CD4 T-cell–specific memory antiviral responses to human immunodeficiency virus type 1 (HIV-1) and cytomegalovirus (CMV) were investigated in 16 patients with documented primary HIV-1 infection (4 of the 16 subjects also had primary CMV infection) and compared with those observed in patients with chronic HIV-1 and CMV coinfection. Virus-specific memory CD4 T cells were characterized on the basis of the expression of the chemokine receptor CCR7. HIV-1– and CMV-specific interferon-γ–secreting CD4 T cells were detected in patients with primary and chronic HIV-1 and CMV coinfection and were mostly contained in the cell population lacking expression of CCR7. The magnitude of the primary CMV-specific CD4 T-cell response was significantly greater than that of chronic CMV infection, whereas there were no differences between primary and chronic HIV-1–specific CD4 T-cell responses. A substantial proportion of CD4+CCR7− T cells were infected with HIV-1. These results advance the characterization of antiviral memory CD4 T-cell response and the delineation of the potential mechanisms that likely prevent the generation of a robust CD4 T-cell immune response during primary infection.
Introduction
Previous studies performed in mice and humans have clearly highlighted the critical role of virus-specific CD4 T cells in the control of chronic viral infections.1-13 In particular, CD4 proliferative responses are generally not detected during primary infection3,14 or in patients with chronic infection, with the exception of patients with nonprogressive disease (“long-term nonprogressors”).1-5,15 Recent studies have linked the lack of immune control of human immunodeficiency virus 1 (HIV-1) infection to the absence of CD4 proliferation, that is, the helper function.3 Initiation of antiviral treatment during the early phase of acute infection may lead to restoration of the virus-specific CD4 helper response and may be associated with partial control of virus replication during chronic infection.3Despite the lack of virus-specific CD4 helper response, HIV-1–specific CD4 T cells have been identified even in patients with progressive disease on the basis of their ability to secrete interferon (IFN)-γ after a short period (6 hours) of stimulation with HIV-1 antigens.5 The discordance between the lack of CD4 T cell proliferation and the detection of IFN-γ–secreting CD4 T cells can be explained by the stimulation of different populations of antigen-specific CD4 T cells in the 2 assays. Stimulation of precursors of antigen-specific memory T cells occurs in the 6-day lymphoproliferative assay, whereas the short-term (6-hours) stimulation IFN-γ flow cytometry assay likely detects functionally differentiated antigen-specific T cells. This interpretation is supported by a series of recent studies that indicate that memory T cells progressively lose their capacity to proliferate after differentiation and acquisition of effector function.16
With regard to primary infection, little is known about the presence of IFN-γ–secreting CD4 T cells, and no characterization of the populations of memory CD4 T cells has been performed. In the present study, to characterize the primary CD4 T-cell immune response, we performed phenotypic and functional analysis of blood virus–specific memory CD4 T cells, as defined by the expression of the chemokine receptor CCR7, in 16 subjects with documented primary HIV-1 infection. Previous studies have demonstrated that the expression of CCR7 distinguishes populations of memory CD4 and CD8 T lymphocytes with different homing and function capacities and at different stages of differentiation.16-18 We also studied cytomegalovirus (CMV)-specific CD4 T-cell responses in the same group of patients, and we compared primary HIV-1– and CMV-specific CD4 T-cell responses with those of patients with chronic HIV-1 and CMV coinfection. Finally, we analyzed the distribution of HIV-1 viral load in different subsets of memory CD4 T cells in a subgroup of subjects with primary HIV-1 infection.
Patients and methods
Patients
The 16 patients in this study, all of whom had primary HIV-1 infection19 (Table 1), had been enrolled in a clinical therapeutic trial. All had experienced an acute viral syndrome of variable severity. The criteria for diagnosis of primary HIV-1 infection at the first visit (baseline) included (1) the presence of HIV-1 RNA in the plasma; (2) negative or weakly positive antibody tests (HIV 1/2 enzyme-linked immunosorbent assay); and (3) negative or weakly positive (< 3 bands) HIV-1 Western blot. Four of the patients also had primary CMV infection (Table2). The criteria for the diagnosis of primary CMV infection were the presence of immunoglobulin (Ig)M but not IgG anti-CMV antibodies followed by the appearance of IgG antibodies at later time points. Eight patients had chronic CMV infection, as indicated by the presence of high-avidity IgG anti-CMV antibodies. The patients with primary CMV infection had detectable CMV DNA in their plasma, whereas CMV DNA was not detectable (< 10 CMV DNA copies per milliliter of plasma) in those with chronic CMV infection.
Clinical, virologic, and immunologic characteristics of the 16 patients with primary HIV-1 infection
| Patient . | Age, y . | Sex . | Days from onset of symptoms . | CD4, % . | CD4 count, cells/μL . | Viremia, copies/mL of plasma . | CMV serology . | IFN-γ–secreting cells, %* . | Lymphoproliferation (stimulation index)† . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p55 . | CMV . | ||||||||||||||
| HIV-1 RNA . | CMV DNA . | CD4+ CCR7− . | CD4+ CCR7+ . | CD4+ CCR7− . | CD4+ CCR7+ . | p24 . | p55 . | CMV . | |||||||
| 1 | 32 | M | 8 | 3.5 | 79 | 58 081 400 | 980 | IgM | 0.37 | 0.03 | 4.05 | 0.09 | < 5 | < 5 | < 5 |
| 2 | 30 | M | 4 | 6.5 | 706 | 701 000 | 10 350 | IgM | 1.62 | 0.05 | 2.75 | 0.05 | < 5 | < 5 | < 5 |
| 3 | 20 | M | 21 | 18 | 591 | 66 700 | 361 | IgM | 0.38 | 0.08 | 5.24 | 0.27 | < 5 | < 5 | < 5 |
| 4 | 28 | M | 45 | 11 | 1500 | 551 000 | 1 455 | IgM | 0.54 | 0.12 | 16.57 | 2.57 | ND | < 5 | < 5 |
| 5 | 23 | M | 21 | 6.8 | 231 | 105 000 | < 10 | IgG | 0.12 | < 0.03 | 0.06 | 0.07 | ND | ND | ND |
| 6 | 34 | M | 26 | 19.6 | 838 | 400 291 | < 10 | IgG | < 0.03 | < 0.03 | < 0.03 | < 0.03 | < 5 | < 5 | ND |
| 7 | 35 | M | 10 | 14.6 | 342 | 256 667 | < 10 | IgG | 0.10 | < 0.03 | 2.30 | 0.03 | < 5 | < 5 | ND |
| 8 | 20 | F | 7 | 15.2 | 468 | 373 693 | < 10 | IgG | 0.95 | 0.04 | 0.56 | 0.03 | < 5 | < 5 | < 5 |
| 9 | 33 | M | 35 | 19.6 | 435 | 150 000 | < 10 | ND | 0.06 | 0.20 | 0.13 | 0.14 | 6 | 12 | < 5 |
| 10 | 27 | M | 14 | 12 | 165 | 839 000 | < 10 | IgG | 0.07 | 0.05 | 0.72 | 0.12 | < 5 | < 5 | < 5 |
| 11 | 29 | M | 14 | 27.5 | 1005 | > 750 000 | ND | IgG | ND | ND | ND | ND | ND | ND | ND |
| 12 | 21 | M | 14 | 14.4 | 153 | 54 722 | < 10 | Negative | 0.32 | 0.03 | < 0.03 | < 0.03 | < 5 | < 5 | < 5 |
| 13 | 33 | M | 9 | 51.7 | 596 | 26 500 000 | < 10 | Negative | 0.40 | 0.03 | < 0.03 | < 0.03 | < 5 | < 5 | < 5 |
| 14 | 33 | M | 10 | 18 | 286 | 1 190 000 | ND | Negative | 1.00 | 0.28 | < 0.03 | < 0.03 | < 5 | 5.4 | < 5 |
| 15 | 34 | F | 28 | 10 | 419 | 505 000 | ND | Negative | ND | ND | ND | ND | ND | ND | ND |
| 16 | 35 | M | 12 | 24 | 405 | 4 000 000 | ND | Negative | ND | ND | ND | ND | ND | ND | ND |
| Patient . | Age, y . | Sex . | Days from onset of symptoms . | CD4, % . | CD4 count, cells/μL . | Viremia, copies/mL of plasma . | CMV serology . | IFN-γ–secreting cells, %* . | Lymphoproliferation (stimulation index)† . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p55 . | CMV . | ||||||||||||||
| HIV-1 RNA . | CMV DNA . | CD4+ CCR7− . | CD4+ CCR7+ . | CD4+ CCR7− . | CD4+ CCR7+ . | p24 . | p55 . | CMV . | |||||||
| 1 | 32 | M | 8 | 3.5 | 79 | 58 081 400 | 980 | IgM | 0.37 | 0.03 | 4.05 | 0.09 | < 5 | < 5 | < 5 |
| 2 | 30 | M | 4 | 6.5 | 706 | 701 000 | 10 350 | IgM | 1.62 | 0.05 | 2.75 | 0.05 | < 5 | < 5 | < 5 |
| 3 | 20 | M | 21 | 18 | 591 | 66 700 | 361 | IgM | 0.38 | 0.08 | 5.24 | 0.27 | < 5 | < 5 | < 5 |
| 4 | 28 | M | 45 | 11 | 1500 | 551 000 | 1 455 | IgM | 0.54 | 0.12 | 16.57 | 2.57 | ND | < 5 | < 5 |
| 5 | 23 | M | 21 | 6.8 | 231 | 105 000 | < 10 | IgG | 0.12 | < 0.03 | 0.06 | 0.07 | ND | ND | ND |
| 6 | 34 | M | 26 | 19.6 | 838 | 400 291 | < 10 | IgG | < 0.03 | < 0.03 | < 0.03 | < 0.03 | < 5 | < 5 | ND |
| 7 | 35 | M | 10 | 14.6 | 342 | 256 667 | < 10 | IgG | 0.10 | < 0.03 | 2.30 | 0.03 | < 5 | < 5 | ND |
| 8 | 20 | F | 7 | 15.2 | 468 | 373 693 | < 10 | IgG | 0.95 | 0.04 | 0.56 | 0.03 | < 5 | < 5 | < 5 |
| 9 | 33 | M | 35 | 19.6 | 435 | 150 000 | < 10 | ND | 0.06 | 0.20 | 0.13 | 0.14 | 6 | 12 | < 5 |
| 10 | 27 | M | 14 | 12 | 165 | 839 000 | < 10 | IgG | 0.07 | 0.05 | 0.72 | 0.12 | < 5 | < 5 | < 5 |
| 11 | 29 | M | 14 | 27.5 | 1005 | > 750 000 | ND | IgG | ND | ND | ND | ND | ND | ND | ND |
| 12 | 21 | M | 14 | 14.4 | 153 | 54 722 | < 10 | Negative | 0.32 | 0.03 | < 0.03 | < 0.03 | < 5 | < 5 | < 5 |
| 13 | 33 | M | 9 | 51.7 | 596 | 26 500 000 | < 10 | Negative | 0.40 | 0.03 | < 0.03 | < 0.03 | < 5 | < 5 | < 5 |
| 14 | 33 | M | 10 | 18 | 286 | 1 190 000 | ND | Negative | 1.00 | 0.28 | < 0.03 | < 0.03 | < 5 | 5.4 | < 5 |
| 15 | 34 | F | 28 | 10 | 419 | 505 000 | ND | Negative | ND | ND | ND | ND | ND | ND | ND |
| 16 | 35 | M | 12 | 24 | 405 | 4 000 000 | ND | Negative | ND | ND | ND | ND | ND | ND | ND |
ND indicates not determined.
A percentage of IFN-γ–secreting cells lower than 0.03% was considered negative.
A stimulation index more than 5-fold over the negative control (unstimulated PBMC cultures) was considered positive.
CMV antibody responses in the 4 patients with primary HIV-1 and CMV coinfection
| . | IgG-ELFA (4-6 IU/mL)* . | IgM-EIA index (0.9-01.1)† . |
|---|---|---|
| Patient 1 | ||
| Baseline | < 4 | 2.4 |
| Day 15 | 27 | 4.9 |
| Patient 2 | ||
| Baseline | 6 | 3.3 |
| Day 70 | 73 | 1.4 |
| Patient 3 | ||
| Baseline | < 4 | 0.8 |
| Day 18 | 25 | 10 |
| Patient 4 | ||
| Baseline | < 4 | 2.2 |
| Day 8 | 8 | 5.8 |
| . | IgG-ELFA (4-6 IU/mL)* . | IgM-EIA index (0.9-01.1)† . |
|---|---|---|
| Patient 1 | ||
| Baseline | < 4 | 2.4 |
| Day 15 | 27 | 4.9 |
| Patient 2 | ||
| Baseline | 6 | 3.3 |
| Day 70 | 73 | 1.4 |
| Patient 3 | ||
| Baseline | < 4 | 0.8 |
| Day 18 | 25 | 10 |
| Patient 4 | ||
| Baseline | < 4 | 2.2 |
| Day 8 | 8 | 5.8 |
In patients 1, 3, and 4, the absence of CMV IgG antibodies and the presence of IgM antibodies (borderline positive in patient 3) at baseline, together with the seroconversion at later time points, are unequivocal evidence of primary CMV infection. In patient 2, the borderline level and low avidity (19%) of IgG and the presence of IgM at baseline also suggest a primary CMV infection. The substantial increase in both IgG levels and avidity (60%) at day 70 further supports the diagnosis of primary CMV infection. IU indicates international units.
Determination of the IgG CMV antibodies was performed by an enzyme-linked fluorescent assay (ELFA). Range indicates positive borderline values.
Determination of the IgM CMV antibodies was performed by an enzyme immunoassay (EIA). Range indicates positive borderline values.
The 7 patients with chronic HIV-1 infection were naive to antiviral therapy (CD4 T-cell count ≥ 250 cells/μL, plasma viremia ≥ 5000 HIV-1 RNA copies per milliliter) and were coinfected with CMV. The patients with primary and chronic CMV infections had no clinical signs of CMV-associated organ disease. Both patients with primary HIV-1 infection and those with chronic HIV-1 infection were enrolled in therapeutic clinical trials with antiretroviral regimens comprising 2 nucleoside reverse transcriptase inhibitors and 1 or 2 protease inhibitors.20 These trials were open-label, observational, nonrandomized prospective studies carried out at a single site (Lausanne, Switzerland). These studies were approved by the local institutional review board, and all subjects gave written informed consent.
Fluorescence-activated cell sorter (FACS) analysis and sorting
Cells that had been cryopreserved in liquid nitrogen were thawed for staining and analysis.16 Cells were stained for a panel of cell surface markers. Rat anti-human CCR7 antibody (3D12, rat IgG2a) staining was followed by goat anti-rat IgG(H+L) −fluorescein isothiocyanate (FITC) or −phycoerythrin (PE) (Southern Biotechnologies Associates, Birmingham, AL). The following mouse antihuman antibodies were used in different combinations for cell surface staining and sorting: anti-CD4 CyChrome and/or allophycocyanin (APC) and anti-CCR5 PE and/or CyChrome (Becton Dickinson, Franklin, NJ). For intracellular Ki67 analysis, following surface marker labeling, cells were fixed and permeabilized with ORTHO PermeaFix (Ortho Diagnostic Systems, Raritan, NJ) as per manufacturer's instructions, prior to intracellular staining with anti-Ki67 FITC (IgG1, MIB-1) (Immunotech). Data were acquired on a FACSCalibur system and analyzed with CellQuest software (Becton Dickinson). In all the flow analyses, at least 106 events were acquired and gated on lymphocytes. Cell sorting was performed on a FACSVantage (Becton Dickinson). The purity of the sorted cell populations was higher than 97%.
Determination of CMV DNA in plasma and of HIV-1 cellular viral load
CMV viremia was measured with a modified version of the Amplicor CMV Monitor test (Roche Diagnostic, Indianapolis, IN), with a limit of detection of 10 DNA copies per milliliter of plasma.21
Cell-associated HIV-1 DNA and RNA in sorted CD4+CCR7+ and CD4+CCR7− T-cell populations were determined as previously described.21
HIV-1– and CMV-specific lymphoproliferation assays
Peripheral blood mononuclear cells (PBMCs) were thawed and resuspended at 37°C in RPMI 1640 Gutamax-1 medium containing 2% inactivated AB human serum. PBMCs were plated at 1 × 105cells per well in U-bottom 96-well cell culture plates (Costar, Bucks, United Kingdom) and incubated with HIV-1 p55 and p24 gag proteins (1 μg per well) and/or CMV lysates (1:2000 final concentration) for 5 days. Then cell cultures were pulsed with [3H]thymidine (1.0 μCi [.037 MBq] per well) for 18 hours. Cell cultures with a stimulation index of 5 or higher compared with the unstimulated control cell cultures were considered to be positive for HIV-1– and/or CMV-specific proliferation.
Cytokine detection
Intracellular cytokine production was assessed as previously described.5 PBMCs (3-4 × 106 cells in 2 mL) were stimulated with 10 μg (5 μg per mL final concentration) of p55 or gp160 HIV-1 proteins (Protein Sciences, Meriden, CT) and/or 1:200 final dilution CMV lysates (Bio Whittaker Verviers, Belgium) and/or 200 ng/mL staphylococcal enterotoxin B (positive control), or phosphate-buffered saline (PBS) for unstimulated negative controls for 6 hours at 37°C. Stimulation was performed in the presence 0.5 μg/mL of purified anti-CD28 antibody (Becton Dickinson) and, as of the second hour, with 10 μg/mL Brefeldin A (Sigma, St Louis, MO). Cell surface staining was completed as described following the 6-hour in vitro activation. Cells were then permeabilized with Intrastain (Dako, Glostrup, Denmark) and labeled with antihuman IFN-γ APC (IgG1, B27; Pharmingen, San Diego, CA). Simultaneously, activation was assessed by staining with anti-CD69 FITC (Becton Dickinson). Since the mean percentage background in the unstimulated cultures was less than 0.02, background levels were not subtracted.
Statistical analysis
Statistical significance (P values) of the results was calculated by 2-tailed t test.
Results
Patients
All the patients included in the present study experienced an acute viral clinical syndrome of variable severity19 at the time of primary HIV-1 infection. The most common symptoms were fever, asthenia, fatigue, diarrhea, and lymphoadenopathy. The laboratory criteria for the diagnosis of primary infection were the presence of HIV-1 RNA plasma viremia, negative or weakly positive antibody tests, and less than 3 bands at Western blot. These stringent laboratory criteria allowed us to identify the patients during the early phase of primary infection, as indicated by mean number of days (17.4) from the onset of symptoms to the time of the diagnosis and by the high levels of HIV-1 plasma viremia (mean 6.251.000 HIV-1 RNA copies per milliliter of plasma) (Table 1).
CMV serology and CMV plasma viremia were determined in the majority of patients; CMV serology was not determined in 1 patient. On the basis of CMV serology we identified 3 groups of patients: (1) patients with negative serology for CMV (5 of 15); (2) patients with high-avidity IgG anti-CMV, typical of chronic CMV infection (6 of 15) and no history of CMV-associated disease; and (3) patients with IgM anti-CMV and no IgG antibodies, an antibody profile typical of CMV seroconversion (4 of 15) (Table 2). The 4 patients with CMV seroconversion also had substantial levels of CMV viremia at the time of the diagnosis of primary infection (Table 1). These latter patients had no signs of CMV-associated organ disease, although there was a trend toward statistical significance for a lower percentage of CD4 T cells (mean 9.75% ± 6.3%, n = 4) compared with the patients with no CMV primary infection (mean 19.6% ± 11.7%, n = 12,P = .07) (Table 1). Therefore, these results indicate that these 4 patients experienced primary HIV-1 and CMV coinfection.
Phenotypic analysis of CD4 T cells
Previous studies have shown that the chemokine receptor CCR7 defines distinct subsets of naive and memory T lymphocytes with different homing and effector capacities.16-18 In particular, CD4 and CD8 T lymphocyte populations lacking CCR7 contain the majority of cells with potential effector function as defined by the secretion of IFN-γ and by the expression of perforin.18 In this regard, it has been recently shown that CD8 T lymphocytes are mostly contained within the CCR7− T lymphocyte subset.16
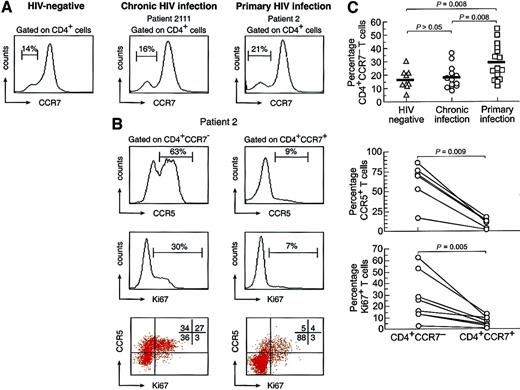
To characterize the CD4 T-cell response during primary HIV-1 infection, in preliminary experiments we analyzed PBMCs obtained from 9 HIV-negative subjects, 13 HIV-1–infected subjects with chronic infection, and 15 subjects with primary infection with monoclonal antibodies to CD4 and CCR7. These analyses were performed on PBMCs collected prior to the initiation of therapy. In Figure1A, representative examples for the 3 groups studied are shown. These analyses showed that the CD4 cell population lacking CCR7 appeared to be increased in the patients with primary HIV-1 infection. The mean percentage of CD4+CCR7− cells was 29.8% ± 13.5% in patients with primary HIV-1 infection (n = 15), 18.5% ± 8.6% in HIV-1–infected patients with chronic infection (n = 13), and 15.3% ± 5.7% in HIV-negative patients (n = 9). The differences were statistically significant in the group of patients with primary infection vs the group with chronic infection (P = .008) and vs HIV-1–negative subjects (P = .008), but the differences observed between the latter 2 groups were not (P> .05) (Figure 1A).
Distribution of chemokine receptors and of cycling capacity of CD4 T cells.
(A) Representative flow cytometry profiles of the distribution of CCR7 on gated CD4 T cells in HIV-negative and HIV-1–infected patients with chronic and primary infection. The individual data of the different cohorts analyzed are also shown. (B) Distribution of CCR5 and Ki67 on gated CD4+CCR7− and CD4+CCR7+ cells of a representative patient with primary HIV-1 infection. A large proportion of CD4+CCR7− cells expressed CCR5 and Ki67. The individual data of the different patients analyzed are also shown. Blood mononuclear cells were stained with anti-CD4 APC, anti-CCR5 CyChrome, anti-CCR7 PE, and anti-Ki67 FITC antibodies.
Distribution of chemokine receptors and of cycling capacity of CD4 T cells.
(A) Representative flow cytometry profiles of the distribution of CCR7 on gated CD4 T cells in HIV-negative and HIV-1–infected patients with chronic and primary infection. The individual data of the different cohorts analyzed are also shown. (B) Distribution of CCR5 and Ki67 on gated CD4+CCR7− and CD4+CCR7+ cells of a representative patient with primary HIV-1 infection. A large proportion of CD4+CCR7− cells expressed CCR5 and Ki67. The individual data of the different patients analyzed are also shown. Blood mononuclear cells were stained with anti-CD4 APC, anti-CCR5 CyChrome, anti-CCR7 PE, and anti-Ki67 FITC antibodies.
A large percentage of CD4+CCR7− cells expressed CCR5, the main coreceptor for HIV-1,22 as compared with a minority of CD4+CCR7+ cells (Figure 1B). The preferential expression of CCR5 in memory T lymphocyte populations is in agreement with previous studies.18,23Furthermore, a substantial proportion (30%) of CD4+CCR7− cells expressed Ki67, a nuclear antigen associated with all phases of the cell cycle with the exception of G0 (Figure 1B).24 Four-color flow cytometry demonstrated that Ki67 was expressed by about 50% of CD4+CCR7−CCR5+ cells (Figure 1B). Therefore, a large proportion of CD4 T cells with potential effector function expressed the main coreceptor for HIV-1 and were actively cycling.
Functional analysis of different populations of virus-specific memory CD4 T cells
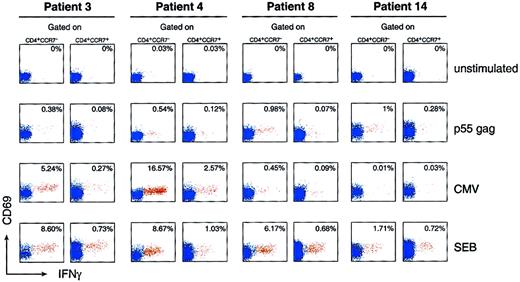
As mentioned above, CD4 and CD8 T cells with effector function are contained within the cell populations lacking CCR7.16-18To delineate the antiviral-specific primary CD4 T-cell–mediated immune response, we assessed the ability of the different populations of CD4 T cells, as defined by the expression and/or the absence of CCR7, to secrete IFN-γ. Patients 1 through 4 also had primary CMV infection (Tables 1 and 2). IFN-γ–secreting cells were detected in 12 of 13 patients studied (Table 1). The assessment of HIV-1–specific CD4 T cells was mostly performed following stimulation of blood mononuclear cells with p55 gag HIV-1 protein; assessment of CMV-specific CD4 T cells was performed following stimulation with CMV lysates. As shown in Figure 2 and Table 1, in 4 representative patients selected on the basis of CMV serology (CMV primary infection [patients 3 and 4], chronic CMV infection [patient 8], and negative CMV serology [patient 14]), the majority (> 90%) of CD4+ IFN-γ–secreting cells were contained within the CCR7− cell population. The mean percentage of CD4+CCR7− IFN-γ–secreting cells was significantly lower (0.49% ± 0.47%, n = 12; Table 1) than the mean percentage of CMV-specific CD4+CCR7−T cells observed in the 4 patients with CMV primary infection (mean percentage 7.2% ± 6.3%, n = 4, P = .003; Table 1).
Analysis of HIV-1– and CMV-specific CD4 T cells within different populations of memory cells defined by the expression of CCR7.
Patients 3 and 4 had primary HIV-1 and CMV coinfection, patient 8 had primary HIV-1 infection and chronic CMV infection, and patient 14 had only primary HIV-1 infection. Blood mononuclear cells were stimulated with p55 gag and CMV lysates and analyzed for the expression of CD4, CCR7, CD69, and IFN-γ (intracellular expression). The data show the expression of CD69 and IFN-γ within CD4+CCR7− and CD4+CCR7+ T-cell populations. Negative control: unstimulated blood mononuclear cells. Positive controls: blood mononuclear cells stimulated with staphylococcal enterotoxin B. The cluster of events shown in red corresponds to the responder CD4 T cells (ie, coexpressing CD69 and IFN-γ), whereas the cluster of events in blue corresponds to the nonresponder CD4 T cells. The data are expressed as the percentage of cells coexpressing CD69 and IFN-γ within CD4+CCR7− and CD4+CCR7+ T-cell populations. Following in vitro antigen-specific stimulation, blood mononuclear cells were stained with anti–IFN-γ APC, anti-CD69 FITC, anti-CCR7 PE, and anti-CD4 CyChrome.
Analysis of HIV-1– and CMV-specific CD4 T cells within different populations of memory cells defined by the expression of CCR7.
Patients 3 and 4 had primary HIV-1 and CMV coinfection, patient 8 had primary HIV-1 infection and chronic CMV infection, and patient 14 had only primary HIV-1 infection. Blood mononuclear cells were stimulated with p55 gag and CMV lysates and analyzed for the expression of CD4, CCR7, CD69, and IFN-γ (intracellular expression). The data show the expression of CD69 and IFN-γ within CD4+CCR7− and CD4+CCR7+ T-cell populations. Negative control: unstimulated blood mononuclear cells. Positive controls: blood mononuclear cells stimulated with staphylococcal enterotoxin B. The cluster of events shown in red corresponds to the responder CD4 T cells (ie, coexpressing CD69 and IFN-γ), whereas the cluster of events in blue corresponds to the nonresponder CD4 T cells. The data are expressed as the percentage of cells coexpressing CD69 and IFN-γ within CD4+CCR7− and CD4+CCR7+ T-cell populations. Following in vitro antigen-specific stimulation, blood mononuclear cells were stained with anti–IFN-γ APC, anti-CD69 FITC, anti-CCR7 PE, and anti-CD4 CyChrome.
A possible explanation for the differences in the magnitude of HIV-1– and CMV-specific CD4 T-cell responses was that HIV-1–specific CD4 T-cell response might be directed against HIV-1 proteins other than gag. To test this hypothesis, PBMCs were also stimulated with gp160 envelope HIV-1 protein. No evidence was found for the presence of a substantial percentage of env-specific CD4 T cells in patients with primary infection (data not shown). Because only a percentage higher than 0.03% of IFN-γ–secreting cells is considered positive, it is not possible to exclude the possibility that env-specific CD4 T cells are present at very low frequency. The lack of evidence of substantial HIV-1 env-specific CD4 T-cell responses is in agreement with previous studies.5
Another possible explanation of the difference in magnitude of primary HIV-1– and CMV-specific CD4 T-cell responses that we observed is that CMV viral lysate was used to assess CMV-specific responses, whereas either p55 or gp160 proteins were used to assess HIV-specific responses. It has been shown that CD4+ T-cell responses to the CMV pp65 protein often account for as little as 10% of the response obtained by stimulation with viral lysates,25despite the fact that pp65 is known to be the major target of the CMV T-cell response. Therefore, it is not possible to exclude the possibility that the observed differences in magnitude of CMV and primary immune responses were dependent upon the type of antigen stimulation (eg, viral lysate vs protein).
We then analyzed whether there were differences between primary CMV- and HIV-1–specific CD4 T-cell responses and those in patients with chronic CMV and HIV-1 coinfection. CMV-specific memory CD4 T-cell responses were determined in 13 patients with chronic CMV infection. We determined that 6 patients with primary HIV-1 infection and 7 with chronic HIV-1 infection also had chronic CMV infection. The patients with chronic HIV-1 infection were at early stages of the disease and had had no previous antiviral treatment; the mean plasma viremia was 32 000 HIV-1 RNA copies per milliliter, the mean CD4 T-cell count was 952 ± 254 per μL of blood, and the mean CD4 T-cell percentage was 36.3% ± 7%. CMV-specific CD4+ IFN-γ–secreting cells were detected in 12 of the 13 patients with chronic CMV infection and, as for HIV-specific CD4+ T cells, most of the IFN-γ–secreting cells were CCR7− (Figure 2 and Table1). The detection of CMV-specific memory CD4 T-cell responses in patients with HIV-1 infection is consistent with previous studies.26 27 These analyses showed that the percentage (mean 7.2% ± 6.3%, n = 4) of CMV-specific CD4+CCR7− T cells in the patients with CMV primary infection was significantly higher (P = .005) than that observed in patients with chronic CMV infection (mean 1.3% ± 1.4%, n = 12). There were no differences (P = .1) in the percentage of HIV-1–specific CD4 T cells between patients with primary infections (0.49% ± 0.47%, n = 12) and those with chronic infections (0.25% ± 0.08%, n = 7).
Therefore, these results indicate that a major expansion of virus-specific CD4 T cells is associated with primary CMV but not with HIV-1 infection. The magnitude of primary HIV-1–specific CD4 T-cell response is indeed not significantly different from that observed during chronic infection. The differences in magnitude between primary and chronic HIV-1– and CMV-specific immune responses may translate into differences in effectiveness of the 2 antiviral responses in the control of virus replication. In this regard, it is important to underscore that CMV replication was efficiently (within 2 to 4 weeks) suppressed in the patients experiencing CMV primary infection even in the absence of specific anti-CMV therapy, and CMV viremia was rapidly below 10 DNA copies per milliliter of plasma (data not shown).
With regard to the antigen-specific proliferative responses, blood mononuclear cells were stimulated with p24 and p55 gag HIV-1 proteins and with CMV lysates. HIV-1–specific proliferative responses were rarely detected (2 of 13 patients) during primary infection (Table1). Similarly, CMV-specific proliferative responses were not detected in the 13 patients with primary HIV-1 infection (Table 1). The lack of detection of virus-specific proliferative responses during primary HIV-1 infection is not surprising. Previous studies performed by the Walker and McElrath groups3 14 have clearly demonstrated that these responses are suppressed during primary HIV-1 infection and are eventually restored only after early initiation of antiviral therapy and effective suppression of HIV-1 replication and resolution of the acute phase of infection.
Analysis of HIV-1 viral load in different populations of memory CD4 T cells
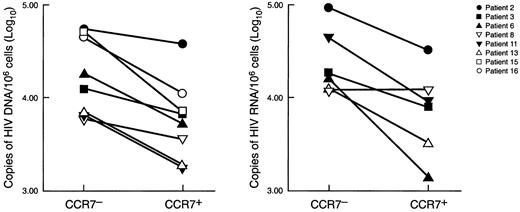
Blood CD4+CCR7− and CD4+CCR7+ T-cell populations were isolated by cell sorting and analyzed for cellular HIV-1 viral load. We found that a substantial proportion of CD4+CCR7− T cells were infected with HIV-1, as indicated by the presence of viral DNA and RNA (Figure 3). The levels of viral load were consistently found to be higher (in the range of 0.5 log for both HIV-1 DNA and RNA, P = .02) in the CD4+CCR7− cell population than in the CD4+CCR7+ cell subset.
Analysis of HIV-1 cellular viral load in different CD4 T-cell populations.
HIV-1 DNA and RNA viral load was determined in sorted CD4+CCR7− and CD4+CCR7+ T-cell populations isolated from patients with primary HIV-1 infection. HIV-1 DNA was determined in 8 patients and HIV-1 RNA in 6 patients. Data are expressed as copies (log10) of HIV-1 DNA and RNA per 106CD4+CCR7− and CD4+CCR7+ cells. The purity of the sorted populations was higher than 97%. Determination of HIV-1 DNA and RNA was performed as previously described.21
Analysis of HIV-1 cellular viral load in different CD4 T-cell populations.
HIV-1 DNA and RNA viral load was determined in sorted CD4+CCR7− and CD4+CCR7+ T-cell populations isolated from patients with primary HIV-1 infection. HIV-1 DNA was determined in 8 patients and HIV-1 RNA in 6 patients. Data are expressed as copies (log10) of HIV-1 DNA and RNA per 106CD4+CCR7− and CD4+CCR7+ cells. The purity of the sorted populations was higher than 97%. Determination of HIV-1 DNA and RNA was performed as previously described.21
Discussion
Previous studies of patients with chronic HIV-1 infection have shown the presence of HIV-1–specific CD4 T cells as measured by the secretion of IFN-γ following stimulation with p55 gag HIV-1 protein.5 The presence of IFN-γ–secreting CD4 T cells is generally not associated in chronic infection with the detection of proliferative responses.4 Similarly, proliferative responses as well as other antigen-specific CD4 T-cell responses are not detected during primary HIV-1 infection and are eventually rescued after antiviral treatment and effective suppression of virus replication.3,14 Primary CD4 T-cell responses have been only partially characterized3 14 and, more important, little is known about the composition of the pool of memory CD4 T cells.
In the present study, we have taken advantage of the recent characterization of the complexity of the antigen-specific memory CD4 T-cell response in HIV-1–negative healthy subjects17,18and the delineation of the memory CD8 T-cell response.16To assess the memory CD4 T-cell response, we used the expression of the chemokine receptor CCR7 as a tool to identify different populations of memory virus-specific CD4 T cells. With this strategy, we have demonstrated that the majority of CD4 T cells, as measured by the ability to secrete IFN-γ in response to stimulation with p55 gag, were contained within the CD4 T-cell population lacking CCR7. The finding that CD4+CCR7− T cells are able to secrete IFN-γ indicates that these cells potentially mediate effector function. These results are consistent with previous studies that have demonstrated that both antigen-specific CD4 and CD8 T cells with effector function, that is, IFN-γ–secreting cells, are contained within the memory CD4 T-cell population lacking CCR7.16-18Because HIV-1–specific IFN-γ–secreting CD4+CCR7− cells were found in both primary and chronic HIV-1 infection even in the absence of helper CD4 T cells, these findings indicate that HIV-1–specific CD4 T cells consist mostly of cells with effector function. This is certainly supported by the observation that HIV-1–specific CD4 T-cell proliferation is not restored in patients with chronic infection even after antiviral therapy and long-term suppression of HIV-1 replication.28 29
Furthermore, previous studies showed that CCR7− memory CD4 and CD8 T cells contain the long-lived memory T cells.17,18 Because almost the totality of HIV-1–specific IFN-γ–secreting CD4 T cells are CCR7−, this finding may explain why they may persist for long time during chronic infection. Interestingly, we have also shown that the majority of CD4+CCR7− cells expressed CCR5, the coreceptor for HIV-1,22 and the Ki67 nuclear antigen, a marker of cell division.24 The observation that a substantial proportion of CD4+CCR7− cells expressed Ki67 nuclear antigen can be explained on the basis of previous studies demonstrating that the massive T-cell proliferation observed in viral infections is the result of a bystander cell activation driven by cytokines rather than a T-cell receptor, eg, antigen-specific, mediated phenomenon.30 Therefore, the results in the present study provide substantial advancement in the characterization of the pool HIV-1–specific memory CD4 T-cell responses in primary and chronic infection.
Four of 16 patients experienced primary HIV-1 and CMV infections, as is unequivocally shown by the pattern of the CMV antibody response (Table2). The results show a substantial difference in the magnitude of the effector HIV-1–specific T-cell response (significantly lower) compared with the CMV-specific CD4 T-cell response. However, as mentioned above, the possibility cannot be ruled out that these differences resulted from the different types of antigen stimulation used for CMV (eg, viral lysates) and HIV-1 (eg, p55 or gp160 proteins). Recent studies have indicated that stimulation with viral lysates may be more efficient than stimulation with proteins.25 There was no evidence for an expansion of effector HIV-1–specific CD4 T cells during primary infection, since their percentage was comparable to that found during chronic infection. In contrast, the pool of effector CMV-specific CD4 T cells was significantly expanded during primary CMV infection.
These results raise the issue of the potential mechanisms responsible for the observed lack of expansion of HIV-1–specific CD4 T cells during the primary immune response. One potential mechanism may be related to poor immunogenicity of HIV-1, which, in turn, may result in a weak CD4 T-cell immune response. This explanation, however, is not supported by the massive expansion of HIV-1–specific CD8 T cells invariably associated with primary HIV-1 infection.31 Another possibility is that HIV-1–specific CD4 T cells are accumulated in the lymphoid tissue. Previous studies have shown, however, that there is no evidence for accumulation in the lymphoid tissue of HIV-1–specific CD8 T cells at the time of primary infection.32 A more likely hypothesis is that a larger proportion of effector HIV-1–specific CD4 T cells are infected with HIV-1 during primary infection. This hypothesis was consistent with the finding that a substantial proportion of HIV-1–specific CD4 T cells were contained within the CCR7− T-cell populations (Figure2 and Table 1) and that a large percentage of CD4+CCR7− cells are CCR5+ and Ki67+ (Figure 1). Therefore, the expression of the main coreceptor for HIV-1 and the cycling ability may potentially render CD4+CCR7− cells highly susceptible targets to infection with HIV-1.
The differences in viral load between the 2 cell populations appear too small to propose that one population may serve as the preferential reservoir for HIV-1. However, these results certainly provide evidence that a large proportion of CD4+CCR7− T cells, which contain the majority of HIV-1–specific CD4 T cells, are infected with HIV-1 during primary infection. Ideally, the assessment of HIV-1 viral load would have been performed on purified HIV-1–specific effector CD4 T cells. However, technical constraints associated with fixation of cells to detect IFN-γ secretion may significantly impair the efficiency of DNA and RNA amplification. Even in the absence of direct information on the HIV-1 viral load in the HIV-1–specific effector CD4 T cells, the finding of wide spreading of HIV-1 within the CD4+CCR7− cell population supports the possibility that HIV-1–specific CD4 T cells may become infected and be rapidly eliminated.
On the basis of the data from the present study, the likely scenario is that the expansion of effector HIV-1–specific CD4 T cells during primary infection is aborted by the infection of a large proportion of virus-specific CD4 T cells. Interestingly, HIV-1–specific CD4 T cells are depleted preferentially, because HIV-1 infection does not seem to prevent the expansion of CMV-specific CD4 T cells. However, 2 recent studies in mice have clearly demonstrated that both CD4 and CD8 T cells with effector function accumulate in the target organ of the pathogen.33,34 The target organ of HIV-1 is the lymphoid tissue.35 Thus HIV-1–specific CD4 T cells are preferentially eliminated, since they mediate their effector function in the anatomic site that serves as the primary site of virus replication.35
Lungs, salivary glands, cervix, and/or retina are the target organs of CMV. Therefore, on the basis of the studies in mice we would expect a substantial proportion of HIV-1–specific CD4 and CD8 T cells with effector function to accumulate in the lymphoid tissue (eg, lymph nodes), whereas CMV-specific CD4 and CD8 T cells with effector function should accumulate away from the lymphoid tissue. Therefore, the likely reason CMV-specific CD4 T cells can expand is because they will rapidly exit from the lymphoid tissue to migrate to the anatomic sites where CMV replicates (lungs, salivary glands, cervix, and/or retina) and therefore will escape HIV infection. In this regard, we recently compared the distribution of HIV- and CMV-specific CD4 and CD8 T cells in blood and lymph nodes of HIV-1– and CMV-coinfected individuals with chronic infection (all the patients studied had CD4 T-cell counts > 500/μL and were naive to antiretroviral therapy). This analysis clearly demonstrated that a very low proportion of CMV-specific CD4 and CD8 T lymphocytes reside in the lymph nodes as compared with the blood (the frequency of CMV-specific T cells was at least 1 log higher in blood).35 In contrast, the distribution of HIV-specific CD4 and CD8 T cells was almost identical between blood and lymph nodes.36
These results further support the critical role played by CD4 T cells in antiviral immune response,1-13 provide advances in the delineation of virus-specific memory CD4 T-cell responses and in the potential mechanisms of the defect of the CD4 T-cell response in HIV-1 infection, and reinforce the importance of the initiation of antiviral therapy in primary HIV-1 infection to preserve virus-specific CD4 T-cell immune response.3
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2001-11-0080.
Supported by research grants from the Swiss National Foundation (3100-66788-01) and from the National Institutes of Health (001 A1-G1535)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giuseppe Pantaleo, Laboratory of AIDS Immunopathogenesis, Department of Medicine, Centre Hospitalier Universitaire Vaudois, University of Lausanne, 1011 Lausanne, Switzerland; e-mail: giuseppe.pantaleo@chuv.hospvd.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal