Cloned T9-C2 glioma cells transfected with membrane macrophage colony-stimulating factor (mM-CSF) never formed subcutaneous tumors when implanted into Fischer rats, whereas control T9 cells did. The T9-C2 cells were completely killed within 1 day through a mechanism that resembled paraptosis. Vacuolization of the T9-C2 cell's mitochondria and endoplasmic reticulum started within 4 hours after implantation. By 24 hours, the dead tumor cells were swollen and terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL)–positive. Bcl2-transduced T9-C2 cells failed to form tumors in rats. Both T9 and T9-C2 cells produced cytokine-induced neutrophil chemoattractant that recruited the granulocytes into the tumor injection sites, where they interacted with the tumor cells. Freshly isolated macrophages killed the T9-C2 cells in vitro by a mechanism independent of phagocytosis. Nude athymic rats treated with antiasialo GM1 antibody formed T9-C2 tumors, whereas rats treated with a natural killer cell (NK)–specific antibody failed to form tumors. When treated with antipolymorphonuclear leukocyte (anti-PMN) and antimacrophage antibodies, 80% of nude rats formed tumors, whereas only 40% of the rats developed a tumor when a single antibody was used. This suggests that both PMNs and macrophages are involved in the killing of T9-C2 tumor cells. Immunocompetent rats that rejected the living T9-C2 cells were immune to the intracranial rechallenge with T9 cells. No vaccinating effect occurred if the T9-C2 cells were freeze-thawed, x-irradiated, or treated with mitomycin-C prior to injection. Optimal tumor immunization using mM-CSF–transduced T9 cells requires viable tumor cells. In this study optimal tumor immunization occurred when a strong inflammatory response at the injection of the tumor cells was induced.
Introduction
In some tumor models the way tumor cells die, either by apoptosis or necrosis, provides a critical dichotomy that determines whether or not the induction of lasting tumor immunity occurs. When tumor cells are killed via apoptosis by the use of ultraviolet light, chemotherapy, or x-irradiation, the immune response is not stimulated, despite a massive release of tumor-derived material. Several groups have shown that macrophages or dendritic cells that phagocytize apoptotic cells increase their synthesis of antiinflammatory cytokines such as prostaglandin E (PGE), transforming growth factor–β (TGF-β), platelet activating factor (PAF), or interleukin (IL) 10,1-3 or there is a down-regulation of the expression of costimulatory molecules.4 This results in the induction of immune tolerance or anergy toward the tumor. In contrast, when antigen-presenting cells (APCs) are stimulated with necrotic tumor cells or tumor cells expressing heat shock proteins, there is lasting antitumor immunity.5 APCs activated by necrotic cells are more effective,6,7 express more costimulatory molecules,2 and produce more inflammatory cytokines.3 Macrophages stimulated with necrotic cells display better antitumor activities than those stimulated by apoptotic cells.8
A third way that cells die, called paraptosis, has been reported.9 Paraptosis is a form of programmed cell death that does not involve DNA fragmentation and traditional apoptotic body formation. This killing resists various caspase inhibitors and bcl-xL treatment, which distinguishes it from apoptosis. Paraptotic death is characterized by cytoplasmic vacuolization, which begins with progressive swelling of the mitochondria and endoplasmic reticulum. Little is known of the evolution of this process in vivo and nothing is known of the manner in which this form of cell death affects the immune system.
Our previous in vitro studies showed that bone marrow–derived macrophages directly kill living malignant T9 glioma cells (T9-C2) transduced with the membrane form of macrophage colony-stimulating factor (mM-CSF) via direct phagocytosis.10,11 This macrophage-mediated process is independent of the Fas–Fas ligand pathway, because macrophages that do not express Fas ligand also kill these cells in vitro.12 T9 cells expressing the secreted form of macrophage colony stimulating factor (sM-CSF) are not killed in vitro. When mM-CSF–transfected T9 cells are injected either subcutaneously or intracranially they are rejected, while the sM-CSF–transduced cells form tumors.13 Rats that spontaneously reject mM-CSF also generate lasting immunity in the form of CD3+ T cells. The mM-CSF–transfected cells therefore lead to both spontaneous destruction of the vaccinating tumor and the development of lasting tumor immunity. The T9 glioma cell line, also called 9L,14 is one of the most widely used gliomas in experimental neuro-oncology. T9 glioma cells are considered an immunogenic glioma cell line.15,16 We have obtained similar results using either weakly immunogenic rat MADB106 breast cancer17 or nonimmunogenic mouse Hepa1-6 hepatomas18 transduced with mM-CSF. Both of these nongliomatous tumors are rejected in vivo and immunize the animals against their parental tumor. These results with 3 different tumor cells show that cloned mM-CSF–transfected tumor cells can successfully stimulate a strong immune response.
Large numbers of the mM-CSF–transfected T9-C2 cells (eg, 107 cells) can be injected subcutaneously into rats without forming tumors.13 Even though cloned mM-CSF–transfected MADB106 and Hepa1-6 tumors express amounts of mM-CSF as T9-C2 tumor cells, we did occasionally (10% of the time) observe that these 2 tumors did grow. These observations suggest that some unique properties of the mM-CSF–transduced T9 glioma cells allow them to be rejected rapidly without forming tumors, while other such cells can sometimes form tumors even when smaller numbers are injected.
In this report, we show that the rat T9 glioma cells expressing mM-CSF do have some intrinsic properties that make them more susceptible to in vivo rejection, and this perhaps contributes to making these cells more immunogenic. These observations may have important ramifications for developing better tumor vaccines. First, the T9 tumors make cytokine-induced neutrophil chemoattractant (CINC, the rat IL-8 equivalent) that allows polymorphonuclear leukocytes (PMNs) to be recruited into the freshly implanted tumor. This influx of PMNs helps macrophages establish a microenvironment in which the T9-C2 tumor is destroyed, leading to the development of anti-T9 immunity. Second, the combined effects of PMNs and macrophages destroy the T9-C2 cells within a single day in vivo, via a swelling and vacuolization process that resembles paraptosis. Third, intact living T9-C2 cells are required to induce immunization, because x-irradiation, mitomycin-C, or freeze-thawing prior to injection does not vaccinate against the tumor cells. Thus, the development of tumor immunity requires active interaction between the tumor and the immune system.
Materials and methods
Cell lines and cell culture
The derivation of the mM-CSF–transduced T9-C2 and sM-CSF–transduced T9-H1 cells has been previously described.10 The cells were grown in RPMI-1640 media supplemented with 5% fetal bovine serum (Gemini Bioproducts, Calabassas, CA). All culture supplies were screened and selected on the basis of being endotoxin-free. All cells were routinely determined to be mycoplasma-free with the aid of the Stratagene (San Diego, CA) polymerase chain reaction (PCR) detection kit.
Reverse transcriptase–PCR
Total RNA was isolated with Tri-Reagent (Sigma Chemical, St Louis, MO) from tumor cells that had been refed 4 hours earlier. The rat CINC primers 5′CTCCAGCCACACTCCAACAGA3′ and 5′CACCCTAACACAAAACACGAT3′ and β-actin primers were synthesized by MWG Biotech (High Point, NC). One microgram of total RNA was incubated with the primers and incubated in the Life Technologies (Grand Island, NY) One Step RT-PCR mix. The PCR was performed with a Techne Progene (Princeton, NJ) Thermal Cycler according to the methods described by Mawet et al.19 After the 20 cycles were completed, the PCR products were electrophoresed on a 2% agarose gel. The gel was visualized and the PCR products were confirmed to be the correct size (670 base pairs) for CINC and for β-actin (764 base pairs).
Intracellular flow cytometry staining of CINC-producing cells
CINC-positive cells were identified by previously described methods.20 The cells were fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) solution, washed, and permeabilized in 0.1% saponin/PBS solution for 1 hour at room temperature. The antibodies (rabbit antirat CINC antibody [Assays Designs, Ann Arbor, MI] or a control rabbit serum) were added. The cells were washed twice with PBS, followed by the application of a fluorescein isothiocyanate (FITC)–labeled antirabbit antibody (Vector Labs, Burlingame, CA) in permeabilization buffer. Data from 104 cells were collected on an EPICS Profile flow cytometer (Beckman-Coulter, Miami, FL) and analyzed with the Phoenix Flow Systems (San Diego, CA) Multi2D program.
Animals
Female Fischer-344 (F344) rats were obtained from Charles River (Wilmington, MA). Nude athymic rats were purchased from Harlan Sprague Dawley (Indianapolis, IN).
Tumor growth in animals
The rats were injected subcutaneously on the ventral side with the tumor cells in a volume of 50 μL. Palpable tumors were measured with metric calipers for length, width, and height. Tumor volume was calculated as height × width × length × π/6. The data are expressed as mean tumor volume ± SEM.
Adoptive transfer studies
Rats that had been immunized subcutaneously with 3 × 105 T9-C2 cells 2 weeks earlier were killed and their spleens were removed. The splenocytes (one spleen equivalent) were injected intraperitoneally into naive rats that were intracranially implanted with 104 T9 cells.
In vivo leukocyte depletion
Nude athymic rats were used for these experiments. Natural killer (NK) cells were depleted with either antiasialo GM1 antibody (Wako Biochemicals, Richmond, VA) or an NK cell–specific monoclonal ascites antibody generously provided by Dr William Chambers (University of Pittsburgh, Pittsburgh, PA). The 100-g rats were injected 4 times intraperitoneally with 50 μL of the antiasialo GM1 antibody or 12.5 μL of the anti-NK antibody over a 2-week period. This procedure eliminated more than 95% of NK activity against the NK-sensitive YAC cells in a 6-hour 51Cr release–based cytotoxicity assay. Granulocytes and macrophages were eliminated with polyclonal rabbit antirat PMN or antirat macrophage antiserum (Accurate Scientific and Chemical, Westbury, NY). Preliminary tests using these antibodies confirmed that the circulating levels of granulocytes and macrophages were markedly reduced by 4 intraperitoneal injections with their respective antibodies over a 7-day period.
Differences in tumor growth were determined by one-tailed Fisher exact test, and analysis of variance was used to analyze the data at each time point (P < .05 was considered significant).
Bcl2 transduction of T9-C2 cells
To prevent the T9-C2 cells from dying of apoptosis, we transduced the antiapoptotic gene, bcl2, into the T9-C2 cells. The retroviral transfection of the bcl2 gene into T9-C2 cells was achieved with a retroviral construct, AM12-bcl2,5 generously supplied by Dr Richard Vile (Mayo Clinic, Minneapolis, MN). After the transfection, the cells were selected in 5 μg/mL of puromycin. Cells were tested immunohistochemically to ensure that they were expressing thebcl2 transgene by using an anti-bcl2 antibody (Santa Cruz Antibodies, Santa Cruz, CA). These cotransduced cells also proved to be resistant to apoptosis-inducing drugs such as staurosporine and camptothecin.
Immunohistology
Tumor tissue was excised from the killed animals and sectioned in a cryostat as previously described.14 The various antirat primary antibodies were obtained from Pharmingen (San Diego, CA); these included CD4, CD8, NKRP-1, HIS48 (granulocytes), 1C7 (mononuclear phagocyte-specific), and antidendritic cell antibodies. The rabbit anti-iNOS antibody was purchased from Affinity BioReagents (Golden, CO). The rabbit anti–heat shock protein-70 antibody was obtained from Chemicon International (Temecula, CA). Rabbit anti-bcl2 antibody was obtained from Santa Cruz Antibodies (Santa Cruz, CA). Biotinylated goat antiprimary antibody (Pharmingen) and Strept-avidin conjugated peroxidase (DAKO, Carpinteria, CA) were also used.
TUNEL staining
The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) was performed with Promega's Death-End Colorimetric Apoptosis Detection kit (Madison, WI) according to the manufacturer's instructions.
Electron microscopic studies
Tissues taken at 2, 4, and 20 hours after injection were fixed for 1 to 2 hours with 2.5% glutaraldehyde at pH 7.3 in either 0.1 M Sorensen phosphate buffer or 0.1 M sodium cacodylate buffer. The tissues were then rinsed, postfixed for 1 hour in 1% OsO4, and rinsed again. Following dehydration with cold ethanol and subsequent transfer to propylene oxide, the tissues were infiltrated with Spurr resin over a period of 36 hours. Sections were stained with uranyl acetate and lead citrate and then observed with a JEOL-1200EX II transmission electron microscope (Peabody, MA).
Freshly isolated macrophages and PMNs
Macrophages were collected from the rats that had been injected with 2 mL of a sterile 3% thioglycolate solution 3 days earlier. The animals were killed and the peritoneum was washed out. Alveolar macrophages were obtained by standard lavage techniques.21 Endotoxin-free PBS was used throughout all the procedures. By flow cytometry, these macrophage preparations were more than 92% Ox-41, Ed1, and CD18, consistent with a macrophage phenotype.
Cytotoxicity studies
Results
Several types of leukocytes are present within the subcutaneous T9-C2 rejection site
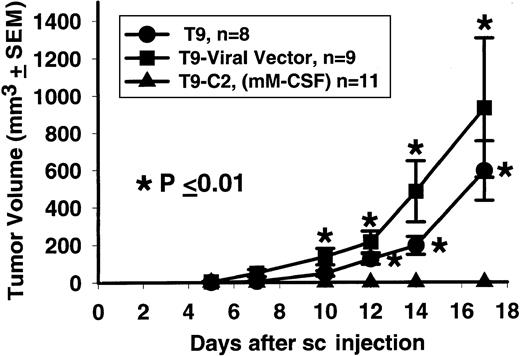
When 2 × 105 T9-C2 cells were injected subcutaneously into the syngeneic F344 rats, they did not form tumors, while the viral vector T9 and parental T9 cells consistently formed tumors in all rats (Figure 1). This experiment confirms previous work showing that T9-C2 cells never grow at subcutaneous sites, even when concentrations as high as 107 cells are used.13 Over the last 5 years in our facility, none of the Fischer rats (n > 200) injected with living T9-C2 cells have ever developed a subcutaneous tumor.
Fisher 344 rats will reject the mM-CSF bearing T9-C2 cells.
Tumor cells, T9 parental, T9 viral vector, and T9-C2 (2 × 105) were injected subcutaneously and monitored for the next 3 weeks. An asterisk indicates that the P value between the rats injected with the T9-C2 cells and the other rats treated with the T9 cells was less than or equal to .01.
Fisher 344 rats will reject the mM-CSF bearing T9-C2 cells.
Tumor cells, T9 parental, T9 viral vector, and T9-C2 (2 × 105) were injected subcutaneously and monitored for the next 3 weeks. An asterisk indicates that the P value between the rats injected with the T9-C2 cells and the other rats treated with the T9 cells was less than or equal to .01.
Three days after subcutaneous injection of viable T9-C2 cells, only eosinophilic tumor cell remnants were seen histologically. In contrast, the corresponding T9 and T9–viral vector cells were relatively easy to find after 3 days, because these cells were actively growing. We therefore examined the histology at earlier time points to determine the kinetics of the mM-CSF–transfected tumor destruction. Within 20 hours of injection, numerous mature polymorphonuclear cells (PMNs) and macrophagelike cells were seen in close proximity to the tumor cells. Tumor cells were observed dying as individual cells and were not actively phagocytized by macrophages. PMNs were also seen with the T9 gliomas, but never to the same extent as was seen with the T9-C2 tumors.
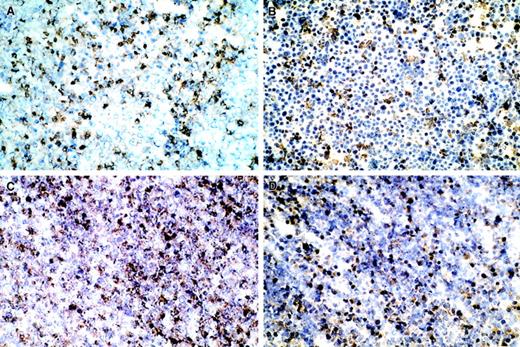
Immunohistology confirmed that granulocytes were present within the leukocytic infiltrate. Figure 2A shows numerous HIS48+ stained granulocytes. Immunohistochemical analysis revealed that 1C7+ staining macrophages also infiltrated the T9-C2 tumor cells (data not shown). The PMNs and macrophages were present in greater quantities (25%-50%) in the T9-C2 tumors in the T9 tumor (data not shown). A few NK cells were observed within the T9-C2 cell mass, but they were more commonly seen along the margins. Dendritic cells were present only in scattered regions at a low density. Immunostaining labeled the inducible form of nitric oxide synthase (iNOS) in some leukocytes (Figure 2B). Staining of the tissue with the antinitrotyrosine antibody revealed that the dead T9-C2 cells were heavily stained, suggesting that these cells interacted with peroxynitrite (Figure 2C). T9-C2 cells were also stained with a HSP-70 antibody (Figure 2D). The expression of the last 3 antibodies was 10% to 25% greater than that found in association with T9 cells implanted under identical conditions.
Immunohistology of the T9-C2 injection site in a subcutaneous site.
Five million T9-C2 cells were injected in a subcutaneous site for 20 hours. The T9-C2 cells were excised and frozen sections were prepared. (A) Cells stained for granulocytes; (B) cells stained for iNOS; (C) cells stained with antinitrotyrosine; (D) cells stained for anti–HSP-70. All micrograms are × 200.
Immunohistology of the T9-C2 injection site in a subcutaneous site.
Five million T9-C2 cells were injected in a subcutaneous site for 20 hours. The T9-C2 cells were excised and frozen sections were prepared. (A) Cells stained for granulocytes; (B) cells stained for iNOS; (C) cells stained with antinitrotyrosine; (D) cells stained for anti–HSP-70. All micrograms are × 200.
T9 and T9-C2 cells produce CINC, the rat IL-8 equivalent, which recruits PMNs
The neutrophil infiltrate within both the T9 and the T9-C2 tumors suggested that a soluble mediator attracted the granulocytes to the tumor injection site. Our previous work (C.D., M.R.J., unpublished data, May 2001) failed to detect any colony-stimulating factor (eg, granulocyte (G)-CSF or granulocyte-macrophage (GM)-CSF) made by T9 or T9-C2 cells in vitro that could account for the presence of the granulocytes in vivo. This prompted us to examine possible chemokines, because human gliomas22 and rat C6 gliomas23produce IL-8 and the rodent IL-8 equivalent known as CINC, respectively. By RT-PCR, both T9 and T9-C2 cells produced CINC-specific mRNA (Figure 3). To confirm the RT-PCR data, we stained permeabilized T9 and T9-C2 cells with anti-CINC antibody. Nonpermeabilized tumor cells failed to stain (Figure4A,B), whereas permeabilized cells displayed positive fluorescence (Figure 4C,D). This finding is consistent with CINC's secretory properties. Both the T9 and T9-C2 cells possessed the same amount of intracellular CINC, as quantitated by identical mean peak channel numbers.
T9 and T9-C2 cells produce CINC (IL-8) mRNA as detected by RT/ PCR.
The T9 and T9-C2 cells were refed with fresh tissue culture media and after 4 hours' incubation the total RNA was isolated. One microgram of total RNA was incubated with the CINC primers and was amplified.
T9 and T9-C2 cells produce CINC (IL-8) mRNA as detected by RT/ PCR.
The T9 and T9-C2 cells were refed with fresh tissue culture media and after 4 hours' incubation the total RNA was isolated. One microgram of total RNA was incubated with the CINC primers and was amplified.
Intracellular flow cytometry of T9 and T9-C2 cells making CINC (IL-8).
One million T9 and T9-C2 cells were allowed to react with the anti–IL-8 antibody or isotypic control antibody. Nonpermeabilized cells are shown in panels A (T9) and B (T9-C2) and permeabilized cells in panels C (T9) and D (T9-C2). Ten thousand cells were analyzed on the flow cytometer.
Intracellular flow cytometry of T9 and T9-C2 cells making CINC (IL-8).
One million T9 and T9-C2 cells were allowed to react with the anti–IL-8 antibody or isotypic control antibody. Nonpermeabilized cells are shown in panels A (T9) and B (T9-C2) and permeabilized cells in panels C (T9) and D (T9-C2). Ten thousand cells were analyzed on the flow cytometer.
Electron microscopy reveals that the T9-C2 cells are killed through a pathway that resembles paraptosis
Two hours after subcutaneous injection of tumor cells, PMNs and monocytes were found within blood vessels immediately adjacent to the tumor. At this time these leukocytes were beginning to invaginate through the endothelial cells lining the venule but were not infiltrating the tumor cells. The tumor cells appeared healthy and viable (data not shown).
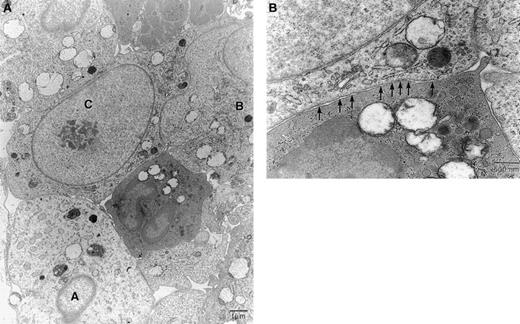
By 4 hours, many PMNs physically contacted the tumor cells. In regions where there was a high density of PMNs, the tumor cells were usually dying. We estimate that 50% to 75% of the T9-C2 cells were either dead or dying. T9-C2 cells immediately adjacent to the granulocytes and macrophages showed morphologic changes. Frequently, there appeared to be a lowered content of primary granules within the PMNs adjacent to the tumor cells. Tumor cells displayed swelling and vacuolization, with distention of the mitochondria and the endoplasmic reticulum (ER). Figure 5A shows a central granulocyte physically adjacent to 3 tumor cells in various stages of cell death. Tumor cell A is swollen and nonviable, with numerous vacuoles. Tumor cell B is also in the process of dying but shows less severe cellular pathology than cell A. Tumor cell C is just beginning to show signs of pathology; at higher magnification many clathrin-coated pits are visible immediately adjacent to the granulocyte (Figure 5B). Such a response was often observed in viable tumor cells that were in contact with PMNs and macrophages. An increase in the number of clathrin-coated pits was seen only in cells that had not begun to swell. This observation suggests pinocytosis by tumor cells of something being released by myeloid cells. Both tumor cells and granulocytes showed swelling of mitochondria. Similar results were noted in some of the control T9 tumor cells, except that the damage done to the T9 cells was never as severe or complete.
Electron micrographs examined of the T9-C2 cells after 4 hours' subcutaneous implantation.
Five million T9-C2 cells were injected in a subcutaneous site for 4 hours. Panel A shows 3 tumor cells, labeled A, B, and C, in various stages of swelling and death. Panel B shows a higher magnification of the central granulocyte and cell C. The arrows indicate clathrin-coated pits in cell C. Panels stained with uranyl acetate and osmium tetroxide. Original magnifications: Panel A, × 3100; Panel B, × 12 500.
Electron micrographs examined of the T9-C2 cells after 4 hours' subcutaneous implantation.
Five million T9-C2 cells were injected in a subcutaneous site for 4 hours. Panel A shows 3 tumor cells, labeled A, B, and C, in various stages of swelling and death. Panel B shows a higher magnification of the central granulocyte and cell C. The arrows indicate clathrin-coated pits in cell C. Panels stained with uranyl acetate and osmium tetroxide. Original magnifications: Panel A, × 3100; Panel B, × 12 500.
By 20 hours, all the T9-C2 cells were dead, swollen, and vacuolated, with rare membrane blebbing (zeiosis). The T9-C2 cells seldom possessed nuclear crescents, fragments, or the heavy condensation of nuclear chromatin that is characteristic of apoptotic cells. These dead tumor cells rarely formed smaller apoptotic bodies. The electron micrographs suggest that the process of tumor cell death most closely resembled paraptosis9 rather than apoptosis or necrosis (data not shown).
Killed T9-C2 cells were TUNEL-positive at 20 hours
We performed a TUNEL assay to determine whether DNA fragmentation had occurred within these dead T9-C2 tumor cells. The T9 cells failed to show any TUNEL staining (data not shown), whereas the T9-C2 injection site exhibited strong brown staining localized to the cell nucleus (not shown). No TUNEL staining was observed in T9-C2 cells sampled 4 hours after injection, when morphologic changes were documented by electron microscopy.
Bcl2-transduced T9-C2 cells also fail to form tumors in vivo
Disruption of mitochondria liberates cytochrome-c, which activates the effector caspases that translocate into the nucleus, where DNA cleavage occurs and initiates apoptosis. To study whether or not the presence of bcl2 could prevent apoptosis and thereby inhibit the killing of cells by the PMNs and macrophages, we transduced T9-C2 cells retrovirally with the bcl2 gene and then selected for optimal bcl2 expression. The bcl2-transduced cells showed growth rates and mM-CSF levels identical to those of the original T9-C2 cells. When these cells were injected into rats, these tumor cells failed to grow even after 3 months. Thus, this work eliminates the possibility that the killing process requires an apoptosis-dependent pathway.
Freshly isolated macrophages can kill the T9-C2 cells
The immunohistology and electron microscopy findings suggested that the mechanism of killing of the T9-C2 cells in vivo was different from the mechanism in previous studies done with bone marrow–derived macrophages, where direct phagocytosis of living T9-C2 cells occurred.10 11 To reconcile the differences between these in vivo and in vitro assays, we used freshly isolated macrophages, which we speculated might have different killing properties than bone marrow–derived macrophages. Thioglycolate-elicited peritoneal macrophages and alveolar macrophages both specifically killed the mM-CSF–transduced T9-C2 cells but not the parental T9 or the secreted M-CSF–producing T9-H1 cloned cells (Table1). Upon visual inspection of the macrophage-tumor cultures after 24 hours' incubation, it appeared that the tumor cells were not disappearing as we had previously seen with bone marrow–derived macrophages. Instead, numerous dead unattached cells were floating in the tissue culture media.
Freshly isolated rat peritoneal and alveolar macrophages kill mM-CSF-transfected T9-C2 cells but not T9 or T9-H1 (sM-CSF)-transfected cells
| Macrophage:T9 ratio . | Specific release, %, ± SD . | ||
|---|---|---|---|
| T9 parental cells . | sM-CSF T9-H1 clone . | mM-CSF T9-C2 . | |
| Peritoneal macrophages | |||
| 10:1 | 10 ± 0 | Not tested | 32 ± 0* |
| 5:1 | 6 ± 0 | Not tested | 22 ± 1* |
| 2.5:1 | 5 ± 0 | Not tested | 18 ± 0* |
| Alveolar macrophages | |||
| 10:1 | 8 ± 1 | 2 ± 1 | 23 ± 5* |
| 5:1 | 5 ± 0 | 1 ± 2 | 21 ± 6* |
| 2.5:1 | 4 ± 2 | 0 ± 1 | 16 ± 0* |
| Macrophage:T9 ratio . | Specific release, %, ± SD . | ||
|---|---|---|---|
| T9 parental cells . | sM-CSF T9-H1 clone . | mM-CSF T9-C2 . | |
| Peritoneal macrophages | |||
| 10:1 | 10 ± 0 | Not tested | 32 ± 0* |
| 5:1 | 6 ± 0 | Not tested | 22 ± 1* |
| 2.5:1 | 5 ± 0 | Not tested | 18 ± 0* |
| Alveolar macrophages | |||
| 10:1 | 8 ± 1 | 2 ± 1 | 23 ± 5* |
| 5:1 | 5 ± 0 | 1 ± 2 | 21 ± 6* |
| 2.5:1 | 4 ± 2 | 0 ± 1 | 16 ± 0* |
Freshly isolated rat macrophages were added to the T9, T9-H1, and T9-C2 target cells and the assay was harvested after 24 hours.
These values were significantly different (P< .05) from the results of the T9 tumor cell killing.
Thioglycolate-elicited macrophages cultured with the T9-C2 cells produced a respiratory burst, as detected by the luminol technique. This respiratory burst occurred within 30 minutes, and it was about 10% the strength of the burst produced by phorbol myristate acetate (PMA)–stimulated macrophages. No respiratory burst occurred when the macrophages were cultured with the T9 cells. Thus, freshly isolated macrophages are specifically able to kill the mM-CSF–transduced cells via reactive oxygen intermediates.
T9-C2 cells can grow in nude athymic rats only when certain leukocyte subsets are depleted
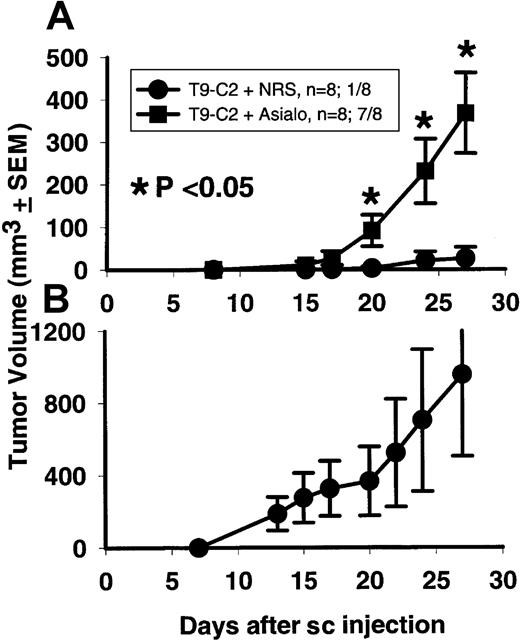
To prove which cell types are responsible for mM-CSF tumor destruction in vivo, we used athymic nude rats depleted of their various leukocyte subsets. When antiasialo GM1 antibody was used, 7 of 8 antiasialo GM1–treated rats formed T9-C2 tumors (Figure6A), while only 1 of 8 nude rats treated with normal rabbit serum formed a tumor. Starting at day 20, these 2 groups of animals were statistically significant (P <.05) . This experiment shows that the T9-C2 cells can grow in vivo under the right conditions. The data also indicate that some leukocytes were actively inhibiting the growth of the T9-C2 cells in rats.
mM-CSF–transfected T9-C2 cells will grow in nude athymic rats previously treated with antiasialo GM1 antibody.
Rats (8 per group) were injected with either the antiasialo GM1 antibody or normal rabbit serum (NRS) 4 times, 2 weeks prior to subcutaneous injection with 2 × 105 T9-C2 cells on day 0. Tumors were measured for the next 27 days (panel A). Panel B shows the growth of 2 × 105 T9 cells within all the NRS-treated rats that rejected the T9-C2 cells.
mM-CSF–transfected T9-C2 cells will grow in nude athymic rats previously treated with antiasialo GM1 antibody.
Rats (8 per group) were injected with either the antiasialo GM1 antibody or normal rabbit serum (NRS) 4 times, 2 weeks prior to subcutaneous injection with 2 × 105 T9-C2 cells on day 0. Tumors were measured for the next 27 days (panel A). Panel B shows the growth of 2 × 105 T9 cells within all the NRS-treated rats that rejected the T9-C2 cells.
When the normal rabbit serum–treated control athymic rats that rejected the T9-C2 cells were rechallenged with the parental T9 gliomas, all the rats developed subcutaneous T9 tumors (Figure 6B). Therefore, there is no inherent immune memory response generated against the T9 glioma in athymic rats that reject T9-C2 cells.
Besides being able to bind NK cells, the antiasialo GM1 antibody can bind to granulocytes, activated T cells, and activated macrophages.24-27 Thus, it was possible that the antiasialo GM1 antibody may have depleted the nude rat's NK cells, PMNs, and macrophages and therefore allowed the T9-C2 cells to grow. We next used more restricted antibodies against NK cells, granulocytes, and macrophages specifically to deplete these leukocytes. None of the rats treated with a control antibody or with the anti-NK antibody formed a T9-C2 tumor after 45 days (Table2). Of the rats treated with either anti-PMN or antimacrophage antibodies, 2 (40%) of the 5 in each group formed T9-C2 tumors. Of the nude rats that simultaneously received both the anti-PMN and antimacrophage antibodies, 80% formed T9-C2 tumors. By Fisher exact test, these results were significantly different from the results for the normal rabbit serum–treated rats (P = .015). This work demonstrates that both macrophages and granulocytes are required to kill the T9-C2 cells in vivo.
Treatment of nude athymic rats with antigranulocyte and antimacrophage antibodies allows some T9-C2 tumors to grow
| Treatment . | No. with tumors/total no. of rats injected (% with tumors) . |
|---|---|
| Normal rabbit serum | 0/6 (0) |
| Anti-NK antibody | 0/5 (0) |
| Anti-PMN antibody | 2/5 (40)* |
| Antimacrophage antibody | 2/5 (40)* |
| Anti-PMN and antimacrophage | 4/5 (80)† |
| Treatment . | No. with tumors/total no. of rats injected (% with tumors) . |
|---|---|
| Normal rabbit serum | 0/6 (0) |
| Anti-NK antibody | 0/5 (0) |
| Anti-PMN antibody | 2/5 (40)* |
| Antimacrophage antibody | 2/5 (40)* |
| Anti-PMN and antimacrophage | 4/5 (80)† |
The rats were injected 5 times over a 10-day period. Two injections occurred following subcutaneous injection of 105T9-C2 cells. After 30 days, the presence or absence of T9-C2 tumors was noted.
The result was not significantly different (by Fisher exact test) from the results for rats injected with normal rabbit serum (P = .182).
The result was significantly different (by Fisher exact test) from the results for rats injected with normal rabbit serum (P = .015) or anti-NK antibody (P = .048).
Immunocompetent rats that reject the living mM-CSF–transfected T9-C2 tumor cells resist an intracranial rechallenge with parental tumor T9 cells
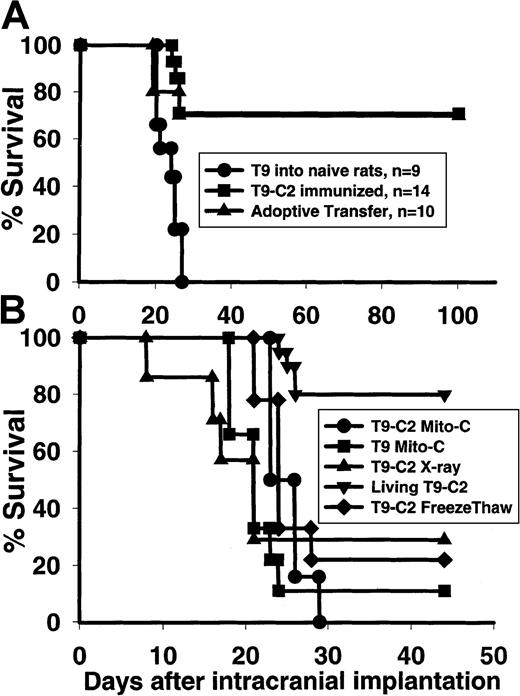
Fully immunocompetent F344 rats that rejected the subcutaneous T9-C2 cells were intracranially rechallenged with the parental T9 tumor cells to assess the immunization status of the rats. Seventy-one percent of the immunized rats rejected the intracranial tumor (Figure7A), while none of the control rats implanted with the same inoculum of T9 cells survived. Adoptive transfer of the splenocytes from rats that were immunized subcutaneously with the T9-C2 cells protected 70% of the naive rats against a lethal intracranial challenge of the T9 tumor. If rats had been immunized 2 weeks earlier with 3 × 105 T9-C2 cells that were x-irradiated, mitomycin-C–treated, or freeze-thawed, no protective immunity was generated (Figure 7B). Thus, living mM-CSF expressing T9-C2 cells that are killed via paraptosis by the innate immune system generates protective immunity against an intracranial glioma.
Rats that spontaneously reject mM-CSF T9-C2 cells are immune to intracranial T9 gliomas.
Rats were immunized for 2 weeks with 3×105 living T9-C2 cells and then implanted with 104 T9 cells intracranially (A). Data are also shown from rats that were immunized with living T9-C2 cells for 2 weeks, after which they were killed. Splenocytes were then removed and adoptively transferred into naive rats that were implanted with 104 T9 cells intracranially. (B) Rats were injected with 3×105 T9-C2 cells that were untreated (n = 20), x-irradiated with 10G (n = 7), treated with mitomycin-C (n = 6), or killed by freeze-thawing (n = 9). As a comparison, T9 cells treated with mitomycin-C were injected into 9 rats. After 2 weeks, the various immunized rats were implanted with 104 T9 cells intracranially. The rats that had been immunized with the living T9-C2 cells were significantly different (P < .001) from all other groups.
Rats that spontaneously reject mM-CSF T9-C2 cells are immune to intracranial T9 gliomas.
Rats were immunized for 2 weeks with 3×105 living T9-C2 cells and then implanted with 104 T9 cells intracranially (A). Data are also shown from rats that were immunized with living T9-C2 cells for 2 weeks, after which they were killed. Splenocytes were then removed and adoptively transferred into naive rats that were implanted with 104 T9 cells intracranially. (B) Rats were injected with 3×105 T9-C2 cells that were untreated (n = 20), x-irradiated with 10G (n = 7), treated with mitomycin-C (n = 6), or killed by freeze-thawing (n = 9). As a comparison, T9 cells treated with mitomycin-C were injected into 9 rats. After 2 weeks, the various immunized rats were implanted with 104 T9 cells intracranially. The rats that had been immunized with the living T9-C2 cells were significantly different (P < .001) from all other groups.
Discussion
Our previous in vitro studies showed that bone marrow–derived macrophages directly killed living malignant T9 glioma cells (T9-C2) transduced with mM-CSF, but not sM-CSF, via direct phagocytosis.10,11 Preliminary in vivo studies confirmed the results of the in vitro work.13 Not only were these mM-CSF–transfected T9 cells prevented from growing in vivo, both subcutaneously and intracranially, but the rats generated lasting immunity in the form of CD3+ T cells against the parental T9 gliomas.13 The use of mM-CSF–transfected cells therefore led to both spontaneous destruction of the vaccinating tumor and the development of lasting tumor immunity. We have seen similar results when weakly immunogenic rat MADB106 breast cancer or nonimmunogenic mouse Hepa1-6 hepatomas were used. This work showed that mM-CSF–transfected tumor cells can be used successfully to stimulate an immune response against a variety of cancer cells.
One distinguishing feature of our tumor models is that PMNs heavily infiltrated the T9-C2 tumor cells within 1 day. Previously, we had not seen these inflammatory cells, because we failed to look at earlier times.13 Their presence within both T9 and T9-C2 tumors results from the production of CINC (Figures 7 and 8), the rat IL-8 equivalent, a chemokine that attracts both PMNs and T cells.28 Lejeune et al29 have reported that CINC possesses antitumor properties against rat colon cancer, but it was not directly associated with PMN cytotoxicity in vivo. PMNs can kill some tumor cells through the release of several mediators, such as reactive oxygen species, defensins, or other soluble factors.30,31 Peripheral blood PMNs did not kill the T9 or T9-C2 cells, so the precise antitumor properties that the PMNs have in the T9 tumor model are still unknown. Graf et al32 reported that PMNs helped reject subcutaneously injected IL-6–transduced T9 cells in situ through an unknown mechanism. PMNs have the ability to attract various T cells and dendritic cells via the release of defensins.33 PMNs also act as antigen-presenting cells to preactivated T cells.34Thus, PMNs can produce a Th1 T cell immune response against some tumors and intracellular bacteria.35 36 These findings may help to explain why T9 cells are considered to be immunogenic.
Macrophages were seen among the dead T9-C2 cells after 18 hours, because mM-CSF was made by the T9-C2 cells. The histology of the T9-C2 tumor cells revealed that macrophages were not killing the tumor cells via phagocytosis. Soluble cytotoxic factors released by the macrophages include reactive oxygen intermediates, nitric oxide (NO) or peroxynitrite. We saw that freshly isolated peritoneal macrophages produced a respiratory burst when cultured with T9-C2 cells but not with T9 cells. Immunohistology revealed more iNOS staining in response to the T9-C2 cells. The antinitrotyrosine antibody staining indicated that peroxynitrite was generated. Xia and Zweier37 showed that iNOS catalyzes the production of NO when high concentrations of arginine are present, but with low concentrations of arginine, iNOS forms both NO and superoxide molecules. When the superoxide anions combine with NO, it forms peroxynitrite, which becomes more toxic than NO.38
The end result of the coordinated attack by the PMNs and macrophages is that the T9-C2 cells died through a pathway that resembled paraptosis.9 Here the tumor cell mitochondria began to swell and form vacuoles. These organelles resembled megamitochondria, previously described in several cells responding to various free radicals, including reactive oxygen intermediates and hydrogen peroxide.39,40 Eventually, the entire tumor cell becomes swollen, as evidenced by the loss of ground substance by electron microscopy. Some cells had condensed chromatin, but the dying cells rarely formed nuclear crescents or exhibited membrane blebbing. After 20 hours, but not after 4 hours, the cells displayed a TUNEL-positive phenotype. Positive TUNEL staining has been considered to be evidence of apoptosis, although nonapoptotic pathways can result in a positive TUNEL stain and DNA fragmentation does not necessarily equal apoptosis.41,42 To eliminate the possibility that the mM-CSF-transduced cells were dying of apoptosis, we cotransduced the T9-C2 cells with the anti–apoptosis-inducing gene, bcl2. Bcl2-cotransfected T9-C2 cells were still prevented from growing in the skin. It is possible to stimulate apoptosis via a bcl2-independent pathway via Fas-Fas ligand and tumor necrosis factor (TNF) binding via tumor necrosis factor–related apoptosis-inducing ligand (TRAIL). We have shown in unpublished studies that T9 cells are Fas and Fas ligand–negative by flow cytometry and that recombinant TNF will not kill the T9 cells in vitro. This macrophage-mediated process is independent of the Fas–Fas ligand pathway, because macrophages that do not express Fas ligand also kill these cells in vitro.12 Thus, we have eliminated this non–bcl2-related apoptosis route.
Once the T9-C2 cells were killed in the immunocompetent F344 rats, the rats resisted lethal intracranial challenge by the T9 cells. Adoptive transfer of the immunized splenocytes also protected the naive rats against a lethal intracranial challenge. This work shows that protective cellular immunity was generated and that immunized splenocytes protected naive rats against the T9 tumor. If the rats were vaccinated with x-irradiated, mitomycin-C–treated, or freeze-thawed T9-C2 cells 2 weeks prior to intracranial challenge, no protective immunity was generated. Therefore, something within the treated T9-C2 cell has changed, making these cells less immunogenic. Mitomycin-C cross-links DNA, preventing the DNA from separating during DNA replication. X-irradiation induces DNA damage, culminating in apoptosis. Freeze-thawing immediately killed the tumor cells. These treatments probably modified the T9-C2 cells, rendering them incapable of making either mRNA or protein and preventing any intracellular responses by the T9-C2 cells. We speculate that living mM-CSF–transduced cells must be injected into the animal to induce the PMN and macrophage responses that generate the intracellular signals necessary to generate the optimal immunization conditions. By electron microscopy, we saw that the T9-C2 cells responded to the granulocytes and macrophages by forming clathrin-coated pits, suggesting that the living tumor cells are reacting to some leukocyte-derived product. This tumor response is consistent with the hypothesis that paraptosis is also a type of programmed cell destruction that requires intracellular signaling and that leads toward vacuolization of the killed tumor cell.9
The mechanism of killing of mM-CSF–transduced tumor cells is extremely important for therapeutic purposes. In a recent study,20we have seen that rats immunized with T9-C2 cells also produce immunity against other syngeneic gliomas, such as RT2 and F98. We believe that the best clinical use of mM-CSF would be as an allogeneic transfected tumor vaccine. After the tumor has been surgically resected and radiation has been given, when the tumor burden is at its least, would be the best time to be vaccinated. A subcutaneous injection would be the best strategy, because it allows the immune system to be fully exposed to the tumor antigens. By the time an autologous tumor was established, transfected, and cloned, the primary tumor would have recurred and any immunotherapy would be useless. We have seen a similar phenomenon when we injected a mM-CSF–transduced human MG-U251 glioma into either nude or NIH-bg-nu-xid mice. The viral vector tumor cells grew as subcutaneous tumors, while 3 × 106cloned mM-CSF–transfected cells failed to form tumors in all immunodeficient mice (Y.C. et al, manuscript in preparation). Additionally, human monocytes specifically killed the mM-CSF–transfected cells in vitro. If our hypothesis that paraptosis occurs is correct, then this killing process should lead toward tumor immunity. Thus methods that lead toward tumor cell killing by paraptosis should be investigated and used. Not only will the tumor be killed, but the killing process could lead to conditions that generate stronger immunity against the tumor.
We appreciate Dr Maria DaCosta-Iyer's helpful suggestions of ways to improve the immunohistochemical staining. We thank Sundra Crooms and Lynn Carmon (Diagnostic and Molecular Medicine, Histology Section) for their help in cutting the various histology samples. We thank Walt Thill (Medical Media, Veterans Affairs Medical Center, Long Beach, CA) for his help with photography. We also thank Dr Michael Selsted (Department of Pathology, University of California, Irvine) for his helpful discussions regarding neutrophils and the antitumor properties of defensins. We also deeply appreciate the help of Ms Terri Bondy, who proofread our paper.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/2002-01-0174.
Supported in part by grants from the Veterans Affairs Medical Center; the National Institutes of Health (grant CA77802 R01); the Avon Foundation, via the University of California at Irvine Cancer Research Program; and the Chiron Corporation, Emeryville, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martin R. Jadus, Box 113, Diagnostic and Molecular Health Care Group, Veterans Affairs Medical Center, 5901 E 7th St, Long Beach, CA 90822; e-mail: martin.jadus@med.va.gov orjadus.martin@long-beach.va.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal