 4 | 10 y | 2 | CTX | 0.9 |
| 5 | 3 mo | 3 | ATG/CSA | 1 |
| 6 | 8 y | 4 | ATG/CSA | 0.52 |
| 7 | 4 mo | 20 | ATG/CSA | 0.36 |
| 8 | 8 y | 3 | CTX | 0.54 |
| 9 | 2.5 mo | 4 | CTX | 0.28 |
| 10 | 4 y | 11 | ATG/CSA | 0.58 |
| 11 | 3 mo | 2 | ATG/CSA | 0.39 |
| 12 | 3 mo | 9 | ATG/CSA | 1 |
| 13 | 3 mo | 3 | ATG/CSA | 1 |
| 14 | 3 wk | 3 | ATG/CSA | 1 |
| 15 | 1 mo | 2 | ATG/CSA | 0.7 |
| 16 | 2 wk | 4 | ATG/CSA | 0.8 |
| 17 | 3 mo | 5 | ATG/CSA | 1.2 |
| 18 | 2 mo | 4 | ATG/CSA | 1 |
| 19 | 1 mo | 6 | ATG/CSA | 1.1 |
| 20 | 2 mo | 1 | ATG/CSA | 0.66 |
| 21 | 2 wk | 8 | ATG/CSA | 0.89 |
| 22 | 2 wk | 3 | ATG/CSA | 0.7 |
| 23 | 3 wk | 4 | ATG/CSA | 2.8* |
| 24 | 2 wk | 5 | ATG/CSA | 1.0 |
Patient was scored as positive, remained positive following treatment, but responded to second course of immunotherapy.
Relation of IFN-γ to response in immunosuppressive therapy in treatment-naive patients
| . | Positive IFN-γ . | Negative IFN-γ . |
|---|---|---|
| Response | 27 | 11 |
| Treatment failure | 1 | 23 |
| . | Positive IFN-γ . | Negative IFN-γ . |
|---|---|---|
| Response | 27 | 11 |
| Treatment failure | 1 | 23 |
Sixty patients with a diagnosis of AA were assayed for PB T-cell IFN-γ prior to first treatment with immunosuppressive therapy.
Fisher exact test, P < .00001.
Comparison of IFN-γ intracellular content in previously untreated responding and unresponding patients with severe AA, and healthy controls.
Lymphocytes from 62 previously untreated patients with severe AA (SAA) and 30 healthy volunteers were surface stained with CD4-TC and CD8-PE mAbs and intracellularly stained with IFN-γ–FITC mAb. Patients with SAA received immunosuppressive therapy (either ATG/CSA or CTX/CSA) and were designated as responders or nonresponders on the basis of improvement of BM function by 6 months following treatment to the point that the patient no longer fulfilled severity criteria. The ratio of the MCFsample to the MCFisotype of staining of PB lymphocytes is shown.
Comparison of IFN-γ intracellular content in previously untreated responding and unresponding patients with severe AA, and healthy controls.
Lymphocytes from 62 previously untreated patients with severe AA (SAA) and 30 healthy volunteers were surface stained with CD4-TC and CD8-PE mAbs and intracellularly stained with IFN-γ–FITC mAb. Patients with SAA received immunosuppressive therapy (either ATG/CSA or CTX/CSA) and were designated as responders or nonresponders on the basis of improvement of BM function by 6 months following treatment to the point that the patient no longer fulfilled severity criteria. The ratio of the MCFsample to the MCFisotype of staining of PB lymphocytes is shown.
IFN-γ intracellular cytokine content in CD8 cells of patients with severe AA before and after successful immunosuppressive treatment.
A subset of 27 patients was tested before and 6 months after treatment with either CTX/CSA or ATG/CSA. In 17 of 27 responding patients, IFN-γ was detectable in CD4 and CD8 cells and declined following treatment. None of the 10 nonresponding patients showed elevated IFN-γ lymphocyte staining prior to therapy. The ratio of the MCFsample to the MCFisotype.of staining of CD8 cells before and following treatment is shown.
IFN-γ intracellular cytokine content in CD8 cells of patients with severe AA before and after successful immunosuppressive treatment.
A subset of 27 patients was tested before and 6 months after treatment with either CTX/CSA or ATG/CSA. In 17 of 27 responding patients, IFN-γ was detectable in CD4 and CD8 cells and declined following treatment. None of the 10 nonresponding patients showed elevated IFN-γ lymphocyte staining prior to therapy. The ratio of the MCFsample to the MCFisotype.of staining of CD8 cells before and following treatment is shown.
Relapse occurs frequently in AA. For 53 patients with stable recovery, IFN-γ was detected in only 4 cases. Seventeen patients were observed at the time of relapse. IFN-γ was detected in 14, and all but 1 of these responded to reinstitution of immunosuppression. Only one patient who was negative for IFN-γ responded to treatment. In 5 patients who were followed serially by intracellular cytokine testing, recurrence of a positive assay preceded pancytopenia by 1 to 2 weeks (Figure5). Four patients who were treated with a second course of immunosuppressive therapy after failure to respond to the first course were all negative for IFN-γ, and all failed to respond to further treatment.
IFN-γ lymphocyte content in a patient with AA with hematogic response to treatment, relapse, and retreatment.
This patient had severe AA at presentation, at which time lymphocyte staining was positive for IFN γ. He was treated with ATG/CSA; a hematologic response was observed within 4 months of treatment, when PB lymphocyte staining was negative for IFN γ. The patient experienced a relapse at 14 months and lymphocyte staining was again positive for IFN γ; the CSA dose was increased. The patient subsequently had a second response, and lymphocyte staining became negative for IFN γ. ARC (dotted line) and platelet counts (solid line); inserts show examples of scattergrams of CD8 lymphocytes stained with IFN-γ–FITC mAb at varying times during the clinical course.
IFN-γ lymphocyte content in a patient with AA with hematogic response to treatment, relapse, and retreatment.
This patient had severe AA at presentation, at which time lymphocyte staining was positive for IFN γ. He was treated with ATG/CSA; a hematologic response was observed within 4 months of treatment, when PB lymphocyte staining was negative for IFN γ. The patient experienced a relapse at 14 months and lymphocyte staining was again positive for IFN γ; the CSA dose was increased. The patient subsequently had a second response, and lymphocyte staining became negative for IFN γ. ARC (dotted line) and platelet counts (solid line); inserts show examples of scattergrams of CD8 lymphocytes stained with IFN-γ–FITC mAb at varying times during the clinical course.
Comparison of BM and peripheral blood lymphocyte IFN-γ expression
Intracellular CD8 IFN-γ of PB was compared with cytokine measurements in CD8 lymphocytes derived from BM in 30 severe AA patients on presentation, and 20 showed the presence of this cytokine. All 20 patients whose BM CD8 lymphocytes stained positive for IFN-γ responded to therapy, whereas all nonresponders failed to demonstrate any IFN-γ staining in their BM T cells (Fisher exact test, P < .0001) (Figure 6; Table 4). In 2 responding patients, IFN-γ was detected in the marrow and not in the blood. IFN-γ was not present in marrow CD8 cells from 8 healthy donors.
IFN-γ content of BM CD4 and CD8 cells in patients with SAA.
Lymphocytes from 30 previously untreated patients with severe AA and 7 healthy volunteers were surface stained with mAbs to CD4-TC and CD8-PE and intracellularly stained with antibody to IFN-γ–FITC mAb. Patient with SAA received immunosuppressive therapy (either ATG/CSA or cyclophosphamide) and were designated responders or nonresponders. The ratio of the MCF for IFN-γ staining is shown for each group.
IFN-γ content of BM CD4 and CD8 cells in patients with SAA.
Lymphocytes from 30 previously untreated patients with severe AA and 7 healthy volunteers were surface stained with mAbs to CD4-TC and CD8-PE and intracellularly stained with antibody to IFN-γ–FITC mAb. Patient with SAA received immunosuppressive therapy (either ATG/CSA or cyclophosphamide) and were designated responders or nonresponders. The ratio of the MCF for IFN-γ staining is shown for each group.
Relation of bone marrow CD8+ cell IFN-γ to response to immunosuppressive therapy
| . | Positive IFN-γ . | Negative IFN-γ . |
|---|---|---|
| Response to therapy | 20 | 0 |
| Treatment failure | 0 | 10 |
| . | Positive IFN-γ . | Negative IFN-γ . |
|---|---|---|
| Response to therapy | 20 | 0 |
| Treatment failure | 0 | 10 |
Samples of PB and BM were obtained from 26 patients with severe AA before and after immunosuppressive treatment and were analyzed according to response to therapy. Fisher exact test showed a significant difference between responders and nonresponders;P < .00002.
Discussion
Our results have implications related to the basic immunology of AA, the role of the TH1/TH2 dichotomy in a human disease, and the practical management of patients with severe BM failure. First, our data are consistent with a model of TH1/Tc1 autoimmune disease. Flow cytometric analysis of intracellular cytokines has shown differences in T-cell responses between infants and adults17; during allergic reactions17; under a number of conditions in which the frequency of cytokine expression in T cells was assessed18,19; and, in a few studies, in some human diseases. As examples of the last item, a TH1-dominant immune response appears to characterize multiple sclerosis,20 and a TH2 response, systemic lupus erythematosis.21
Reported cytokine abnormalities in AA, highly suggestive of a TH1/Tc1–dominant immune response, are now confirmed by intracellular cytokine staining of stimulated cells. Other researchers have reported an increased TH1-to-TH2 ratio in stimulated lymphocytes from patients with AA.23 In this study, patients showed increased IFN-γ content in circulating cells when their disease is active; hematologic improvement correlated with a marked reduction in IFN-γ but with stable or increased IL-4 content. In contrast to reports in other diseases, we found that IFN-γ was measurable in circulating cells without the requirement for in vitro stimulation of lymphocytes, as with a phorbol ester. Such a “preactivated” status for T cells is consistent with earlier experiments examining lymphocyte function.24 As in other autoimmune states, activated T cells appear to localize in the target tissue.7,25,26 While circulating cells may reflect BM activity, in hematologic disease intracellular cytokine detection of BM T cells may be preferable to testing PB, as these cells scored positive in some patients in whom IFN-γ was not detected in blood cells (see below); other measurements,7 such as flow cytometric determination of lymphocyte cell surface expression of activation markers and gene amplification to detect IFN-γ mRNA,12 are also consistent with marrow localization of immune system activity. The finding of substantial numbers of IFN-γ–containing T cells in AA is consistent with results of other types of analysis of lymphocyte function in this disease, especially recent data indicating that some patients, especially those dependent on continued cyclosporine administration to maintain blood counts, show marked skewing of Vβ chain use,27 suggestive of a specific antigenic response. Possibly, activated T cells may reflect an underlying viral infection, such as could occur in posthepatitis AA,28 or, more generally, commitment of significant numbers of lymphocytes to the underlying pathophysiologic immune process. In either case, if inactivated lymphocytes represent residual normal cells, lymphopenia has been underestimated by simple blood counts and differentials.
If our results are confirmed, measurement of intracellular cytokines would have practical utility. Predictive tests based on hematopoietic colony formation with and without added T cells,13,29 in vitro response of progenitor cells to ATG,30 IFN-γ mRNA measurements of marrow cells,31 the detection of ill-defined marrow cells by flow cytometry,32 and measurement of early progenitor cell numbers after ATG,33but no laboratory test has to date been generally accepted to prospectively distinguish the approximately 30% of patients who fail immunosuppressive treatments. In the current series, the presence of intracellular IFN-γ was a powerful predictor of response, and this simple test might allow earlier application of high-risk treatments such as alternative donor transplantation from unrelated matched8,34 or haplo-identical family35 donors. Alternative donor transplantation is currently undertaken only after a course of immunosuppression and a 3-month or longer waiting period; alloimmunization and the frequent intercurrent occurrence of serious infections may adversely affect the outcome of subsequent transplantation. Repeated futile application of immunosuppressive drugs in a refractory patient might also be avoided. Among our responding patients, some did not show intracellular cytokine staining for IFN-γ in circulating T cells. In all those tested, such cells were detected in marrow samples, suggesting that application of this assay in the target organ may improve its sensitivity. Although most responding patients showed a marked decrease or disappearance of IFN-γ cells with hematologic improvement, in a few cases, abnormal cells persisted, consistent with a low level of immunological activity in marrow during remission—a likely explanation for both the frequent necessity for retreatment of patients with drugs active on the immune system2 and the long-term dependence of a subset of AA patients on continued administration of CSA.36 In some patients whose blood was assayed serially, increased intracellular staining for IFN-γ preceeded a relapse in blood counts by weeks. Ideally, intracellular cytokine staining, because it is simple to perform, reproducible, and quantitative, could be applied to the routine management of patients with AA, and possibly also to other cytokine-mediated autoimmune diseases.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-01-0035.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elaine Sloand, Building 10, Room 7C103, National Institutes of Health, 9000 Rockville Pike, Bethesda MD 20892-1652; e-mail: sloande@gwgate.nhlbi.nih.gov.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal